Abstract
We performed a systematic sampling and analysis of airborne SARS-CoV-2 RNA in different hospital areas to assess viral spread.
Systematic air filtration was performed in rooms with COVID-19 infected patients, in corridors adjacent to these rooms, to rooms of intensive care units, and to rooms with infected and uninfected patients, and in open spaces. RNA was extracted from the filters and real-time reverse transcription polymerase chain reaction was performed using the LightMix Modular SARS-CoV-2 E-gene.
The highest occurrence of RNA was found in the rooms with COVID-19 patients (mean 2600 c/m3) and the adjacent corridor (mean 4000 c/m3) which was statistically significant more exposed (p < 0.01). This difference was related to the ventilation systems. As is commonly found in many hospitals, each of the rooms had an individual air inlet and outlet, while in the corridors these devices were located at the distance of every four rooms. There was a significant transfer of viruses from the COVID-19 patients’ rooms to the corridors. The airborne SARS-CoV-2 RNA in the corridors of ICUs with COVID-19 patients or care rooms of uninfected patients were ten times lower, averages 190 c/m3 and 180 c/m3, respectively, without presenting significant differences. In all COVID-19 ICU rooms, patients were intubated and connected to respirators that filtered all exhaled air and prevented virus release, resulting in significantly lower viral concentrations in adjacent corridors.
The results show that the greatest risk of nosocomial infection may also occur in hospital areas not directly exposed to the exhaled breath of infected patients. Hospitals should evaluate the ventilation systems of all units to minimize possible contagion and, most importantly, direct monitoring of SARS-CoV-2 in the air should be carried out to prevent unexpected viral exposures.
Keywords: SARS-CoV-2, Nosocomial infection, Hospital infections, Covid-19 virus disease, Aerosols, Indoor air quality
1. Introduction
More than a year after January 30, 2020, when the WHO declared the COVID-19 outbreak a public health emergency of international concern, the pandemic has yet to be brought under control. Vaccination has significantly improved limiting the spread of the virus among the general population in countries where it has been widely implemented. Unfortunately, this currently only represents a small portion of the world's population, which raises concern about the large number of individuals susceptible to infection and also about the recurrent generation of viral variants that may carry risks of reinfection in already vaccinated populations.
Both in countries with high and low degree of vaccinated population, hospitals are at the forefront in the treatment of this disease where SARS-CoV-2 is brought in by infected patients seeking treatment. Strict precautions are needed to prevent these institutions from becoming local hot spots for the spread of the virus.
SARS-CoV-2 RNA has been found in the near and distant air of patients (Birgand et al., 2020). In addition to transmission through respiratory droplets (Bourouiba, 2020), aerosols generated by the evaporation of microdroplets can also lead to long-range viral dispersion and infection (Yuan et al., 2020; Song et al., 2020). Virions are stable in airborne particles with half-lives of more than 1 h (van Doremalen et al., 2020). Infections in a host can even be caused by a single virus (Nicas et al., 2010), so inhalation by susceptible individuals can involve contagion and further spread of the disease (Asadi et al., 2020).
A comprehensive assessment of the distribution of this virus in indoor air (Morawska et al., 2020) is needed to avoid the spread of infection. The transmission of COVID-19 from asymptomatic hosts (Bai et al., 2020; Feng et al., 2020) emphasizes the need to understand the airborne distribution of this virus in indoor environments to design the necessary measures to prevent the spread of the infection. Indoor spaces can have extremely complex flows as a consequence of ventilation systems and thermally driven flow effects (Craven and Settle, 2006; Licina et al., 2014). Hospitals add further complexity to the aerial distribution of SARS-CoV-2 due to the location requirements of the COVID-19 patients, either those with varying degrees of infection or asymptomatic. Several studies in hospitals have included air samples for SARS-CoV-2 (Birgand et al., 2020; Yuan et al., 2020; Jie Zhou et al., 2020), but the knowledge about the factors that determine the airborne transmission is still incomplete.
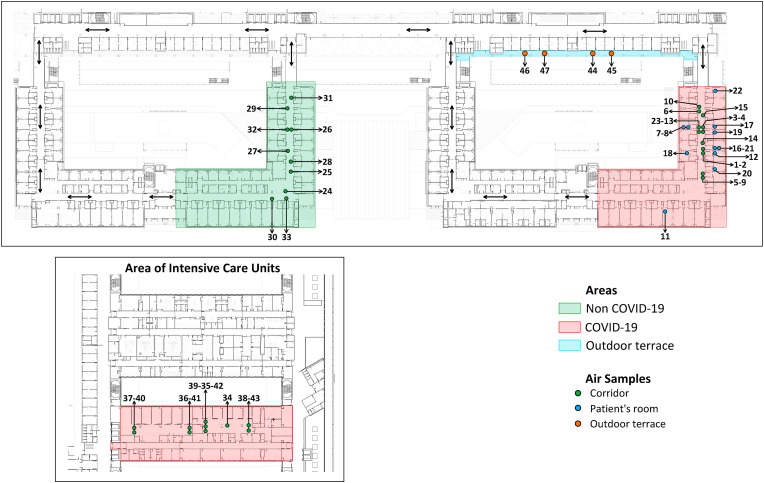
In the present study, we report the results of a systematic sampling in rooms with COVID-19 infected and uninfected patients, in the corridors adjacent to these rooms and in intensive care units (ICUs), and in reference open spaces (Fig. 1). The results have shown statistically significant differences in terms of the airborne occurrence of the virus between the areas examined, which can be used to generate reference measures to prevent nosocomial infection.
Fig. 1.
Representation of the air samples collected in the hospital areas: rooms with COVID-19 infected and uninfected patients, corridors adjacent to these rooms and intensive care units, and reference open spaces.
2. Experimental methods
Samples were collected between November 11 and December 15, 2020 in Hospital Son Espases, the major public hospital in the Island of Mallorca which was inaugurated in May 2011. Air was collected with an Aircheck XR5000 pump (SKC, Eighty Four, PA, USA). The pump was located at about 1.5 m above ground. In the COVID-19 patient rooms it was located 2 m away from the beds. The pumps were provided with a SureSeal Cassette Blanks composed of three 37 mm diameter styrene clear pieces. This cassette contained a PTFE membrane filter of 37 mm diameter and 0.3 μm pore size. Air was collected at 4.5 L min−1 during 4 h. The pump was calibrated before and after each air intake with a Defender 510-L primary flow calibrator (Bios International, NJ, USA). This system was used for filtration of the air contained in different hospital areas. The reported data in copies/m3 (Table 1 ) refer to the filtered air volume which is approximately 1.08 m3.
Table 1.
Summary of the RNA SARS-CoV-2 measurements in the air of the monitored hospital areas.
| Covid-19 patients | Location | n | Positive identification |
Cycle threshold (Ct) |
Copies/m3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | min | max | average | sd | median | min | max | |||
| Yes | Roomsa | 11 | 100 | 33.24 | 36.13 | 2600 | 1500 | 2500 | 700 | 5800 |
| Yes | Room corridora | 12 | 100 | 33.03 | 34.49 | 4400 | 1300 | 4400 | 2300 | 6200 |
| Yes | ICU corridor a | 10 | 30 | 35.82 | 36.56 | 190 | 320 | 0b | 0 | 880 |
| No | Room corridora | 10 | 50 | 36.54 | 44.25 | 180 | 240 | 0 | 0 | 550 |
| No | Outdoor terracea | 4 | 25 | 37.47 | 37.47 | 73 | 150 | 0 | 0 | 290 |
Location of the samples shown in Fig. 1
As mentioned in the positive identification column, not all samples showed airborne RNA. In these samples a value of 0 copies/m3 was assigned.
After sampling, the filters were introduced into Genesis vials containing 3 mL of KaiBiL Viral Transport Medium (VTM; Biomed Global, Kuala Lumpur, Malaysia). RNA extraction was performed from 400 μL of the VTM solution, using the KingFisher purification system (Thermo Fisher Scientific, Waltham, Massachusetts, USA) based on magnetic beads. Once extracted, a real-time PCR was performed from 10 μL of the RNA eluate, using LightMix Modular SARS-CoV-2 E-gene (TIB MOLBIOL, Berlin, Germany) that detects the presence of the E (Envelope) gene of Sarbecoviruses. The polymerase chain reaction (PCR) was performed in a CFX96 Touch Real-Time PCR thermal cycler (Bio-Rad, Hercules, CA, USA). An internal control of the extraction system was performed with the 77b fragment of the Equine Arteritis virus. The recoveries of the extraction and PCR replication cycles were nearly 100%. Genetic material was detected at amplifications up to 44.25 cycle thresholds (Ct). Beyond this value the measurements were negative and 0 was introduced in the database.
This research was approved by the Ethics Committee of the Balearic Islands.
Means, medians, quartiles, maximum and minimum values were used for descriptive statistics. Given the contrast between measurements both in the rooms with COVID-19 patients and the corridor of these rooms (positive airborne RNA identification in all cases) and those from the corridors of the ICU rooms and rooms with non-COVID-19 patients and from the outdoor terrace (30%, 50% and 25% positive identifications, respectively; Table 1) both parametric (t-test) and non-parametric (Tukey) tests were performed. The hypothesis testings were devoted to identify whether the positive measurements in each hospital area were significantly different from the others. The standard deviations of the measurements in the rooms with COVID-19 patients and corridor of these rooms were not significantly different according to the F test (p < 0.01).
3. Results
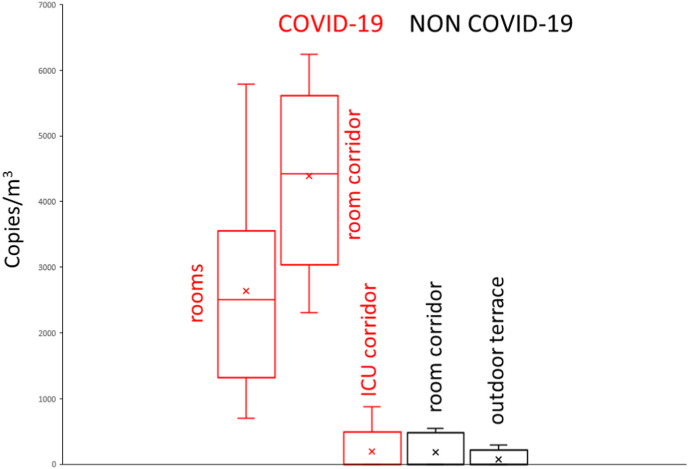
The results of the air analyses of SARS-CoV-2 RNA in the rooms with COVID-19 patients in need of regular care and in the corridor where these rooms are located (Fig. 1 ) are summarized in Table 1. The airborne RNA concentrations in the corridors with ICU rooms for COVID-19 patients and those with rooms for non-COVID-19 patients (Fig. 1) are also reported in this Table and in the box plots of Fig. 2 . The concentrations of the analyses of air from an open environment (terrace; Fig. 1) located next to the cafeteria are also summarized in Table 1 and Fig. 2.
Fig. 2.
Box plots showing the distribution of SARS-CoV-2 RNA in the air of the studied hospital areas. X indicates the mean value of each distribution.
Samples with airborne SARS-CoV-2 RNA were found in all these hospital units. The air collected in rooms with COVID patients showed detectable RNA concentrations in all cases (100%) with average of 2600 copies/m3 and maximum value of 5800 c/m3 (Table 1). In the corridor where these rooms are located, SARS-CoV-2 RNA was also detected in all cases and the concentrations were higher, mean 4400 c/m3 and maximum value 6200 c/m3; Table 1), than in the rooms. As shown in Table 2 , the differences between these two groups of samples was significant when comparing both the means (p < 0.01; t-test) and the medians (p < 0.05; Tukey test).
Table 2.
Statistical significance values resulting from the parametric (means t-test) and non-parametric (Tukey) tests of the airborne distributions of SARS-CoV-2 RNA in the studied hospital areas (Fig. 1).
| Rooms with COVID-19 patients | Corridor with COVID-19 rooms | Corridor with COVID-19 ICUS | Corridor with non COVID-19 rooms | Outdoor terrace | |
|---|---|---|---|---|---|
| Rooms with COVID-19 patients | p < 0.01a p < 0.05b |
p < 0.0001 p < 0.0005 |
p < 0.0001 p < 0.0005 |
p < 0.01 p < 0.0005 |
|
| Corridor with COVID-19 rooms | p < 0.001 p < 0.0005 |
p < 0.0001 p < 0.0005 |
p < 0.0001 p < 0.0005 |
||
| Corridor with COVID-19 ICUS | no difference |
no difference | |||
| Corridor with non COVID-19 rooms | no difference |
Parametric.
non parametric.
The frequencies of detectable SARS-CoV-2 RNA in the air of the corridor of the ICU rooms with COVID-19 patients or in the corridors with rooms for care of non COVID-19 patients were much lower, 30% and 50%, respectively, than those from the previously described hospital areas. In these cases, the means were ten times lower, 190 c/m3 and 180 c/m3, respectively, than in the above described hospital areas. Their SARS-CoV-2 airborne distributions did not display significant differences between them in the t-tests and non-parametric test (Table 2).
The samples from the outdoor terrace showed lower airborne SARS-CoV-2 RNA frequencies than in the indoor areas, 25%, and the mean was also lower, 73 c/m3. The t- and non-parametric test did not allow to discriminating the airborne distributions of the terrace from those of corridors with rooms occupied with non-COVID-19 patient or COVID-19 ICU rooms (Table 2).
4. Discussion
The highest occurrence of SARS-CoV-2 is found in two related units, one composed by the rooms with COVID-19 patients receiving regular care and the other the adjacent corridor (Fig. 1) which was significantly more exposed to airborne SARS-CoV-2 RNA. The difference was statistically significant both when considering parametric (p values smaller than 0.01, 0.001 and 0.0001) and non-parametric tests (p < 0.0005) (Table 2).
The airborne transmission route is associated with small droplets that are suspended and transported in air currents. Most of these droplets evaporate within a few seconds (Xie et al., 2007) and the remaining nuclei consist of virions and solid residue (Vejerano and Marr, 2018) but water may never be completely removed (Mezhericher et al., 2010). These droplet nuclei are submicron in size and can remain suspended in air for hours. Transport of droplet nuclei over greater distances is driven primarily by environmental flows that pose a particular challenge for disease transmission. The importance of ventilation in the control of airborne transmission of infections is important (Tang et al., 2006; Li et al., 2007) and must be modeled by fluid dynamics (Thatiparti et al., 2017; Yang et al., 2018; Yu et al., 2018).
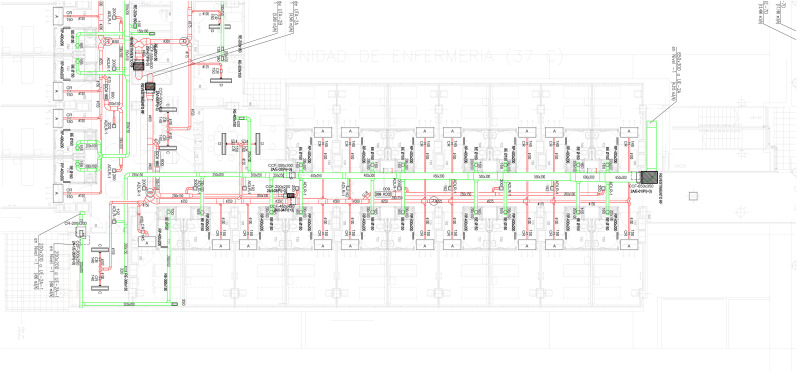
The higher concentration of SARS-CoV-2 RNA in the corridor than in the rooms could a priori be unexpected because the viral source is located in the rooms. Each of the rooms has an individual air inlet and outlet, while the corridors have only one air inlet and one extraction device at the distance of every four rooms (Fig. 3 ). These ventilation differences are typical of many hospitals because the system generally enhances aeriation of the patient rooms. Therefore, there is a significant transfer of virus from the rooms to the corridors, either when opening the doors or adhering to the clothing of the healthcare personnel working in the rooms, which is eventually released into the corridor.
Fig. 3.
Detailed description of the ventilation system in the Covid-19 and non-Covid-19 areas of Fig. 1. The green and red lines indicate the air inlet and outlet pipes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The corridors of ICUs with COVID-19 patients had much lower SARS-CoV-2 airborne occurrence. In all ICU rooms patients were intubated and connected to respirators that filtered all exhaled air. This filtration system retained the release of virus with exhaled breath, and therefore the corridors located next to these rooms had significantly lower airborne SARS-CoV-2 concentrations. The low values of these corridors were similar to those of the corridors of the rooms with non-COVID patients or the outdoor site. No statistically significant differences were observed between them.
Previous studies of airborne SARS-CoV-2 RNA in hospital areas have found a wide diversity of results, encompassing no positive samples (Ong et al., 2020), a very small number of positive samples (Ding et al., 2021; Guo et al., 2020) or RNA levels (Yuan et al., 2020) or various positive samples (Chia et al., 2019; Ding et al., 2021; Jiang et al., 2019; Liu et al., 2020; Santarpia et al., 2020). Positive results have also been found in hotels containing quarantine rooms for COVID-19 patients (Jiang et al., 2020). However, no previous study has identified such regular distribution of indoor airborne SARS-CoV-2 RNA as the one identified here. This regular distribution of airborne RNA in the studied hospital is related to the location of COVID-19 patients but the observed highest RNA concentrations in the corridor of the COVID-19 rooms indicates that the ventilation systems should be supervised to avoid unexpected SARS-CoV-2 exposures. The present case study which corresponds to a modern hospital with advanced technology may constitute a representative case of other settings which may remain unnoticed.
While the measured RNA is not per se an active SARS-CoV-2 marker, the present findings show that the risk of virus infection cannot be excluded in some areas of the corridors and to prevent nosocomial infection, staff should be strict in the use of protective elements or ventilation procedures should be modified.
One of the strengths of this study is the active sampling and analysis of indoor air in the various hospital areas. Another strength is the strong consistency of the results, showing an RNA distribution that may be related to the location of COVID-19 patients and reveal an unexpected pattern. One weakness of the study is the relatively small number of samples. Another weakness refers to the fact that the observation of the airborne RNA of SARS-CoV-2 does not imply a direct risk of contagion by viable viral activity. However, the precautionary principle recommends to implement adequate strategies to avoid the occurrence of airborne SARS-CoV-2 RNA in hospital areas.
5. Conclusions
The greatest risk of nosocomial infection can occur in hospital areas not directly exposed to the exhaled breath of infected patients. Hospitals should evaluate the ventilation systems of all units to minimize possible contagion and, most importantly, direct monitoring of SARS-CoV-2 in the air should be carried out to prevent unexpected viral exposures.
Credit author statement
Joan O. Grimalt: Conceptualization. Overall project study. Investigation. Writing. Francisco Fanjul: Conceptualization. Overall project study. Investigation. Organization of sampling. Sampling. Discussion of results. Pablo A. Fraile-Ribot: Analyses of RNA. Discussion of results. Esther Marco: Design of sampling. Drawing maps. Discussion of results. Helem Vilchez: Sampling. Discussion of results. Antoni Campins: Sampling. Discussion of results. Barend L. Van Drooge: Design of sampling. Discussion of results. Jaime Orfila: Organization of sampling. Discussion of results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Financial support from the European Union Project EDCMET (H2020-HEALTH/0490–825762) is acknowledged.
References
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. J. Am. Med. Assoc. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgand G., Peiffer-Smadja N., Fournier S., Kerneis S., Lescure F.-X., Lucet J.-C. Assessment of air contamination by SARS-CoV-1 in hospital settings. J. Am. Med. Assoc. 2020;3(12) doi: 10.1001/jamaetworkopen.2020.33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions potential implications for reducing transmission of COVID-19. J. Am. Med. Assoc. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Chia Po Ying, Coleman K.K., Tan Y.-K., Ong S.W.X., Gum M., Lau S.K., et al. Novel Coronavirus Outbreak Research Team. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2019;11:2800. doi: 10.1038/s41467-020-16670-2. doi.org/10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven B.A., Settles G.S. A computational and experimental investigation of the human thermal plume. Trans. ASME J. Fluids Eng. 2006;128:1251–1258. [Google Scholar]
- Ding Zhen, Qian Hua, Xu Bin, Huang Ying, Miao Te, Yen Hui-Ling, et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021;753:141710. doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Ye, Xu Shicai, Rong Zhihua, Xu Ronghua, Liu Xiaowei, Deng Pingfu, et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int. J. Infect. Dis. 2020;94:133–138. doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Zhen-Dong, Wang Zhong-Yi, Zhang Shou-Feng, Xiao Li, Lin Li, Li Chao, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, wuhan, China, 2020. Emerg. Infect. Dis. 2020;26:1586–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F.C., Jiang X.-L., Wang Z.-G., Meng Z.-H., Shao S.-F., Anderson B.D., et al. Detection of severe acute respiratory syndrome coronavirus 2 RNA on surfaces in quarantine rooms. Emerg. Infect. Dis. 2020;26:2162–2164. doi: 10.3201/eid2609.201435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Leung G.M., Tang J.W., Yang X., Chao C.Y., Lin J.Z., et al. Role of ventilation in airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Licina D., Pantelic J., Melikov A., Sekhar C., Kwok Wai Tham. Experimental investigation of the human convective boundary layer in a quiescent indoor environment. Build. Environ. 2014;75:79–91. [Google Scholar]
- Liu Yang, Yan Li-Meng, Wan Lagen, Xiang Tian-Xin, Le Aiping, Liu Jia-Ming, Peiris Malik, Poon Leo L.M., Zhang Wei. Viral dynamics in mild and severe cases of COVID-19. Lancet/Infection. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezhericher M., Levy A., Borde I. Theoretical models of single droplet drying kinetics: a review. Dry. Technol. 2010;28:278–293. [Google Scholar]
- Morawska L., Tang J.W., Bahnfleth W., Bluyssend P.M., Boerstrae A., Buonanno G., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Env Pat. 2010;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X, Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Su M., Wong Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Tang, Mao Yixin, Jones Rm, Tana Qiyue, Jid Js, Lia Na, et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106039. 106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatiparti D.S., Ghia U., Mead K.R. Computational fluid dynamics study on the influence of an alternate ventilation configuration on the possible flow path of infectious cough aerosols in a mock airborne infection isolation room. Sci. Technol. Built Environ. 2017;23:355–366. doi: 10.1080/23744731.2016.1222212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:16. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Li Y., Chwang A.T.Y., Ho P.L., Seto W.H. How far droplets can move in indoor environments – revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Yang L., Li X., Yan Y., Tu J. Effects of cough-jet on airflow and contaminant transport in an airliner cabin section. J. Comput. Multiph. Flows. 2018;10:72–82. [Google Scholar]
- Yu H., Mui K., Wong L. Numerical simulation of bioaerosol particle exposure assessment in office environment from MVAC systems. J. Comput. Multiph. Flows. 2018;10:59–71. [Google Scholar]
- Yuan Liu, Ning Zhi, Chen Yu, Guo Ming, Liu Yingle, Kumar Gali Nirmal, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Zhou Jie, Otter J.A., Price J.R., Cimpeanu C., Garcia D.N., Kinross J., et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis. ciaa905. 2020 doi: 10.1093/cid/ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]