Abstract
Introduction
There is ongoing debate regarding the role of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in asthma exacerbation, and its long-term impact on the lung function of individuals with asthma. In contrast, the potential impact of coronavirus disease 2019 (COVID-19) vaccination on asthma is entirely unexplored.
Case study
This study examined a challenging case of severe asthma exacerbation in a 28-year-old female following two doses of the mRNA-based vaccine BNT162b2 (Pfizer-BioNTech) at IRCCS Policlinico San Matteo in Pavia, Italy. The patient, a fourth-year resident at the hospital, was vaccinated in early 2021. She was an occasional smoker with a 10-year history of asthma and seasonal allergic rhinitis. She tested negative for SARS-CoV-2 on several molecular swabs and serology tests.
Results
After receiving the second dose of vaccine, the patient started to experience worsening of respiratory symptoms. Following several episodes and a severe asthma attack, the patient required treatment with mepolizumab, a biologic drug (interleukin-5) antagonist monoclonal antibody.
Conclusion
This single case study is insufficient to draw conclusions about the association between asthma exacerbation and the COVID-19 vaccine. While the cause–effect link between vaccination against SARS-CoV-2 and worsening of asthmatic disease might only be suggested at present, this case is a valuable prompt for further investigation. This is particularly true from the perspective of mass vaccination of adolescents and children currently underway across the globe.
Keywords: SARS-CoV-2, Asthma exacerbation, Monoclonal antibody against interleukin-5, COVID-19 vaccination, Long COVID, Mass vaccination
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) may cause worsening of asthmatic symptoms, in common with other viral infections (Novak and Cabanillas, 2020), but no evidence exists to support the hypothesis that patients with bronchial asthma are at increased risk of an exacerbation when receiving coronavirus disease 2019 (COVID-19) vaccination.
Although the cause–effect link is fairly intractable, this critical topic will be investigated here by examining a case study. This may be of considerable relevance given the high prevalence of bronchial asthma, especially among teenagers and school children (Dharmage et al., 2019), most of whom have yet to be vaccinated. Although the benefits of COVID-19 vaccination are unquestionable, it is argued that being informed about its potential impact on asthma may be equally valuable for both patients and physicians.
Case presentation
Written informed consent for publication of clinical data was obtained from the patient.
Once approved by the US Food and Drug Administration and the European Medicines Agency, the first doses of the mRNA-based vaccine BNT162b2 (Pfizer-BioNTech) were administered in Italy on 27 December 2020. At IRCCS Policlinico San Matteo in Pavia, Italy, healthcare workers (HCWs) were vaccinated in early January 2021.
A 28-year-old Caucasian female, a fourth-year resident of the Infectious Diseases Division, completed the two-dose schedule in February 2021. Due to the surveillance programmes on HCWs, she was known to have had several negative molecular swabs for SARS-CoV-2, and had not been infected with SARS-CoV-2.
The case was an occasional smoker with a 10-year history of asthma and seasonal allergic rhinitis. She had not experienced any asthma exacerbation requiring hospitalization, and she used an inhaled short-acting bronchodilator as needed, generally before exercising, which provided complete relief.
Approximately 10 h after the second dose of vaccine, she complained of some ‘COVID-like symptoms’, including fever (axillary temperature up to 39°C), fatigue and dry cough. Although occurring with high frequency, the symptoms receded entirely after 5 days, which was slightly longer than reported by vaccine national surveillance systems (AIFA, 2021). Approximately 3 weeks after the second dose of vaccine, she started to experience worsening of respiratory symptoms, with mild dyspnoea during physical activity and some expiratory wheezing, mainly at night. Therefore, she started daily controller therapy, with budesonide 160 μg and formoterol 4.5 μg t.i.d. two times daily.
Notably, at that time, the flowering of grasses, which typically triggers allergic asthma and rhinitis, had not yet commenced. Nevertheless, her symptoms did not recede but instead worsened, mainly at night, with frequent awakenings.
Being a physician herself, the patient independently started three short-term cycles of oral steroid therapy, with prednisone 50 mg/day for 5 days, subsequently tapering off for a further 5 days, and doubled the antihistaminic dosage up to a daily dosage of ebastine 10 mg b.i.d before agreeing to a specialistic evaluation.
After 1 month, in April 2021, as her asthma had failed to come back under control, the patient was referred to the Operational Unit of Allergology at the study hospital. On physical examination, she had symptoms of rhinitis, with copious clear nasal discharge and continual drip, red eyes (as a result of allergic conjunctivitis) and sneezing. Her lungs were clear on auscultation, with some mild bilateral basal wheeze. Peripheral oxygen saturation (SpO2) was 100% in room air.
Routine blood tests were performed while taking steroid therapy for the third and last self-prescribed cycle, and did not show any eosinophilia, with a total leukocyte count of 9300 and a negative C-reactive protein. Moreover, total IgE was 800 kU/L, and the IgE-positive response was significant for grasses and other types of pollens, cat allergens and crustaceans.
Fractional exhaled nitric oxide (FeNO) was 40 ppb, and spirometry showed an obstructive pattern [forced expiratory volume in 1 s (FEV1) 1.97 L (60%); forced vital capacity (FVC) 3.55 L (91%); FEV1/FVC 55%]. As expected, the airway obstruction was reversible [prebronchodilator FEV1, 1.97 L (60%); postbronchodilator FEV1, 2.21 L (67%); reversibility, 12%].
After that visit, the patient was recommended a multi-drug regimen which comprised budesonide 320 μg + formoterol 9 μg b.i.d., tiotropium bromide 2.5 μg and montelukast 10 mg/day.
Due to prolonged steroid consumption, the physician attempted to boost the doses of other drugs while trying to avoid the use of prednisone. However, some days later, the patient experienced another acute exacerbation of asthma, with wheezing and dyspnoea, requiring intravenous steroid treatment (a bolus of methylprednisolone 1 mg/kg). Therefore, she resumed the use of oral steroids, with benefit. At this time, she also performed a molecular swab for SARS-CoV-2 detection and an anti-N serology test, which showed negative results.
Subsequently, chest radiography and computed tomography were performed, both of which showed normal results.
To exclude any other comorbidity favouring asthma exacerbation, the patient also underwent nasal endoscopy, which only showed mild turbinate congestion. However, this condition improved quickly with daily application of a nasal corticosteroid and antihistamine spray.
Common infectious causes were also excluded, given the negative serology for antibodies anti-Chlamydia pneumoniae and anti-Mycoplasma pneumoniae, the negative SARS-CoV-2 anti-N protein IgG, and the complete absence of symptoms such as fever, productive cough or other organ-specific recalls. Autoimmune disorders were also investigated and disregarded. In addition, an echocardiogram and electrocardiogram were obtained to rule out any other cardiological causes of breathlessness.
Furthermore, the patient did not have any contact with potential allergic triggers, such as crustaceans or cats, that could have caused the asthmatic crises.
Significantly, the typical allergic symptoms (i.e. rhinitis and conjunctivitis) diminished and then vanished completely during treatment, whereas the bronchial hyper-reactivity remained unchanged.
Although the patient was not dyspnoeic during her daily routine, and she continued working normally without any other unusual symptomatology, she was not physically active and had frequent nocturnal awakenings. This was despite compliance with the prescribed treatment, which included steroids.
However, in June 2021, during the umpteenth scaling of steroid therapy, the patient suffered a more severe asthma attack which prompted admission to the emergency department. She was frankly dyspnoeic with SpO2 <90% and was instantly given oxygen (5 L/min), inhaled therapy with bronchodilators, and simultaneous intravenous treatment with 1 g hydrocortisone.
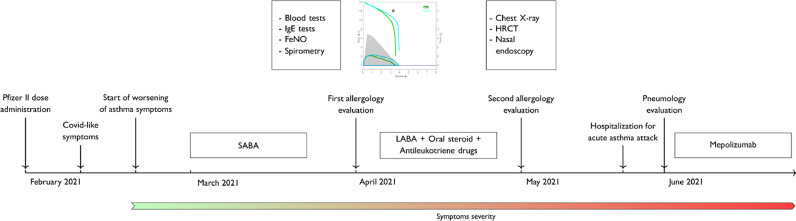
At this stage, the patient was referred to the Pneumology Unit at the study hospital, where the decision was made to start treatment quickly with an humanized, monoclonal antibody against interleukin-5. Specifically, mepolizumab was started 3 days later, although eosinophilia with >300 eosinophils/µL [the first of the mepolizumab eligibility criteria according to the National Institute for Health and Care Excellence guidance (Bermejo et al., 2018)] was not fulfilled. The timeline of events is described in Figure 1 .
Figure 1.
Timeline of the patient's symptoms and examinations performed. SABA, short-acting bronchodilator; LABA, long-acting bronchodilator; HRCT, high-resolution computed tomography.
Discussion
SARS-CoV-2 is an acute viral infection which might be expected to flare up a chronic pulmonary disease, such as asthma (Yang et al., 2020), in common with other viral respiratory infections, including other types of coronaviruses (Edwards et al., 2012). As a result, moderate-to-severe asthma has been listed as a risk factor for COVID-19 morbidity and mortality.
However, the rate of asthma disease has been reported as relatively low among patients with COVID-19, especially in observational case series from China, Europe and South America (Skevaki et al., 2020). In contrast, a higher prevalence of asthma among patients hospitalized with COVID-19 was reported in studies from the USA, UK, Ireland, Korea and Australia (De Boer et al., 2021). Hence, it remains unclear whether or not asthma may constitute a risk factor for severe COVID-19.
There is also a theoretical risk that SARS-CoV-2 could be a trigger for asthma exacerbations. To date, there are no data to support or refute this. Likewise, a decrease in asthma exacerbations during the COVID-19 lockdown measures has been reported in patients with asthma (De Boer et al., 2021), but these data may also be attributable to social distancing and reduced environmental exposures.
Some researchers found that asthmatic patients had reduced levels of angiotensin‐converting enzyme 2, which has been identified as a SARS‐CoV‐2 cellular receptor (Kimura et al., 2020). Although this finding suggests a potential protective role of the asthmatic phenotype on SARS-CoV-2 infection and, consequently, the severity of COVID-19 (Jackson et al., 2020), other authors have suggested higher expression of transmembrane protease serine 2 among asthmatic individuals, which might facilitate SARS‐CoV‐2 cell entry by its role in cleavage of the spike protein (Radzikowska et al., 2020).
Moreover, mast cells may have an antiviral role (Marshall et al., 2019), and eosinopenia has been proposed as a marker of severe COVID-19 (Rosenberg and Foster, 2021), as opposed to eosinophilia which is known to be a marker of allergic asthma. More thorough multi-centre investigations are needed to truly establish the complex relationship between SARS-CoV-2 infection and asthma.
The impact of SARS-CoV-2 on asthmatic disease after the acute phase of the viral infection (i.e. the long-term consequences of COVID-19, if any, on asthmatic individuals) remains unknown.
As SARS-CoV-2 is a respiratory virus, lung injury can be expected. Concordantly, there is evidence of impaired lung function following COVID-19, which belongs to a cluster of recently recognized tissue damage referred to as ‘long COVID’ (Torres-Castro et al., 2021, Zhao et al., 2020).
A study also discovered defective pulmonary gas-exchange function among patients with COVID-19 who had been discharged from hospital compared with healthy individuals (Li et al., 2021).
However, to the best of the authors’ knowledge, only Eggert et al. (2021) have attempted to tackle this ongoing debate. They found no difference in time to resolution of lower respiratory symptoms between asthmatics and non‐asthmatics after 3 months of follow-up. Nevertheless, this single retrospective study is insufficient to fulfil all the current doubts and uncertainties on the topic.
Relatively few data are available on the long-term impact of SARS-CoV-2 infection on asthma, and the effect of COVID-19 vaccination on this condition is entirely unexplored.
Many studies have shown that mRNA vaccines are likely to induce production of interferon I (IFN-1) (Cagigi and Loré, 2021), and it is known that the bronchial epithelium of asthmatics produces less IFN-I in response to a viral infection (Edwards et al., 2017). However, hyperactive IFN-I production during asthma exacerbations, associated with viral infection, has been described (Bergauer et al., 2017). As such, the authors believe that there is a need to consider the impact of COVID vaccination on asthma, especially in the run-up to mass vaccination of adolescents and children.
Conflict of interest statement
None declared.
Acknowledgments
Funding
None.
Ethical approval
Written informed consent for publication of clinical data was obtained from the patient.
References
- AIFA. Rapporto sulla Sorveglianza dei vaccini COVID-19. 2021. Available at: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_6.pdf.
- Bergauer A, Sopel N, Kroß B, Vuorinen T, Xepapadaki P, Weiss ST, et al. IFN-α/IFN-λ responses to respiratory viruses in paediatric asthma. Eur Respir J. 2017;49 doi: 10.1183/13993003.00006-2017. [DOI] [PubMed] [Google Scholar]
- Bermejo I, Stevenson M, Cooper K, Harnan S, Hamilton J, Clowes M, et al. Mepolizumab for treating severe eosinophilic asthma: an Evidence Review Group perspective of a NICE Single Technology Appraisal. Pharmacoeconomics. 2018;36:131–144. doi: 10.1007/s40273-017-0571-8. [DOI] [PubMed] [Google Scholar]
- Cagigi A, Loré K. Immune responses induced by MRNA vaccination in mice, monkeys and humans. Vaccines. 2021;9:61. doi: 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer G, Braunstahl GJ, Hendriks R, Tramper-Stranders G. Asthma exacerbation prevalence during the COVID-19 lockdown in a moderate-severe asthma cohort. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2020-000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MR, Bartlett NW, Hussell T, Openshaw P, Johnston SL. The microbiology of asthma. Nat Rev Microbiol. 2012;10:459–471. doi: 10.1038/nrmicro2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140:909–920. doi: 10.1016/j.jaci.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert LE, He Z, Collins W, Lee AS, Dhondalay G, Jiang SY, et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy Eur J Allergy Clin Immunol. 2021 doi: 10.1111/all.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DJ, Busse WW, Bacharier LB, Kattan M, O'Connor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin. 2020;146:203–206. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhao X, Wang Y, Lou X, Chen S, Deng H, et al. Damaged lung gas exchange function of discharged COVID-19 patients detected by hyperpolarized 129Xe MRI. Sci Adv. 2021;7 doi: 10.1126/sciadv.abc8180. eabc8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Portales-Cervantes L, Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci. 2019;20:4241. doi: 10.3390/ijms20174241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N, Cabanillas B. Viruses and asthma: the role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology. 2020;161:83–93. doi: 10.1111/imm.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Foster PS. Eosinophils and COVID-19: diagnosis, prognosis, and vaccination strategies. Semin Immunopathol. 2021;43:383–392. doi: 10.1007/s00281-021-00850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skevaki C, Karsonova A, Karaulov A, Xie M, Renz H. Asthma-associated risk for COVID-19 development. J Allergy Clin Immunol. 2020;146:1295–1301. doi: 10.1016/j.jaci.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy Eur J Allergy Clin Immunol. 2020 doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27:328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y-M, Shang Y-M, Song W-B, Li Q-Q, Xie H, Xu QF, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]