Abstract
This work attempts to shed light on whether the COVID-19 pandemic rides on airborne pollution. In particular, a two-city study provides evidence that PM2.5 contributes to the timing and severity of the epidemic, without adjustment for confounders. The publicly available data of deaths between March and October 2020, updated it on May 30, 2021, and the average seasonal concentrations of PM2.5 pollution over the previous years in Thessaloniki, the second-largest city of Greece, were investigated. It was found that changes in coronavirus-related deaths follow changes in air pollution and that the correlation between the two data sets is maximized at the lag time of one month. Similar data from Tehran were gathered for comparison. The results of this study underscore that it is possible, if not likely, that pollution nanoparticles are related to COVID-19 fatalities (Granger causality, p < 0.05), contributing to the understanding of the environmental impact on pandemics.
Keywords: Particulate matter, Iron-bearing nanoparticles, Airborne, COVID-19, Pandemics
Graphical abstract
1. Introduction
Direct person-to-person transmission, which generally requires prolonged face-to-face or other close contact, is believed to be the most common route for the spread of infectious diseases, although airborne transmission over longer distances cannot be ruled out (Al Huraimel et al., 2020; Wang et al., 2021). This is in addition to the significant public health threats posed by air pollution, especially the fine <2.5 μm particulate matter (PM2.5) which, due to their small dimensions, can penetrate deep into the lungs and access the brain and the cardiovascular system, causing oxidative stress and inflammation (Cachon et al., 2014). Interestingly, this somehow parallels the symptoms of SARS-CoV-2 infection in humans. In this regard, the COVID-19 pandemic has offered the opportunity to conduct an intensive examination of virus transmission through PM carriers, also considering the real world features, which has come with many surprises. For example, despite the fact that nearly one in four individuals in India tested positive for antibodies to SARS-CoV-2 in December 2020 (Murhekar et al., 2021), and about 10% of India's population were vaccinated at the time, the extraordinary surge in cases from March 2021 onwards took most analysts off guard, as everyone thought India was doing well and would escape a deadly second wave. On the contrary, based on environmental pollution facts it was expected that “(in India) the number of daily new deaths will peak again during the turning of the year” as mentioned in a preprint on December 11, 2020 [see supplemental file in (Martinez-Boubeta and Simeonidis, 2020). Sadly, the truth turned out to be as predicted. Hence, this paper revisits our previous discussion of the finding that air pollution Granger causes COVID-19 deaths.
In this context, a large body of literature has consistently shown an association between PM and an increase in the numbers of deaths from cardiopulmonary disease, especially among the elderly and those with comorbidities (Seaton et al., 1995), with more than 3 million mortalities per annum (Anenberg et al., 2010). It has been also suggested that air pollution is an important cofactor increasing the risk of mortality from coronavirus. For instance, analysis of the first severe acute respiratory syndrome coronavirus SARS-CoV-1 outcomes in 2003, demonstrated that in heavily polluted regions the risk of dying from the disease was >80% higher compared with areas with relatively clean air (Cui et al., 2003; Kan et al., 2005). Furthermore, a correlation -but not necessarily a causal link-between exposure to NO2 from the burning of fuel and COVID-19 cases has been reported (Ogen, 2020; Liang et al., 2020). Yet, those studies cannot rule out the possibility that NO2 is serving as a proxy for other PM vehicle pollution such as soot and metal particles. In this regard, we (Kermenidou et al., 2020) and others (Petrovský et al., 2013), have provided evidence for the ubiquitous presence of magnetite in PM. Iron oxide nanoparticles may pose a potential threat, as they have been frequently and consistently associated with reactive oxygen species (ROS) activity and inflammatory cytokine release (Saffari et al., 2014). Moreover, the increased mutagenic activity of PM extracts related to the presence of magnetite may have a great effect on virus infectivity (Morris et al., 1995). Iron overload is a risk factor for many viral infections (Drakesmith and Prentice, 2008), and probably one of the most important predisposing conditions for many co-morbidities associated with severe COVID-19 (Whiteside and Herndon, 2020). Thus, constituting a biologically plausible pathway through which airborne magnetite may impact SARS-CoV-2 transmission.
On the other hand, while there has been ample evidence for a relationship between long-term air pollution exposure and the severity of COVID-19 outcomes (Pozzer et al., 2020; Cole et al., 2020; Hou et al., 2021), one aspect that has yet to be described is the effect of seasonal variances on excess mortality from COVID-19. This is of paramount importance since reinfections by other seasonal coronaviruses occur most frequently every 12 months (Edridge et al., 2020), and SARS-CoV-2 might share this feature. Therefore, one key question that needs to be assessed is whether air pollution showing seasonal variations is capable of modulating COVID-19 severity in different regions.
On the heels of our previous article, here we aim to investigate the effects of seasonal exposure to PM2.5 on the impact of pandemic waves. To the best of our knowledge, it is the first evidence provided on the linkage between air pollution and the seasonal variability of COVID-19.
2. Methods
The major hypothesis of this work is that seasonal patterns of PM, along with certain climatic conditions, can be the main driver of the global pandemic. Consequently, the pandemic's impact was investigated from two perspectives, considering different seasonality and considering different polluted areas. First, we investigated qualitatively the spread of disease in a medium-sized city in Europe, using Thessaloniki, Greece, with over 1 million inhabitants, as an example. Since the effects of PM are related to their chemical composition and size, we focused on the magnetic properties of PM2.5. Next, a mathematical model was employed to quantify the association between the COVID-19 outbreaks and air pollution in Tehran, with a population above 10 million, the largest city in West Asia.
Estimation of exposure of inhabitants of Thessaloniki to airborne magnetite was determined by a combination of magnetic measurements and electron microscopy, as described in detail elsewhere (Kermenidou et al., 2020). Briefly, aerosol samples were collected on PTFE PM2.5 filters by a low-volume sampler. The filters were weighed before and after sampling using an analytical balance after stabilizing in constant temperature and humidity. Dust particles were characterized by scanning electron microscopy and the average elemental analysis was obtained by Energy Dispersive X-ray Spectroscopy. The filters were cut into pieces and some parts rinsed in a mixed solution of acetone and polyvinyl alcohol in order to collect the magnetic fraction by means of permanent magnets. Samples were drop casted onto a carbon coated copper grid for observation by high-resolution transmission electron microscopy (TEM). Quasi-static magnetic properties were measured using a superconducting quantum interference device. Seasonal arithmetic mean concentrations were calculated over the period from February 2015 to October 2018.
Because the composition and health risks of PM2.5 might vary widely in different geographical and climatic areas, a complimentary statistical analysis was performed on data from Tehran, based on Nabavi et al. (2019). The monthly pattern of PM2.5 mass concentrations during the period (2011–2016) was compared to confirmed deaths from COVID-19 in Iran, as reported by the “Worldometer” website, updated with daily frequency (https://www.worldometers., 2020). It must be said that epidemiological data were not available at city level. The time-series data were interpolated to evenly spaced observations (Dean and Dunsmuir, 2016). Lead-lag relationships were investigated by cross-correlation analysis of monthly data during the period from March to October. Granger causality was computed by testing the null hypotheses in the Free Statistics Software from Wessa.net (Wessa, 2016).
3. Results
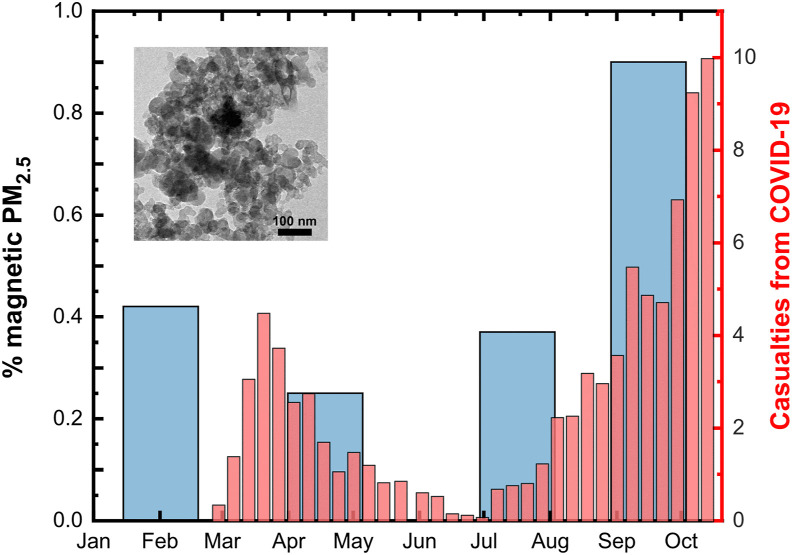
Magnetic traces were identified on in vivo human nasal swab specimens collected in late autumn 2019 from patients in the Otorhinolaryngology Clinic of the General Hospital “Papageorgiou” (Kermenidou et al., 2020). The magnetically responsive PM is made of aggregates in which particles with rounded morphology and a mean size around 15 nm (inset in Fig. 1 ) appear basically made of magnetite (and minor contribution of Fe3+ rich shell and substituted heavy metals such as Cr, Mn, Co or V).
Fig. 1.
The disease progression timeline and the seasonal variability of the airborne magnetite pollutant. The thin red bars represent the 7-period moving average for new daily confirmed deaths from COVID-19 in Greece. Note that there is about a four weeks delay from first symptoms after infection to death. The thick blue bars depict the percentage of magnetic material estimated from PM2.5 observations at urban site in Thessaloniki, representatively for the years 2015–2018. The inset shows a representative TEM image of the magnetic dust. We ascribe these particles to high-temperature anthropogenic processes, e.g. industrial and traffic sources, but can also result from natural fires, desert dust plumes and cosmic flux. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These are particles derived from combustion (Lighty et al., 2000), typically for relative humidity conditions below 65% (Willeke and Whitby, 1975), which, given their tiny size, remain airborne for a long time (Stadnytskyi et al., 2020), and can penetrate the respiratory tract down into the alveolar space (Tellier et al., 2019), where ACE2 is highly expressed. Thereby, magnetite could then tentatively offer a Trojan horse docking for virus infectivity, potentially facilitating its transmission efficiency (Godri Pollitt et al., 2020). In fact, recent studies revealed that iron oxide nanoparticles may interact efficiently with the SARS-CoV-2 spike binding proteins (Abo-zeid et al., 2020). In support of this hypothesis are findings that viable SARS-CoV-2 aerosols, from hospitals in Wuhan (China), were mainly found in the submicrometre <0.5 μm aerodynamic diameter range (Liu et al., 2020).
In this framework, it was analyzed whether the chronic seasonal exposure to airborne magnetite has positive qualitative effects on coronavirus mortality. Results for Greece are shown in Fig. 1. Visual inspection suggests that COVID-19 death rates, up to the end of October 2020, mimic the same lagged pattern in magnetic PM2.5 over the past years. Two main conclusions can be drawn from this chart: first, given the epidemiological situation, schools re-opening on 14th September brought a significant negative impact. The figure above (Fig. 1) shows that the country was making progress in bringing down the curve of new deaths from coronavirus until families returned to the big cities from their summer vacation and, consequently, air pollution peaked. Statistical and dynamic modelling approaches have shown that school closures can substantially help reduce the burden of viral diseases (Adda, 2016; Davies et al., 2021). This is consistent with reported increased risks of infection in households with children (Harris et al., 2021). It also adds to evidence that adolescents can seed clusters of COVID-19 cases (Schwartz et al., 2020).
Secondly, the air pollution conditions may had been favourable for the virus circulation since late September 2019, much earlier than the first reported cases. In this regard, we note that WHO declared the pandemic on March 11, 2020. Officially, a 38-year-old woman who returned to Thessaloniki from Milan by air on February 23, 2020 became Greece's first coronavirus. Though, a growing body of evidence suggests that the new SARS-CoV-2 virus had already been circulating unnoticed in the community a few months before the first reported case in Wuhan City, China, by December 2019 (van Dorp et al., 2020).
It is also worth noting that during the winter and spring times the mean PM2.5 concentration in Thessaloniki exceeds the European ambient air quality standard of 25 μg/m3 (Kermenidou et al., 2020). It would mean that the attributable fraction of COVID-19 mortality due to the long-term exposure to ambient fine particulate air pollution could be as high as ∼50% (Pozzer et al., 2020). Even though the present data must be interpreted with caution, the amount of airborne magnetite, which is at its peak during the autumn, might potentially explain the rise in the incidence of the second wave of the pandemic outbreak. Concomitantly, magnetic particulate matter shows maximum values during autumn months (0.8 % wt.), compared to <0.4% in spring, meaning that doubling the concentration of airborne pollutants leads to a ten-to 20-fold increase in the number of deaths linked to COVID-19. This goes in line with other studies, which have found that the association between the pollutant and the health outcome is log-linear (Schwartz et al., 2008; Borro et al., 2020; De Angelis et al., 2021). Consequently, our research strongly suggests that magnetic parameters can be used as an efficient proxy to assess the urban atmospheric quality and the impact of COVID-19 in Thessaloniki, and potentially in any other region in the world. Therefore, further investigations focusing on the development of COVID-19 outbreaks over highly polluted areas are encouraged.
In this respect, it should be noted that deaths from COVID-19 vary markedly across countries and at different paces. Changes in the geographical and temporal distribution of disease occurrence may occur as impact of both the concentration and composition of the PM2.5 mixture. For example, in a worldwide cross-sectional and longitudinal data analysis Damialis et al. (2021) hypothesized that airborne pollen concentrations could explain the SARS-CoV-2 infection rate variability, however, such correlations are only evident during springtime, when high concentrations of tree pollen occur, but cannot explain the several outbreaks in a year. It is most likely that the contents of water-soluble metal ions in PM2.5 compromise the viability of human lung cells by modifying the ROS and inflammatory cytokines response (e.g. representative TNF-α and IL-6) (Pang et al., 2020), thus facilitating the virus infection. A paradigmatic example is the occurrence of elevated PM2.5 levels over the Po Valley, Italy, for the 12-days period preceding the pandemic onset (De Angelis et al., 2021).
For the while let us, for the sake of History, focus on Iran since the ancient Persian and Greek cultures intermingled when Alexander the Great conquered most of that region by 330 BC. This may further be related to genomic results demonstrating similarities in the populations of Neolithic Iran-like ancestry and Helladic Bronze Age culture (Clemente et al., 2021). Interest arises from the fact that, when it comes to number of new deaths per day, the third wave of coronavirus in Iran is the worst yet (Fig. 2 ). But most importantly for our discussion is that the climate in Thessaloniki is warm and temperate (Köppen climate classification: Cfa) due to its proximity to the sea, while Tehran has a cold semi-arid steppe climate (Köppen climate classification: BSk), typically found at some distance from the sea. On this matter, Iran as a developing country is facing severe problems in terms of air quality. Urban air pollution due to PM2.5 in Tehran and other regions of the country has been reported by many researchers (Nabavi et al., 2019; FarajiGhasemi et al., 2020; Hadei et al., 2020; Zallaghi et al., 2020; Kermani et al., 2020). Accordingly, most cities in Iran have PM2.5 concentrations above the WHO air quality guideline value, which in turn are magnified by frequent desert dust storms during the summer months as temperatures rise and rainfall reaches a minimum (Shahsavani et al., 2020). However, the highest amounts of PM2.5 are normally recorded during winter. The increase of PM concentration in the cold period of the year is due not only to the increased emissions from additional sources, such as domestic heating, but rather also to the decrease in the thickness of the mixing layer (Murthy et al., 2019). These episodes may result in notable health consequences. As such, the highest number of deaths due to exposure to fine particles was estimated to occur in Iran's largest city, Tehran, where motor vehicles play a major role (Oroji et al., 2018; Broomandi et al., 2020). Other episodes that may be linked to human activity include the significant drop of PM in the spring caused by Tehran's minimal traffic flow during Nowruz holidays. Consequently, we assume that the mean seasonal concentrations of magnetite would roughly track the trends in PM2.5 levels.
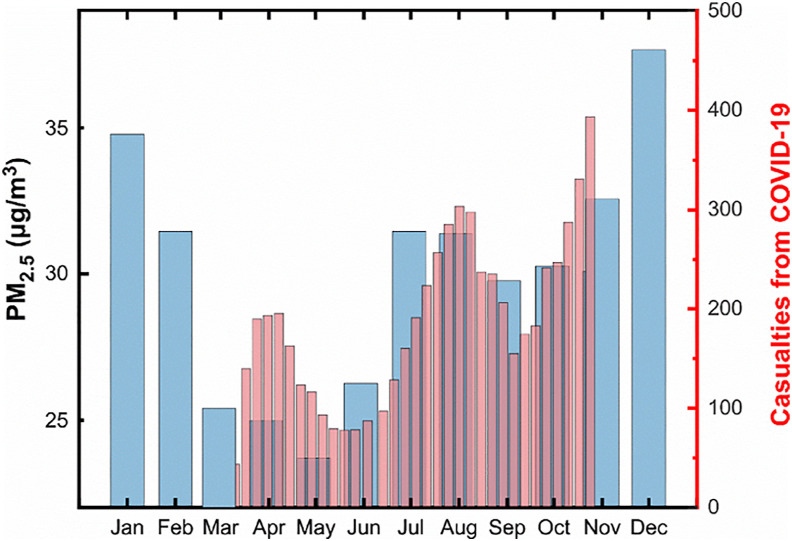
Fig. 2.
Two or more ‘camel humps' in Iran. The thick blue bars depict the monthly pattern of PM2.5 mass concentrations during the period (2011–2016) in Tehran. The thin red bars represent the 7-period moving average for new daily confirmed deaths from COVID-19 in Iran, as of October 28, 2020. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The figure above (Fig. 2) depicts that the coronavirus casualties follow the seasonal variation of air pollution closely, except that the PM2.5 waves start sometime early. This plot is of special interest since the data can be used to validate the causal association between pollution and COVID-19 severity. Techniques exist for establishing the direction of this association and have previously been used primarily in fields such as econometrics (Granger, 1988). For instance, to say “one variable X Granger causes another variable Y” means that past values of X contain information that helps better predict future Y rather than just using past values of Y. Therefore, the prediction of Y is significantly improved by including X as a predictor. Let Y = daily deaths be the potential outcome in the population of a country exposed to X = pollution concentration. The Granger causality was computed under the assumption that the null hypotheses of X does not Granger-cause Y, and vice versa, are true. The F-test and its associated p-value are presented in Table 1 .
Table 1.
Iranian values of the constructed proxies for PM2.5 pollution (X) and the health impact (Y) on COVID-19, and both cross-correlation and Granger causality summaries identifying significant causal relationships between X→Y. Significance level **p < 0.05.
| X | Y | lag | ρ(Y [t], X [t + lag]) |
|---|---|---|---|
| 25 | 65 | −4 | −0.12 |
| 23.7 | 140 | −3 | 0.10 |
| 26.3 | 74 | −2 | 0.53 |
| 31.5 | 98 | −1 | 0.71** |
| 31.4 | 199 | 0 | 0.51 |
| 29.8 | 223 | 1 | 0.16 |
| 30.3 | 159 | 2 | 0.04 |
| 30.1 | 262 | 3 | −0.27 |
| Granger-Causal | Y = f(X) | X = f(Y) | |
| F | 8.33 | 0.04 | |
| P | 0.04 | 0.86 | |
There is a remarkable correlation between the two datasets. Accepting a lag of one month, the correlation coefficient >0.7, which is reasonable given that there are significant delays between infection, the onset of symptomatic disease, and recovery or death. These estimates imply that people who died this week were most likely infected a month ago (Sanche et al., 2020). In the same fashion, it is assumed it would take a month before any effect of vaccination on deaths is observed (England and Public Health. I, 2020).
Moreover, this analysis suggests that the PM2.5 level is predictive of the subsequent SARS-CoV-2 related deaths: the F statistic was 8.33 and the p-value was 0.04. The null hypothesis that X does not cause Y can be rejected at the 5% significance level. On the other hand, the p-value of 0.86 allows us to accept the null for X = f(Y). The fact that the first hypothesis was rejected and the second was not, means that X can be used to forecast Y. Sensitivity analyses (not shown) using mid-month values on the 15th day, and log-transformed data, found little effect on results. Consequently, it is safe to conclude that a portion of the variability of the clinical outcomes of COVID-19 could be affected by environmentally driven variance of nasal infectivity (Hou et al., 2020).
4. Discussion
4.1. Virus impact and air pollution linkage
This finding is consistent with the result by Sharma et al. (2021), which states that pollutant PM2.5 exhibited a statistically significant impact on the Covid-19 deaths among the world's top 10 infected countries (including Brazil, Chile, India, Iran, Italy, Peru, Russia, Spain, the UK, and the USA), from February 1, 2020 through June 30, 2020, while our study includes a more extended timeline.
Moreover, our results indicate that there is a one-way link from PM2.5 concentrations to COVID-19 deaths, which is also supported by Delnevo et al. (2020) and Mele et al. (Mele and Magazzino, 2020), who reported that there is a Granger causal relationship between daily PM2.5 values and new daily COVID-19 infections in Emilia-Romagna, Italy, and a similar linkage between PM2.5 concentration and COVID-19 mortality in 25 cities in India, respectively. Another study in Peru shows the higher rates of spread of COVID-19 in Lima were associated with the increasing levels of exposure to PM2.5 in the previous years (2012–2016) (Vasquez-Apestegui et al., 2020).
Thus, the relationship to COVID-19 mortality in Iran is not unexpected, considering past evidence of association between patterns of PM2.5 mass concentrations and, for example, cases of cardiovascular mortality: a study of the 2008–2017 period showed that 2009 and 2010 were the most polluted and unhealthy years, the lowest being 2014, coinciding with the minimum and maximum of solar cycle 24, respectively (Zallaghi et al., 2020). Incidentally, note that a small amount of iron oxides arrives as cosmic flux (Kapper et al., 2020). In this regard, the dust concentration in ice cores retrieved from Greenland show a modulation with a period of about 11 years all the way back from at least 100,000 yrs BP, which points to the solar cycle (Ram et al., 1997). It is interesting to note that the idea that the Sun influences the weather has been discussed time and again for over a century (Meldrum, 1873). Likely, the solar cycle modulates the solar wind and affects, also, the rainfall and seasonal temperature patterns through alteration of atmospheric circulation on decadal timescales (Leamon et al., 2021), and, therefore, also the dust modulation. Indeed, a recent paper has linked the increasing levels of PM2.5 to the worst drought that has hit Taiwan in more than a half-century (Chen et al., 2021). Besides, analysis performed in the summer season during the period 2001–2012 revealed that the largest number of dust storms in southeastern Iran occurred in June 2008, attributed to the influence of specific meteorological conditions as the abnormal enhanced cyclonic circulation over northern Arabian Sea (Rashki et al., 2015). A further confirmation comes from the recent work by Cooper et al. (2021). In fact, both the COVID-19 pandemic and the new solar cycle 25 officially began in December 2019. It is to be seen whether the magnetic state of the Sun can affect PM2.5 trends and may play a role in pandemics (Hope-Simpson, 1978; Nicastro et al., 2020; Nasirpour et al., 2021). Although the exact nature of the link cannot be established at present, additional studies are needed to reject the coincidence entirely (Towers, 2017).
4.2. Possible mechanisms
While our study supports a link between a month-lag PM2.5 and COVID-19, the mechanisms underlying this relationship are complex, and beyond the scope of this paper. Several studies have previously found similar effects of PM on infectious diseases such as influenza (Chen et al., 2018) and coronavirus outbreaks (Villeneuve and Goldberg, 2020). In a recent review, Domingo et al. (2020) have collected evidence that supports a clear association between concentrations of various air pollutants and the airborne transmission of SARS-CoV-2 and the severity of disease outbreaks. Enhanced persistence of the virus in the air, promotion of a pro-inflammatory state, and increased expression of the viral receptor ACE-2, are all factors that have been proposed as possible links between air pollution and COVID-19 (Borro et al., 2020). Indeed, Watzky, et al. (Watzky et al., 2021) have recently found that many chemicals in PM can regulate ACE2 expression, which is important for SARS-CoV-2 entry into the cells. In parallel, there is consistent, strong evidence that the airborne route is likely the dominant mode of transmission for SARS-CoV-2 (Greenhalgh et al., 2021). Consequently, several studies have been reported that explored the presence of SARS-CoV-2 in the air, both in hospital (Zhou et al., 2020; Chia et al., 2020) and non-medical environments. In particular, Setti et al. (2020) analyzed the outdoor open-air from streets in Bergamo, Italy. In contrast, Hadei et al. (2020) detected viral RNA associated with ambient indoor PM in Tehran's public places and transport vehicles. Both may give support to our findings. Even so, Belosi et al. (2021) estimated very low (<1 RNA copy/m3) average outdoor concentrations of SARS-CoV-2 in Lombardia, which means a very low probability of airborne transmission provided that large gatherings of people are avoided, though they admit that it could be more relevant for indoor environments due to the combination of virus-laden aerosols and nanometric PM (around 0.01 μm in diameter, like in our case). This parallels the well-established pathogenic role of the microbial component of PM (see below) (Griffin, 2007).
By exploiting data from two countries, which differ in several respects, it was possible to successively infer the potential link. But the association of pandemic outbreaks with different PM seasonality in various regions of the world does not prove that PM alone is necessarily the mediating mechanism in COVID-19 lethality. It is possible that these trends are also correlated with other atmospheric variables (Paraskevis et al., 2021), which leaves the possibility of confounding (Ito et al., 2007). For example, Wang and Wang (2021) founded a nonlinear relationship between COVID-19 and air pollutants, and the temperature has a significant impact on the correlation between these two variables. In this regard, it has been speculated that cool and dry weather contributes to the transmission of the Covid-19 pandemic, as it influences socialising patterns and encourages indoor activities (Sharma et al., 2021). At the same time, the relative humidity of the environment has been shown to have a dramatic effect on respiratory droplet transport and evaporation (Dbouk and Drikakis, 2021; Brain and Valberg, 1979). And it was previously suggested that the combination of temperature and humidity modulates the virus survival, transmission and seasonality (Marr et al., 2019). These conditions are particularly dangerous at under 60% humidity and temperatures around 20 °C, also typical for indoors (Biryukov et al., 2020). In addition, sunlight can induce anoxic conditions that stabilize ROS in iron-containing organic-rich aerosol particles, thus affecting the lifetimes of aerosol-bound pathogens and their health-related impacts (Alpert et al., 2021). Also consistent with our findings is the report by Karimi et al. (2020) on the seasonal concentrations of airborne biome and associated PM2.5 in Isfahan, the third largest city in Iran. All of these together provide a sound explanation for the positive (negative) relationship between PM2.5 concentration with air temperature and relative humidity (the presence of clouds, precipitation, wind speed and UV radiation) observed at Isfahan from March 2019 to March 2020 (Kermani et al., 2020). Correspondingly, the presence of magnetite in PM extracts has been previously shown to strongly correlate also with meteorological data, in particular, the number of sunny hours and the relative humidity (Muxworthy et al., 2001).
On the other hand, Pauluhn and Wiemann (2011) showed that the repeated inhalation exposure to iron oxides cause nonspecific pulmonary inflammation as well as ferritin protein expression, which shows a clear dependence on the particle overload. This is the result of the degradation of magnetic nanoparticles and the natural strategy to limit the toxicity of free iron ions (Kolosnjaj-Tabi et al., 2016), which may act as trigger for infection. In this regard, magnetic characterization data cited in our previous publication can be used to estimate in several μg per day the airborne magnetite inhaled dose (Kermenidou et al., 2020). Though, note that PM inhaled dose is a function of both pollutant concentration and the inhaled volume of air, which depends on individual characteristics, such as age, sex and physical activity (Greenwald et al., 2019). The possibility that elevated iron status aggravates virus infections (e.g. HIV-1 and hepatitis C) is reviewed in Drakesmith and Prentice (2008). Evidence shows that inflammation, oxidative stress and altered iron homeostasis are linked (Orino et al., 2001), and play a potential role in the pathogenesis of COVID-19 (Edeas et al., 2020). This notion is supported by the fact that COVID-19 patients with elevated levels of ferritin are much more likely to experience severe symptoms and possibly increased mortality rates (Casas Rojo et al., 2020; Shoenfeld, 2020; Chaudhary et al., 2021; Burugu et al., 2020; Perricone et al., 2020; Habib et al., 2021). Therefore, it is envisaged that magnetite in PM could participate in most of the typical complications caused by COVID‐19.
4.3. Limitations of analysis
Nonetheless, the present study has several limitations that should be considered when interpreting its findings. First, our modelling of ‘Granger-Causality’ is designed to handle pairs of variables, in which only linear relationships between predictor (pollution exposure) and target variable (casualties) are considered, despite associations between air pollution and health outcome are rarely linear. In addition, it may suffer an inherent limitation if a third variable affects both the exposure and the outcome. For example, climatic factors (such as temperature, rainfall and prevailing winds) exert their effects on the vertical profiles of air pollutants, but also modulates human activities and would subsequently impact the relationship between PM and the spread of infections. As mentioned above, despite the role of the meteorological parameters is quite evident in this study, these have not been quantified. Also, we were unable to assess other important potential cofounders; for example, De Angelis et al. (De Angelis et al., 2021) concluded that pandemic's diffusion patterns and mortality are influenced by a multiplicity of environmental, social and economic indicators, to which other researchers also added dietary (Perez-Araluce et al., 2021) and genetic factors (Vietzen et al., 2021). In particular, Bontempi et al. (Bontempi et al., 2020, 2021; Bontempi and Coccia, 2021) provide further insights into the interplay between international trade data and the impact of COVID-19 in society. In this context, it is important to remember that characteristics that can stand in for environmental-to-human proxies (e.g. data on air pollution) can always be associated with other determinants related to person-to-person transmission; for instance, economic factors, such as the import and export transactions to and from big cities with high population density and intensive transport, can be seen as a reflection of social interactions that leads, concurrently, to higher pollution.
A second limitation of this study is missing data. On the one hand, the detailed identification of the mechanisms underlying this effect is limited by air quality measurement protocols, since to date ultrafine particles (<100 nm) and the occurrence of magnetite in the atmosphere are neither monitored nor regulated (Maher et al., 2020). Another limitation is the spatial resolution of COVID-19 cases, as it was mostly not available at the city level, and inference of the epidemic dynamics at the nationwide level may be misleading and should be taken with care. Furthermore, COVID-19 deaths are probably underreported in almost every country (Institute for Health Metr, 2021). Despite its limitations, reported deaths are likely to be more reliable than new case data. Given the above considerations, future studies are needed involving a larger number of observations.
Indeed, final mention must go to the fact that our results are provisional, based on epidemiological data collected up to the beginning of November 2020, and a comprehensive evaluation will need to follow after the COVID-19 pandemic. These limitations notwithstanding, at the time of revisiting this manuscript -early June 2021- it seems that mortality rates trends follow the same pattern as in the previous year despite the many restrictive measures (masking, vaccines, lockdowns, etc.) put in place in response to the COVID-19 crisis. Indeed, it was widely suggested that staying at home policy did not play a dominant role in reducing COVID-19 transmission (Savaris et al., 2021; Boretti, 2020), nor did it significantly influence PM2.5 pollution (Broomandi et al., 2020).
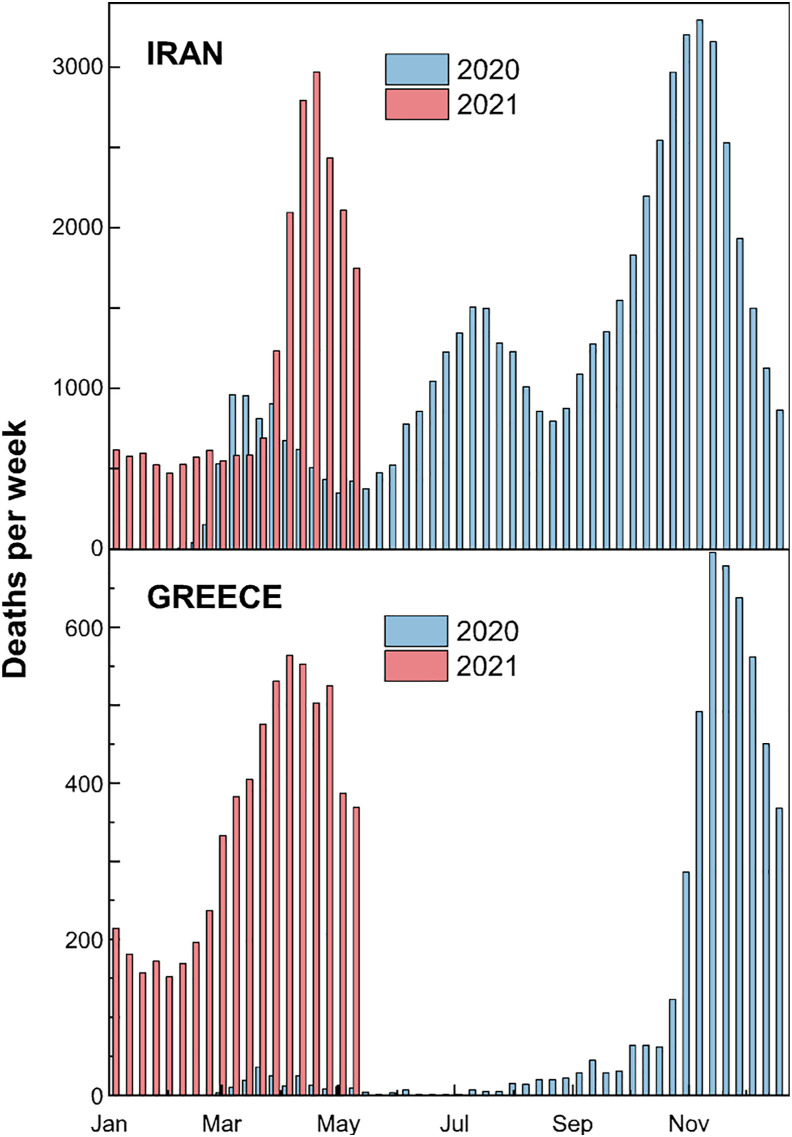
Below, the number of weekly deaths in 2020 is compared with the ongoing pandemic wave (Fig. 3 ). It suggests that the relationship between PM level and pandemic mortality that we observe is maintained over time. In fact, recent literature has already proposed the use of air pollution data in 2018 to separate the effect of human mobility and control measures in the COVID-19 pandemic during 2020 (Coccia, 2021). In this regard, Edridge et al. (2020) detected evidence that protection against reinfection by all human coronaviruses declined within a year. Obviously, for each epidemic wave, the growth and decay regimes depend on the baseline incidence and the implementation of various lockdown restrictions. However, country-to-country discrepancies in the wave's characteristics are likely due to geographic heterogeneity, being some regions (Greece) in temperate climate with distinct fall and spring blooms, while others (Iran) located in the subtropical region suffer also occasional summer outbreaks. For example, the summer outbreak in Iran (not observed in Greece) from June to September corresponds, and it is at the time when the concentration of PM and bacterial colonies are most prevalent in the ambient air of Ilam, in the western part of the country, due to dust events (Amarloei et al., 2020). It is also very interesting to highlight that the most severe dust transport episodes in Greece usually appear during the spring and autumn, which parallels more or less the advent of COVID-19, while minimum values are observed in the summer due to the prevailing northerly wind patterns (Mitsakou et al., 2008). Another telling example is Saudi Arabia, a country with a hot climate, which has been characterized so far by a single wave per year (Ben Maatoug et al., 2021; https://www.worldometers., 2021). In this regard, a hypothesis has been set that desert dust intrusions have modulated the spreading and virulence of COVID-19 (Rohrer et al., 2020). Those dust outbreaks come loaded with bioaerosols (Griffin, 2007; Polymenakou et al., 2008; Gorbushina et al., 2007; Hu et al., 2020), which could possibly have a synergistic impact on human health. And they are aligned with regional weather regimes (as defined e.g. by the summer North Atlantic Oscillation index (Salvador et al., 2014)) that are suspected –again- to be globally coupled to solar activity (Laken and Stordal, 2016).
Fig. 3.
The weekly counts in 2020 (blue bars) are compared to deaths for the same weeks in 2021 (red bars). Top: In the Islamic Republic of Iran, as of May 24, 2021, there have been 2.855.396 confirmed cases of COVID-19 with 79.056 deaths, and 3.141.577 vaccine doses have been administered (https://covid19.who.int/region/emro/country/ir). Bottom: In Greece, from January 3, 2020 to May 16, 2021, there have been 389.804 confirmed cases of COVID-19 with 11.772 deaths, reported to WHO (https://covid19.who.int/region/euro/country/gr). A total of 4.337.001 vaccine doses have been administered. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In contrast, we note that mathematical models of SARS-CoV-2 transmission assume that physical distancing can mitigate pandemics and prevent successive waves, however, once these social measures are reduced, the case-fatality rate will again increase. In fact, using one such model to describe the propagation of COVID-19 in Spain resulted in disparate predictions (Castro et al., 2020). Similar attempts in the Republic of Korea have not really grasped the timing of outbreaks (Kim et al., 2020), leaving us with the impression that the dynamics of the COVID-19 pandemic may be essentially unpredictable. In this sense, we believe patterns of air pollution may further assist in forecasting.
In summary, our analysis identifies that exposure to PM2.5 in excess have a significant impact on the development of lethal SARS-CoV-2 infections and provides a quantitative prediction of the link between airborne toxic metals, and concentrations during the previous month, on increased rates of mortality. These findings not only explain large parts of regional and seasonal differences in death rates from COVID-19 but also confirm those of many very recent contributions and call for policies aimed at a rapid reduction in air pollution. It is to be seen whether our initial conclusions remain appropriate and would be relevant to the public health response to future outbreaks.
Credit author statement
C.M.-B. did the conceptualization of the work, the design of the methodology, data curation and evaluation and the writing of the draft. K.S. supported experimental section as well as writing, review and editing of the manuscript.
Contributors
Both authors conceived of the idea for the study. Both authors designed the study. CMB conducted data analysis and drafted the manuscript, which KS critically reviewed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent for publication
Not required.
Provenance and peer review
Not commissioned; externally peer reviewed.
Data availability statement
All data are available from public repositories, in particular the Worldometers Database (https://www.worldometers.info/coronavirus), referenced studies and/or from the corresponding author on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the collaboration of Diego Martinez from DMcreatividad (https://www.dmcreatividad.com) for his contribution to the Graphical Abstract.
References
- Abo-zeid Y., Ismail N.S., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharmaceut. Sci. 2020 doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adda J. Economic activity and the spread of viral diseases: evidence from high frequency data. Q. J. Econ. 2016 doi: 10.1093/qje/qjw005. [DOI] [Google Scholar]
- Al Huraimel K., Alhosani M., Kunhabdulla S., Stietiya M.H. SARS-CoV-2 in the environment: modes of transmission, early detection and potential role of pollution. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert P.A., Dou J., Corral Arroyo P., et al. Photolytic radical persistence due to anoxia in viscous aerosol particles. Nat. Commun. 2021 doi: 10.1038/s41467-021-21913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarloei A., Fazlzadeh M., Jafari A.J., Zarei A., Mazloomi S. Particulate matters and bioaerosols during Middle East dust storms events in Ilam, Iran. Microchem. J. 2020 doi: 10.1016/j.microc.2019.104280. [DOI] [Google Scholar]
- Anenberg S.C., Horowitz L.W., Tong D.Q., West J.J. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ. Health Perspect. 2010 doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosi F., Conte M., Gianelle V., Santachiara G., Contini D. On the concentration of SARS-CoV-2 in outdoor air and the interaction with pre-existing atmospheric particles. Environ. Res. 2021 doi: 10.1016/j.envres.2020.110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Maatoug A., Triki M.B., Fazel H. How do air pollution and meteorological parameters contribute to the spread of COVID-19 in Saudi Arabia? Environ. Sci. Pollut. Res. Int. 2021 doi: 10.1007/s11356-021-13582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov J., Boydston J.A., Dunning R.A., et al. mSphere; 2020. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M. International trade as critical parameter of COVID-19 spread that outclasses demographic, economic, environmental, and pollution factors. Environ. Res. 2021 doi: 10.1016/j.envres.2021.111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Vergalli S., Squazzoni F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M., Vergalli S., Zanolettia A. Can commercial trade represent the main indicator of the COVID-19 diffusion due to human-to-human interactions? A comparative analysis between Italy, France, and Spain. Environ. Res. 2021 doi: 10.1016/j.envres.2021.111529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretti A. After less than 2 months, the simulations that drove the world to strict lockdown appear to be wrong, the same of the policies they generated. Health Serv. Res. Manag. Epidemiol. 2020 doi: 10.1177/2333392820932324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borro M., Di Girolamo P., Gentile G., et al. Evidence-based considerations exploring relations between SARS-CoV-2 pandemic and air pollution: involvement of PM2.5-mediated up-regulation of the viral receptor ACE-2. Int. J. Environ. Res. Publ. Health. 2020 doi: 10.3390/ijerph17155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain J.D., Valberg P.A. Deposition of aerosol in the respiratory tract. Am. Rev. Respir. Dis. 1979 doi: 10.1164/arrd.1979.120.6.1325. [DOI] [PubMed] [Google Scholar]
- Broomandi P., Karaca F., Nikfal A., et al. Impact of COVID-19 event on the air quality in Iran. Aerosol Air Qual. Res. 2020 doi: 10.4209/aaqr.2020.05.0205. [DOI] [Google Scholar]
- Burugu H.R., Kandi V., Kutikuppala L., Suvvari T.K. Activities of serum ferritin and treatment outcomes among COVID-19 patients treated with vitamin C and dexamethasone: an uncontrolled single-center observational study. Cureus. 2020 doi: 10.7759/cureus.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachon B.F., Firmin S., Verdin A., et al. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM2.5 and PM>2.5) collected from Cotonou. Benin. Environ. Pollut. 2014 doi: 10.1016/j.envpol.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Casas Rojo J.M., Antón Santos J.M., Millán-Núñez-Cortés J., et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMI-COVID-19 Registry. Rev. Clínica Española. 2020 doi: 10.1016/j.rceng.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M., Ares S., Cuesta J.A., Manrubia S. The turning point and end of an expanding epidemic cannot be precisely forecast. Proc. Natl. Acad. Sci. U.S.A. 2020 doi: 10.1073/pnas.2007868117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary R., Garg J., Houghton D.E., et al. Thrombo-inflammatory biomarkers in COVID-19: systematic review and meta-analysis of 17,052 patients. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021 doi: 10.1016/j.mayocpiqo.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.W., Hsieh Y.H., Su H.C., Wu J.J. Causality test of ambient fine particles and human influenza in Taiwan: age group-specific disparity and geographic heterogeneity. Environ. Int. 2018 doi: 10.1016/j.envint.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Wang S.-H., Min Q., et al. Aerosol impacts on warm-cloud microphysics and drizzle in a moderately polluted environment. Atmos. Chem. Phys. 2021 doi: 10.5194/acp-21-4487-2021. [DOI] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020 doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente F., Unterländer M., Dolgova O., et al. The genomic history of the Aegean palatial civilizations. Cell. 2021 doi: 10.1016/j.cell.2021.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the dejà vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-020-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.A., Ozgen C., Strobl E. IZA Institute of Labor Economics; 2020. Air Pollution Exposure and COVID-19.https://www.iza.org/publications/dp/13367/air-pollution-exposure-and-covid-19 DP No. 13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A., Turney C.S.M., Palmer J., et al. A global environmental crisis 42,000 years ago. Science. 2021 doi: 10.1126/science.abb8677. [DOI] [PubMed] [Google Scholar]
- Cui Y., Zhang Z.-F., Froines J., Zhao J., Wang H., Yu S.-Z., Detels R. Air pollution and case fatality of SARS in the People's Republic of China: an ecologic study. Environ. Health. 2003 doi: 10.1186/1476-069x-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damialis A., Gilles S., Sofiev M., et al. Higher airborne pollen concentrations correlated with increased SARS-CoV-2 infection rates, as evidenced from 31 countries across the globe. Proc. Natl. Acad. Sci. U.S.A. 2021 doi: 10.1073/pnas.2019034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk T., Drikakis D. Fluid dynamics and epidemiology: seasonality and transmission dynamics. Phys. Fluids. 2021 doi: 10.1063/5.0037640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis E., Renzetti S., Volta M., et al. COVID-19 incidence and mortality in Lombardy, Italy: an ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021 doi: 10.1016/j.envres.2021.110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R.T., Dunsmuir W.T.M. Dangers and uses of cross-correlation in analyzing time series in perception, performance, movement, and neuroscience: the importance of constructing transfer function autoregressive models. Behav. Res. Methods. 2016 doi: 10.3758/s13428-015-0611-2. [DOI] [PubMed] [Google Scholar]
- Delnevo G., Mirri S., Roccetti M. Particulate matter and COVID-19 disease diffusion in emilia-romagna (Italy). Already a cold case? Computation. 2020. [DOI]
- Domingo J.L., Marquès M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008 doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- Edeas M., Saleh J., Peyssonnaux c. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edridge A.W.D., Kaczorowska J., Hoste A.C.R., et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020 doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- England, Public Health Impact of COVID-19 vaccines on mortality in england, december 2020 to March 2021. https://www.gov.uk/government/publications/phe-monitoring-of-the-effectiveness-of-covid-19-vaccination Available from.
- Faraji Ghasemi F., Dobaradaran S., Saeedi R., et al. Levels and ecological and health risk assessment of PM2.5-bound heavy metals in the northern part of the Persian Gulf. Environ. Sci. Pol. 2020 doi: 10.1007/s11356-019-07272-7. [DOI] [PubMed] [Google Scholar]
- Godri Pollitt K.J., Peccia J., Ko A.I., et al. COVID-19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Hum. Genom. 2020 doi: 10.1186/s40246-020-00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbushina A.A., Kort R., Schulte A., et al. Life in Darwin's dust: intercontinental transport and survival of microbes in the nineteenth century. Environ. Microbiol. 2007 doi: 10.1111/j.1462-2920.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Granger C.W.J. Some recent development in a concept of causality. J. Econom. 1988 doi: 10.1016/0304-4076(88)90045-0. [DOI] [Google Scholar]
- Greenhalgh T., Jimenez J.L., Prather, et al. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021 doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R., Hayat M.J., Dons E., et al. Estimating minute ventilation and air pollution inhaled dose using heart rate, breath frequency, age, sex and forced vital capacity: a pooled-data analysis. PloS One. 2019 doi: 10.1371/journal.pone.0218673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007 doi: 10.1128/CMR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib H.M., Ibrahim S., Zaim A., Ibrahim W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021 doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadei M., Shahsavani A., Krzyzanowski M., et al. Burden of mortality attributed to PM2.5 exposure in cities of Iran; contribution of short-term pollution peaks. Atmos. Environ. 2020 doi: 10.1016/j.atmosenv.2020.117365. [DOI] [Google Scholar]
- Harris R.J., Hall J.A., Zaidi A., et al. Impact of vaccination on household transmission of SARS-COV-2 in England. 2021. https://www.gov.uk/government/news/one-dose-of-covid-19-vaccine-can-cut-household-transmission-by-up-to-half Available from. [DOI] [PMC free article] [PubMed]
- Hope-Simpson R. Sunspots, flu: a correlation Nature. 1978 doi: 10.1038/275086a0. [DOI] [Google Scholar]
- Hou Y.J., Okuda K., Edwards C.E., et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020 doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Qin Y., Wang G., Liu Q., Yang X., Wang H. Impact of a long‐term air pollution exposure on the case fatality rate of COVID‐19 patients—a multicity study. J. Med. Virol. 2021 doi: 10.1002/jmv.26807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.worldometers.info/coronavirus/country/iran, last retrieved on October 28th 2020. The same applies to data from Greece, https://www.worldometers.info/coronavirus/country/greece.
- https://www.worldometers.info/coronavirus/country/saudi-arabia, last retrieved on August 4th 2021.
- Hu W., Murata K., Fan C., et al. Abundance and viability of particle-attached and free-floating bacteria in dusty and nondusty air. Biogeosciences. 2020 doi: 10.5194/bg-17-4477-2020. [DOI] [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME) May 6, 2021. http://www.healthdata.org/node/8660 Available from.
- Ito K., Thurston G.D., Silverman R.A. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J. Expo. Sci. Environ. Epidemiol. 2007 doi: 10.1038/sj.jes.7500627. [DOI] [PubMed] [Google Scholar]
- Kan H.D., Chen B.H., Fu C.W., Yu S.Z., Mu L.N. Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomed. Environ. Sci. 2005 [PubMed] [Google Scholar]
- Kapper K.L., Bautista F., Goguitchaishvili A., Bógalo M.F., Cejudo-Ruíz R., Solano M.C. The use and misuse of magnetic methods to monitor environmental pollution in urban areas. Bol. Soc. Geol. Mex. 2020 doi: 10.18268/BSGM2018v72n1a111219. [DOI] [Google Scholar]
- Karimi H., Nikaeen M., Gholipour S., et al. PM2.5-associated bacteria in ambient air: is PM2.5 exposure associated with the acquisition of community-acquired staphylococcal infections? J. Environ. Health Sci. Eng. 2020 doi: 10.1007/s40201-020-00522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani M., Jonidi Jafari A., Gholami M., et al. Investigation of relationship between particulate matter (PM2.5) and meteorological parameters in Isfahan, Iran. J. Air Pollut. Health. 2020 doi: 10.18502/japh.v5i2.4238. [DOI] [Google Scholar]
- Kermenidou M., Balcells Ll, Martinez-Boubeta C., et al. Magnetic nanoparticles: an indicator of health risks related to anthropogenic airborne particulate matter. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.116309. [DOI] [PubMed] [Google Scholar]
- Kim S., Ko Y., Kim Y.-J., Jung E. The impact of social distancing and public behavior changes on COVID-19 transmission dynamics in the Republic of Korea. PLoS ONE. 2020 doi: 10.1371/journal.pone.0238684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosnjaj-Tabi J., Lartigue L., Javed Y., et al. Biotransformations of magnetic nanoparticles in the body. Nano Today. 2016 doi: 10.1016/j.nantod.2015.10.001. [DOI] [Google Scholar]
- Laken B.A., Stordal F. Are there statistical links between the direction of European weather systems and ENSO, the solar cycle or stratospheric aerosols? R. Soc. Open Sci. 2016 doi: 10.1098/rsos.150320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamon R.J., McIntosh S.W., Marsh D.R. Termination of solar cycles and correlated tropospheric variability. Earth Space Sci. 2021 doi: 10.1029/2020EA001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Shi L., Zhao J., et al. The Innovation; 2020. Urban Air Pollution May Enhance COVID-19 Case-Fatality and Mortality Rates in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighty J., Veranth J., Sarofim A. Combustion aerosols: factors governing their size and composition and implications to human health. J. Air Waste Manage. 2000 doi: 10.1080/10473289.2000.10464197. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Maher B.A., González-Maciel A., Reynoso-Robles R., Torres-Jardón R., Calderón-Garcidueñas L. Iron-rich air pollution nanoparticles: an unrecognised environmental risk factor for myocardial mitochondrial dysfunction and cardiac oxidative stress. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr L.C., Tang J.W., Van Mullekom J., Lakdawala S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface. 2019 doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Boubeta C., Simeonidis K. Airborne magnetic nanoparticles: environmental risk factors for the transmission of SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.12.10.20247130. [DOI] [Google Scholar]
- Meldrum C. Periodicity of rainfall. Nature. 1873 doi: 10.1038/008547c0. [DOI] [Google Scholar]
- Mele M., Magazzino C. Pollution, economic growth, and COVID-19 deaths in India: a machine learning evidence. Environ. Sci. Pollut. Res. 2020 doi: 10.1007/s11356-020-10689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsakou C., Kallos G., Papantoniou N., et al. Saharan dust levels in Greece and received inhalation doses. Atmos. Chem. Phys. 2008 doi: 10.5194/acp-8-7181-2008. [DOI] [Google Scholar]
- Morris W.A., Versteeg J.K., Bryant D.W., et al. Preliminary comparisons between mutagenicity and magnetic susceptibility of respirable airborne particulate. Atmos. Environ. 1995 doi: 10.1016/1352-2310(95)00203-B. [DOI] [Google Scholar]
- Murhekar M.V., Bhatnagar T., Thangaraj J.W.V., et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020-January 2021. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy B.S., Latha R., Tiwari A., Rathod A., Singh S., Beig G. Impact of mixing layer height on air quality in winter. J. Atmos. Sol. Terr. Phys. 2019 doi: 10.1016/j.jastp.2019.105157. [DOI] [Google Scholar]
- Muxworthy A.R., Matzka J., Petersen N. Comparison of magnetic parameters of urban atmospheric particulate matter with pollution and meteorological data. Atmos. Environ. 2001 doi: 10.1016/S1352-2310(01)00250-3. [DOI] [Google Scholar]
- Nabavi S.O., Haimberger L., Abbasi E. Assessing PM 2.5 concentrations in Tehran, Iran, from space using MAIAC, deep blue, and dark target AOD and machine learning algorithms. Atmos. Pollut. Res. 2019 doi: 10.1016/j.apr.2018.12.017. [DOI] [Google Scholar]
- Nasirpour M.H., Sharifi A., Ahmadi M., Ghoushchi S.J. Revealing the relationship between solar activity and COVID-19 and forecasting of possible future viruses using multi-step autoregression (MSAR) Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-13249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro F., Sironi G., Antonello E., et al. iScience; 2020. Forcing Seasonality of Influenza-like Epidemics with Daily Solar Resonance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orino K., Lehman L., Tsuji Y., et al. Ferritin and the response to oxidative stress. Biochem. J. 2001 doi: 10.1042/bj3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroji B., Solgi E., Sadighzadeh A. Recognition of the source and nature of atmospheric aerosols in tehran, Iran. Aerosol Air Qual. Res. 2018 doi: 10.4209/aaqr.2018.03.0098. [DOI] [Google Scholar]
- Pang Y., Huang W., Luo X.-S., et al. In-vitro human lung cell injuries induced by urban PM2.5 during a severe air pollution episode: variations associated with particle components. Ecotoxicol. Environ. Saf. 2020 doi: 10.1016/j.ecoenv.2020.111406. [DOI] [PubMed] [Google Scholar]
- Paraskevis D., Kostaki E.G., Alygizakis N., et al. A review of the impact of weather and climate variables to COVID-19: in the absence of public health measures high temperatures cannot probably mitigate outbreaks. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2020.144578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J., Wiemann M. Siderite (FeCO₃) and magnetite (Fe₃O₄) overload-dependent pulmonary toxicity is determined by the poorly soluble particle not the iron content. Inhal. Toxicol. 2011 doi: 10.3109/08958378.2011.606431. [DOI] [PubMed] [Google Scholar]
- Perez-Araluce R., Martinez-Gonzalez M.A., Fernández-Lázaro C.I., et al. Mediterranean diet and the risk of COVID-19 in the ‘Seguimiento Universidad de Navarra’ cohort. Clin. Nutr. 2021 doi: 10.1016/j.clnu.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone C., Bartoloni E., Bursi R., et al. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. Res. 2020 doi: 10.22541/au.158880283.34604328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovský E., Zbořil R., Grygar T.M., et al. Magnetic particles in atmospheric particulate matter collected at sites with different level of air pollution. Studia Geophys. Geod. 2013 doi: 10.1007/s11200-013-0814-x. [DOI] [Google Scholar]
- Polymenakou P.N., Mandalakis M., Stephanou E.G., Tselepides A. Particle size distribution of airborne microorganisms and pathogens during an intense african dust event in the eastern mediterranean. Environ. Health Perspect. 2008 doi: 10.1289/ehp.10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Munzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram M., Stolz M., Koenig G. Eleven year cycle of dust concentration variability observed in the dust profile of the GISP2 ice core from Central Greenland: possible solar cycle connection. Geophys. Res. Lett. 1997 doi: 10.1029/97GL02521. [DOI] [Google Scholar]
- Rashki A., Kaskaoutis D.G., Francois P., Kosmopoulos P.G., Legrand M. Dust-storm dynamics over Sistan region, Iran: seasonality, transport characteristics and affected areas. Aeolian Res. 2015 doi: 10.1016/j.aeolia.2014.10.003. [DOI] [Google Scholar]
- Rohrer M., Flahault A., Stoffel M. Peaks of fine particulate matter may modulate the spreading and virulence of COVID-19. Earth Syst. Environ. 2020 doi: 10.1007/s41748-020-00184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffari A., Daher N., Shafer M.M., Schauer J.J., Sioutas C. Global perspective on the oxidative potential of airborne particulate matter: a synthesis of research findings. Environ. Sci. Technol. 2014 doi: 10.1021/es500937x. [DOI] [PubMed] [Google Scholar]
- Salvador P., Alonso S., Pey J., et al. African dust outbreaks over the western Mediterranean basin: 11 year characterization of atmospheric circulation patterns and dust source areas. Atmos. Chem. Phys. 2014 doi: 10.5194/acp-14-6759-2014. [DOI] [Google Scholar]
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020 doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaris R.F., Pumi G., Dalzochio J., et al. Stay-at-home policy is a case of exception fallacy: an internet-based ecological study. Sci. Rep. 2021 doi: 10.1038/s41598-021-84092-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schwartz J., Coull B., Laden F., Ryan L. The effect of dose and timing of dose on the association between airborne particles and survival. Environ. Health Perspect. 2008 doi: 10.1289/ehp.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N.G., Moorman A.C., Makaretz A., et al. Adolescent with COVID-19 as the source of an outbreak at a 3-week family gathering — four states, june–july 2020. MMWR. Morb. Mortal. Wkly. 2020 doi: 10.15585/mmwr.mm6940e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton A., MacNee W., Donaldson K., Godden D. Particulate air pollution and acute health effects. Lancet. 1995 doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., et al. SARS-Cov-2RNA found on particulate matter of Bergamo in northern Italy: first evidence. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahsavani A., Tobías A., Querol X., et al. Short-term effects of particulate matter during desert and non-desert dust days on mortality in Iran. Environ. Int. 2020 doi: 10.1016/j.envint.2019.105299. [DOI] [PubMed] [Google Scholar]
- Sharma G.D., Bansal S., Yadav A., et al. Meteorological factors, COVID-19 cases, and deaths in top 10 most affected countries: an econometric investigation. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-12668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U.S.A. 2020 doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019 doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers S. Sunspot activity and influenza pandemics: a statistical assessment of the purported association. Epidemiol. Infect. 2017 doi: 10.1017/S095026881700173X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp L., Acman M., Richard D., et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020 doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Apestegui V., Parras-Garrido E., Tapia V., et al. Association between air pollution in Lima and the high incidence of COVID-19: findings from a post hoc analysis. Res. Sq. 2020 doi: 10.21203/rs.3.rs-39404/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietzen H., Zoufaly A., Traugott M., et al. Deletion of the NKG2C receptor encoding KLRC2 gene and HLA-E variants are risk factors for severe COVID-19. Genet. Med. 2021 doi: 10.1038/s41436-020-01077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020 doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X. Threshold effects of COVID-19-confirmed cases on change in pollutants changes: evidence from the Chinese top ten cities. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-13980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Prather K.A., Sznitman J., et al. Airborne transmission of respiratory viruses. Science. 2021 doi: 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzky M., de Dieuleveult M., Letessier A., Saint-Ruf C., Miotto B. Assessing the consequences of environmental exposures on the expression of the human receptor and proteases involved in SARS-CoV-2 cell-entry. Environ. Res. 2021 doi: 10.1016/j.envres.2020.110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa P. Bivariate granger causality (v1.0.4) in free statistics software (v1.2.1) 2016. http://www.wessa.net/rwasp_grangercausality.wasp
- Whiteside M., Herndon J.M. COVID-19 immunopathology, particle pollution, and iron balance. J. Adv. Med. Med. Res. 2020 doi: 10.9734/jammr/2020/v32i1830654. [DOI] [Google Scholar]
- Willeke K., Whitby K.T. Atmospheric aerosols: size distribution interpretation. J. Air Pollut. Contr. Assoc. 1975 doi: 10.1080/00022470.1975.10470110. [DOI] [Google Scholar]
- Zallaghi E., Goudarzi G., Sabzalipour S., Zarasvandi A. Estimation of PM2.5 pollutant time changes and its effect on ischemic heart disease (IHD) outcome in Ahvaz city, Iran (2008–2017) Toxin Rev. 2020 doi: 10.1080/15569543.2020.1790605. [DOI] [Google Scholar]
- Zhou L., Yao M., Zhang X., et al. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol Sci. 2020 doi: 10.1016/j.jaerosci.2020.105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from public repositories, in particular the Worldometers Database (https://www.worldometers.info/coronavirus), referenced studies and/or from the corresponding author on request.