Abstract
Background
The second wave of COVID-19 pandemic has seen an unprecedented rise in the number of mucormycosis cases worldwide and in India particularly. This otherwise rare fungal infection has become an endemic among patients who have recovered from recent SARS-CoV-2 infection. Among the different types of mucormycosis, rhino-orbital-cerebral involvement has mainly been observed in the recent surge of cases. Very few cases of mucormycosis of mandible have been reported in literature and none in COVID-19 patients. We report a case of isolated mandibular mucormycosis in a COVID- 19 patient, with no other predisposing comorbidities.
Case report.
A 39 year old patient recently recovered from COVID-19 presented with typical symptoms of osteomyelitis which was confirmed using computed tomography of face. He underwent thorough debridement and curettage and tissue was sent for culture, special staining and biopsy.
Result
Diagnosis of mucormycosis was confirmed based on postoperative biopsy and special staining. He was further managed with complete course of appropriate antifungal therapy.
Conclusion
Mucormycosis is a fulminant and aggressive infection which requires prompt diagnosis and intervention. Early referral to a maxillofacial surgeon by physicians and general dental practitioners on seeing signs and symptoms of secondary fungal infections involving maxilla or mandible in patients with history of SARS-CoV-19 infection can improve prognosis.
Keywords: Mandibular mucormycosis, SARS CoV-2, COVID-19, Fungal osteomyelitis, Black fungus
Introduction
The B.1.617.2 (Delta) variant is thought to be responsible for the deadly second wave of SARS-CoV-2 infection in India. Many cases of mucormycosis infection during the second wave of pandemic have surfaced, with numbers approaching epidemic proportions. The exact scale of real-world data is not clear and are under-reported. However, according to news reports, as on 21st July, India reported nearly 45,432 cases of mucormycosis in COVID-19 patients till July 15, bringing global attention to the disease. Among these total reported deaths were 4252. (Over 45,000 cases of mucormycosis reported in country - India News, 2021)
Mucormycosis is a fatal, opportunistic infection with high morbidity and mortality, first coined by Baker in 1957.(Baker, 1957) It is caused by fungi belonging to the Mucoraceae family, comprising species in genera Rhizopus (most common type), Mucor and Lichtheimia.(Blitzer et al., 1980, Roden et al., 2005) Among the different clinical forms of the disease, rhino-orbito-cerebral mucormycosis (ROCM) is most common.(Prakash and Chakrabarti, 2021) ROCM starts in susceptible individuals with nasal involvement through inhalation of spores. Hyphae then germinate and subsequently extend into the paranasal sinuses, orbits and cranium owing to the angioinvasive property and dissemination through vascular channels. The organism have an affinity for vascular invasion causing purulent arteritis and vascular thrombosis. This leads to infarction of surrounding tissues which is responsible for the clinical manifestations of the disease.(Baker, 1957)
Predisposing factors contributing to this opportunistic fungal infection include uncontrolled and acidotic diabetes mellitus, immunocompromised or immunosuppressed condition (steroid use, AIDS, prolonged steroid use etc.), iron overload, haematological malignancy, renal and liver disease.(Chakrabarti and Singh, 2014) The aetiopathogenesis of COVID 19-associated mucormycosis appears to be multifactorial, with many factors interplaying in disease onset and progression. A breach in the structural integrity of mucosal barrier could play a role in inoculation of spores and initiation of disease in susceptible individuals. Hypoxia, low pH, diabetes or steroid-associated hyperglycemia and reduced chemotactic or phagocytic activity provide a suitable environment for fungal hyphae to grow unabated. In the presence of a SARS-CoV-2 infection, the environment is also responsible for other factors that encourage fungal proliferation and virulence such as; (i) raised free serum iron level (due to transferrin and ferritin glycosylation) which acts as a substrate (ii) upregulation of host endothelial glucose regulator protein (GRP78) and its interaction with fungal spore coat protein ligand CotH3, (iii) cytokine storm (iv) free radical injury and endothelial damage (v) lymphocytopenia. (Singh et al., 2021, Jose et al., 2021) All these factors acting together may be responsible for acute rise in number of COVID-19 associated mucormycosis (CAM) cases. Corticosteroid therapy has shown to decrease mortality in COVID-19 patients as demonstrated by the RECOVERY collaborative group trial.(Dexamethasone in Hospitalized Patients with Covid-19, 2021) Widespread use of steroids (oral and IV) and the use of higher dose and duration has been shown to be an independent risk factor for CAM. Steroid use can exacerbate existing diabetes and ketoacidosis and combination of SARS-CoV-2 infection with corticosteroid use can precipitate new onset diabetes. (Yang et al., 2010)
In the wake of second wave of SARS-CoV-2 infection in the Indian subcontinent the sudden surge of mucormycosis cases has brought global attention to this life-threatening disease. The term ‘Black fungus’ is commonly used by the laity to describe this disease due to dark appearance of infected and necrotic tissue. (Gandra et al., 2021) Numerous cases of maxillary involvement in ROCM have been reported during the current wave of the pandemic. The patients can present with complaints of facial pain and/or discoloration, swelling, nasal or intraoral pus discharge, paraesthesia, proptosis, toothache and mobile teeth. Management includes controlling underlying risk factors, medical therapy with antifungal agent and surgical debridement and rehabilitation. The mortality rate of ROCM has been reported to range from 50 to 80% in literature and can reach up to 90% with intracranial extension.(Roden et al., 2005, Sen et al., 2021) Prognosis improves with early diagnosis, aggressive surgery and antifungal therapy.(Blitzer et al., 1980) We report a case of isolated mandibular mucormycosis in a young healthy adult male, as a sequelae of SARS-CoV-2 infection.
Report of a case
A 39 year old male, with recent SARS-CoV-2 infection was referred to our unit with complaints of numbness of left side lower lip for 24 days and pain in left lower jaw since 15 days. He was hospitalized for COVID-19 pneumonia for 27 days. He was managed by supportive oxygen therapy and empirical IV antibiotics for 21 days. Inj. Dexamethasone 6 mg 12 hourly was given for 15 days followed by 6 mg 24 hourly. The patient was also given oral azithromycin, doxycycline and ascorbic acid. He had no history of any medical comorbidities. During day 20 of admission he noticed tingling sensation over left lip. The patient was then discharged after being treated for SARS-CoV-2 infection. He then noticed pain in left side lower back teeth which was insidious in onset, gradually progressive in nature, moderate in intensity and radiating to left jaw and cheek. Computed tomography of face was advised by a local dentist which showed signs of osteomyelitis. Patient was given antibiotics and analgesics by the dentist who then proceeded to extract his left lower third molar.
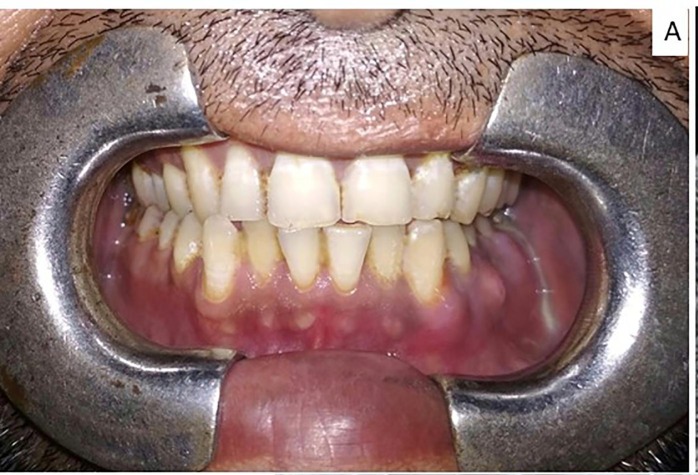
When the patient presented to us, one week after extraction, clinical examination showed diffuse oedema and tenderness over left side submandibular and mandibular body region. He had no medical co-morbidities, nor did he have a familial history of diabetes mellitus. Mouth opening was reduced to 22 mm and intraoral examination showed multiple draining sinuses from 34 to 38 region and inflamed gingiva. (Fig. 1A ) Pus was sent for fungal and bacterial culture which grew Klebsiella oxytoca sensitive to meropenem, but showed no evidence of fungal hyphae or spores in fungal culture.
Fig. 1A.
Clinical presentation with multiple draining sinuses.
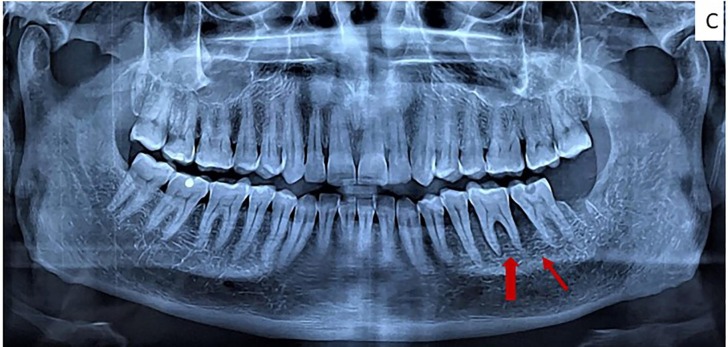
All laboratory investigations (including blood sugar levels) were within normal limits except for C- reactive protein (CRP) 38 mg/L [normal range < 6 mg/L] and D-dimer 716.53 ng/mL (normal < 500 ng/mL). Blood was screened for HIV, Hepatitis B and C using rapid tests which were negative. Diagnostic nasal endoscopy ruled out sinus involvement.. Contrast CT of face and paranasal sinuses revealed multiple air foci in marrow cavity in the body and ramus of mandible with sub-periosteal thickening and bony erosion (Fig. 1B ). Orthopantomogram although not very significant showed some ill-defined areas of bone loss in mandibular body and angle region. (Fig. 1C ) History, clinical and radiographic findings were suggestive of aggressive osteomyelitis of mandible.
Fig. 1B.
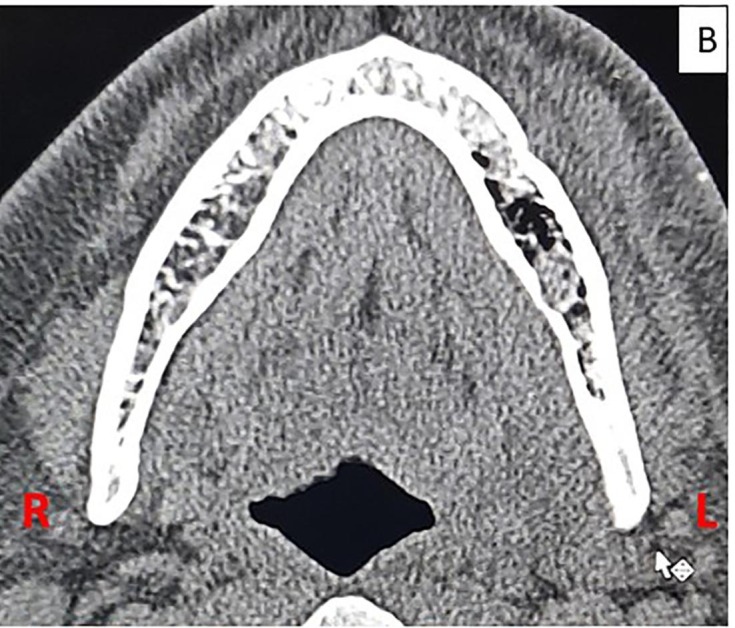
Contrast enhanced CT scan of face showing areas of bone erosion with multiple air foci and hypodense collection in marrow cavity of mandibular ramus and body on left side.
Fig. 1C.
Preoperative panoramic radiograph showing bone loss is between lower first and second molar and periodontal ligament enlargement in first molar and some degree of rarefaction in posterior body-angle region on left side mandible (arrows).
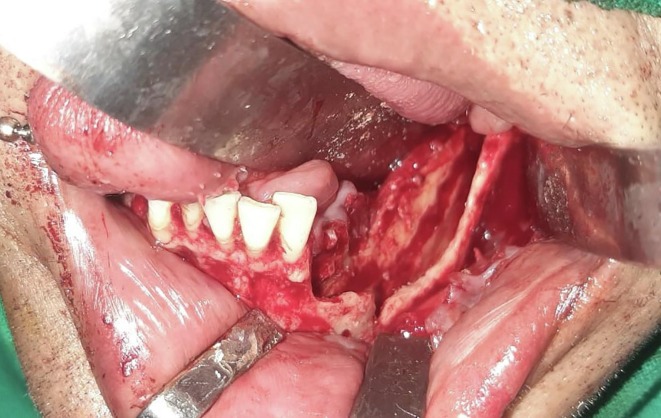
Patient was taken for debridement and curettage under general anaesthesia. Intraoperatively, the mucoperiosteal flap was oedematous with pus pockets and easily separated from underlying bone. All the teeth of left lower quadrant were mobile, infected and were removed. Medullary bone was necrotic and cheese-like in texture which was debrided thoroughly (Fig. 2, Fig. 3 ). The inferior alveolar neurovascular bundle appeared friable and thrombosed. Tissue was sent for histopathology, KOH mount, cultures and CB-NAAT. Postoperatively, the patient was given Inj. Meropenem and Ryle’s tube feed was started.
Fig. 2.
Surgical debridement of left mandible.
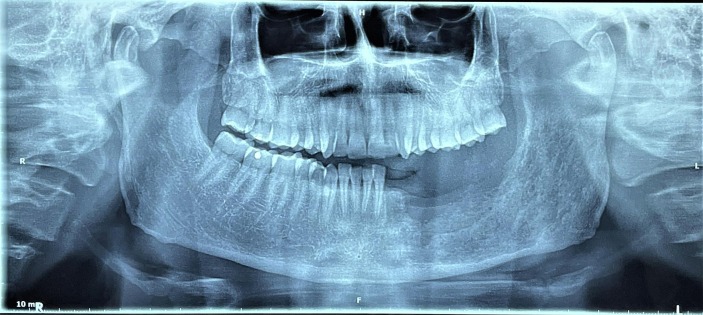
Fig. 3.
Postoperative panoramic radiograph.
Histopathological examination showed fragmented tissue, consisting of abundant fibrocollagenous tissue with vascular proliferation, thrombosis of vessels and mixed inflammatory infiltrate. Necrotic tissue fragments show broad based aseptate hyphae of variable thickness with right angled branching. Fungal hyphae were PAS positive confirming diagnosis of mucormycosis with osteomyelitis of mandible (Fig. 4 ). Therefore, antifungal therapy was started with oral posaconazole 300 mg twice a day for 3 days followed by 300 mg once a day for 45 days. He was discharged from the hospital one week postoperatively after satisfactory wound healing. Minocycline 100 mg twice daily was also added to the treatment regimen as per culture and sensitivity report. Two months follow-up showed good wound healing and no signs of recurrence of infection.
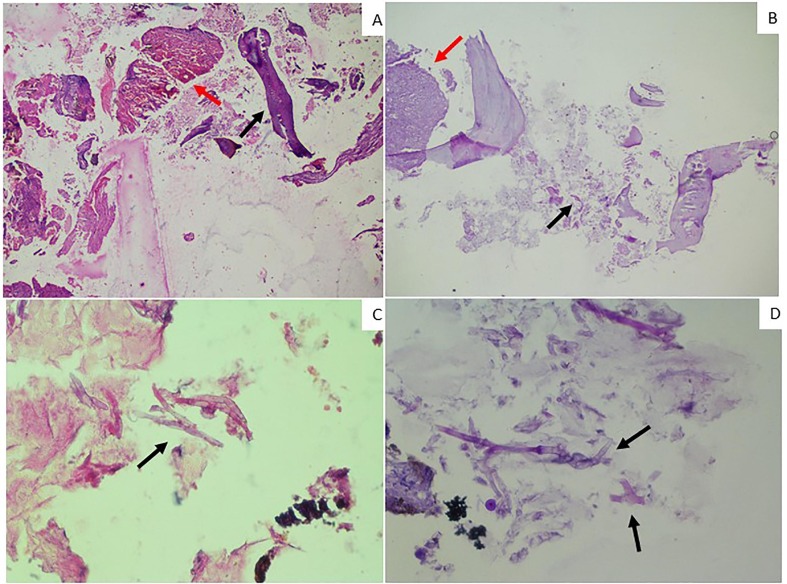
Fig. 4.
Histopathology of debrided necrotic tissue: A) Haematoxylin and Eosin (H and E) images 40x magnification showing multiple haemorrhagic blood vessels surrounded by inflammatory infiltrate (red arrow) and decalcified necrotic bone (black arrow). B) Periodic Acid Schiff (PAS) images showing 40x magnification fibrocollagenous tissue with vascular proliferation and mixed inflammatory infiltrate (red arrow) and fungal hyphae (black arrow). C) H and E images and D) PAS images of 400x magnification showing broad, non-septate fungal hyphae with right angle to obtuse branching (arrows).
Discussion:
Mucormycosis (earlier also referred to as Zygomycosis or Phycomycosis) is an uncommon yet life-threatening infection that rarely prevails in conditions with good host immunity. There were a large number of cases reported in India (almost 70 times the global average), even prior to the pandemic due to high incidence of diabetes mellitus and tropical climate. (Prakash and Chakrabarti, 2021, Chakrabarti and Singh, 2014, Singh et al., 2021) Among these, ROCM was found to be the more common presenting variant. (Chakrabarti and Singh, 2014) Chakrabarti et al enumerated the various risk factors that can cause mucormycosis infection, with diabetes mellitus noted in 53.6%.(Prakash and Chakrabarti, 2021) Other common predisposing conditions are patients with chronic kidney disease, ketoacidosis, neutropenia, corticosteroid therapy to name a few. Mucormycosis in immunocompetent individuals although less common has been reported in literature. The incidence in patients with no underlying condition was reported in 4% by Blitzer et al.(Blitzer et al., 1980)
Patients presenting with a triad of diabetes, steroid therapy and SARS-CoV-2 infection (concomitant or recent) are at a higher risk of contracting the infection. A recent study including 2826 ROCM in COVID-19 patients revealed that 78% had diabetes (controlled or uncontrolled) and 87% had history of corticosteroid therapy.(Sen et al., 2021) The use of steroids (oral or intravenous) was found to be the most common risk factor (among 631 non-diabetics out of total 2826, 89% of cases of COVID-19 associated ROCM had history of steroid therapy). In a patient with underlying disease or risk factors along with COVID-19 infection are more prone to contract secondary fungal infection. However, in COVID-19 patients with no predisposing risk factors like our patient the disease aetiology could be attributed to: (i) immune system dysregulation (ii) COVID-19 associated coagulopathy (Becker, 2020) (iii) vascular invasion by fungal hyphae [raised level of heat shock protein receptor GRP78 on endothelial cells due to stress which interacts with fungal coat protein CotH3 facilitating fungal endocytosis] (Baldin et al., 2017) (iv) prolonged or unregulated steroid use (v) free radical induced endothelial damage and fungal proliferation as a result of raised serum iron level. (Jose et al., 2021) The role of Delta variant in India in current mucormycosis endemic still needs to be investigated. The incidence of CAM outside of India was found to be only 18.8%.(Singh et al., 2021) Inoculation of spores through mucosal microtrauma (due to high flow oxygen), contaminated water in humidifiers or contaminated industrial oxygen cylinders could play an additional role in the dramatic increase of COVID-19 associated ROCM in the country.
The pathogenesis of mucormycosis in mandible (which is mainly dependent on endosteal blood supply) appears to be different from that of involvement of maxilla in ROCM. Isolated mandibular mucormycosis is rare with very few reported cases. We searched the PubMed interface of MEDLINE and Google scholar database to retrieve English language reports/series of mandibular mucormycosis cases using terms like mandibular mucormycosis, oral mucormycosis, and fungal osteomyelitis. Cases with concomitant sino-maxillary involvement were excluded as the pathogenesis and pathway of spread was different than that of isolated mandibular involvement. After exclusion and screening, we found only 17 articles with 23 cases of isolated mandibular mucormycosis. (Kwak et al., 2020, Agarwal et al., 2020, Aras et al., 2012, Cohen et al., 2019, Urs and Singh et al., 2019)Literature search revealed no cases of mandibular mucormycosis in COVID-19 patients. (Table 1 ) Our patient presented with paraesthesia over left side lower lip, pain and pus discharge in relation to left mandibular teeth, suggestive of mandibular involvement. He had a history of recent hospitalization for COVID 19 associated pneumonia and no other underlying medical condition.
Table 1.
Isolated mandibular mucormycosis cases reported in literature.
| Case No. | Authors | Year of publication | Type of Study | Age | Gender | Underlying condition | Outcome |
|---|---|---|---|---|---|---|---|
| 1. | Brown and Finn | 1986 | Case report | 57 | M | DM, Chronic renal failure | Death (due to cardiac arrest) |
| 2. | Jones and colleagues | 1993 | Case series | 43 | M | AML, Acute renal failure,Pancytopenia | Recovery satisfactory |
| 3. | Salisbury and colleagues | 1997 | Case report | 60 | M | AML, Prostate cancer, Heavy alcoholism, Hypertension | Recovery satisfactory |
| 4. | Lador and colleagues | 2006 | 42 | F | Acute lymphoblastic leukemia | Death (due to Leukemia) | |
| 5. | Bakathir and colleagues | 2006 | Case series | 49 | M | DM with ketoacidosis, ALL, dental extraction | Recovery satisfactory |
| 6. | Dogan and colleagues | 2007 | Case report | 7 | M | AML | Incomplete healing (after 4 weeks of debridement and antifungal) |
| 7. | Antonetti and colleagues | 2009 | Case report | 10 | M | Severe burns | Recovery satisfactory |
| 8. | Ojeda-Uribe and colleagues | 2010 | Case report | 55 | F | AML Decompensated DM | Recovery satisfactory |
| 9. | Oswal and colleagues | 2012 | Case series | 68 | F | DM, diabetic nephropathy, hypertension, sleep apnea,dental extraction | Death (due to septicaemia with multiorgan failure) |
| 10. | Aras and colleagues | 2012 | Case series | 6 | M | Neuroblastoma | Death (due to maligancy) |
| 15 | M | AML | |||||
| 11. | Mc Spadden and colleagues | 2016 | Case report | 63 | M | CML, steroid and immunosupresive therapy | Death (due to infection) |
| 12. | Urs and colleagues | 2016 | Case series | 26 | M | Dengue fever, dental extraction | Recovery satisfactory |
| 13 | Cheong and colleagues | 2017 | Case series | 38 | F | Chronic lymphocytic leukemia | Death (due to pneumonia) |
| 14. | Cohen and colleagues | 2019 | Case series | 21 | F | ALL | Recovery satisfactory |
| 14 | M | ALL | |||||
| 41 | M | AML | |||||
| 15. | Elitzur and colleagues | 2019 | Multicentre study (4 cases of mandibular mucor) | Not specified | Not specified | Acute Leukemias | Death due to mucormycosis in 2 out of 4 cases |
| 16. | Agarwal and colleagues | 2020 | Case report | 37 | M | Chronic granulomatous disease,tooth extraction, kidney disease | Recovery satisfactory |
| 17. | Kwak and colleagues | 2020 | Case report | 61 | M | AML, DM, hypertension, dental extraction | Recovery satisfactory |
| 18. | Our case | 2021 | Case report | 39 | M | SARS-CoV-2 infection | Recovery satisfactory |
Primary management includes antifungal therapy with amphotericin B and posaconazole or isavuconazole along with debridement of necrotic tissue. Surgical debridement or radical resection reduces progression of fungal infection and improves survival rate. (Blitzer et al., 1980) The use of Amphotericin B for mucormycosis has been validated by many authors after it was initially isolated in late 1958. Liposomal preparation of this polyene antifungal drug (L-AMB) is less toxic and used as first line of management in mucormycosis infections. L-AMB (5 mg/kg body weight) was advised for our patient. However, due limited resources and increased disease burden during the pandemic, our patient could not be given L-AMB and was stared on oral posaconazone 300 mg once daily for 45 days. Posaconazole has been proven to be an effective salvage therapy in 67% of mucormycosis patients. (Singh et al., 2021) The European Confederation of Medical Mycology has mentioned the use of triazoles like posaconazole (delayed release oral or intravenous use) and intravenous isavuconazole as first-line management of mucormycosis successfully.(Cornely et al., 2019)
ROCM exhibits high mortality rates which depend on early identification and treatment. Mandibular involvement has better prognosis than other forms of this disease. Since mandibular mucormycosis without sino-maxillary component is infrequent, an initial diagnosis of fungal osteomyelitis would be difficult. Moreover, fungal hyphae are not easily detected which can further delay diagnosis. Early diagnosis is crucial as mucormycosis has a rapid and aggressive progression as seen in our case. With the rise in mucormycosis cases and limited knowledge among general practitioners and dentists, the authors experienced many patients reporting late and/or with dental extractions performed. The reported case showed satisfactory healing until 2 months follow-up.
Conclusion:
COVID-19 associated mucormycosis (CAM) is aggressive and locally invasive, therefore early detection and management is imperative to improve prognosis. Management by a multidisciplinary team dedicated to CAM can help in prompt diagnosis and management and help reduce morbidity and disfigurement. Cases with mandibular involvement which were previously rare are predicted to rise due to the pandemic. Early referral and caution before attempting dental extraction (which can worsen ongoing infection) will ensure good outcomes, shorter hospital stay and better rehabilitation.
CRediT authorship contribution statement
Aafiya Ambereen: Conceptualization, Methodology, Writing – original draft. Sajjad A. Rahman: Visualization, Supervision, Writing – review & editing. Suhailur Rehman: Investigation, Validation. Kamran Zaidi: Data curation, Resources. S.H. Arif: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
None.
Patient informed and written consent
Taken.
Funding source
None.
References:
- Over 45,000 cases of mucormycosis reported in country - India News. https://www.indiatoday.in/india/story/over-45-000-cases-of-mucormycosis-reported-in-country-1830590-2021-07-2Accessed September 13, 2021.
- Baker R.D. Mucormycosis—a new disease? J Am Med Assoc. 1957;163(10):805–808. doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- Blitzer A., Lawson W., Meyers B.R., Biller H.F. Patient survival factors in paranasal sinus mucormycosis. Laryngoscope. 1980;90(4):635–648. doi: 10.1288/00005537-198004000-00010. [DOI] [PubMed] [Google Scholar]
- Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., Sein M., Sein T., Chiou C.C., Chu J.H., Kontoyiannis D.P., Walsh T.J. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Prakash H., Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):1–12. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Singh R. Mucormycosis in India: Unique features. Mycoses. 2014;57(s3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A, Singh S, Roychoudhury A, Kholakiya Y, Arya S, Roychoudhury S. Current Understanding in the Pathophysiology of SARS-CoV-2-Associated Rhino-Orbito-Cerebral Mucormycosis: A Comprehensive Review. J Maxillofac Oral Surg. June 2021:1-8. [DOI] [PMC free article] [PubMed]
- Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693-704. [DOI] [PMC free article] [PubMed]
- Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandra S, Ram S, Levitz SM. The “Black Fungus” in India: The Emerging Syndemic of COVID-19-Associated Mucormycosis. Ann Intern Med. June 2021. [DOI] [PMC free article] [PubMed]
- Sen M., Honavar S.G., Bansal R., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin C., Ibrahim A.S., Sheppard D.C. Molecular mechanisms of mucormycosis—The bitter and the sweet. PLoS Pathog. 2017;13(8):e1006408. doi: 10.1371/journal.ppat.100640810.1371/journal.ppat.1006408.g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak E.J., Kim D.J., Nam W., Park W. Mucormycosis in the Jaw: A Report of 2 Cases and Literature Review. Oral Health Prev Dent. 2020;18(1):1011–1016. doi: 10.3290/j.ohpd.a45522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Anand A., Ranjan P., Meena V.P., Ray A., Dutta R., Jadon R.S., Vikram N.K. Case of mucormycosis of mandible after self-extraction of teeth incidentally detected to have chronic granulomatous disease: Case report and literature review. Med Mycol Case Rep. 2020;28:55–59. doi: 10.1016/j.mmcr.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras M.H., Kara M.I., Erkiliç S., Ay S. Mandibular mucormycosis in immunocompromised patients: report of 2 cases and review of the literature. J Oral Maxillofac Surg. 2012;70(6):1362–1368. doi: 10.1016/j.joms.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Cohen A., Shoukair F.L., Korem M., Shaulov A., Casap N. Successful Mandibular Mucormycosis Treatment in the Severely Neutropenic Patient. J Oral Maxillofac Surg. 2019;77(6):1209.e1–1209.e12. doi: 10.1016/j.joms.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Urs, Augustine J, Singh S. Histopathological evaluation of a rare fulminant case of contemporaneous mucormycosis, aspergillosis and actinomycosis. J Oral Maxillofac Pathol. 2019;23(1):144. [DOI] [PMC free article] [PubMed]
- Cornely O.A., Alastruey-Izquierdo A., Arenz D., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]