Abstract
The study aimed to decipher the impact of multiple drought stress on wheat. To that effect, Geumgangmil, PL 337 (1AL.1RS), PL 371 (1BL.1RS), and PL 257 (1DL.1RS) seedlings were subjected to four treatments: G1 (control), G2 (stressed thrice with rewatering), G3 (stressed twice with rewatering), and G4 (single stressful event). The findings provided a comprehensive framework of drought-hardening effect at physiological, biochemical, and gene expression levels of drought-stressed wheat genotypes. The treatments resulted in differentially higher levels of malondialdehyde (MDA), hydrogen peroxide (H2O2), soluble sugar, and proline accumulation, and reduced relative water content (RWC) in wheat plants. Photosynthetic pigment (chlorophyll and carotenoid) levels, the membrane stability index (MSI), and shoot biomass decreased dramatically and differently across genotypes, particularly in G3 and G4 compared to G2. The activity of antioxidant enzymes [ascorbate peroxidase (APX), superoxide dismutase (SOD), and catalase (CAT)] increased with the duration and severity of drought treatment. Furthermore, the relative expression of DREB, LEA, HSP, P5CS, SOD1, CAT1, APX1, RBCL, and CCD1 genes was higher in G2 than in other treatments. Drought hardening increased drought tolerance and adaptability in plants under G2 by enhancing growth and activating defensive mechanisms at the physio-biochemical and molecular levels. The findings of the study indicated that early drought stress exposure-induced acclimation (hardening), which enhanced tolerance to subsequent drought stress in wheat seedlings. The findings of this study will be useful in initiating a breeding program to develop wheat cultivars with improved drought tolerance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02991-6.
Keywords: Drought hardening, Antioxidant defense, Membrane stability, Osmoregulation, Photosynthetic activity transcriptional regulation
Introduction
Globally, drought stress has a major negative impact on plant growth, development, and production (Xoconostle-Cazares et al. 2010). The extent of this impact depends on the magnitude, intensity, and duration of the stress. Plants have various physiological, biochemical, and molecular strategies to mitigate drought damage, such as stomatal closure, hormonal control, membrane stability management and cell adaptation, enhancement of the carbon fixation rate, scavenging of reactive oxygen species (ROS), enhancement of photosynthetic activity, and stress-related gene induction (Ullah et al. 2017; Zhou et al. 2017).
Furthermore, responses to single-episode drought have been well studied in numerous crops, but there is relatively limited information on plant responses to progressive or multiple drought stresses of varying intensity, severity, and duration (Bian and Jiang 2009). Drought and other abiotic stresses occur commonly, and concurrently, especially in the field (Suzuki et al. 2014). Thus, plants subjected to initial water stress during seedling growth may enhance their tolerance to recurrent water stress of severe intensity that may occur later in growth and development, a mechanism known as acclimation or hardening (Selote and Khanna-Chopra 2010). Hardening is considered as the proximal phenotypic response of a single genotype to environmental changes, which includes reprograming development, physiology, and metabolism to achieve a new state that increases fitness and survival (Wilson and Franklin 2002). It is also considered as a feasible and practical approach that involves the use of reduced or partial watering of seedlings to prepare the seedlings to drought stress (Thomas 2009). The hardening approach has been used not only under drought stress conditions, but also under other abiotic stresses such as cold stress in tomato seedlings, chilling in cucumber seedlings, and providing cross-adaptability to heavy metals in barley (Ghanbari and Kordi 2019; Ghanbari and Sayyari 2018; Kacienė et al. 2017; Murshed et al. 2008).
Wheat is an important staple crop grown globally. However, abiotic stress, especially drought, hinders its growth, development, and yield, particularly under semi-arid climatic conditions (Crespo-Herrera et al. 2018). The resulting dramatic impact of drought stress on wheat productivity has necessitated the recent rise in research aimed at improving and developing drought-adaptable genotypes to expand wheat production and distribution and safeguard important genetic resources worldwide (Abhinandan et al. 2018). While responses to a single drought stress cycle have been extensively studied in different plants, there is limited information regarding the impact of multiple drought stresses, especially on wheat. It can be argued that the responses of plants to individual or single episodes of drought stress cannot be directly generalized to the responses of plants to progressive or multiple stresses of varying intensity, magnitude, and length, given that plants are subjected to multiple stress conditions during their growth and development cycle (Abid et al. 2018; Rivero et al. 2014). Furthermore, the relationship between the biochemical, physiological, and transcriptomic responses of wheat to progressive drought stress is unclear.
Identifying the essential responsive mechanisms of the numerous physiological, biochemical, and molecular responses used by plants to overcome drought stress will be important for the selection and breeding of drought-adaptable plant genotypes (Chen et al. 2016; Rivero et al. 2014; Suzuki et al. 2014). Drought stress is associated with a higher accumulation of reactive oxygen species (ROS), which are detrimental to plant growth. Plants induce increased expression of antioxidant enzyme genes (SOD1, CAT1, and APX1), and thus the enzymes themselves (superoxide dismutase [SOD], catalase [CAT], and ascorbate peroxidase [APX]), to mitigate the drastic effects of ROS (Abid et al. 2018; Li et al. 2020a). Furthermore, drought stress tolerance involves the upregulation of many other genes, such as the osmolyte biosynthesis-related gene P5CS (Maghsoudi et al. 2018), the photosynthesis-related gene rbcL (Johnson et al. 2014), the carotenoid cleavage dioxygenase-related gene CCD1 (Park et al. 2015), and the transcription factors DREB, LEA, and HSP (Bielsa et al. 2016; Jacob et al. 2017; Wei et al. 2016). These genes can be used to elucidate the role of multiple drought stress in wheat. This study was conducted to compare the biochemical, physiological, and transcriptional responses of wheat under single and multiple drought stress, in relation to photosynthetic pigment content, leaf and root water relations, proline and soluble sugar accumulation, malondialdehyde level, H2O2 and ROS accumulation, and antioxidant enzyme activity. We hypothesized that exposure of wheat to initial drought treatment (drought hardening) could trigger specific responses that differed from those following a single stress event.
Materials and methods
Plant materials, growing conditions, and treatments
Seeds of the wheat strain ‘Geumgangmil’ (National Agrobiodiversity Centre, RDA, Korea; accession no. IT 213,100) and three recombinant inbred lines (F5:9), denoted 1BL.1RS (PL 337), 1AL.1RS (PL 371), and 1BL.1RS (PL 257) and derived from crosses between ‘Geumgangmil’ (female parent) and TAM201 (PI 578256), Seri82 (PI 591774), and Siete Cerros66 (PI 338921), respectively, were used in this study. The seeds were surface sterilized in 70% ethanol for 2 min, treated with 0.1% sodium hypochlorite for 15 min, and then washed five times with sterile distilled water before planting. The resulting 3-day-old seedlings were transferred and grown in pots (10 × 10 × 8 cm) containing soil (sunshine mix #2) under a photocycle of 13:11 h (day: night), 25–22 °C (day to night), 80% relative humidity, and active photosynthetic radiation at 600 μmol m−2 s−1 for 10 d before the drought stress treatments were initiated. The conditions in the green house were monitored using a HOBO data logger device (Onset Computer Corporation, Bourne, MA, USA).
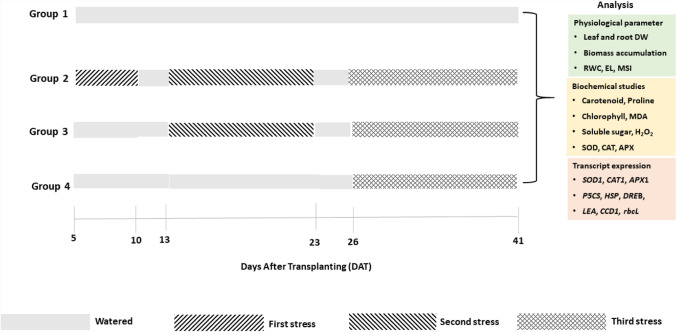
The seedlings of the same shoot length were transferred to soil pots (10 × 10 × 8 cm/top diameter × height × bottom diameter) and separated into four groups: Group 1 (G1) was watered daily (5 DAT to 41 DAT), whereas Group 2 (G2) was stressed three times (5 DAT–10 DAT, 13 DAT–23 DAT, 26 DAT–41 DAT) and rewatered 3 days between stresses. Group 3 (G3) was subjected to two periods of stress (13 DAT–23 DAT, 23 DAT–26 DAT) with 3 days of rewatering in between. Group 4 (G4) was subjected to a single stressful event (26 DAT–41 DAT) (Fig. 1). The findings of previous studies informed our choice of 5-day cyclic water stress intervals (i.e., 5, 10, and 15 days after seedling transfer) (Banik et al. 2016; Khanna-Chopra and Selote 2007; Murshed et al. 2008; Selote and Khanna-Chopra 2010). All analysis were carried out in triplicate. At 41 DAT, leaves and roots from each treatment were collected and frozen in liquid nitrogen for all studies (physiological, biochemical, and gene expression).
Fig. 1.
Schematic experimental setup. Drought stress was applied to four wheat genotype seedlings, Geumgangmil, PL 337, PL 371, and PL 257. Group 1 stands for control plants, Group 2 for plants exposed to first stress for 5 days, second stress for 10 days, and third stress for 15 days, Group 3 for plants exposed to direct second stress for 10 days and third stress for 15 days, and Group 4 denotes plants exposed to direct stress for 15 days, during the 15 days drought period. Drought treatments were terminated by 3 days rewatering regimes. DW dry weight, RWC relative water content, EL electrolyte leakage, MSI membrane stability index, MDA malondialdehyde, SOD/SOD1 superoxide dismutase, CAT/CAT1 catalase, APX/APX1 ascorbate peroxidase, P5CS Δ-1-pyrroline-5-carboxylate synthetase, HSP heat shock protein, DREB dehydration-responsive element-binding, LEA late embryogenesis abundant, RBCL ribulose-bisphosphate carboxylase, CCD1 carotenoid cleavage dioxygenase
Growth parameters and determination of relative water content
The root and shoot dry weights of five plants from each genotype were measured. The relative water content (RWC) was measured as described by Amoah et al., (2019). The fresh weight (FW), turgid weight (TW), and dry weight (DW) of leaves and roots were measured, and the RWC was calculated as follows:
| 1 |
Membrane stability index (MSI) and electrolyte leakage (EL) measurement
The leaves were cut into 2 cm-long pieces, washed with distilled water to remove injured tissues, and ten pieces were incubated in 15 mL of distilled water for 24 h at 25 °C. Subsequently, the initial conductivity (C1) of the solutions was measured. The samples were boiled for 30 min, and the final conductivity (C2) was measured after cooling to room temperature.
| 2 |
| 3 |
Chlorophyll and carotenoid content determination
Leaf chlorophyll (CHL) and carotenoid (CAR) pigments were extracted (approximately 0.2 g) using 80% acetone on a shaker at 25 °C until the tissue was completely bleached. The extract was centrifuged at 12 000 × g for 10 min, and the absorbance of the supernatant was measured at 646, 470, and 663 nm using a spectrophotometer (UV-2550, Shimadzu, Japan). The concentration of each pigment was calculated according to the method described by Warren, (2008). The chlorophyll and carotenoid levels (μmol g−1 FW) were then estimated.
Malondialdehyde (MDA), proline, and soluble sugar determination
The MDA content was determined using the method of Shan et al., (2018). Each 0.1 g leaf sample was homogenized in 1 mL 10% (w/v) trichloroacetic acid (TCA) solution on ice. The homogenate was centrifuged at 12000×g for 10 min at 4 °C, and the supernatant was collected. Then, 1 mL 0.5% thiobarbituric acid was added to a 1 mL aliquot of the supernatant. The mixture was boiled for 25 min and immediately cooled on ice. After centrifugation at 5000×g for 10 min, the absorbance of the supernatant was measured at 532 and 600 nm. The concentration of MDA was calculated using an extinction coefficient of 155 mM/cm.
Proline (PRL) content was measured using the ninhydrin method described by Bates et al., (1973). Each 0.1 g leaf sample was homogenized in 1 mL of 3% sulfosalicylic acid. After centrifugation at 12000×g for 10 min, 0.5 mL of the supernatant was added to a mixture of 0.5 mL of glacial acetic acid and 0.5 mL ninhydrin solution. The mixture was boiled at 100 °C for 30 min and quickly cooled on ice for 20 min, followed by the addition of 1 mL of toluene, extraction of the organic phase, and reading of the absorbance at 520 nm using a UV–Vis spectrometer (UV-2550, Shimadzu, Japan). The proline concentration was determined using a calibration curve and expressed as μmol proline g−1 FW.
Soluble sugar (SS) was extracted and quantified using a modified method described by (Xu et al. 2015). Each 0.1 g leaf sample was extracted with 80% (v/v) ethanol at 80 °C for 25 min, followed by centrifugation at 10000×g for 10 min. The residue was extracted twice using 80% ethanol. The three supernatants were combined, and 80% ethanol was added to make up a total volume of 1 mL. The soluble sugar content was determined spectrophotometrically by reading the absorbance at 620 nm using a UV–Vis spectrometer (UV-2550, Shimadzu, Japan).
Hydrogen peroxide (H2O2) and antioxidant enzyme assays
The H2O2 content was measured using a modified protocol described by Yin et al. (2010). Each 0.1 g frozen leaf sample was homogenized in an ice bath with 1 mL 0.1% (w/v) TCA. The homogenate was centrifuged at 12000×g for 10 min at 4 °C. Afterwards, aliquots of 100 μL from each tube were placed in 96-well plates, and 50 μL of 10 mM potassium phosphate buffer (pH 7.0) and 100 μL of 1 M potassium iodide (KI) were added to each well. Commercial H2O2 was used to generate a standard curve. The plate was vortexed, then incubated at room temperature for 25 min, following which absorbance readings were taken at 390 nm in a microplate reader. The H2O2 content was determined using the standard curve.
For the antioxidant enzyme assays, each 0.1 g leaf sample was homogenized in 1 mL of 0.2 M potassium phosphate buffer (pH 7.0) containing 0.1 mM EDTA at 4 °C. After centrifugation at 12000×g for 20 min, the supernatant was used to determine the antioxidant enzyme (SOD, CAT, and APX) activities. Total protein content was quantified using the Bradford assay (Bradford 1976). APX activity was measured using the method described by Amoah et al., (2019). The reaction mixture comprised 50 mM HEPES, 0.1 mM EDTA, 1 mM H2O2, and 0.6 mM ascorbic acid. The oxidation of ascorbic acid was indicated by decreasing absorbance at 290 nm, 1 min after the start of the reaction (e = 2.6 mM−1 cm−1). CAT activity was assayed spectrophotometrically by monitoring the decrease in the absorbance of H2O2 at 240 nm using the method described by Behnamnia et al., (2009). The assay solution contained 50 mM potassium phosphate buffer (pH 7.0) and 15 mM H2O2. The reaction was initiated by adding 60 μL of the enzyme extract to the reaction mixture. The change in absorbance was determined 1 min after the start of the reaction (e = 43.6 mM−1 cm−1).
Each 2 mL SOD reaction solution comprised 50 μM ρ-nitro blue tetrazolium chloride, 1.3 μM riboflavin, 13 mM methionine, 75 nM EDTA, 50 mM phosphate buffer (pH 7.8), and 20–50 μL of the enzyme extract. Test tubes containing the reaction solution were irradiated with light at 78 μmol m−2 s−1 for 30 min. The absorbance of the irradiated solution was read at 560 nm using a spectrophotometer (APEL PD 303 UV–Vis Spectrophotometer, Tokyo, Japan). A unit of SOD activity was defined as the amount of enzyme that inhibited 50% ρ-nitro blue tetrazolium chloride (NBT) photoreduction ((Amoah et al. 2019).
Analysis of the expression of antioxidant-, photosynthesis-, osmolyte biosynthesis- and stress-related genes
Leaf total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA was quantified spectrophotometrically, and the quality was evaluated by agarose gel electrophoresis. Synthesis of cDNA was performed using a Power cDNA Synthesis Kit (Intron Biotechnology Inc., South Korea).
Quantitative real-time polymerase chain reaction (qPCR) was performed using a CFX 96 Real-Time system (Bio-Rad, Richmond, CA, USA) with SYBR-green fluorescence, and the results were analyzed using the ΔΔCT method. Gene-specific primers (Table 1) for qPCR were used to evaluate the genes’ activity under progressive drought conditions. The thermal cycle employed was 95 °C for 5 min and 40 cycles of 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s. All experiments were conducted with three biological replicates, and the relative transcript levels were standardized using Actin as the internal control.
Table 1.
List of primers used for quantitative real polymerase chain reaction (qPCR)
| Gene name | Gene identity | Primer sequence |
|---|---|---|
| SOD1 | TraesCS7B02G197300.1 |
5ʹ–ACCTCCATGAGTTCGGTGAC–3ʹ 3ʹ–CATTAGGGCCAGTCAAAGGA–5ʹ |
| CAT1 | TraesCS6A02G041700.1 |
5ʹ–TGGATGGACCGTTCTGTGTA–3ʹ 3ʹ–TAAGCTCCAAAGCACCCACT–5ʹ |
| APX1 | TraesCS2A02G082100.1 |
5ʹ–GCCTGAAGGTCGTTAATGGA–3ʹ 3ʹ–CAACCAAATCTCCGTTTCGT–5ʹ |
| P5CS | TraesCS1D02G212400 |
5ʹ–GGGAAAGGTGGAAGATTGGC–3ʹ 3ʹ–AGCCTTCCCATCAAGTTCCA–5ʹ |
| HSP | TraesCS4A02G062800.1 |
5ʹ–CTTCATACGCCAGCTCAACA–3ʹ 3ʹ–GCTTTGACAGGTGCCTTCTC–5ʹ |
| DREB2 | TraesCS3A02G099200.1 |
5ʹ–TGGCTTGGTTCATTCCCTAC–3ʹ 3ʹ–CCCCATTAGACGTCAGCAAT–5ʹ |
| LEA | TraesCS4D02G282400.1 |
5ʹ–GGAGGTGAAGGACAAGGTCA–3ʹ 3ʹ–GTCTGATCGTGCAGGGTTCT–5ʹ |
| CCD1 | TraesCS6A02G271600 |
5ʹ–ATGGAGGGAGCACTTGTCAC–3ʹ 3ʹ–CCTTCCTCCGTCTGTAGCTG–5ʹ |
| RBCL | TraesCS2A02G066800.1 |
5ʹ–CTGGCAAGGCCTAAACTACG–3ʹ 3ʹ–TGCATGCTCGAGAACCTATG–5ʹ |
| Actin | TraesCS1D02G020000 |
5ʹ–ACTGTGCCCATTTACGAAGG–3ʹ 3ʹ–GCAGTCTCCAGCTCCTGTTC–5ʹ |
Statistical analysis
The data were analyzed using R (v.3.5.1). The data were analyzed by two-way analysis of variance (ANOVA) with genotype as the fixed effect and drought treatments as random factors. The differences between means were assessed via Tukey’s multiple range test (P < 0.05) using SIGMAPLOT (v.14.0). Each result was summarized by the mean ± standard error (SE) of three independent experiments. Systematic clustering, and GraphPad Prism 5 and TBtools (Chen et al. 2018) (v1.0971) were used for preparation of the figures.
Results
Changes in physiological and biochemical response parameters of wheat genotypes
Growth, photosynthetic activity, and water content
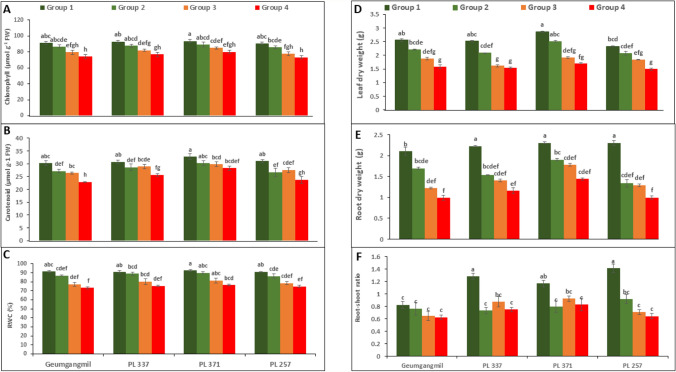
The drought tolerance of four wheat genotypes were analyzed under different drought treatments (G1–4). Significant differences were observed in plant fresh and dry weights, photosynthetic pigments, and water content (Fig. 2). Total CHL, CAR, RWC, LDW, RDW, and RS were shown to be reduced in drought stressed (G2–4) plants than in control (G1) plants. Figure 2 A, B shows the CHL and CAR contents, which determine the photosynthetic performance of the drought treated and control plants. The CHL content ranged from 3% (PL 371 under G2) to 19% (PL 257 under G4), while the CAR level ranged from 4% (PL 371 under G2) to 21% (PL 257 under G4). Furthermore, whereas G1 and G2 treatments in genotypes were statistically comparable in terms of CHL content, only G1 and G2 treatments in PL 371 were statistically similar in terms of CAR content. In addition, CHL and CAR levels were greatly affected by T, but not G and G × T (Table 2).
Fig. 2.
Effect of drought stress on chlorophyll (A), carotenoid (B), and relative water content (C), leaf dry weight (D), root dry weight (E), and root to shoot ratio (F) of four wheat genotypes. Values are means ± standard error (SE) of three independent samples. Different letters on vertical bars indicate significant differences between means at P ≤ 0.05
Table 2.
Analysis of variance (ANOVA) of the physiological, biochemical, and transcript responses of Geumgangmil, PL 337, PL 371, and PL 257 under control and different episodes of drought stress
| Parameters | Source of variations | ||
|---|---|---|---|
| Genotype (G) | Treatment (T) | G × T | |
| (df = 3) | (df = 3) | (df = 5) | |
| Chlorophyll (µmol g−1 FW) | ns | ** | ns |
| Carotenoids (µmol g−1 FW) | ns | ** | ns |
| Relative water content (%) | ** | ** | ** |
| Membrane stability index (%) | ** | ** | ** |
| Malondialdehyde (µmol g−1 FW) | ** | ** | ** |
| Leaf dry weight (g) | ** | ** | ** |
| Root dry weight (g) | ** | ** | ** |
| R–S ratio | ** | ** | ** |
| Soluble sugar (µmol g−1 FW) | ** | ** | ** |
| Proline (µmol g−1 FW) | ** | ** | ** |
| Electrolyte leakage (%) | ** | ** | ** |
| H2O2 (µmol g−1 FW) | ** | ** | ns |
| SOD (µmol min-1 g-1 protein) | ** | ** | ** |
| CAT (µmol min−1 g−1 protein) | ** | ** | ** |
| APX (µmol min−1 g−1 protein) | ** | ** | ** |
| SOD1 | ** | ** | ** |
| CAT1 | ** | ** | ** |
| APX1 | ** | ** | ** |
| P5CS | ** | ** | ** |
| HSP | ** | ** | ** |
| DREB2 | ** | ** | ** |
| LEA | ** | ** | ** |
| CCD1 | ** | ** | ** |
| RBLC | ** | ** | ** |
ns not significant, SOD//SOD1 superoxide dismutase, CAT//CAT1 catalase, APX//APX1 ascorbate peroxidase, P5CS Δ1‑pyrroline‑5‑carboxylate synthase gene, HSP heat shock protein, DREB dehydrin response element binding, LEA late embryonic abundant, CCD1 carotenoid cleavage dioxygenase, RBCL Ribulose-1,5-bisphosphate carboxylase
*, **Significant at the 0.05 and 0.01 probability levels, respectively
RWC, LDW, and RWD reductions were highest under G4, at 17% (PL 257), 19% (Geumgangmil), and 56% (PL 257), respectively (Fig. 2C–D). Interestingly, the RWC and LDW of G1 and G2 were statistically comparable in some genotypes. However, a significant difference was observed between the control and treatments in relation to RDW in genotypes (Fig. 2E). RS was also estimated in G1 and G2–4 plants of wheat genotypes. G1 plants showed a higher RS, except in Geumgangmil. In contrast, a non-significant difference in RS was observed between treatments (G2–4) (Fig. 2F). The RS varied from 7% (Geumgangmil under G2) to 54% (PL 257 under G4). RWC, RDW, LDW, and RS were significantly affected by G, T, and G × T (Table 2).
Membrane status, osmolyte accumulation, and antioxidant enzymes activity
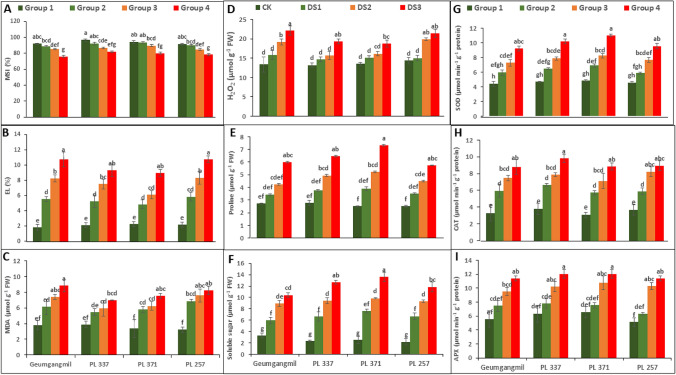
Drought stress resulted in lower MSI values and higher levels of EL, MDA, PRL, SS, H2O2, SOD, CAT, and APX in G2-G4 than in controls (G1) (Fig. 3A–I). G2-treated plants and control plants were statistically similar, while G3 and G4 treatment conditions resulted in 9% and 17% decreased MSI values of PL 337 and Geumgangmil, respectively (Fig. 3A). Furthermore, a higher injury index was observed in G3 and G4 treatment conditions than in G2. Of the four genotypes, PL 371 had significantly less membrane injury than the other genotypes (Fig. 3B). The MSI and EL of wheat genotypes were significantly affected by G and T, but slightly less affected by the G × T interaction (Table 2).
Fig. 3.
Effect of drought stress on membrane stability index (MSI) (A), electrolyte leakage (EL) (B), and malondialdehyde (MDA) content (C), hydrogen peroxide (H2O2) accumulation (D) proline level (E), soluble sugar level (F), superoxide dismutase (SOD) activity (G), catalase (CAT) activity (H), and ascorbate peroxidase (APX) (I) activity of four wheat genotypes. Values are means ± standard error (SE) of three independent samples. Different letters on vertical bars indicate significant differences between means at P ≤ 0.05
Figure 3C, D shows changes in the MDA and H2O2 contents in the G1 and G2–G4 plants of wheat genotypes in response to drought stress. There were significant differences observed between the various treatments compared to the control in genotypes. The magnitude of increase in MDA level was greater in G3 and G4 treated plants than in G2 plants (Fig. 3C). MDA levels ranged from 11 to 17% in PL 257 under G2 and G4 treatment conditions. Similarly, H2O2 levels in G4 treated Geumgangmil plants were 65% higher than in controls (a significant difference), surpassing the 46%, 38%, and 48% greater H2O2 levels in G4 treated PL 337, PL 371, and PL 257 plants. H2O2 levels in G2-treated plants, on the other hand, were statistically similar to those in control (G1) plants (Fig. 3D). G, T, and G × T, all had a significant impact on MDA and H2O2, while G × T did not affect H2O2 levels (Table 2).
Drought stressed (G2–4) plants had significantly higher PRL content than control (G1) plants. However, the PRL content of G2 plants was comparable with that of control (G1) plants, but G3 and G4 treated PL 371 plants showed a significant 51% and 65% increase in PRL, respectively (Fig. 3E). Similarly, SS levels rose with increasing duration of drought stress in G2–4-treated plants of all genotypes, but the magnitude of the difference in this variable was relatively low G2 plants than G3 and G4 (Fig. 3F). The SS level ranged from 2% (Geumgangmil under G2) to 81% (PL 337 under G4). G, T, and G × T, all affected PRL and SS levels (Table 2).
Antioxidant enzyme activity, specifically that of SOD, CAT, and APX, increased with the intensity and duration of drought stress (Fig. 3G–I). SOD activity was greatest in PL 371 at 43%, 62%, and 71% under G2, G3, and G4, respectively, as compared to G1 (Fig. 3 G). Similarly, the PL 371 genotype demonstrated greater CAT activity under drought treatment conditions (G2 by 46%, G3 by 56%, and G4 by 65%) than G1 (Fig. 3 H). In the PL 257 genotype, a non-significant increase in CAT activity was observed between G3 and G4 treatments (Fig. 3H). In Fig. 3I, PL 257 showed greater APX activity, particularly under G3 and G4 conditions. The APX activity ranged from 17% (Geumgangmil under G2) to 61%. (PL 257 under G4). Furthermore, in most genotypes, APX activity was comparable between G1 and G2, as well as G3 and G4 (Fig. 3I). SOD, CAT, and APX activities were greatly affected by G, T, and G × T, according to the two-way ANOVA (Table 2).
Transcriptional expression of antioxidant-, osmolyte biosynthesis-, and stress-related genes
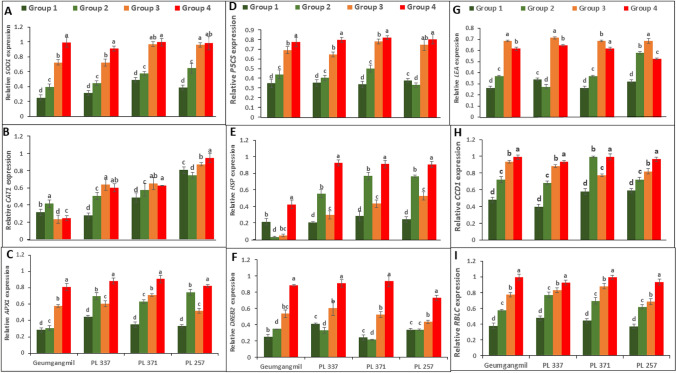
The expression patterns of antioxidant-related (SOD1, CAT1, and APX1), osmolyte biosynthesis-related (P5CS), stress-expressed (LEA, HSP, and DREB2), and photosynthesis-related (CCD1 and RBCL) genes were investigated to gain insight into the regulatory mechanisms of wheat genotypes under G1–4 treatment conditions (Fig. 4). The results indicated that the transcriptional expression levels of SOD1, CAT1, and APX1 were higher in plants treated with drought than in controls (Fig. 4A–C). The expression levels of these genes, however, were higher in G4 and G3 plants than in G2 plants. Furthermore, between G3 and G4 plants of PL 371 and PL 257 (SOD1) and PL 337 and PL 371 (CAT1), there was a non-significant increase in the expression of these genes, whereas G2 plants of Geumgangmil displayed a higher expression of the CAT1 gene in comparison to control (G1) and other treatments (G3 and G4) (Fig. 4A–C). G, T, and G × T influenced SOD1, CAT1, and APX1 expression (Table 2).
Fig. 4.
Relative expression levels of antioxidant-related genes in wheat seedlings of four genotypes (Geumgangmil, PL 337, PL 371, and PL 257) under drought stress. Expression patterns relative to that of Actin are shown for superoxide dismutase (SOD1) (A), catalase (CAT1) (B), and peroxidase (APX1) (C), Δ-1-pyrroline-5-carboxylate synthetase (P5CS) (D), heat shock proteins (HSP) (E), and dehydration-responsive element-binding (DREB) (F), late embryogenesis abundant (LEA) (G), ribulose-bisphosphate carboxylase (RBCL) (H), and carotenoid cleavage dioxygenase (CCD1) (I). Values are means ± standard error (SE) of three independent samples. Different letters on vertical bars indicate significant differences between means at P ≤ 0.05
Similarly, the osmolyte biosynthesis-related gene P5CS1 was found to be more expressed in G2–4 plants than G1 plants (Fig. 4D). P5CS1 expression was higher in G3 and G4 wheat genotypes than in other treatments. However, P5CS1 expression in G3 was similar to that in G4 in PL 257. (Fig. 1D). G, T, and G × T greatly affected the expression of P5CS1 (Table 2). A remarkable difference in the relative expression of HSP, DREB, and LEA were observed. Under G4, the expression of these genes was two–threefold higher than under G2 and G3 treatment conditions (Fig. 4E–G). The difference in expression of HSP and DREB was the greatest, at 4.3-fold (PL 337) and 3.8-fold (PL 371), respectively, under G4 rather than G2 or G3 stress conditions, whereas LEA expression ranged from 1.6-fold (PL 257) to 2.4-fold (PL 371). The two-way ANOVA also showed a significant effect of G, T, and G × T on HSP, DREB, and LEA expression (Table 2).
Also, the expression of CCD1 and RBCL genes were found to be significantly induced in drought stressed plants of genotypes (Fig. 4H–I). Comparatively, the expression of these genes was relatively higher in G3 and G4 plants than in G2 plants. CCD1 expression ranged from 1.2-fold (PL 257 under G2) to 2.3-fold (PL 337 under G4) (Fig. 4H), while RBCL gene expression was minimal at 1.5-fold (Geumgangmil under G2) and maximal at 2.8-fold (Geumgangmil under G4) (Fig. 4I). G, T, and G × T affected CCD1 and RBCL expression (Table 2).
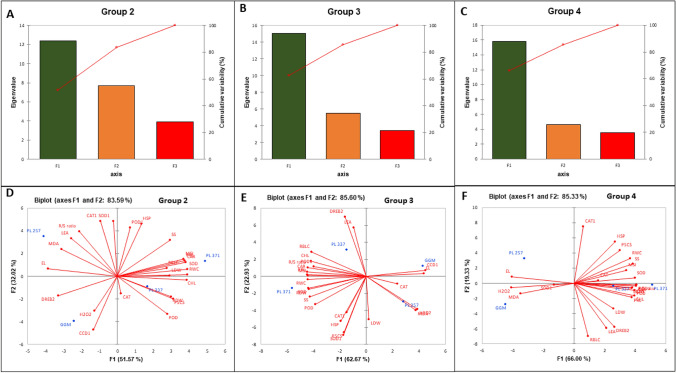
Principal component analysis
Complete data sets, showing the physiological and molecular changes under G2, G3, and G4 treatment conditions, were subjected to principal component analysis. Three principal components were extracted and together these dimensions explained 86.59%, 85.59%, and 85.32% of the total variability under G2, G3, and G4 treatment conditions (Figs. 5A, B, and C and Tables S1-3).
Fig. 5.
Principal component analysis (PCA) for physiological and molecular responses in four wheat genotypes under different drought treatments. Scree plot and biplot of variance explained by each factor of the principal component in Group 2 plants (A/D), Group 3 plants (B/E), and Group 4 plants (C/F). F1–F3 the first to third principal component
Under G2 treatment condition, PC1 was heavily associated with CHL, CAR, RWC, MSI, PRL, and SOD activity; PC2 gave a high weighting to SOD1, CAT1, HSP, and CCD1 (negatively); and PC3 was associated with CAT activity (Fig. 5A and Table S1). Under G3 treatment, PC1 was strongly positively associated with EL and CCD1 expression but negatively associated with CHL, CAR, RWC, RWD, RS, MSI, EL, PRL, MDA, SOD and RBCL expression; PC2 was associated with DREB2 (positively) and SOD1 (negatively) expression; and PC3 was heavily associated with CAT activity (Fig. 5E, Table S2). In addition, under G4, PC1 was positively associated with CHL, CAR, RDW, RS, PRL, SS, SOD, APX, APX, and CCD1 expression but negatively associated with EL, MDA, and H2O2; PC2 was strongly correlated with CAT1 expression (positively) and RBCL expression (negatively); and PC3 was positively correlated with SOD1 but negatively correlated with APX activity (Fig. 5F, Table S3).
The principal components reflected various aspects of physiological and molecular (transcript expression) responses. In addition, the factor scores of each treatment group (G2–4) obtained from the three PCAs were computed to evaluate the variation among the four wheat genotypes in terms of their response to the different drought treatment conditions at the physiological and transcriptional (molecular level) (Table 3). Under G2, PL 371 showed a strongest response, but Geumgangmil showed a weak response. Under G3, PL 337 had the greatest response, while PL 371 showed a weak response. Under G4, PL 371 showed the greatest response, contrary to a weaker response exhibited by Geumgangmil (Table 3).
Table 3.
Total score of physiological and molecular response of wheat genotypes
| Treatment group | Genotype | |||
|---|---|---|---|---|
| Geumgangmil (non-translocated) | PL 337 (1AL.1RS) | PL 371 (1BL.1RS) | PL 257 (1DL.1RS) | |
| Group 2 | − 7.77 | 3.99 | 4.4 | − 0.62 |
| Group 3 | 3.239 | 3.58 | − 8.381 | 1.562 |
| Group 4 | − 6.703 | − 0.726 | 7.101 | 0.328 |
Values represent the total factor score obtained from the three principal components (PC1–3) analysis, under each treatment condition (G2–4). Group 2, plants exposed to three water stress cycles (5, 10, and 15 days) with 3 days rewatering between stress treatments; Group 3, plants exposed to two water stress cycles (10 and 15 days) with 3 days rewatering between treatments; and Group 4, plant exposed to single stressful event (15 days) without rewatering
Correlation analysis between relative expression of transcripts and changes in physiological parameters
Following the different treatments, a correlation analysis was performed to assess the relationship between the physiological indicators of the treatments and the expression patterns of the nine genes (Tables 4, 5, 6). Under G2, SOD1 was strongly associated with RS, CAT1 with RS and APX, APX1 with SS, P5CS with CHL, LDW, RDW, and EL, HSP with proline, DREB2 was negatively correlated with CHL, CAR, LDW, RDW, MSI, EL, H2O2, PRL, and SOD, LEA with MDA, RS, and APX (negative), CCD1 with proline (negative), and RBCL with MSI (positive) and SS (negative) (Table 4). Under G3, APX1 expression was strongly positively associated with CHL, RDW, and MSI, but negatively correlated with EL and CAT, P5CS with LDW, HSP with APX, LEA with LDW, CCD1 with CHL, CAR, RWC, RDW, RS, MSI, EL, H2O2, SOD, and APX (negative), and RBCL with all the physiological parameters except LDW (Table 5). Also, under G4, SOD1 was strongly associated with CAT (negatively), APX1 showed a strong positive or negative correlation with all the physiological parameters except CAT activity, P5CS with RWC, PRL, and SOD, HSP with RWC, MSI, and H2O2 accumulation, and CCD1 with all parameters except MSI, MDA, and CAT activity (Table 6).
Table 4.
Pearson’s correlation analysis between the expression of the nine (9) genes and physiological indicators under group (G2) treatment condition
| Parameter | Relative gene expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOD1 | CAT1 | APX1 | P5CS | HSP | DREB2 | LEA | CCD1 | RBCL | |
| Chlorophyll | − 0.078 | − 0.294 | 0.036 | 0.882** | 0.254 | − 0.877** | − 0.615* | − 0.268 | 0.553 |
| Carotenoid | 0.201 | 0.010 | 0.349 | 0.697* | 0.551 | − 0.925** | − 0.434 | − 0.559 | 0.658* |
| RWC | − 0.056 | − 0.210 | 0.258 | 0.717* | 0.384 | − 0.793* | − 0.666* | − 0.371 | 0.778* |
| LDW | 0.120 | − 0.162 | − 0.184 | 0.895** | 0.169 | − 0.911** | − 0.230 | − 0.231 | 0.048 |
| RDW | − 0.276 | − 0.532 | − 0.438 | 0.997** | − 0.154 | − 0.742* | − 0.542 | 0.108 | 0.102 |
| RS | 0.869** | 0.901** | 0.443 | -0.602* | 0.512 | 0.054 | 0.983** | − 0.549 | − 0.467 |
| MSI | 0.205 | 0.071 | 0.507 | 0.542 | 0.629* | − 0.821** | − 0.463 | − 0.615* | 0.811** |
| EL | 0.161 | 0.368 | 0.010 | − 0.892** | − 0.189 | 0.833** | 0.679* | 0.198 | − 0.570 |
| MDA | 0.603* | 0.660* | 0.087 | − 0.634* | 0.124 | 0.332 | 0.973** | − 0.167 | − 0.752* |
| H2O2 | 0.187 | 0.043 | 0.471 | 0.579 | 0.604* | − 0.839** | − 0.476 | − 0.593 | 0.792* |
| Proline | 0.620* | 0.443 | 0.587 | 0.523 | 0.822** | − 0.923** | 0.013 | − 0.845** | 0.475 |
| SS | − 0.400 | − 0.502 | − 0.927** | 0.324 | − 0.774* | 0.168 | 0.073 | 0.711* | − 0.823** |
| SOD | 0.064 | − 0.120 | 0.272 | 0.742* | 0.451 | − 0.880** | − 0.556 | − 0.451 | 0.698* |
| CAT | -0.518 | − 0.316 | 0.207 | − 0.285 | − 0.134 | 0.470 | − 0.581 | 0.227 | 0.658* |
| APX | − 0.742* | − 0.821** | − 0.338 | 0.722* | − 0.346 | − 0.272 | − 0.988** | 0.376 | 0.558 |
Bold indicates statistical significance at *P ≤ 0.05, **P ≤ 0.01, respectively
RWC, relative water content, LDW leaf dry weight, RDW root dry weight, RS root-shoot ratio, MSI membrane stability index, EL electrolyte leakage, MDA malondialdehyde content, H2O2 hydrogen peroxide level, SS soluble sugar SOD//SOD1 superoxide dismutase, CAT//CAT1 catalase APX//APX1 ascorbate peroxidase, P5CS Δ1‑pyrroline‑5‑carboxylate synthase gene, HSP heat shock protein, DREB dehydrin response element binding, LEA late embryonic abundant, CCD1 carotenoid cleavage dioxygenase, RBCL ribulose-15-bisphosphate carboxylase
*, **Significant at the 0.05 and 0.01 probability levels, respectively
Table 5.
Pearson’s correlation analysis between the expression of the nine (9) genes and physiological indicators under group (G3) treatment condition
| Parameter | Relative gene expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOD1 | CAT1 | APX1 | P5CS | HSP | DREB2 | LEA | CCD1 | RBLC | |
| Chlorophyll | 0.112 | − 0.065 | 0.983** | 0.225 | 0.053 | 0.568 | 0.190 | 0.924** | 0.981** |
| Carotenoid | 0.327 | 0.407 | 0.796* | 0.252 | 0.468 | 0.400 | 0.364 | 0.950** | 0.889** |
| RWC | 0.436 | 0.505 | 0.751* | 0.338 | 0.573 | 0.284 | 0.301 | 0.949** | 0.829** |
| LDW | 0.546 | − 0.195 | 0.198 | 0.802** | 0.026 | − 0.595 | − 0.975** | − 0.097 | − 0.167 |
| RDW | 0.538 | 0.279 | 0.899** | 0.589 | 0.439 | 0.165 | − 0.065 | 0.990** | 0.829** |
| RS | 0.262 | 0.381 | 0.787* | 0.182 | 0.428 | 0.458 | 0.426 | 0.931** | 0.901** |
| MSI | 0.348 | 0.141 | 0.958** | 0.421 | 0.281 | 0.367 | 0.076 | 0.988** | 0.927** |
| EL | − 0.392 | − 0.140 | − 0.957** | − 0.477 | − 0.293 | − 0.314 | − 0.008 | 0.986** | − 0.903** |
| MDA | 0.158 | − 0.104 | − 0.724* | 0.216 | − 0.085 | − 0.776* | − 0.690 | 0.752* | − 0.923** |
| H2O2 | 0.370 | 0.431 | 0.793* | 0.293 | 0.499 | 0.359 | 0.329 | 0.957** | 0.875** |
| Proline | 0.659* | 0.564 | 0.730* | 0.591 | 0.683* | 0.032 | 0.028 | 0.952** | 0.717* |
| SS | 0.135 | − 0.064 | − 0.780* | 0.164 | − 0.065 | − 0.772* | − 0.631* | − 0.788* | − 0.952** |
| SOD | 0.556 | 0.545 | 0.744* | 0.470 | 0.639* | 0.156 | 0.167 | 0.958** | 0.778* |
| CAT | − 0.027 | 0.540 | − 0.860** | − 0.351 | 0.347 | − 0.348 | 0.323 | 0.593 | − 0.665* |
| APX | 0.750* | 0.745* | 0.544 | 0.605* | 0.836** | − 0.126 | 0.033 | − 0.851** | 0.553* |
Bold indicates statistical significance at *P ≤ 0.05, **P ≤ 0.01, respectively
RWC relative water content, LDW leaf dry weight, RDW root dry weight, RS root-shoot ratio, MSI membrane stability index, EL electrolyte leakage, MDA malondialdehyde content, H2O2 hydrogen peroxide level, SS soluble sugar, SOD//SOD1 superoxide dismutase, CAT//CAT1 catalase, APX//APX1 ascorbate peroxidase, P5CS Δ1‑pyrroline‑5‑carboxylate synthase gene, HSP heat shock protein, DREB dehydrin response element binding, LEA late embryonic abundant, CCD1 carotenoid cleavage dioxygenase, RBCL Ribulose-15-bisphosphate carboxylase
*, **Significant at the 0.05 and 0.01 probability levels, respectively
Table 6.
Pearson’s correlation analysis between the expression of the nine (9) genes and physiological indicators under group (G4) treatment condition
| Parameter | Relative gene expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOD1 | CAT1 | APX1 | P5CS | HSP | DREB2 | LEA | CCD1 | RBLC | |
| Chlorophyll | − 0.172 | − 0.073 | 0.972** | 0.629* | 0.454 | 0.791* | 0.623* | 0.963** | 0.382 |
| Carotenoid | − 0.238 | 0.103 | 0.996** | 0.730* | 0.608* | 0.679* | 0.523 | 0.955** | 0.247 |
| RWC | − 0.239 | 0.568 | 0.883** | 0.931** | 0.900** | 0.245 | 0.099 | 0.815** | − 0.216 |
| LDW | 0.415 | − 0.352 | 0.642** | 0.435 | -0.026 | 0.739* | 0.450 | 0.857** | 0.354 |
| RDW | 0.036 | 0.064 | 0.935** | 0.767* | 0.499 | 0.656* | 0.417 | 0.999** | 0.172 |
| RS | − 0.198 | 0.117 | 0.992** | 0.751* | 0.610* | 0.664* | 0.494 | 0.966** | 0.218 |
| MSI | − 0.737* | 0.364 | 0.834** | 0.572 | 0.817** | 0.371 | 0.423 | 0.576 | 0.132 |
| EL | 0.387 | − 0.033 | − 0.993** | − 0.626* | − 0.583 | − 0.732* | − 0.632 | − 0.897** | − 0.362 |
| MDA | 0.744* | − 0.315 | − 0.845** | − 0.547 | − 0.787* | − 0.416 | − 0.469 | − 0.588 | − 0.182 |
| H2O2 | 0.003 | − 0.102 | 0.927** | 0.645* | 0.377 | 0.776* | 0.557 | 0.986** | 0.337 |
| Proline | − 0.232 | 0.474 | 0.928** | 0.909** | 0.850** | 0.349 | 0.193 | 0.868** | − 0.119 |
| SS | 0.389 | − 0.226 | − 0.992** | − 0.743* | − 0.726* | − 0.586 | − 0.481 | − 0.886** | − 0.183 |
| SOD | − 0.130 | 0.235 | 0.973** | 0.837** | 0.676* | 0.560 | 0.366 | 0.967** | 0.080 |
| CAT | − 0.997** | 0.122 | 0.389 | − 0.020 | 0.467 | 0.222 | 0.482 | 0.015 | 0.331 |
| APX | − 0.513 | 0.034 | 0.972** | 0.561 | 0.597 | 0.717* | 0.668* | 0.825** | 0.399 |
Bold indicates statistical significance at *P ≤ 0.05, **P ≤ 0.01, respectively
RWC relative water content, LDW leaf dry weight, RDW root dry weight, RS root-shoot ratio, MSI membrane stability index, EL electrolyte leakage, MDA malondialdehyde content, H2O2 hydrogen peroxide level, SS soluble sugar, SOD//SOD1 superoxide dismutase, CAT//CAT1 catalase, APX//APX1 ascorbate peroxidase, P5CS Δ1‑pyrroline‑5‑carboxylate synthase gene, HSP heat shock protein, DREB dehydrin response element binding, LEA late embryonic abundant, CCD1 carotenoid cleavage dioxygenase, RBCL Ribulose-15-bisphosphate carboxylase
*, **Significant at the 0.05 and 0.01 probability levels, respectively
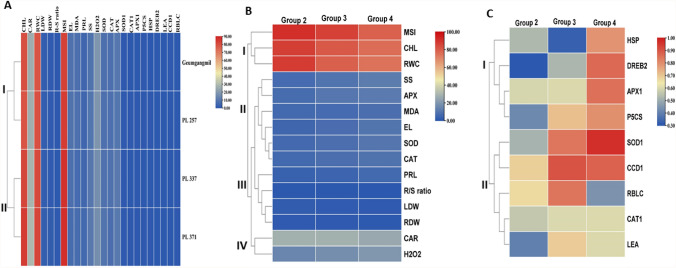
Cluster analysis of the wheat genotypes after different drought treatments
The values of physiological indicators and transcript expression levels were used in a cluster analysis to determine the performance of wheat genotypes under different treatment (G2–4) conditions. It was observed that the four wheat genotypes exposed to the drought treatments were classified into two categories (Fig. 6A). Group I consisted of two genotypes which exhibited less resistance to drought stress, Geumgangmil and PL 257, while Group II consisted of PL 337 and PL 371, which showed greater resistance under different drought conditions (Fig. 6A). Furthermore, from Fig. 6B, the physiological indicators were classified into four categories. Group I consisted of physiological indicators (MSI, RWC, and Chlorophyll) that significantly decreased after first stress. Group II consisted of six members (SS, APX, MDA, EL, SOD, and CAT) that were highly induced after second stress. Group III consisted of four members (Pro, R–S ratio, LDW, and RDW) that were weakly decreased after first stress. Group IV also consisted of two members (Car and H2O2 content) that weakly increased after first stress. In addition, the molecular indicators were clustered into three groups (Fig. 6C). Group I had four genes (HSP, DREB2, APX1, and P5CS) that were significantly upregulated after first stress, while Group II consisted of five genes (SOD1, CCD1, RBCL, CAT1, LEA) that were weakly upregulated after first stress.
Fig. 6.
Heat map of wheat genotypes (A), physiological (B), and molecular (C) indicators under different drought treatment conditions
Drought resistance classification
The drought resistance (D) was estimated to determine the resistance of the wheat genotype to the different treatments. The drought resistance of wheat genotypes was rated using their D value, with higher D values representing greater drought resistance and lower D values representing weaker drought resistance (Li et al. 2020a). Furthermore, the drought resistance coefficient (DC) value of each index was estimated using a PCA, and the eigenvalue vectors, factor loadings, contribution rates, and weights were determined using the contribution rates, and then those values were combined with the membership function values to estimate the metric value D. From Table 8, PL 337 had the highest D value, while PL 257 had the smallest D value, demonstrating that PL 337 (1AL.1RS) was more resistant to drought.
Table 8.
Ranking of wheat genotypes based on drought resistance (D) values
| Genotype | D value | Rank |
|---|---|---|
| Geumgangmil (non-translocated) | 21.181 | 3 |
| PL 337 (1AL.1RS) | 31.540 | 1 |
| PL 371 (1BL.1RS) | 27.819 | 2 |
| PL 257 (1DL.1RS) | 19.460 | 4 |
Discussion
Crops grown in arid climates are vulnerable to recurrent drought cycles. Recently, there has been a greater emphasis placed on crop drought resistance. Crop drought tolerance is a complicated process that requires extensive research at the physiological, biochemical, and molecular levels to understand the mechanisms behind drought tolerance and safeguard valuable genetic resources. As a result, the effect of single and multiple drought stress was compared in four wheat genotypes in the present study to examine drought tolerance and adaptation mechanisms. To achieve these objectives, four wheat genotypes were subjected to three distinct drought treatments (G2–4) and a control treatment (G1). The results showed that exposing wheat seedlings to initial (first) stress (in G2 treatment condition) induced a hardening or acclimation response to subsequent drought stress, contributing to drought resistance in wheat genotypes.
The wheat genotypes studied showed similar patterns of physiological response to drought treatments. Drought decreased chlorophyll, carotenoid, water content, leaf and root dry weights, R–S ratio, MSI, and increased MDA, EL, proline, soluble sugar, H2O2 accumulation, and antioxidant enzymes (SOD, CAT, and APX) activities, which was consistent with previous studies (Du et al. 2020; Govindasamy et al. 2020; Gujjar et al. 2021; Hamurcu et al. 2020; Raja et al. 2020; Upadhyay et al. 2020; Wu et al. 2020) (Figs. 2, 3). Although the genotypes showed similar physiological response patterns under different treatment conditions (G2–4), the degree of these changes differed between genotypes. Most indicators (traits) were significantly influenced by genotype (G), treatment (T), or the interaction of genotype and treatment (G × T) (Table 2).
Furthermore, the correlation analysis between the physiological changes and drought treatment responses indicated that the plants under G2 maintained higher photosynthetic activity (CHL and CAR), water content (RWC and RDW), biomass accumulation (RS), MSI, osmolyte accumulation (SS and PRL), antioxidant (SOD and APX) activity, lower EL, MDA, and H2O2 accumulation contributed to drought tolerance in plants under G2, compared to plants under G3 and G4 treatment conditions (Table 7).
Table 7.
Pearson’s correlation of Correlation analysis between physiological and molecular indicators and drought treatment responses
| Parameter | G2 | G3 | G4 |
|---|---|---|---|
| CHL | 0.938** | − 0.266 | − 0.221 |
| CAR | 0.998** | 0.030 | − 0.011 |
| RWC | 0.994** | − 0.048 | 0.093 |
| LDW | 0.945** | − 0.074 | − 0.881** |
| RDW | 0.880** | 0.092 | − 0.466 |
| R/S ratio | 0.467 | − 0.317 | 0.079 |
| MSI | 0.981** | 0.052 | 0.185 |
| EL | − 0.974** | − 0.166 | 0.154 |
| MDA | − 0.821** | − 0.419 | − 0.389 |
| PRL | 0.991** | 0.069 | − 0.118 |
| SS | 0.943** | − 0.332 | 0.031 |
| H2O2 | − 0.935** | − 0.209 | − 0.288 |
| SOD | 0.997** | − 0.058 | − 0.060 |
| CAT | − 0.071 | 0.183 | 0.981** |
| APX | 0.942** | 0.326 | 0.079 |
| SOD1 | 0.837** | − 0.933** | − 0.270 |
| CAT1 | 0.719** | − 0.876** | 0.429 |
| APX1 | 0.839** | − 0.385 | 0.384 |
| P5CS | 0.658 | − 0.185 | − 0.730 |
| HSP | 0.591 | − 0.718 | 0.367 |
| DREB2 | 0.201 | 0.969** | 0.145 |
| LEA | − 0.446 | − 0.895** | − 0.009 |
| CCD1 | − 0.658 | 0.720** | − 0.221 |
| RBCL | 0.838** | 0.524 | 0.151 |
G2 ~ 4 drought treatment Group 2 ~ 4, CHL chlorophyll content, CAR carotenoid content, RWC relative water content, LDW leaf dry weight, RDW root dry weight, RS root-shoot ratio, MSI membrane stability index, EL electrolyte leakage, MDA malondialdehyde content, H2O2 hydrogen peroxide level, SS soluble sugar, SOD//SOD1 superoxide dismutase, CAT//CAT1 catalase, APX//APX1 ascorbate peroxidase, P5CS Δ1‑pyrroline‑5‑carboxylate synthase gene, HSP heat shock protein, DREB dehydrin response element binding, LEA late embryonic abundant, CCD1 carotenoid cleavage dioxygenase, RBCL Ribulose-15-bisphosphate carboxylase
*, **Significant at the 0.05 and 0.01 probability levels, respectively
A plant’s capacity to maintain a good water status is important for optimal physiological functioning and growth. Previous research has found that high RWC, LDW, RDW, and RS values are associated with drought tolerance (Amoah et al. 2019; Du et al. 2020; Wu et al. 2021), which aligns with our findings on RWC, LDW, and RDW but not RS (Fig. 2C–F). The R–S ratio was higher for control plants of genotypes but showed no significant difference among the treatment groups (G2–4) (Fig. 2F), indicating that RWC, LDW, and RDW, but not RS, may be used as indicators of plant water status, and that exposing wheat seedlings to initial stress (drought hardening) enabled the plants to retain adequate water status, which enhanced their resistance to recurrent drought stress.
Chlorophyll is a photosynthetic pigment that absorbs light and is required for plant photosynthesis. One of the most commonly used metrics for assessing the severity of drought stress is chlorophyll content (Cai et al. 2020). It was observed that the chlorophyll content decreased at a greater magnitude in G3 and G4 plants than in G2 plants (Fig. 2A). Yet, a significant positive correlation was found between the chlorophyll content and G2 treatment condition (Table 7), indicating that first stress treatment (drought-hardening) helped the G2 plants to maintain a higher chlorophyll content against subsequent stress by reducing photo-oxidative damage, which occurs because of the inhibition of photosynthesis and excitation of excess light energy. Similarly, drought hardening (first stress) enabled G2 plant to maintain a higher carotenoid content than other treatment plants (Fig. 2B). Carotenoid plays an essential function in the heat dissipation of excess excitation energy in the photosynthetic system, which aids in the prevention of superoxide formation in plants receiving excess light energy when photosynthesis declines under drought circumstances (Efeoğlu et al. 2009). Carotenoid content has been reported to be associated with drought resistance (Amoah et al. 2019; Zhang et al. 2021), which aligns with our data (Table 2).
Drought stress is directly linked to ROS production, notably H2O2 and O2−, culminating in membrane damage and electrolyte leakage (Kumar et al. 2017; Shanker et al. 2014). MSI, EL, H2O2 accumulation, and lipid peroxidation (MDA level) have all been extensively used to assess the drought tolerance of different plant species (Abid et al. 2018; Raja et al. 2020; Upadhyay et al. 2020). Plants grown under G2 drought conditions had greater MSI and lower EL values than plants grown under G3 and G4 treatment conditions (Fig. 3A–B). Even so, higher MSI and lower EL have been shown to be associated with drought tolerance, which is consistent with our findings (Table 7). The MDA and H2O2 levels were negatively associated with drought tolerance under G2 treatment condition (Table 8). MDA is a lipid peroxidation biomarker commonly used to assess plant membrane status under drought stress conditions (Ayala et al. 2014), whereas H2O2 is a well-known ROS that plays an essential role in stress transduction pathways associated with abiotic stress tolerance (Hossain et al. 2015). Even so, lower concentrations of MDA and H2O2 improved tolerance in soyabean and tomato, which aligns with our results (Guler and Pehlivan 2016; Raja et al. 2020). The findings suggest that the reduced membrane damage in drought hardened (G2) plants may be due to a lower production of ROS, particularly H2O2 (Fig. 3C, D), protecting the G2-treated plants from subsequent stress treatment by improving ROS scavenging or prevention of cellular damage associated with drought stress.
In the G2 plant, initial drought stress increased the content of proline (PRL) and soluble sugars (SS) at a lower magnitude (Fig. 3E, F). Under stress conditions, PRL and SS play osmoregulatory, signaling transduction, redox balance maintenance, and cell structure stability functions in plants (Du et al. 2020; Ghaffari et al. 2019; Kaur and Asthir 2015). Several studies have found that proline and soluble sugars have a role in drought tolerance and adaptation in a wide range of plant species (Abid et al. 2018; Li et al. 2020b; Zhang et al. 2020). PRL and SS increased with increasing drought treatment in G3 and G4 plants more than in G2 plants, according to the findings. Furthermore, PRL and SS levels correlated positively with G2 treatment conditions. Drought hardening enhanced drought resistance in G2 plants against subsequent stress, as evidenced by a lower increased PRL and SS accumulation and improved osmoregulatory function, which is consistent with previous findings in tobacco and cotton (Jie et al. 2020; Khan et al. 2021).
Initial drought stress (drought hardening) improved drought tolerance by enhancing the enzymatic antioxidant defense system in plants under G2 treatment condition (Fig. 3G–I). ROS are inevitable molecules that are produced both under normal and stressed conditions, and they are lethal under stress. In response to an increase in ROS, plants activate their antioxidant enzyme defense system (Das and Roychoudhury 2014; Laxa et al. 2019). The main components of the enzymatic antioxidant defense system, which efficiently scavenges ROS and plays an important role in conferring drought stress tolerance, are SOD, CAT, and APX (Dumanović et al. 2020; Khaleghi et al. 2019). The functions of antioxidant enzymes, notably SOD, CAT, and APX, have been shown to correlate with drought tolerance (Laxa et al. 2019; Rady et al. 2020). Drought-hardening enhanced the enzymatic plant defense system, conferring drought tolerance against subsequent stress, according to the findings, which is consistent with previous findings by (Abdallah et al. 2017) (Fig. 3G–I and Table 8). Plants treated with G2 had a greater ability to reduce oxidative damage, as indicated by lower MDA and H2O2 levels, resulting in enhanced membrane integrity and an improved antioxidant defense system, conferring tolerance to subsequent stresses.
Initial drought (drought hardening) improved the expression of different categories of drought-responsive genes in response to drought stress
In response to drought stress, the expression profile of antioxidant-related genes SOD1, CAT1, and APX1 increased at a lower magnitude under G2 treatment conditions in wheat genotypes. Previous studies showed that SOD1, CAT1, and APX1 gene expression increased in response to drought stress, conferring stress tolerance (Kong et al. 2015; Raja et al. 2020). The expression of SOD1, CAT1, and APX1 were negatively correlated with antioxidant enzymes (SOD, CAT, and APX) activities in wheat genotypes (Table 7). Drought-hardened tomato and cotton plants showed an increased expression of SOD1, CAT1, and APX1 which contradict our findings (Jie et al. 2020; Murshed et al. 2008). From our data, a negative correlation between the antioxidant-related gene expression and their activities indicates under G2 treatment demonstrates that initial drought stress (drought-hardening) was effective in stabilizing the cell membrane against excess ROS production, evidenced by lower MDA levels, H2O2 accumulation, EL values, and enhanced antioxidant enzymes in G2 plants than G3 and G4 (Fig. 2B–D, G–I, Table 7).
Proline accumulation in plants under drought conditions increased stress resistance due to its numerous functions under such conditions (Hamurcu et al. 2020). P5CS, a key gene involved in proline biosynthesis, has been reported to be induced under drought stress in various plants (Adamipour et al. 2020; Maghsoudi et al. 2018; Nasirzadeh et al. 2021; Safari et al. 2021). In the present study, the expression of P5CS was shown to be significantly higher under G3 and G4 treatment conditions than under G2, and its level of expression was found to be positively correlated with the proline level during drought treatment (Fig. 4D, Table 7). The results are consistent with previous findings reported by Dudziak et al., (2019); Maghsoudi et al., (2018), as initial drought treatment improved proline content and P5CS gene expression.
Similarly, in response to drought stress, abundant transcript profile of HSP, LEA, and DREB2 genes were found to increase at a lower magnitude in G2 plants than in G3 and G4 (Fig. 4E–G). LEA proteins have been found in a variety of species to protect protein clumping induced by desiccation or osmotic stress caused by a variety of environmental factors (Kamarudin et al. 2019). The expression HSP and DREB2 gene was detected under drought stress, conferring tolerance to drought in potato and soyabean (El-Esawi and Alayafi 2019; Li et al. 2020a). Based on the findings, the lower increased expression of these genes under G2 treatment conditions indicates that drought-hardening conferred drought tolerance to wheat genotypes in response to subsequent drought stress, which can be attributed to enhanced membrane status, evidenced by low MDA, and an enhanced antioxidant defense system.
The genes, RBCL (photosynthesis-related gene) and CCD1 (carotenoid-biosynthesis gene) are crucial in inducing stress-related genes and thus conferring drought resistance. Diverse genetic approaches and gene regulation are involved in various abiotic stress tolerance and adaptations (Ke et al. 2019; Wang et al. 2012). Previous study has emphasized the importance of the RBCL and CCD1 genes in tomato and carrot drought tolerance and adaptation (Raja et al. 2020; Zhang et al. 2021). The RBCL and CCD1 gene expressions were shown to be positively associated with chlorophyll and carotenoid concentration in these studies. However, in the present study, RBCL and CCD1 expression were shown to be highly expressed under drought treatment conditions, but negatively correlated with the content of these photosynthetic pigments. Interestingly, little is known about the pattern of expression of these genes in crops, particularly wheat under drought stress conditions. The findings shed light on the potential functions of the CCD1 and RBCL genes in drought-hardening and drought stress situations in wheat. In addition, differential expression patterns of the examined genes in various drought-hardening treatments of wheat genotypes under drought stress revealed that each genotype uses different molecular responses under the same conditions.
Identification of wheat drought resistance
Furthermore, a cluster analysis of the physiological and molecular indices divided the four wheat genotypes into two groups: group I drought-sensitive type which consisted of Geumgangmil and PL 257, and group II drought-tolerant type comprised PL 337 and PL 371. The cluster analysis approach has been applied for evaluating the drought tolerance level in various plant plants, such as cotton (Khan et al. 2020), maize (Chen et al. 2016), and wheat (Grzesiak et al. 2019). The findings provided insight into the response to drought hardening among wheat-rye translocation lines; 1AL.1RS (PL 371), 1BL.1RS (PL 337), and 1DL.1RS (PL 257) vs non-translocation lines (Geumgangmil).
Regarding drought resistance, the translocation line, 1AL.1RS (PL 337) was the top ranked genotype. The 1AL.1RS wheat-rye translocation line has been reported to be among widely grown wheat-rye translocation lines due to high yield and resistance to various biotic stresses (Mago et al. 2005; Schlegel1 and Korzun 1997). However, the response of wheat-rye translocation under drought stress and hardening conditions is unknown. Jangra et al., (2017) found that drought stress was related with higher osmoregulation activities in the 1AL.1RS and translocation line than in the non-translocation line, imparting drought resistance under hydroponic conditions, which is consistent with our findings. Here, the drought tolerance may be attributed to the large root biomass of wheat-rye translocations than non-translocation lines. Although the study examined a number of drought stress indicators, it was confined to the seedling development stage in the greenhouse. As a result, more study to determine the responsiveness of wheat genotypes at various stages of development under field conditions may be required. Furthermore, additional molecular study may be required to examine the role of the nine different genes in other abiotic stress conditions. Also, pressure and soil water content measurements are highly suggested for determining the intensity or severity of the drought stress treatment.
Conclusion
The resistance of wheat seedlings to three different drought treatments was investigated using various physiological and biochemical indicators such as chlorophyll content, carotenoid content, RWC, MSI, EL, MDA, Proline, LDW, RDW, R-S ratio, SS, H2O2 level, SOD, CAT, and APX, as well as the relative expression levels of different categories of drought regulation genes. The acclimatization response to subsequent stresses can be induced by initial drought stress (drought hardening). Drought-hardening treatments, particularly G2 and G3, enhanced both enzymatic and antioxidant defense systems, lessening the detrimental effects of oxidative damage.
The relative expression levels of the nine genes were significantly correlated with several physiological indicators under G2 treatment condition and were identified as major components in the principal component analysis. As a result, SOD1, CAT1, APX1, P5CS, DREB2, HSP, CCD1, LEA, and RBCL can be used as molecular indicators to identify drought resistance wheat genotypes. This procedure is quick and accurate, and it can serve as a foundation for more in-depth research into the drought resistance mechanism. Based on numerous indicators, we identified drought-tolerant [PL 371 (1BL.1RS) and PL 337 (1AL.1RS)] and drought-sensitive [Geumgangmil and PL 257 (1DL.1RS)] genotypes to provide reference resources for breeding applications (Table 8).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to extend their appreciation to the Korean government, through the National Institute of International Education (NIIED) for supporting their work through the Global Korea Scholarship (GKS-G-2020) program.
Author contributions
JNA designed the experiment, analyzed the data, and wrote the manuscript with support and valuable contributions from YWS. All authors have discussed the results and approved the final manuscript.
Funding
This work was conducted with support from the ‘Next Generation of BioGreen21 Program for Agriculture and Technology Development (Project No. PJ01324401), Rural Development Administration, Republic of Korea.
Declarations
Conflict of interest
No potential conflict of interest is reported by the authors.
References
- Abdallah MB, Methenni K, Nouairi I, Zarrouk M, Youssef NB. Drought priming improves subsequent more severe drought in a drought-sensitive cultivar of olive cv. Chétoui. Sci Hort. 2017;221:43–52. doi: 10.1016/j.scienta.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abhinandan K, Skori L, Stanic M, Hickerson NMN, Jamshed M, Samuel MA. Abiotic stress signaling in wheat—an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L) Sci Rep. 2018;8:4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamipour N, Khosh-Khui M, Salehi H, Razi H, Karami A, Moghadam A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol Biochem. 2020;155:105–113. doi: 10.1016/j.plaphy.2020.07.028. [DOI] [PubMed] [Google Scholar]
- Amoah JN, Ko CS, Yoon JS, Weon SY. Effect of drought acclimation on oxidative stress and transcript expression in wheat (Triticum aestivum L.) J Plant Interact. 2019;14:492–505. doi: 10.1080/17429145.2019.1662098. [DOI] [Google Scholar]
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik P, Zeng W, Tai H, Bizimungu B, Tanino K. Effects of drought acclimation on drought stress resistance in potato (Solanum tuberosum L.) genotypes. Environ Expt Bot. 2016;126:76–89. doi: 10.1016/j.envexpbot.2016.01.008. [DOI] [Google Scholar]
- Bates L, Waldren S, Teare I. Rapid determination of proline for water-stressed studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Behnamnia M, Kalantari KM, Ziaie J. The effects of brassinosteroid on the induction of biochemical changes in Lycopersicon esculentum under drought stress. Turk J Bot. 2009;33:417–428. [Google Scholar]
- Bian S, Jiang Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hort. 2009;120:264–270. doi: 10.1016/j.scienta.2008.10.014. [DOI] [Google Scholar]
- Bielsa B, Leida C, Rubio-Cabetas MJ. Physiological characterization of drought stress response and expression of two transcription factors and two LEA genes in three Prunus genotypes. Sci Hort. 2016;213:260–269. doi: 10.1016/j.scienta.2016.11.006. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai K, Chen X, Han Z, Wu X, Zhang S, Li Q, Nazir MM, Zhang G, Zeng F. Screening of worldwide barley collection for drought tolerance: the assessment of various physiological measures as the selection criteria. Front Plant Sci. 2020;11:1159. doi: 10.3389/fpls.2020.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wang S, Cao B, Cao D, Leng G, Li H, Yin L, Shan L, Deng X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front Plant Sci. 2016;6:1241. doi: 10.3389/fpls.2015.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen H, He Y, Xia R (2018) TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 289660
- Crespo-Herrera L, Crossa J, Huerta-Espino J, Vargas M, Mondal S, Velu G, Payne T, Braun H, Singh R. Genetic gains for grain yield in CIMMYT's semi-arid wheat yield trials grown in suboptimal environment. Crop Sci. 2018;58:1890–1898. doi: 10.2135/cropsci2018.01.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem. 2020;146:1–12. doi: 10.1016/j.plaphy.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Dudziak K, Zapalska M, Börner A, Szczerba H, Kowalczyk K, Nowak M. Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci Rep. 2019;9:2743. doi: 10.1038/s41598-019-39154-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V. The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci. 2020;11:552969. doi: 10.3389/fpls.2020.552969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeoğlu B, Ekmekçi Y, Çiçek N. Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot. 2009;75:34–42. doi: 10.1016/j.sajb.2008.06.005. [DOI] [Google Scholar]
- El-Esawi MA, Alayafi AA. Overexpression of StDREB2 transcription factor enhances drought stress tolerance in cotton (Gossypium barbadense L) Genes (basEl) 2019 doi: 10.3390/genes10020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari H, Tadayon MR, Nadeem M, Cheema M, Razmjoo J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol Plant. 2019;41:23. doi: 10.1007/s11738-019-2815-z. [DOI] [Google Scholar]
- Ghanbari F, Kordi S. Hardening pretreatment by drought and low temperature enhanced chilling stress tolerance of cucumber seedlings. Acta Sci Pol Hortorum Cultus. 2019;18:29–37. doi: 10.24326/asphc.2019.2.4. [DOI] [Google Scholar]
- Ghanbari F, Sayyari M. Controlled drought stress affects the chilling-hardening capacity of tomato seedlings as indicated by changes in phenol metabolisms, antioxidant enzymes activity, osmolytes concentration and abscisic acid accumulation. Sci Hort. 2018;229:167–174. doi: 10.1016/j.scienta.2017.10.009. [DOI] [Google Scholar]
- Govindasamy V, George P, Kumar M, Aher L, Raina SK, Rane J, Annapurna K, Minhas PS. Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L) Moench] 3 Biotech. 2020;10:1–14. doi: 10.1007/s13205-019-2001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak S, Hordyńska N, Szczyrek P, Grzesiak MT, Noga A, Szechyńska-Hebda M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I—selection approaches. J Plant Interact. 2019;14:30–44. doi: 10.1080/17429145.2018.1550817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujjar RS, Roytrakul S, Chuekong W, Supaibulwatana K. A synthetic cytokinin influences the accumulation of leaf soluble sugars and sugar transporters, and enhances the drought adaptability in rice. 3 Biotech. 2021;11:1–14. doi: 10.1007/s13205-021-02908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler NS, Pehlivan N. Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biol Hung. 2016;67:169–183. doi: 10.1556/018.67.2016.2.5. [DOI] [PubMed] [Google Scholar]
- Hamurcu M, Khan MK, Pandey A, Ozdemir C, Avsaroglu ZZ, Elbasan F, Omay AH, Gezgin S. Nitric oxide regulates watermelon (Citrullus lanatus) responses to drought stress. 3 Biotech. 2020;10:1–14. doi: 10.1007/s13205-020-02479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015;6:420. doi: 10.3389/fpls.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Hirt H, Bendahmane A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J. 2017;15:405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra S, Mishra A, Kamboj D, Yadav NR, Yadav RC. Plant biotechnology: recent advancements and developments. Springer; 2017. Engineering abiotic stress tolerance traits for mitigating climate change; pp. 59–73. [Google Scholar]
- Jie Z, We H, Li Y-X, He J-Q, Zhu H-H, Zhou Z-G. Screening of drought resistance indices and evaluation of drought resistance in cotton (Gossypium hirsutum L.) J Int Agric. 2020;19:495–508. doi: 10.1016/S2095-3119(19)62696-1. [DOI] [Google Scholar]
- Johnson SM, Lim F-L, Finkler A, Fromm H, Slabas AR, Knight MR. Transcriptomic analysis of Sorghum bicolor responding to combined heat and drought stress. BMC Genom. 2014;15:456. doi: 10.1186/1471-2164-15-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacienė G, Juknys R, Januškaitienė I. The role of oxidative stress in spring barley cross-adaptation to different heavy metals. Arch Agron Soil Sci. 2017;63:1037–1048. doi: 10.1080/03650340.2016.1256474. [DOI] [Google Scholar]
- Kamarudin ZS, Yusop MR, Ismail MR, Tengku Muda Mohamed M, Harun AR, Yusuff O, Magaji U, Fatai A. LEA Gene Expression Assessment in Advanced Mutant Rice Genotypes under Drought Stress. Int J Genome. 2019;2019:1–8. doi: 10.1155/2019/8406036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Asthir B. Proline: a key player in plant abiotic stress tolerance. Biol Plant. 2015;59:609–619. doi: 10.1007/s10535-015-0549-3. [DOI] [Google Scholar]
- Ke Q, Kang L, Kim HS, Xie T, Liu C, Ji CY, Kim SH, Park WS, Ahn M-J, Wang S. Down-regulation of lycopene ε-cyclase expression in transgenic sweetpotato plants increases the carotenoid content and tolerance to abiotic stress. Plant Sci. 2019;281:52–60. doi: 10.1016/j.plantsci.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Khaleghi A, Naderi R, Brunetti C, Maserti BE, Salami SA, Babalar M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-55889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Ma X, Shah S, Wu X, Shaheen A, Xiao L, Wu Y, Wang S. Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. 2020;20:486. doi: 10.1186/s12870-020-02688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Ma X, Zhang J, Wu X, Iqbal A, Wu Y, Zhou L, Wang S. Circular drought-hardening confers drought tolerance via modulation of the antioxidant defense system, osmoregulation, and gene expression in tobacco. Physiol Plant. 2021;172:1073–1088. doi: 10.1111/ppl.13402. [DOI] [PubMed] [Google Scholar]
- Khanna-Chopra R, Selote DS. Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-susceptible wheat cultivar under field conditions. Environ Expt Bot. 2007;60:276–283. doi: 10.1016/j.envexpbot.2006.11.004. [DOI] [Google Scholar]
- Kong L, Huo H, Mao P. Antioxidant response and related gene expression in aged oat seed. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Beena A, Awana M, Singh A. Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front Plant Sci. 2017;8:1151. doi: 10.3389/fpls.2017.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxa M, Liebthal M, Telman W, Chibani K, Dietz K-J. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8:94. doi: 10.3390/antiox8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Feng Y, Zong Y, Zhang D, Hao X, Li P. Elevated CO2-induced changes in photosynthesis, antioxidant enzymes and signal transduction enzyme of soybean under drought stress. Plant Physiol Biochem. 2020;154:105–114. doi: 10.1016/j.plaphy.2020.05.039. [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Zhang Y, Wang R, Guo Z, Xie Z. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor) Acta Physiol Plant. 2020;42:1–11. doi: 10.1007/s11738-019-2990-y. [DOI] [Google Scholar]
- Maghsoudi K, Emam Y, Niazi A, Pessarakli M, Arvin MJ. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J Plant Interact. 2018;13:461–471. doi: 10.1080/17429145.2018.1506516. [DOI] [Google Scholar]
- Mago R, Miah H, Lawrence G, Wellings C, Spielmeyer W, Bariana H, McIntosh R, Pryor A, Ellis J. High-resolution mapping and mutation analysis separate the rust resistance genes Sr31, Lr26 and Yr9 on the short arm of rye chromosome 1. Theor Appd Gen. 2005;112:41–50. doi: 10.1007/s00122-005-0098-9. [DOI] [PubMed] [Google Scholar]
- Murshed R, Lopez-Lauri F, Keller C, Monnet F, Sallanon H. Acclimation to drought stress enhances oxidative stress tolerance in Solanum Lycopersicum L. fruits. Plant Stress. 2008;2:145–151. [Google Scholar]
- Nasirzadeh L, Sorkhilaleloo B, Hervan EM, Fatehi F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res Commun. 2021;49:83–89. doi: 10.1007/s42976-020-00085-2. [DOI] [Google Scholar]
- Park S-C, Kim SH, Park S, Lee H-U, Lee JS, Park WS, Ahn M-J, Kim Y-H, Jeong JC, Lee H-S, Kwak S-S. Enhanced accumulation of carotenoids in sweetpotato plants overexpressing IbOr-Ins gene in purple-fleshed sweetpotato cultivar. Plant Physiol Biochem. 2015;86:82–90. doi: 10.1016/j.plaphy.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Rady MM, Belal HE, Gadallah FM, Semida WM. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci Hort. 2020;266:109290. doi: 10.1016/j.scienta.2020.109290. [DOI] [Google Scholar]
- Raja V, Qadir SU, Alyemeni MN, Ahmad P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech. 2020;10:1–18. doi: 10.1007/s13205-020-02206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Mestre TC, Mittler R, Rubio F, Garcia-Sanchez F, Martinez V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014;37:1059–1073. doi: 10.1111/pce.12199. [DOI] [PubMed] [Google Scholar]
- Safari M, Mousavi-Fard S, Nejad AR, Sorkheh K, Sofo A (2021) Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int J Environ Sci Tech 1–16
- Schlegel R, Korzun V. About the origin of 1RS. 1BL wheat-rye chromosome translocations from Germany. Plant Breed. 1997;116:537–540. doi: 10.1111/j.1439-0523.1997.tb02186.x. [DOI] [Google Scholar]
- Selote DS, Khanna-Chopra R. Antioxidant response of wheat roots to drought acclimation. Protoplas. 2010;245:153–163. doi: 10.1007/s00709-010-0169-x. [DOI] [PubMed] [Google Scholar]
- Shan C, Zhang S, Ou X. The roles of H2S and H2O2 in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplas. 2018;255:1257–1262. doi: 10.1007/s00709-018-1213-5. [DOI] [PubMed] [Google Scholar]
- Shanker AK, Maheswari M, Yadav S, Desai S, Bhanu D, Attal NB, Venkateswarlu B. Drought stress responses in crops. Funct Int Genom. 2014;14:11–22. doi: 10.1007/s10142-013-0356-x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203:32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- Thomas D. Survival and growth of drought hardened Eucalyptus pilularis Sm. seedlings and vegetative cuttings. New Fores. 2009;38:245–259. doi: 10.1007/s11056-009-9144-9. [DOI] [Google Scholar]
- Ullah A, Sun H, Yang X, Zhang X. Drought coping strategies in cotton: increased crop per drop. Plant Biotechnol J. 2017;15:271–284. doi: 10.1111/pbi.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay D, Budhlakoti N, Singh AK, Bansal R, Kumari J, Chaudhary N, Padaria JC, Sareen S, Kumar S. Drought tolerance in Triticum aestivum L. genotypes associated with enhanced antioxidative protection and declined lipid peroxidation. 3 Biotech. 2020;10:281. doi: 10.1007/s13205-020-02264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-Y, Bai M-Y, Oh E, Zhu J-Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Ann Review Gene. 2012;46:701–724. doi: 10.1146/annurev-genet-102209-163450. [DOI] [PubMed] [Google Scholar]
- Warren C. Rapid measurement of chlorophylls with a microplate reader. J Plant Nutr. 2008;31:1321–1332. doi: 10.1080/01904160802135092. [DOI] [Google Scholar]
- Wei T, Deng K, Liu D, Gao Y, Liu Y, Yang M, Zhang L, Zheng X, Wang C, Song W. Ectopic expression of DREB transcription factor, AtDREB1A, confers tolerance to drought in transgenic Salvia miltiorrhiza. Plant Cell Physiol. 2016;57:1593–1609. doi: 10.1093/pcp/pcw084. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Franklin CE. Testing the beneficial acclimation hypothesis. Trends Ecol Evol. 2002;17:66–70. doi: 10.1016/S0169-5347(01)02384-9. [DOI] [Google Scholar]
- Wu X, Fan Y, Li L, Liu Y. The influence of soil drought stress on the leaf transcriptome of faba bean (Vicia faba L.) in the Qinghai–Tibet Plateau. 3 Biotech. 2020;10:1–16. doi: 10.3390/biotech10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chang Y, Wang L, Wu J, Wang S. Genetic dissection of drought resistance based on root traits at the bud stage in common bean. Theor Appd Gene. 2021;134:1047–1061. doi: 10.1007/s00122-020-03750-6. [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cazares B, Ramirez-Ortega FA, Flores-Elenes L, Ruiz-Medrano R. Drought tolerance in crop plants. Am J Plant Physiol. 2010;5:241–256. doi: 10.3923/ajpp.2010.241.256. [DOI] [Google Scholar]
- Xu W, Cui K, Xu A, Nie L, Huang J, Peng S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant. 2015;37:9. doi: 10.1007/s11738-014-1760-0. [DOI] [Google Scholar]
- Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Plant. 2010;231:609–621. doi: 10.1007/s00425-009-1075-3. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu H, Wu X, Wang W. Identification of drought tolerant mechanisms in a drought-tolerant maize mutant based on physiological, biochemical and transcriptomic analyses. BMC Plant Biol. 2020;20:1–14. doi: 10.1186/s12870-019-2170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R-R, Wang Y-H, Li T, Tan G-F, Su T-P, X-J, Xu Z-S, Tian Y-S, and Xiong A-S, Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma. 2021;258:379–390. doi: 10.1007/s00709-020-01570-5. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yu X, Ottosen CO, Rosenqvist E, Zhao L, Wang Y, Yu W, Zhao T, Wu Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017;17:1–13. doi: 10.1186/s12870-016-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.