Abstract
Since the emergence of SARS-CoV-2 in Wuhan, China more than a year ago, it has spread across the world in a very short span of time. Although, different forms of vaccines are being rolled out for vaccination programs around the globe, the mutation of the virus is still a cause of concern among the research communities. Hence, it is important to study the constantly evolving virus and its strains in order to provide a much more stable form of cure. This fact motivated us to conduct this research where we have initially carried out multiple sequence alignment of 15359 and 3033 global dataset without Indian and the dataset of exclusive Indian SARS-CoV-2 genomes respectively, using MAFFT. Subsequently, phylogenetic analyses are performed using Nextstrain to identify virus clades. Consequently, the virus strains are found to be distributed among 5 major clades or clusters viz. 19A, 19B, 20A, 20B and 20C. Thereafter, mutation points as SNPs are identified in each clade. Henceforth, from each clade top 10 signature SNPs are identified based on their frequency i.e. number of occurrences in the virus genome. As a result, 50 such signature SNPs are individually identified for global dataset without Indian and dataset of exclusive Indian SARS-CoV-2 genomes respectively. Out of each 50 signature SNPs, 39 and 41 unique SNPs are identified among which 25 non-synonymous signature SNPs (out of 39) resulted in 30 amino acid changes in protein while 27 changes in amino acid are identified from 22 non-synonymous signature SNPs (out of 41). These 30 and 27 amino acid changes for the non-synonymous signature SNPs are visualised in their respective protein structure as well. Finally, in order to judge the characteristics of the identified clades, the non-synonymous signature SNPs are considered to evaluate the changes in proteins as biological functions with the sequences using PROVEAN and PolyPhen-2 while I-Mutant 2.0 is used to evaluate their structural stability. As a consequence, for global dataset without Indian sequences, G251V in ORF3a in clade 19A, F308Y and G196V in NSP4 and ORF3a in 19B are the unique amino acid changes which are responsible for defining each clade as they are all deleterious and unstable. Such changes which are common for both global dataset without Indian and dataset of exclusive Indian sequences are R203M in Nucleocapsid for 20B, T85I and Q57H in NSP2 and ORF3a respectively for 20C while for exclusive Indian sequences such unique changes are A97V in RdRp, G339S and G339C in NSP2 in 19A and Q57H in ORF3a in 20A.
Keywords: COVID-19, Clade, Deleterious mutations, Non-Synonymous Signature SNP, SARS-CoV-2
1. Introduction
The first case of SARS-CoV-2 was registered in Wuhan China, 2019 and it quickly took over the normal functioning of human lives. In the initial phases, lock-down was implemented to limit the spread of infection. It is well known that virus mutations take place in the form of single nucleotide variants, deletions and large structural variants [1] mainly due to replication and some hotspot mutations having severe impact on the host. Many cities around the globe have gone through staggered phases of lockdown in order to avoid the spread of the different strains of SARS-CoV-2. Among these strains, B.1.1.7 (Alpha) is found to be highly transmissible [2] and causes more severe pathogenic infection in young people [3]. Another major variant B.1.351 (Beta) which has emerged from South Africa [4], [5] had also led to a sudden surge in the total number of cases. The efficacy of therapeutic monoclonal antibodies (mAbs) are known to be reduced against another variant P.1 (Gamma). It is estimated to be 2.6 times more transmissible [6]. Another variant B.1.617.2 (Delta) is known for the surge in cases in India during the 2nd wave of the pandemic.
SARS-CoV-2 is a 29.9 kb long single-stranded genomic RNA [7], [8], [9], [10], [11], [12]. It covers 11 coding regions where ORF1ab occupies majority of the genomic sequence while Spike (S), ORF3a, Envelope, Membrane, ORF6, ORF7a, ORF7b, ORF8, Nucleocapsid and ORF10 constitute the rest of the sequence [8], [9], [13]. Through various studies it is found that the South African B.1.351 variant strain consists of mutation in three prominent places in Spike (S) protein, they being K417N, E484K and N501Y [4] whereas the UK variant B.1.1.7 which was found to be part of the 20B clade contains multiple mutations with a combination of N501Y in Spike (S) protein [2] and the 69-70del which have been circulating within the community for months. Hence, it is incumbent that such frequent mutations be given special focus by the scientific community to trace and tackle the challenges posed by the mutations.
Tang et al. [14] investigated the extent of molecular divergence between SARS-CoV-2 and other related coronaviruses by analysing 103 SARS-CoV-2 genomes and reported two major lineages, L and S. Several other mutations are also identified in the last few months which demands re-purposing of the current methods to deal with the virus. Wang et al. [15] have proposed a h-index mutation ratio criteria to evaluate the non-conserved and conserved proteins with the help of more than 15000 sequences. Consequently, nucleocapsid, spike and papain-like protease are found to be highly non-conserved while envelope, main protease, and endoribonuclease protein are relatively conservative. They have further identified the mutations on 40% of nucleotides in nucleocapsid, thereby indicating potential impacts on the ongoing development of various COVID-19 diagnosis and cure. Such similar analysis conducted by Yuan et al. [16] with 11183 sequences revealed 119 high frequency substitutions or Single Nucleotide Polymorphism (SNP) around the globe. Among the nucleotide changes in SNPs, C to T is the major one indicating adaptation and evolution of the virus in the human host which can pose new challenges. On the other hand, Chen et al. [17] focused on the binding of free energy changes between the angiotensin converting enzyme 2 (ACE2) receptor with the frequently changing Spike protein of SARS-CoV-2 considering algebraic topology-based machine learning model and found 3 sub-type of the virus with slightly high infectivity. 570 SARS-CoV-2 sequences were analysed and 10 distinct hotspot mutations points from China, India, USA, Europe which might affect the replication-relevant proteins were identified by Weber et al. [18]. Further, they found that these mutations can effect the secondary structure of the RNA molecule of SARS-CoV-2 and its repertoire which are essential for viral and cellular proteins. Moreover, Nagy et al. [19] computed the direct effect of mutations over clinical outcome with the help of Chi-square test, in which they found mutations in ORF8, NSP6, ORF3a, NSP4 and nucleocapsid regions are associated with mild effects while inferior outcomes were mapped in spike, RNA-dependent RNA polymerase, ORF3a, NSP3, ORF6 and nucleocapsid. Further, they concluded that mutations in ORF3a and NSP7 can lead to severe outcomes but with low prevalence. Cheng et al. [20] identified 5 major mutation points, C28144T, C14408T, A23403G, T8782C and C3037T in almost all strains for the month of April 2020. Their functional analysis show that these mutations lead to a decrease in protein stability and eventually a reduction in the virulence of SARS-CoV-2 but A23403G mutation increases the Spike-ACE2 interaction leading to an increase in its infectivity. Whole-genome analysis of 837 Indian SARS-CoV-2 genomes were carried out by Sarkar et al. [21] which revealed 33 different mutations, out of which 18 were unique to India. Based on their co-existing mutations, the Indian isolates were classified into 22 groups. Their study highlighted the evolution of divergent SARS-CoV-2 strains and also co-circulation of multiple strains in India.
Motivated by the aforementioned studies, in this work we have analysed 18392 SARS-CoV-2 genomes for 71 countries where 15359 global dataset without Indian (Dataset A) and dataset of 3033 exclusive Indian (Dataset B) SARS-CoV-2 genomes are taken separately to identify clade specific signature SNPs. To achieve this, multiple alignment using fast fourier transform (MAFFT) [22] is used for multiple sequence alignment (MSA) followed by Nextstrain [23] for performing phylogenetic analysis to identify virus clades. As a result, the virus strains are found to be distributed among 5 different clades viz. 19A, 19B, 20A, 20B and 20C. Subsequently, mutation points as SNPs are identified in each clade. Furthermore, top 10 signature SNPs based on their frequency are then identified in each clade resulting in a total of 50 such SNPs each for Dataset A and Dataset B. Out of 50 signature SNPs for Dataset A, 39 unique SNPs are identified among which 25 non-synonymous signature SNPs resulted in 30 amino acid changes in protein while for Dataset B, 22 non-synonymous signature SNPs are identified from 41 unique SNPs resulting in 27 amino acid changes. These 30 and 27 amino acid changes for the non-synonymous signature SNPs are visualised in their respective protein structure as well. Furthermore, in order to judge the characteristics of the identified clades, the non-synonymous signature SNPs are considered to evaluate the changes in proteins as biological functions with the sequences using PROVEAN and PolyPhen-2 while I-Mutant 2.0 is used to evaluate their structural stability. As a consequence, for Dataset A, G251V in ORF3a in clade 19A, F308Y and G196V in NSP4 and ORF3a in 19B are the unique amino acid changes which are responsible for defining each clade as they are all deleterious and unstable. Such changes which are common for both Datasets A and B are R203M in Nucleocapsid for 20B, T85I and Q57H in NSP2 and ORF3a respectively for 20C while for Dataset B such unique changes are A97V in RdRp, G339S and G339C in NSP2 in 19A and Q57H in ORF3a in 20A.
2. Material and Methods
In this section, the collection of the dataset for SARS-CoV-2 genomes and the proposed pipeline are discussed.
2.1. Data acquisition
The collection of the dataset can be summarised as below:
-
•
For phylogenetic analyses, Dataset A with 15359 sequences and Dataset B with 3033 SARS-CoV-2 genomes are collected from Global Initiative on Sharing All Influenza Data (GISAID)2 while the Reference Genome (NC_045512.2)3 is collected from National Center for Biotechnology Information (NCBI).
-
•
The 18392 SARS-CoV-2 sequences for 71 countries are mostly distributed from December 2019 to December 2020. The average and maximum lengths of the sequences are 29820 and 29903 respectively.
-
•
Further, to map the changes of amino acid in proteins, PDB of the proteins are collected from Zhang Lab4 and other reliable sources.
-
•
All these analysis are performed on High Performance Computing facility of NITTTR, Kolkata and the codes are written in MATLAB R2019b.
2.2. Pipeline of the work
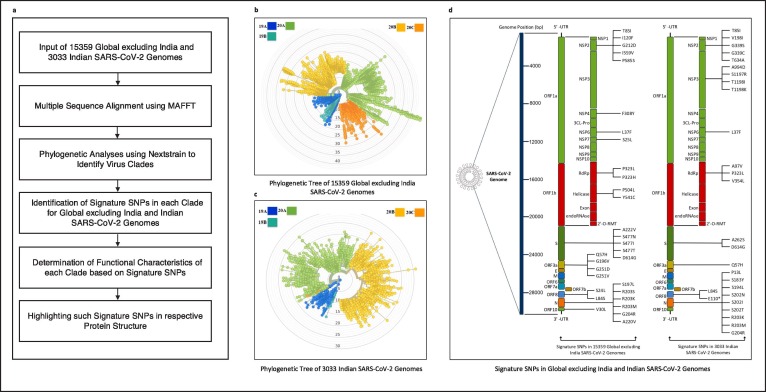
The pipeline of the work is provided in Fig. 1 (a). Initially, multiple sequence alignment of Datasets A and B are carried out using MAFFT followed by phylogenetic analyses using Nextstrain which resulted in the identification of virus clades. The corresponding phylogenetic trees are shown in Fig. 1(b) and Fig. 1(c) respectively. MAFFT has two novel techniques: Fast Fourier Transform (FFT) rapidly identifies homologous regions and a simple scoring system to reduce the CPU time [22]. MAFFT merges local and global algorithms for MSA and uses two different heuristic methods such as progressive (FFT-NS-2) and iterative refinements (FFT-NS-i). FFT-NS-2 is used to calculate all-pairwise distances to create a provisional MSA from which refined distances are calculated. FFT-NS-i is then performed to get the final MSA. The use of fast fourier transform in MAFFT makes it outperform other alignment techniques [22]. Thus, MAFFT is used in this work for multiple sequence alignment.
Fig. 1.
Pipeline of the work.
On the other hand, Nextstrain is a collection of open-source tools which is useful for understanding the evolution and spread of pathogen, particularly during an outbreak. Using Nextstrain, proper and meaningful visualisation of a large number of virus sequences can be achieved. It consists of “auspice” which is a web-based visualisation program used to present and interact with phylogenomic and phylogeographic data. There are a substantial number of tools in Nextstrain which perform phylodynamic analysis [24] which ranges from subsampling, alignment, phylogenetic inference to temporal dating of ancestral nodes and discrete trait geographic reconstruction and inference of the most likely transmission events. The spread and evolution of virus genomes can be visualised at nextstrain.org using auspice. By taking the advantage of this tool, in this work the evolution and geographic distribution of SARS-CoV-2 genomes are visualised by creating the metadata in our High Performance Computing environment.
Once the virus clades are identified using Nextstrain, clade specific aligned sequences are used to identify the mutation points as substitutions specifically SNPs in each clade. Following this, amino acid changes in the virus proteins corresponding to the SNPs are identified using the codon table. Thereafter, individually for Datasets A and B, top 10 signature SNPs are identified in each clade based on their frequencies or number of occurrences in the virus genome. Such frequencies can also be quantified by considering entropy values as well, the calculation of which is described in details in [25]. It is to be noted that the amino acid changes for the SNPs can be either synonymous or non-synonymous. Thereafter, the amino acid changes in the non-synonymous signature SNPs are considered to evaluate their functional characteristics. These amino acid changes are visualised in the respective protein structure as well. The amino acid changes for the non-synonymous signature SNPs in the different coding regions for both Datasets A and B are visualised graphically in Fig. 1(d).
3. Results
3.1. Phylogenetic analyses
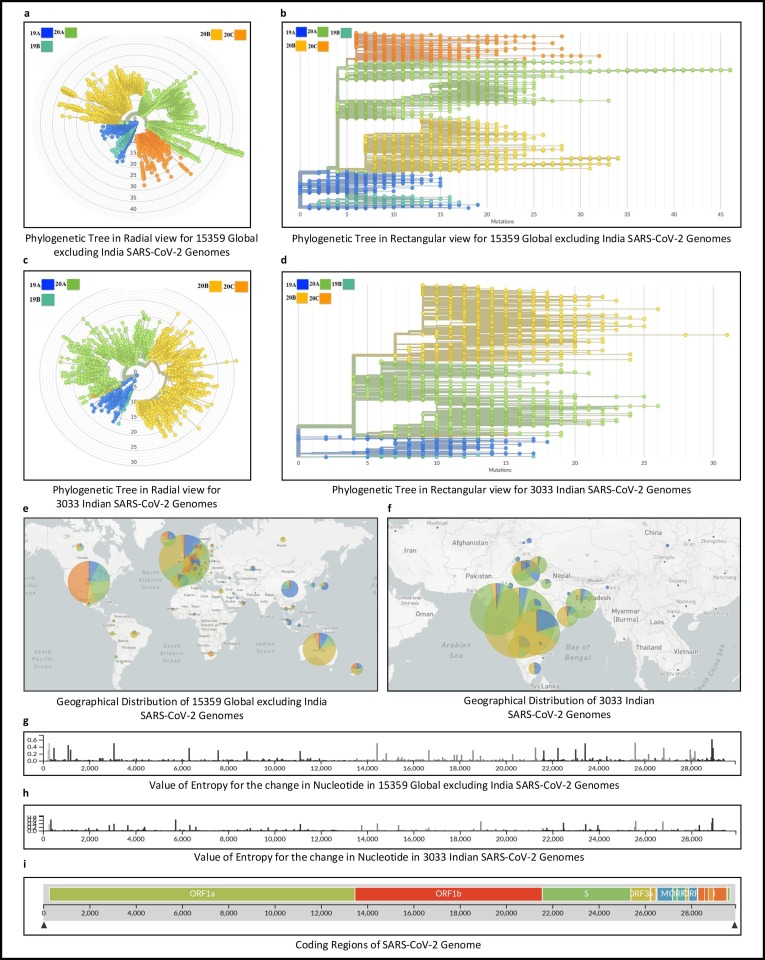
The experiments in this work are carried out according to the pipeline as provided in Fig. 1(a). In this regard, initially multiple sequence alignment of Dataset A with 15359 and Dataset B with 3033 SARS-CoV-2 genomic sequences are carried out using MAFFT followed by their phylogenetic analysis using Nextstrain. The results from the phylogenetic analyses are as follows:
-
•
As a result of phylogenetic analysis by Nextstrain, 5 clades are identified viz. 19A, 19B, 20A, 20B and 20C. Subsequently, mutation points as SNPs are identified in each clade for both Dataset A and Dataset B.
-
•
As a result, 2060 mutation points as SNPs are identified in clade 19A for 2194 genomic sequences for Dataset A. 1015 sequences belonging to clade 19B have 865 SNPs while for 5134, 4627 and 2389 sequences in clades 20A, 20B and 20C respectively, the number of SNPs are 4292, 3695 and 2280. The corresponding phylogenetic tree in radial and rectangular view is shown in Fig. 2 (a)-(b).
-
•
For Dataset B, 467 and 125 SNPs in clades 19A and 19B respectively are identified covering 309 and 48 genomic sequences while 2212 and 2311 SNPs are covered in clades 20A and 20B for 1322 and 1342 genomic sequences respectively. Finally, clade 20C consists of 12 sequences and has 33 SNPs. The phylogenetic tree for Dataset B is shown in Fig. 2(c)-(d).
-
•
The clade wise distribution of 15359 and 3033 sequences for 70 countries in Dataset A and statewise for India in Dataset B are reported in Table 1, Table 2 respectively. For example, as reported in Table 1, USA has 263, 530, 707, 244 and 1628 sequences distributed in clades 19A, 19B, 20A, 20B and 20C respectively. Thus, most of the variants in USA belongs to clade 20C.
-
•
For India as given in Table 2, the most dominant clade in Maharashtra is 20B while for Gujarat it is 20A. It can be further concluded from Table S2 that most of the variants in India belong to clades 20A and 20B.
-
•
These clade wise distributions of Datasets A and B are visualised in Fig. 2(e) and (f) respectively.
-
•
The clade wise evolution of all 18392 global including India (country wise) and separately 3033 Indian (state wise) SARS-CoV-2 genomes for each month is shown in the form of pie charts in supplementary Tables S1 and S2 respectively.
-
•
The month wise evolution of such genomes for each clade is reported in supplementary Tables S3 and S4 respectively. The corresponding colour representation for the five major clades and the months are shown in supplementary Figure S1.
Fig. 2.
Phylogenetic analyses of 15359 Global excluding India and 3033 Indian SARS-CoV-2 genomes.
Table 1.
Clade wise distribution of 15359 sequences for Global Dataset without Indian sequences (Dataset A).
| Country | 19A | 19B | 20A | 20B | 20C | Country | 19A | 19B | 20A | 20B | 20C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | 263 | 530 | 707 | 244 | 1628 | Thailand | 2 | 16 | 1 | 3 | 4 |
| England | 336 | 7 | 981 | 1137 | 43 | Northern Ireland | 8 | 0 | 5 | 10 | 0 |
| Australia | 190 | 84 | 143 | 1499 | 116 | Norway | 8 | 0 | 6 | 1 | 5 |
| Wales | 97 | 0 | 1174 | 176 | 10 | Austria | 3 | 0 | 8 | 3 | 4 |
| Scotland | 109 | 14 | 514 | 212 | 22 | Chile | 0 | 8 | 5 | 1 | 1 |
| Netherlands | 141 | 8 | 241 | 126 | 41 | Colombia | 2 | 3 | 6 | 2 | 2 |
| Belgium | 100 | 1 | 185 | 226 | 27 | Indonesia | 12 | 0 | 3 | 0 | 0 |
| China | 394 | 90 | 23 | 12 | 13 | Estonia | 0 | 0 | 3 | 8 | 2 |
| Iceland | 95 | 16 | 152 | 91 | 66 | Senegal | 3 | 1 | 8 | 0 | 0 |
| Portugal | 33 | 11 | 128 | 189 | 8 | Croatia | 1 | 0 | 5 | 2 | 3 |
| France | 33 | 2 | 147 | 18 | 73 | Georgia | 4 | 1 | 4 | 1 | 1 |
| Spain | 17 | 129 | 97 | 19 | 4 | Malaysia | 10 | 1 | 0 | 0 | 0 |
| New Zealand | 43 | 10 | 63 | 77 | 56 | Romania | 0 | 0 | 5 | 6 | 0 |
| Sweden | 9 | 0 | 62 | 113 | 38 | Ireland | 3 | 0 | 2 | 5 | 0 |
| Switzerland | 19 | 0 | 71 | 48 | 25 | Kenya | 2 | 0 | 7 | 1 | 0 |
| Italy | 7 | 0 | 84 | 35 | 0 | Latvia | 5 | 0 | 2 | 2 | 0 |
| Luxembourg | 5 | 1 | 79 | 2 | 24 | Nigeria | 7 | 0 | 1 | 0 | 1 |
| Denmark | 2 | 0 | 32 | 9 | 66 | Kuwait | 5 | 0 | 1 | 1 | 0 |
| Japan | 71 | 4 | 7 | 24 | 0 | Slovakia | 1 | 0 | 4 | 1 | 0 |
| Canada | 7 | 25 | 31 | 16 | 23 | Tunisia | 0 | 0 | 5 | 1 | 0 |
| Brazil | 5 | 2 | 6 | 80 | 1 | Bangladesh | 1 | 0 | 0 | 3 | 0 |
| Germany | 23 | 2 | 5 | 14 | 25 | Greece | 0 | 1 | 0 | 3 | 0 |
| Singapore | 31 | 1 | 18 | 3 | 1 | Qatar | 4 | 0 | 0 | 0 | 0 |
| Russia | 0 | 0 | 9 | 41 | 2 | Turkey | 2 | 0 | 2 | 0 | 0 |
| South Africa | 1 | 0 | 11 | 5 | 34 | Argentina | 0 | 0 | 2 | 1 | 0 |
| Kazakhstan | 26 | 18 | 2 | 0 | 3 | Belarus | 2 | 0 | 1 | 0 | 0 |
| Israel | 1 | 0 | 8 | 31 | 0 | Hungary | 0 | 0 | 2 | 1 | 0 |
| Poland | 2 | 0 | 14 | 21 | 3 | Saudi Arabia | 1 | 0 | 2 | 0 | 0 |
| Oman | 16 | 0 | 6 | 16 | 1 | Slovenia | 2 | 0 | 1 | 0 | 0 |
| Mexico | 1 | 10 | 15 | 8 | 2 | Pakistan | 2 | 0 | 0 | 0 | 0 |
| South Korea | 17 | 19 | 0 | 0 | 0 | Serbia | 0 | 0 | 1 | 1 | 0 |
| Peru | 0 | 0 | 2 | 31 | 0 | Cambodia | 1 | 0 | 0 | 0 | 0 |
| Czech Republic | 0 | 0 | 9 | 20 | 3 | Morocco | 0 | 0 | 0 | 1 | 0 |
| Vietnam | 5 | 0 | 2 | 22 | 2 | Nepal | 1 | 0 | 0 | 0 | 0 |
| Finland | 3 | 0 | 13 | 4 | 6 | Panama | 0 | 0 | 1 | 0 | 0 |
Table 2.
Clade wise distribution of 3033 exclusive Indian sequences (Dataset B).
| State | 19A | 19B | 20A | 20B | 20C |
|---|---|---|---|---|---|
| Maharashtra | 39 | 8 | 289 | 808 | 0 |
| Gujarat | 16 | 12 | 559 | 21 | 3 |
| Telangana | 94 | 0 | 59 | 311 | 2 |
| West Bengal | 9 | 14 | 154 | 15 | 0 |
| Delhi | 55 | 1 | 79 | 19 | 2 |
| Karnataka | 25 | 2 | 25 | 51 | 0 |
| Odisha | 6 | 10 | 28 | 45 | 4 |
| Haryana | 15 | 0 | 44 | 29 | 1 |
| Uttarakhand | 2 | 1 | 40 | 25 | 0 |
| Madhya Pradesh | 10 | 0 | 25 | 1 | 0 |
| Tamil Nadu | 15 | 0 | 1 | 15 | 0 |
| Uttar Pradesh | 4 | 0 | 16 | 1 | 0 |
| Rajasthan | 4 | 0 | 2 | 0 | 0 |
| Punjab | 4 | 0 | 1 | 0 | 0 |
| Ladakh | 5 | 0 | 0 | 0 | 0 |
| Bihar | 2 | 0 | 0 | 0 | 0 |
| Assam | 2 | 0 | 0 | 0 | 0 |
| Andhra Pradesh | 1 | 0 | 0 | 1 | 0 |
| Jammu and Kashmir | 1 | 0 | 0 | 0 | 0 |
| Total | 309 | 48 | 1322 | 1342 | 12 |
Moreover, the entropy values for the nucleotide changes for Datasets A and B are shown respectively in Fig. 2(g)-(h). Furthermore, the coding regions of the SARS-CoV-2 genome are visualised in Fig. 2(i).
3.2. Signature SNPs in each Clade
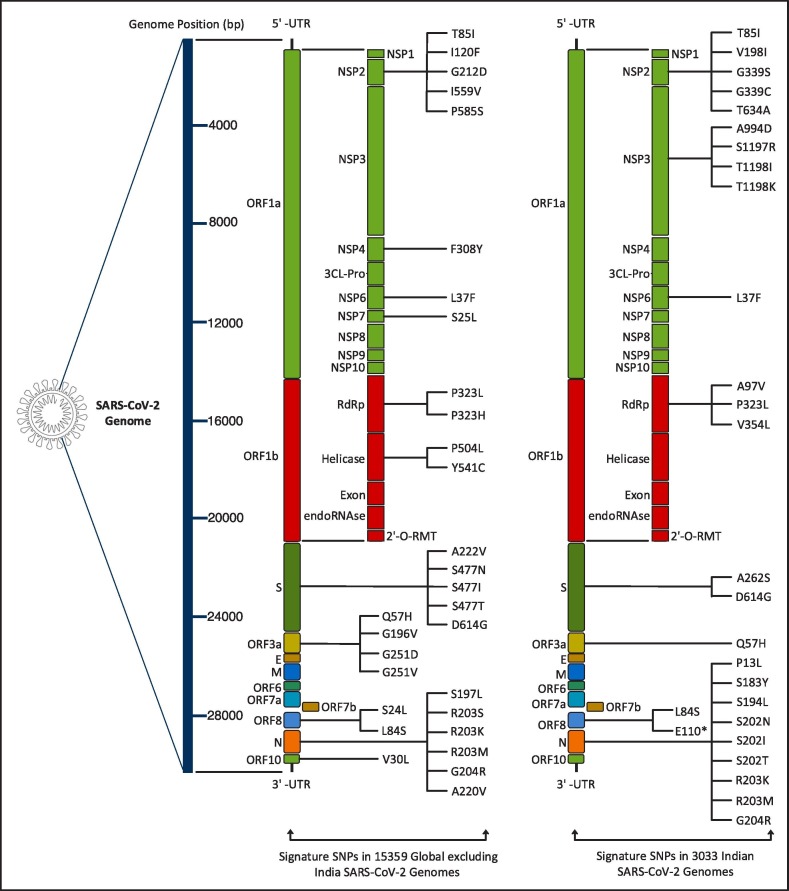
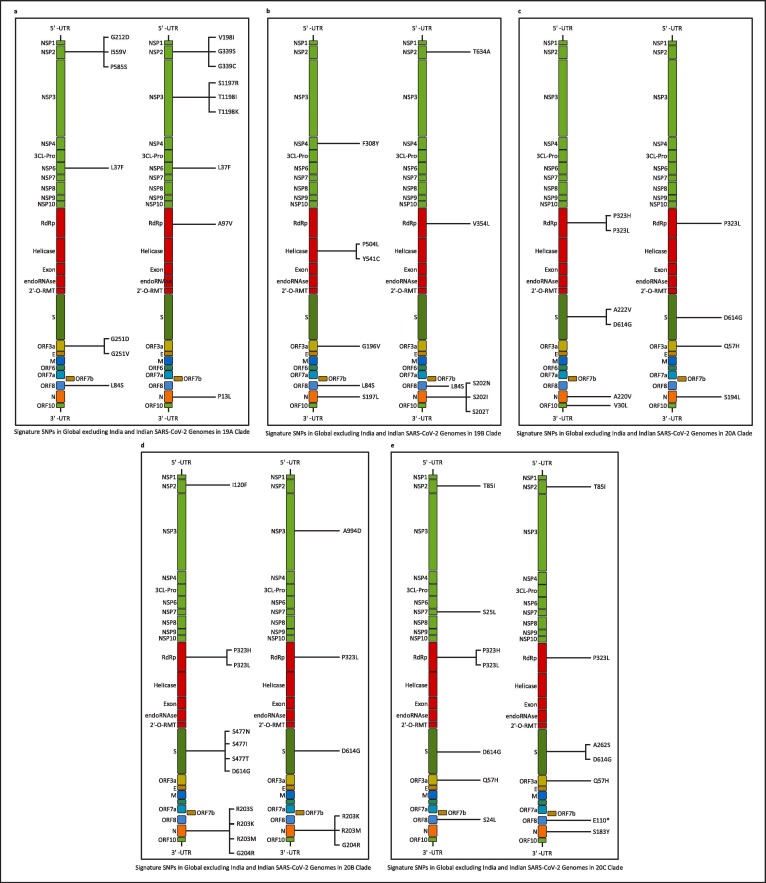
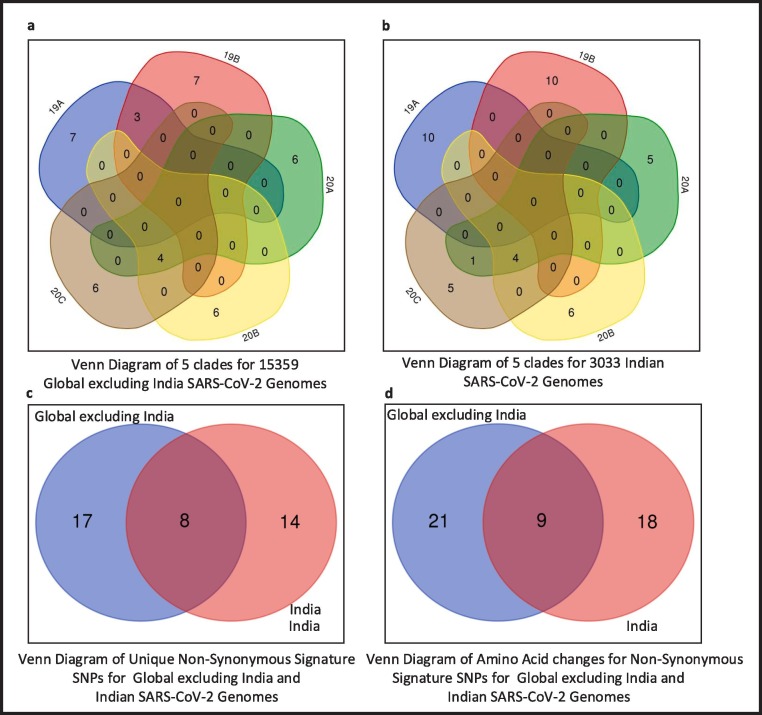
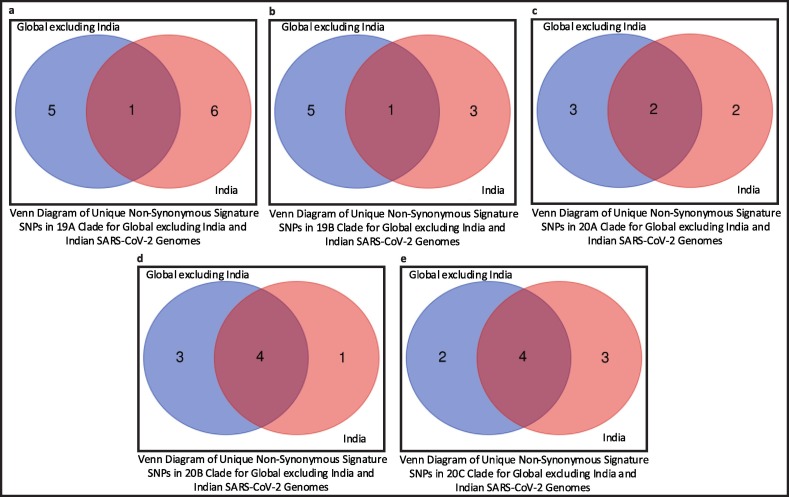
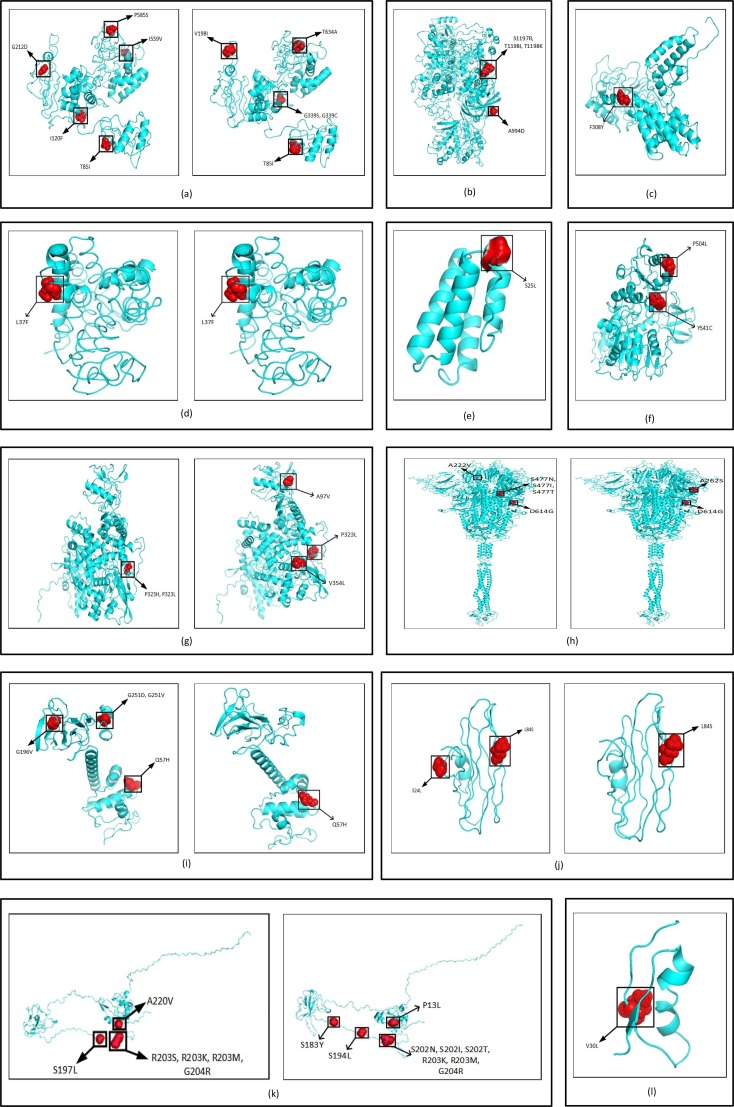
Once the mutation points as SNPs are determined, top 10 signature SNPs are identified in each clade for both Datasets A and B, thus resulting in 50 signature SNPs for each category as reported in Table 3 . For example, for Dataset A, G11083T with a frequency of 939 is the top signature SNP in clade 19A while for Dataset B the top signature SNP is C13730T with a frequency of 274. Thereafter, 39 and 41 unique signature SNPs are identified for each category. For Dataset A, these 39 signature SNPs result in 25 non-synonymous signature SNPs with 30 amino acid changes in protein. On the other hand, for Dataset B, 41 unique signature SNPs have 22 non-synonymous signature SNPs with 27 amino acid changes. The non-synonymous signature SNPs are reported in Table 3 and their amino acid changes in protein are shown in Fig. 3 . The corresponding clade-wise distribution is shown in Fig. 4 . To depict the common signature SNPs in the five clades for both Datasets A and B, visualisation in the form of Venn diagram is shown in Fig. 5 (a) and (b). As can be seen from the figures, there are no common SNPs in all the five clades for both Datasets A and B, thereby confirming the fact that such signature SNPs are features which indeed define each of the clades. For Datasets A and B, the visualisation of unique and common non-synonymous signature SNPs are shown in Fig. 5(c) while unique and common amino acid changes in protein are given in Fig. 5(d). Fig. 5(c) depicts 17 and 14 unique non-synonymous signature SNPs in Datasets A and B while the number of common non-synonymous signature SNPs are 8. Fig. 5(d) shows that there are 21 and 18 unique amino acid changes in Datasets A and B while 9 amino acid changes are common in both. Furthermore, clade wise unique and common non-synonymous signature SNPs are visualised in Fig. 6 . It can be observed from the figure that 1, 1, 2, 4 and 4 non-synonymous signature SNPs are common for both Datasets A and B in clades 19A, 19B, 20A, 20B and 20C respectively. In 19A, the number of such unique SNPs are 5 and 6 for Datasets A and B while for 19B they are 5 and 3. For 20A and 20B such statistics are 3, 2, 3 and 1 while for 20C, the number of unique SNPs are 2 and 3. All the amino acid changes for the non-synonymous signature SNPs in the respective protein structure are visualised in Fig. 7 . All the detailed results are provided in Supplementary Table S5.
Table 3.
List of Signature SNPs in each clade for 15359 Global Dataset without India (Dataset A) and 3033 exclusive Indian (Dataset B) SARS-CoV-2 Genomes.
| Signature SNPs in 15359 Global excluding India sequences |
Signature SNPs in 3033 Indian sequences |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clade | Genomic | Frequency | Change in | Change in | Mapped with | Genomic | Frequency | Change in | Change in | Mapped with |
| Coordinate | Nucleotide | Amino Acid | Coding and Non-Coding Region | Coordinate | Nucleotide | Amino Acid | Coding and Non-Coding Regions | |||
| 11083 | 939 | GT | L37F | NSP6 | 13730 | 274 | CT | A97V | RdRp | |
| 26144 | 751 | GA, GT | G251D, G251V | ORF3a | 11083 | 268 | GA, GT | Synonymous, L37F | NSP6 | |
| 14805 | 655 | CT | Synonymous | RdRp | 28311 | 264 | CT | P13L | Nucleocapsid | |
| 17247 | 308 | TC | Synonymous | Helicase | 6312 | 263 | CT, CA | T1198I, T1198K | NSP3 | |
| 19A | 2558 | 235 | C T | P585S | NSP2 | 23929 | 261 | CT | Synonymous | Spike |
| 2480 | 215 | AG | I559V | NSP2 | 19524 | 60 | CT | Synonymous | Exon | |
| 28144 | 199 | TC | L84S | ORF8 | 6310 | 55 | CA, CT | S1197R, Synonymous | NSP3 | |
| 29742 | 188 | GA, GT | Not Present | 3’-UTR | 1820 | 26 | GA, GT | G339S, G339C | NSP2 | |
| 8782 | 166 | CT | Synonymous | NSP4 | 1397 | 21 | GA | V198I | NSP2 | |
| 1440 | 163 | GA | G212D | NSP2 | 28688 | 21 | TC | Synonymous | Nucleocapsid | |
| 28144 | 1010 | TC | L84S | ORF8 | 28144 | 48 | TC | L84S | ORF8 | |
| 8782 | 993 | CT | Synonymous | NSP4 | 8782 | 47 | CT | Synonymous | NSP4 | |
| 18060 | 638 | CT | Synonymous | Exon | 28878 | 45 | GA, GT, GC | S202N, S202I, S202T | Nucleocapsid | |
| 17858 | 626 | AG | Y541C | Helicase | 22468 | 44 | GT | Synonymous | Spike | |
| 19B | 17747 | 610 | CT | P504L | Helicase | 29742 | 44 | GA, GC | Not Present | 3’-UTR |
| 9477 | 190 | TA | F308Y | NSP4 | 7945 | 13 | CT | Synonymous | NSP3 | |
| 14805 | 190 | CT | Synonymous | RdRp | 2705 | 7 | AG | T634A | NSP2 | |
| 28657 | 189 | CT | Synonymous | Nucleocapsid | 14500 | 7 | GT | V354L | RdRp | |
| 28863 | 187 | CT | S197L | Nucleocapsid | 29830 | 6 | GT, GC | Not Present | 3’-UTR | |
| 25979 | 184 | GT | G196V | ORF3a | 24358 | 6 | CA | Synonymous | Spike | |
| 14408 | 5133 | CT, CA | P323L, P323H | RdRp | 23403 | 1313 | AG | D614G | Spike | |
| 23403 | 5131 | AG | D614G | Spike | 241 | 1295 | CT | Not Present | 5’-UTR | |
| 241 | 5128 | CT | Not Present | 5’-UTR | 3037 | 1294 | CT | Synonymous | NSP3 | |
| 3037 | 5123 | CT | Synonymous | NSP3 | 14408 | 1248 | CT | P323L | RdRp | |
| 20A | 21255 | 1870 | GA, GT, GC | Synonymous, Synonymous, Synonymous | 2’-O-RMT | 18877 | 633 | CT | Synonymous | Exon |
| 26801 | 1869 | CT, CG | Synonymous, Synonymous | Membrane | 26735 | 624 | CT | Synonymous | Membrane | |
| 22227 | 1864 | CT | A222V | Spike | 25563 | 611 | GA, GT, GC | Synonymous, Q57H, Q57H | ORF3a | |
| 6286 | 1863 | CT | Synonymous | NSP3 | 28854 | 506 | CT | S194L | Nucleocapsid | |
| 29645 | 1863 | GT | V30L | ORF10 | 22444 | 473 | CT | Synonymous | Spike | |
| 28932 | 1862 | CT | A220V | Nucleocapsid | 15324 | 281 | CT | Synonymous | RdRp | |
| 241 | 4623 | CT | Not Present | 5’-UTR | 241 | 1341 | CT | Not Present | 5’-UTR | |
| 23403 | 4623 | AG | D614G | Spike | 3037 | 1340 | CT | Synonymous | NSP3 | |
| 28882 | 4621 | GA, GT | Synonymous, R203S | Nucleocapsid | 23403 | 1340 | AG | D614G | Spike | |
| 28883 | 4621 | GA, GC | G204R, G204R | Nucleocapsid | 28881 | 1337 | GA, GT | R203K, R203M | Nucleocapsid | |
| 20B | 28881 | 4620 | GA, GT | R203K, R203M | Nucleocapsid | 28882 | 1337 | GA | Synonymous | Nucleocapsid |
| 3037 | 4614 | CT | Synonymous | NSP3 | 28883 | 1337 | GA, GC | G204R, G204R | Nucleocapsid | |
| 14408 | 4613 | CT, CA | P323L, P323H | RdRp | 14408 | 1331 | CT | P323L | RdRp | |
| 1163 | 1486 | AT | I120F | NSP2 | 5700 | 923 | CA | A994D | NSP3 | |
| 22992 | 1421 | GA, GT, GC | S477N, S477I, S477T | Spike | 313 | 912 | CT | Synonymous | NSP1 | |
| 18555 | 1395 | CT | Synonymous | Exon | 4354 | 170 | GA | Synonymous | NSP3 | |
| 1059 | 2389 | CT | T85I | NSP2 | 241 | 12 | CT | Not Present | 5’-UTR | |
| 14408 | 2388 | CT, CA | P323L, P323H | RdRp | 1059 | 12 | CT | T85I | NSP2 | |
| 3037 | 2387 | CT | Synonymous | NSP3 | 3037 | 12 | CT | Synonymous | NSP3 | |
| 23403 | 2387 | AG | D614G | Spike | 14408 | 12 | CT | P323L | RdRp | |
| 20C | 25563 | 2381 | GT, GC | Q57H, Q57H | ORF3a | 23403 | 12 | AG | D614G | Spike |
| 241 | 2379 | CT | Not Present | 5’-UTR | 25563 | 12 | GA, GT, GC | Synonymous, Q57H, Q57H | ORF3a | |
| 27964 | 380 | CT | S24L | ORF8 | 16260 | 6 | CT | Synonymous | Helicase | |
| 11916 | 190 | CT | S25L | NSP7 | 28821 | 6 | CA | S183Y | Nucleocapsid | |
| 29553 | 130 | GA | Not Present | 3’-UTR | 22346 | 4 | GT | A262S | Spike | |
| 29540 | 126 | GT, GA | Not Present | 3’-UTR | 28221 | 2 | GT | E110* | ORF8 | |
Fig. 3.
Amino acid changes in the proteins for the non-synonymous signature SNPs of Global excluding India and Indian SARS-CoV-2 genomes.
Fig. 4.
Amino acid changes in the proteins for the non-synonymous signature SNPs of Global excluding India and Indian SARS-CoV-2 genomes in 5 clades.
Fig. 5.
Venn diagrams of Global excluding India and Indian genomes to represent common signature SNPs.
Fig. 6.
Venn diagrams of Global excluding India and Indian genomes to represent common signature SNPs in clades (a) 19A (b) 19B (c) 20A (d) 20B (e) 20C.
Fig. 7.
Highlighted amino acid changes in the protein structures for the non-synonymous signature SNPs of (a) NSP2 for Global excluding India and India, (b) NSP3 for India, (c) NSP4 for Global excluding India, (d) NSP6 for Global excluding India and India, (e) NSP7 for Global excluding India, (f) Helicase for Global excluding India, (g) RdRp for Global excluding India and India, (h) Spike for Global excluding India and India, (i) ORF3a for Global excluding India and India, (j) ORF8 for Global excluding India and India, (k) Nucleocapsid for Global excluding India and India, (l) ORF10 for Global excluding India.
4. Discussion
SARS-CoV-2 has resulted in a mass meltdown throughout the globe. Recently, the mutated variants of the virus are turning out to be another major concern for the researchers. Thus, the identification of the virus strains is very crucial in this scenario. In this regard, we have analysed Datasets A and B with 15359 and 3033 SARS-CoV-2 genomes respectively which resulted in the identification of five major clades for both Datasets A and B and consequently top 10 signature SNPs in each clades.
Initially, multiple sequence alignment of Dataset A with 15359 and Dataset B with 3033 SARS-CoV-2 genomes using MAFFT are performed followed by phylogenetic analyses using Nextstrain to identify virus clades. Thereafter, mutation points as SNPs in each clade are identified. Subsequently, top 10 signature SNPs with high frequency are identified in each clade, the details of which are provided in the Results section.
Structural changes in amino acid residues often lead to alterations in the protein translations which can lead to functional instability of the proteins. In this regard, to judge the characteristics of the identified clades, non-synonymous signature SNPs of Datasets A and B are considered to evaluate the changes in proteins as biological functions using PROVEAN (Protein Variation Effect Analyser) [26] and PolyPhen-2 (Polymorphism Phenotyping) [27] while I-Mutant 2.0 [28] is used to evaluate their structural stability. The results are reported in Table 4 . PROVEAN5 works on sequence based prediction algorithm while the prediction of Polyphen-26 is based on sequence, structural and phylogenetic information of a SNP. On the other hand, I-Mutant 2.07 uses support vector machine (SVM) for the automatic prediction of protein stability changes upon single point mutations. PROVEAN and PolyPhen-2 are used to find the deleterious or damaging non-synonymous SNPs. The threshold value of PROVEAN is set at −2.5. If the PROVEAN score of a SNP is equal to or below this threshold, the corresponding non-synonymous mutation is considered to be deleterious. For Polyphen-2, this range is between 0 to 1. If the score is closer to 1, mutations are more confidently considered to be damaging. Considering the predictions of both PROVEAN and Polyphen-2, for Dataset A it can be seen from Table 4 that out of 30 unique amino acid changes, 11 unique changes are damaging or deleterious while for Dataset B, 9 unique changes are damaging out of 27 unique changes. Furthermore, protein stability is important to determine the functional and structural activity of a protein. Protein stability dictates the conformational structure of the protein, thereby determining its function. Any change in protein stability may cause misfolding, degradation or aberrant conglomeration of proteins [29]. The protein stabilities corresponding to the non-synonymous signature SNPs are determined using I-Mutant 2.0. The changes in the protein stability in I-Mutant 2.0 tool is predicted using free energy change values (DDG). A negative value of DDG indicates that the stability of a protein is decreasing. The result from I-mutant 2.0 concludes that out of the 11 and 9 unique damaging changes for Datasets A and B, 6 changes for both decrease the stability of the protein structures respectively. Consequently, for Dataset A, G251V in ORF3a in clade 19A, F308Y and G196V in NSP4 and ORF3a in 19B are the unique amino acid changes which are responsible for defining each clade as they are all deleterious and unstable. Such changes which are common for both Datasets A and B are R203M in Nucleocapsid for 20B, T85I and Q57H in NSP2 and ORF3a respectively for 20C while for Dataset B such unique changes are A97V in RdRp, G339S and G339C in NSP2 in 19A and Q57H in ORF3a in 20A. All of them are marked in bold in Table 4. It is to be noted that for Indian non-synonymous signature SNPs, there are 26 amino acid changes as opposed to 27 such changes in Table 3, the discarded change being E110* in ORF8 as the amino acid change leads to a stop codon.
Table 4.
Characteristics of non-synonymous signature SNPs for Global Dataset without India (Dataset A) and exclusive Indian (Dataset B) SARS-CoV-2 genomes.
| Non-synonymous signature SNPs for Global excluding India sequences | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clade | Change in | Mapped with | PROVEAN |
PolyPhen-2 |
I-Mutant 2.0 |
|||
| Amino Acid | Coding Regions | Prediction | Score | Prediction | Score | Stability | DDG | |
| L37F | NSP6 | Neutral | -1.369 | Benign | 0.027 | Decrease | -0.05 | |
| G251D | ORF3a | Deleterious | -6.933 | Probably Damaging | 1.000 | Increase | 0.02 | |
| G251V | ORF3a | Deleterious | −8.581 | Probably Damaging | 1.000 | Decrease | −0.54 | |
| 19A | P585S | NSP2 | Neutral | 0.442 | Benign | 0.005 | Decrease | −1.58 |
| I559V | NSP2 | Neutral | 0.444 | Benign | 0.003 | Decrease | −0.28 | |
| L84S | ORF8 | Neutral | 2.333 | Benign | 0.002 | Decrease | −2.87 | |
| G212D | NSP2 | Neutral | 0.704 | Benign | 0.013 | Decrease | −1.15 | |
| L84S | ORF8 | Neutral | 2.333 | Benign | 0.002 | Decrease | −2.87 | |
| Y541C | Helicase | Deleterious | -8.863 | Probably Damaging | 1.000 | Increase | 0.67 | |
| 19B | P504L | Helicase | Deleterious | -8.158 | Probably Damaging | 0.993 | Increase | 0.16 |
| F308Y | NSP4 | Deleterious | −2.663 | Probably Damaging | 0.998 | Decrease | −0.68 | |
| S197L | Nucleocapsid | Neutral | -2.221 | Probably Damaging | 0.994 | Increase | 0.26 | |
| G196V | ORF3a | Deleterious | −6.581 | Probably Damaging | 1.000 | Decrease | −0.80 | |
| P323H | RdRp | Neutral | 1.146 | Benign | 0.005 | Decrease | −2.09 | |
| P323L | RdRp | Neutral | -0.865 | Benign | 0.005 | Decrease | −0.80 | |
| 20A | D614G | Spike | Neutral | 0.598 | Benign | 0.004 | Decrease | −1.94 |
| A222V | Spike | Neutral | -0.096 | Benign | 0.000 | Increase | 0.48 | |
| V30L | ORF10 | Deleterious | -3.000 | Not Generated | Not Generated | Decrease | −1.31 | |
| A220V | Nucleocapsid | Neutral | -0.140 | Probably Damaging | 0.999 | Increase | 0.76 | |
| D614G | Spike | Neutral | 0.598 | Benign | 0.004 | Decrease | −1.94 | |
| R203S | Nucleocapsid | Neutral | -2.374 | Probably Damaging | 0.994 | Decrease | −2.10 | |
| G204R | Nucleocapsid | Neutral | -1.656 | Probably Damaging | 1.000 | No change | 0.00 | |
| R203K | Nucleocapsid | Neutral | -1.604 | Probably Damaging | 0.969 | Decrease | −2.26 | |
| 20B | R203M | Nucleocapsid | Deleterious | −3.305 | Probably Damaging | 0.998 | Decrease | −1.52 |
| P323H | RdRp | Neutral | 1.146 | Benign | 0.005 | Decrease | −2.09 | |
| P323L | RdRp | Neutral | -0.865 | Benign | 0.005 | Decrease | −0.80 | |
| I120F | NSP2 | Neutral | -1.333 | Benign | 0.393 | Decrease | −1.85 | |
| S477N | Spike | Neutral | -0.034 | Benign | 0.014 | Increase | 0.01 | |
| S477I | Spike | Neutral | -1.310 | Probably Damaging | 0.531 | Increase | 0.34 | |
| S477T | Spike | Neutral | -0.336 | Benign | 0.066 | Decrease | −0.49 | |
| T85I | NSP2 | Deleterious | −4.090 | Probably Damaging | 0.998 | Decrease | −1.71 | |
| P323H | RdRp | Neutral | 1.146 | Benign | 0.005 | Decrease | −2.09 | |
| 20C | P323L | RdRp | Neutral | -0.865 | Benign | 0.005 | Decrease | −0.80 |
| D614G | Spike | Neutral | 0.598 | Benign | 0.004 | Decrease | −1.94 | |
| Q57H | ORF3a | Deleterious | −3.286 | Probably Damaging | 0.966 | Decrease | −1.12 | |
| S24L | ORF8 | Neutral | -1.833 | Benign | 0.013 | Increase | 0.53 | |
| S25L | NSP7 | Deleterious | -4.272 | Probably Damaging | 0.600 | Increase | 0.21 | |
| Non-synonymous signature SNPs for Indian sequences | ||||||||
| Clade | Change in | Mapped with | PROVEAN |
PolyPhen-2 |
I-Mutant 2.0 |
|||
| Amino Acid | Coding Regions | Prediction | Score | Prediction | Score | Stability | DDG | |
| A97V | RdRp | Deleterious | −3.611 | Probably Damaging | 0.990 | Decrease | −0.53 | |
| L37F | NSP6 | Neutral | -1.369 | Benign | 0.027 | Decrease | -0.05 | |
| P13L | Nucleocapsid | Neutral | -1.230 | Probably Damaging | 1.000 | Increase | 0.11 | |
| T1198I | NSP3 | Neutral | -0.085 | Probably Damaging | 0.998 | Decrease | -0.72 | |
| 19A | T1198K | NSP3 | Neutral | -0.353 | Not generated | Not generated | Decrease | -1.37 |
| S1197R | NSP3 | Neutral | -0.835 | Not generated | Not generated | Decrease | -0.88 | |
| G339S | NSP2 | Deleterious | −3.130 | Probably Damaging | 1.000 | Decrease | −1.57 | |
| G339C | NSP2 | Deleterious | −4.874 | Probably Damaging | 1.000 | Decrease | −1.91 | |
| V198I | NSP2 | Neutral | 0.307 | Benign | 0.006 | Increase | 0.18 | |
| L84S | ORF8 | Neutral | 2.333 | Benign | 0.002 | Decrease | -2.87 | |
| S202N | Nucleocapsid | Neutral | -0.404 | Probably Damaging | 0.994 | Decrease | -0.80 | |
| 19B | S202I | Nucleocapsid | Deleterious | -3.308 | Probably Damaging | 0.998 | Increase | 0.22 |
| S202T | Nucleocapsid | Neutral | −1.428 | Probably Damaging | 0.986 | Decrease | -0.53 | |
| T634A | NSP2 | Neutral | -0.004 | Benign | 0.106 | Decrease | -1.13 | |
| V354L | RdRp | Deleterious | -2.581 | Probably Damaging | 0.997 | Decrease | -1.95 | |
| D614G | Spike | Neutral | 0.598 | Benign | 0.004 | Decrease | -1.94 | |
| 20A | P323L | RdRp | Neutral | -0.865 | Benign | 0.005 | Decrease | -0.80 |
| Q57H | ORF3a | Deleterious | −3.286 | Probably Damaging | 0.966 | Decrease | −1.12 | |
| S194L | Nucleocapsid | Deleterious | -4.272 | Probably Damaging | 0.994 | Increase | 0.45 | |
| D614G | Spike | Neutral | 0.598 | Benign | 0.004 | Decrease | -1.94 | |
| R203K | Nucleocapsid | Neutral | -1.604 | Probably Damaging | 0.969 | Decrease | -2.26 | |
| 20B | R203M | Nucleocapsid | Deleterious | −3.305 | Probably Damaging | 0.998 | Decrease | −1.52 |
| G204R | Nucleocapsid | Neutral | -1.656 | Probably Damaging | 1.000 | No change | 0.00 | |
| P323L | RdRp | Neutral | -0.865 | Benign | 0.005 | Decrease | -0.80 | |
| A994D | NSP3 | Neutral | -1.103 | Not generated | Not generated | Decrease | -0.78 | |
| T85I | NSP2 | Deleterious | −4.090 | Probably Damaging | 0.998 | Decrease | −1.71 | |
| P323L | RdRp | Neutral | -0.865 | Benign | 0.005 | Decrease | -0.80 | |
| 20C | D614G | Spike | Neutral | 0.598 | Benign | 0.004 | Decrease | -1.94 |
| Q57H | ORF3a | Deleterious | −3.286 | Probably Damaging | 0.966 | Decrease | −1.12 | |
| S183Y | Nucleocapsid | Deleterious | -2.750 | Probably Damaging | 0.998 | No change | 0.00 | |
| A262S | Spike | Neutral | 0.154 | Not generated | Not generated | Decrease | -0.95 | |
Table 5 provides a comparative study of all the signature SNPs identified in our work with that of literature [30], [16], [31], [18], [19], [32], [33], [20], [34], [35], [36]. In [30], the authors have performed whole-genome sequencing of 303 Indian isolates and have reported 11 important genetic mutations as a part of Clade I/A3i which are dominant in most of the states in India. Yuan et al. [16] have performed genomic analysis of 11,183 genomes from around the globe and have reported 9 SNPs with high frequency. In their work, Goswami et al. [31] have identified 18 high frequency genomic co-ordinates viz. hot-spots to investigate inverted repeat loci and CpG islands. They concluded that these points are indicative of genomic instability of SARS-CoV-2. Genomic analysis of 570 SARS-CoV-2 genomes from China, Europe, US and India have been carried out in [18] where they have identified at least 10 hotspot mutations which are present in more than 80% of viral genomes. In [19], Nagy et al. have mapped 3733 non-silent mutations to amino acid changes for 4566 patients where they identified 17 mutations related to hospitalisation, severe and deadly outcomes as well. Rahimi et al. [32] have reported 17 high frequency mutations that are reported in other literatures as well. In [33], the authors have worked with 1566 SARS-CoV-2 genome sequences across ten Asian countries and have clustered and characterized them based on the clade they belong to. Cheng et al. [20] have considered 1809 SARS-CoV-2 genomes and identified 1017 and 512 non-synonymous and synonymous mutations respectively. In their work, they have reported 7 dominant mutations for each month from January to April 2020. In [34], Hamdan et al. have considered 11 SARS-CoV-2 isolates from Lebanon to identify new mutations which have not been reported till then in Lebanon. Yang et al. [35] have performed phylodynamic analysis of 247 genomic sequences to identify four genetic clusters called super-spreaders. SS1 was widely circulating in Asia and the US whereas SS4 was responsible for the pandemic in Europe. In [36] have reported 9 newly evolved SARS-CoV-2 SNPs that have undergone a rapid increase or decrease in frequency for 30–80% in the initial four months. It can be seen from the table that out of the 48 unique genomic coordinates identified in the literature, 29 signature SNPs are common with our work. Out of these 29 common signature SNPs, C14805T, T17247C, C17747T, A17858G, C18060T and G26144T are present in Dataset A. Such signature SNPs present only in Indian genomes are C6310A, C6312A, C13730T, G22346A, C28311T, T28688C, C28854T and G28878A while C241T, C1059T, G1397A, C3037T, C8782T, G11083T, C14408T, A23403G, G25563T, T28144C, G28881A, G28882A, G28883C and G29742A are common in both Datasets A and B. Furthermore, for Dataset A, G26144T which corresponds to G251V in ORF3a in clade 19A is damaging and shows a decrease in stability while C13730T which corresponds to A97V in RdRp for 19A, C1059T corresponding to T85I in NSP2 for clade 20C and G25563T corresponding to Q57H in ORF3a for 20C are damaging and exhibits shrinking stability for both Datasets A and B.
Table 5.
Comparative study of our work with the literature.
| Genomic coordinate | Change in Nucleotide | Change in Amino acid | Coordinate of Amino Acid in Protein | Mapped with Coding and Non-coding Region | Banu et al. [30] | Yuan et al. [16] | Goswami et al. [31] | Weber et al. [18] | Nagy et al. [19] | Rahimi et al. [32] | Sengupta et al. [33] | Cheng et al. [20] | Abou-Hamdan et al. [34] | Yang et al. [35] | Zhu et al. [36] | Our work |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 241 | CT | NA | NA | 5’-UTR | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 1059 | CT | TI | 85 | NSP2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 1190 | CT | PS | 129 | NSP2 | ✓ | |||||||||||
| 1397 | GA | VI | 378 | ORF1a | ✓ | ✓ | ✓ | |||||||||
| 1440 | GA | GD | 212 | NSP2 | ✓ | ✓ | ||||||||||
| 1605 | AC | NT | 267 | NSP 1ab | ✓ | |||||||||||
| 1917 | CT | TI | 371 | NSP2 | ✓ | |||||||||||
| 2891 | GR, GA | AT | 58 | NSP3 | ✓ | ✓ | ||||||||||
| 3037 | CT | Synonymous | 105, 106 | NSP3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 4402 | TC | Synonymous | 561 | NSP3 | ✓ | |||||||||||
| 5062 | GT | LF | 781 | NSP3 | ✓ | |||||||||||
| 6310 | CA | SR | 1197 | NSP3 | ✓ | ✓ | ||||||||||
| 6312 | CA | TK | 1198, 2016 | NSP3, ORF1a | ✓ | ✓ | ✓ | |||||||||
| 6446 | GA | VI | 1243 | ORF1ab | ✓ | |||||||||||
| 8782 | CT | Synonymous | 75, 76 | NSP4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 9438 | CT | TI | 295 | NSP4 | ✓ | |||||||||||
| 9924 | CT | AV | 3220 | ORF1a | ✓ | |||||||||||
| 11083 | GT | LF | 37, 3606 | NSP6, ORF1a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 12053 | CT | LF | 71 | NSP7 | ✓ | |||||||||||
| 13730 | CT | AV | 97 | RdRp | ✓ | ✓ | ✓ | |||||||||
| 14408 | CT | PL | 314, 323 | RdRp | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 14805 | CT | Synonymous | 446, 455 | RdRp | ✓ | ✓ | ✓ | |||||||||
| 17247 | TC | Synonymous | 337 | NSP13 | ✓ | ✓ | ||||||||||
| 17747 | CT | PL | 504 | Helicase | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 17858 | AG | YC | 541 | Helicase | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 18060 | CT | Synonymous | 6, 7 | Exon | ✓ | ✓ | ✓ | ✓ | ||||||||
| 21724 | GT | LF | 54 | Spike | ✓ | ✓ | ||||||||||
| 21859 | CT | Synonymous | 99 | Spike | ✓ | |||||||||||
| 22346 | GA | AT | 262 | Spike | ✓ | ✓ | ||||||||||
| 22661 | GT | VF | 367 | Spike | ✓ | |||||||||||
| 23403 | AG | DG | 614 | Spike | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 24047 | GA | AT | 829 | Spike | ✓ | |||||||||||
| 25088 | GT | VF | 1176 | Spike | ✓ | |||||||||||
| 25563 | GT | QH | 57 | ORF3a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 26144 | GT | GV | 251 | ORF3a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 26149 | TC | SP | 253 | ORF3a | ✓ | |||||||||||
| 27299 | TC | IT | 33 | ORF6 | ✓ | |||||||||||
| 28144 | TC | LS | 84 | ORF8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 28311 | CT | PL | 13 | Nucleocapsid | ✓ | ✓ | ✓ | |||||||||
| 28688 | TC | LL | 129 | Nucleocapsid | ✓ | ✓ | ||||||||||
| 28854 | CT | SL | 194 | Nucleocapsid | ✓ | ✓ | ✓ | ✓ | ||||||||
| 28878 | GA | SN | 202 | Nucleocapsid | ✓ | ✓ | ✓ | |||||||||
| 28881 | GA | RK | 203 | Nucleocapsid | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 28882 | GA | Synonymous | 203 | Nucleocapsid | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 28883 | GC | GR | 204 | Nucleocapsid | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 29095 | CT | FF | 274 | Nucleocapsid | ✓ | |||||||||||
| 29148 | TC | IT | 292 | Nucleocapsid | ✓ | |||||||||||
| 29742 | GT, GR | NA | NA | 3’-UTR | ✓ | ✓ | ✓ |
5. Conclusion
In this work, multiple sequence alignment of 18392 SARS-CoV-2 sequences is carried out using MAFFT followed by phylogenetic analyses using Nextstrain where 15359 global dataset without Indian (Dataset A) and dataset of 3033 exclusive Indian (Dataset B) SARS-CoV-2 genomes are considered separately to identify the virus clades. Consequently, the virus strains are found to be distributed among five major clades viz. 19A, 19B, 20A, 20B and 20C. Subsequently, mutation points as SNPs are identified in each clade. Thereafter, clade specific signature SNPs are identified by considering top 10 SNPs with high frequency, resulting in 50 such signature SNPs each for Datasets A and B. Out of each 50 signature SNPs, 39 and 41 unique SNPs are identified among which 25 non-synonymous signature SNPs (out of 39) resulted in 30 amino acid changes in protein while 27 changes in amino acid are identified from 22 non-synonymous signature SNPs (out of 41). These 30 and 27 amino acid changes for the non-synonymous signature SNPs are visualised in their respective protein structures as well. The sequence and structural homology-based prediction of biological functions along with the protein structural stability of such amino acid changes are also determined to judge the characteristics of the identified clades. Consequently, for Dataset A, G251V in ORF3a in clade 19A, F308Y and G196V in NSP4 and ORF3a in 19B are the unique amino acid changes which are responsible for defining each clade as they are all deleterious and unstable. Such changes which are common for both Datasets A and B are R203M in Nucleocapsid for 20B, T85I and Q57H in NSP2 and ORF3a respectively for 20C while for Dataset B such unique changes are A97V in RdRp, G339S and G339C in NSP2 in 19A and Q57H in ORF3a in 20A. Moreover, a comparative study is also put forth to show the correctness of our work. We hope this work will better equip the researchers in their path of designing anti-viral therapeutics to mitigate COVID-19. As a future scope of research, consensus of SNPs can be considered by taking more than one multiple sequence alignment techniques. Also, investigation of the characteristics of these signature SNPs of SARS-CoV-2 on human hosts can be conducted with the help of virologists. The authors are working in these directions.
Ethics approval and consent to participate
The ethical approval or individual consent was not applicable.
Availability of data and materials
All the files which include dataset (raw and aligned sequences, metadata for Nextstrain and JSON files as outputs of Nextstrain), codes, supplementary PDF and videos of clade specific virus evolution and transmission in 71 countries are available at “http://www.nitttrkol.ac.in/indrajit/projects/COVID-Evolution-SignatureSNPs-18 K/”.
Consent for publication
Not applicable.
Funding
This work has been partially supported by CRG short term research grant on COVID-19 (CVD/2020/000991) from Science and Engineering Research Board (SERB), Department of Science and Technology, Govt. of India. However, it does not provide any publication fees.
CRediT authorship contribution statement
Nimisha Ghosh: Conceptualization, Methodology, Data curation, Formal analysis, Software, Validation, Writing - original draft. Indrajit Saha: Conceptualization, Data curation, Supervision, Funding acquisition, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing - review & editing. Suman Nandi: Conceptualization, Formal analysis, Software, Validation, Visualization, Writing - review & editing. Nikhil Sharma: Conceptualization, Formal analysis, Software, Validation, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank all those who have contributed sequences to GISAID database.
https://provean.jcvi.org/index.php
http://genetics.bwh.harvard.edu/pph2/
http://folding.biofold.org/i-mutant/i-mutant2.0.html
Supplementary data associated with this article can be found, in the online version, athttps://doi.org/10.1016/j.ymeth.2021.09.005.
https://www.gisaid.org/
https://www.ncbi.nlm.nih.gov/nuccore/1798174254
https://zhanglab.ccmb.med.umich.edu/COVID-19/
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nesta A., Tafur D., Beck C. Hotspots of human mutation. Trends Genet. 2020 doi: 10.1016/j.tig.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J., Tambyah P., Hui D. Emergence of a new sars-cov-2 variant in the uk. J Infection. 2020 doi: 10.1016/j.jinf.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookman S., Cook J., Zucherman M., et al. Effect of the new sars-cov-2 variant b. 1.1. 7 on children and young people. Lancet Child Adolescent Health. 2021 doi: 10.1016/S2352-4642(21)00030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makoni M. South africa responds to new sars-cov-2 variant. The Lancet. 2021;397:267. doi: 10.1016/S0140-6736(21)00144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S., Saxena S.K. Structural and molecular perspectives of sars-cov-2. Methods. 2021 doi: 10.1016/j.ymeth.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm E., Kronig I., Neher R.A. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clinical Microbiol Infection. 2021;27(8):1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D. Kim, J.-Y. Lee, J.-S. Yang, et al., The architecture of sars-cov-2 transcriptome, Cell 181 (04 2020). doi:10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed]
- 8.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon D.E., Jang G.M., Bouhaddou M., et al. A sars-cov-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu I.-N., Muller C.P., He F.Q. Applying next-generation sequencing to unravel the mutational landscape in viral quasispecies. Virus Res. 2020;283 doi: 10.1016/j.virusres.2020.197963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin C. Genotyping coronavirus sars-cov-2: methods and implications. Genomics. 2020;112(5):3588–3596. doi: 10.1016/j.ygeno.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfredonia I., Nithin C., Ponce-Salvatierra A., et al. Genome-wide mapping of SARS-CoV-2 RNA structures identifies therapeutically-relevant elements. Nucleic Acids Res. 2020;48(22):12436–12452. doi: 10.1093/nar/gkaa1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha I., Ghosh N., Pradhan A., et al. Whole genome analysis of more than 10 000 SARS-CoV-2 virus unveils global genetic diversity and target region of NSP6. Briefings in Bioinformatics. 2021;22(2):1106–1121. doi: 10.1093/bib/bbab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang X., Wu C., Li X., et al. On the origin and continuing evolution of SARS-CoV-2. National Sci. Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R. Wang, Y. Hozumi, C. Yin, et al., Decoding sars-cov-2 transmission, evolution and ramification on covid-19 diagnosis, vaccine, and medicine, J. Chem. Inform. Modeling XXXX (06 2020). doi:10.1021/acs.jcim.0c00501. [DOI] [PMC free article] [PubMed]
- 16.F. Yuan, L. Wang, Y. Fang, et al., Global SNP analysis of 11,183 SARS-CoV-2 strains reveals high genetic diversity, Transboundary Emerging Diseases (11 2020). doi:10.1111/tbed.13931. [DOI] [PMC free article] [PubMed]
- 17.J. Chen, R. Wang, M. Wang, et al., Mutations strengthened sars-cov-2 infectivity, J. Mol. Biol. 432 (07 2020). doi:10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed]
- 18.Weber S., Ramirez C., Doerfler W. Signal hotspot mutations in sars-cov-2 genomes evolve as the virus spreads and actively replicates in different parts of the world. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy A., Pongor S., Gyõrffy B. Different mutations in sars-cov-2 associate with severe and mild outcome. Int. J. Antimicrob. Agents. 2020;57 doi: 10.1016/j.ijantimicag.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L., Han X., Zhu Z., et al. Functional alterations caused by mutations reflect evolutionary trends of sars-cov-2. Briefings Bioinformatics. 2021:1–9. doi: 10.1093/bib/bbab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar R., Mitra S., Chandra P., et al. Comprehensive analysis of genomic diversity of SARS-CoV-2 in different geographic regions of India: an endeavour to classify Indian SARS-CoV-2 strains on the basis of co-existing mutations. Arch. Virol. 2021;166(3):801–812. doi: 10.1007/s00705-020-04911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.K. Katoh, K. Misawa, K. Kuma, et al., MAFFT: A novel method for rapid multiple sequence alignment based on fast fourier transform, Nucleic Acids Research 30 (14) (2002) 3059–3066. doi:https://doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed]
- 23.Hadfield J., Megill C., Bell S., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics (Oxford, England) 2018;34 doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volz E.M., Koelle K., Bedford T. Viral phylodynamics. PLoS Computer Biol. 2013;9(3) doi: 10.1371/journal.pcbi.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha I., Ghosh N., Maity D., et al. Genome-wide analysis of indian sars-cov-2 genomes for the identification of genetic mutation and snp. Infection, Genetics and Evolution. 2020;85 doi: 10.1016/j.meegid.2020.104457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi Y., Chan A.P. Provean web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31(16):2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adzhubei I.A., Schmidt S., Peshkin L., et al. A method and server for predicting damaging missense mutations. Nature Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capriotti E., Fariselli P., Casadio R. I-mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acid Res. 2005;33:306–310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain M.S., Roy A.S., Islam M.S. In silico analysis predicting effects of deleterious snps of human rassf5 gene on its structure and functions. Sci. Rep. 2020;10:14542. doi: 10.1038/s41598-020-71457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.S. Banu, B. Jolly, P. Mukherjee, et al., A Distinct Phylogenetic Cluster of Indian Severe Acute Respiratory Syndrome Coronavirus 2 Isolates, Open Forum Infectious Diseases 7 (11) (09 2020). doi:10.1093/ofid/ofaa434. [DOI] [PMC free article] [PubMed]
- 31.P. Goswami, M. Bartas, M. Lexa, et al., SARS-CoV-2 hot-spot mutations are significantly enriched within inverted repeats and CpG island loci, Briefings in Bioinformatics (12 2020). doi:10.1093/bib/bbaa385. [DOI] [PMC free article] [PubMed]
- 32.A. Rahimi, A. Mirzazadeh, S. Tavakolpour, Genetics and genomics of sars-cov-2: A review of the literature with the special focus on genetic diversity and sars-cov-2 genome detection, Genomics 113 (1, Part 2) (2021) 1221–1232. doi:10.1016/j.ygeno.2020.09.059. [DOI] [PMC free article] [PubMed]
- 33.Sengupta A., Hassan S.S., Choudhury P.P. Clade gr and clade gh isolates of sars-cov-2 in asia show highest amount of snps. Infection, Genetics Evol. 2021;89 doi: 10.1016/j.meegid.2021.104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abou-Hamdan M., Hamze K., Sater A.A., et al. Variant analysis of the first lebanese sars-cov-2 isolates. Genomics. 2020:892–895. doi: 10.1016/j.ygeno.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X., Dong N., Chan E.W.C., et al. Genetic cluster analysis of sars-cov-2 and the identification of those responsible for the major outbreaks in various countries. Emerging Microbes Infections. 2020;9(1):1287–1299. doi: 10.1080/22221751.2020.1773745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Z. Zhu, G. Liu, K. Meng, et al., Rapid Spread of Mutant Alleles in Worldwide SARS-CoV-2 Strains Revealed by Genome-Wide Single Nucleotide Polymorphism and Variation Analysis, Genome Biology and Evolution 13 (2) (01 2021). doi:10.1093/gbe/evab015. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the files which include dataset (raw and aligned sequences, metadata for Nextstrain and JSON files as outputs of Nextstrain), codes, supplementary PDF and videos of clade specific virus evolution and transmission in 71 countries are available at “http://www.nitttrkol.ac.in/indrajit/projects/COVID-Evolution-SignatureSNPs-18 K/”.