Abstract
Objectives
To investigate the response of the immune system (and its influencing factors) to vaccination with BNT162b2 or mRNA-1273.
Methods
531 vaccinees, recruited from healthcare professionals, donated samples before, in between, and after the administration of the two doses of the vaccine. T- and B-cell responses were examined via interferon-γ (IFN-γ) release assay, and antibodies against different epitopes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (S1 and NCP) were detected via ELISA and surrogate neutralization assay. Results were correlated with influencing factors such as age, sex, prior infection, vaccine received (BNT162b2 or mRNA-1273), and immunosuppression. Furthermore, antinuclear antibodies (ANAs) were measured to screen for autoimmune responses following vaccination with an mRNA vaccine.
Results
No markers of immunity against SARS-CoV-2 were found before the first vaccination. Two weeks after it, specific responses against SARS-CoV-2 were already measurable (median ± median absolute deviation (MAD): anti-S1 IgG 195.5 ± 172.7 BAU/mL; IgA 6.7 ± 4.9 OD; surrogate neutralization 39 ± 23.7%), and were significantly increased two weeks after the second dose (anti-S1 IgG 3744 ± 2571.4 BAU/mL; IgA 12 ± 0 OD; surrogate neutralization 100 ± 0%, IFN-γ 1897.2 ± 886.7 mIU/mL). Responses were stronger for younger participants (this difference decreasing after the second dose). Further influences were previous infection with SARS-CoV-2 (causing significantly stronger responses after the first dose compared to unexposed individuals (p ≤ 0.0001)) and the vaccine received (significantly stronger reactions for recipients of mRNA-1273 after both doses, p < 0.05–0.0001). Some forms of immunosuppression significantly impeded the immune response to the vaccination (with no observable immune response in three immunosuppressed participants). There was no significant induction of ANAs by the vaccination (no change in qualitative ANA results (p 0.2592) nor ANA titres (p 0.08) from pre-to post-vaccination.
Conclusions
Both vaccines elicit strong and specific immune responses against SARS-CoV-2 which become detectable one week (T-cell response) or two weeks (B-cell response) after the first dose.
Keywords: BNT162b2, COVID-19, Immune response, mRNA-1273, SARS-CoV-2, Vaccination
Introduction
Since its appearance in late 2019 [1], the worldwide spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to increasingly drastic public health measures with immense social, political, and economic consequences [[2], [3], [4]]. To achieve a sustainable containment of the pandemic, several vaccines against SARS-CoV-2 have been developed, BNT162b2 and mRNA-1273 being the first to be approved and administered since December 2020. While clinical data of these vaccines—both from the clinical trials [[5], [6], [7]] and ‘real-world’ data from their application in the field [[8], [9], [10]]—appear promising, their effects on the recipients' immune systems remain to be studied further. On the one hand, the efficacy of the vaccines, as measured by the detection of assumed correlates (both humoral and cellular) of immunity against SARS-CoV-2, needs to be well characterized. On the other hand, possible autoimmune events associated with the vaccines should be closely monitored, especially as there is considerable uncertainty among the general population concerning mRNA-based vaccines owing to the novelty of their use in humans [11] as well as the unusually rapid process of their development and approval. To dispel this uncertainty, as well as to cast further light on the development of assumed immunity against SARS-CoV-2 after vaccination, is the goal of this study.
To this end, we examined the serological status of 531 vaccinees before, during, and after vaccination against SARS-CoV-2, measuring different components of the immune response to the vaccination: binding antibodies against different target antigens of SARS-CoV-2 (the S1-subunit of the spike protein (anti-S1) as well as the nucleocapsid protein (anti-NCP)), the neutralizing capacity anti-S1 IgG, and the specific T-cell response (via a SARS-CoV-2-specific interferon-γ release assay (IGRA)). Further, we examined antinuclear antibodies (ANAs) as a broad screening for the detection of antibodies against nuclear antigens, including mRNA. We hypothesized that: (a) prior to the vaccination, the seroprevalence would be low, (b) vaccinees would develop antibodies specifically against the S1-subunit of the spike protein (and not against NCP), (c) in addition to the antibody response, vaccinees would develop a specific T-cell response against SARS-CoV-2, and (d) vaccinees would show no development of antibodies against nuclear antigens, including RNA, as a consequence of the vaccination.
Methods
General design of the study
The study consisted of a series of tests performed on samples donated by the participants at different time points during the course of their vaccination against SARS-CoV-2. At the first time point (t1), shortly before or after the first dose of the vaccine, the sera were tested for anti-SARS-CoV-2 antibodies, ANAs, as well as the T-cell response (via IGRA). For analytical purposes, all samples collected within 6 days after the first dose of vaccine were considered as t1 (as not all participants were able to donate samples prior to the first dose). At a second time point (t2), 2 weeks after the first dose of the vaccine, anti-SARS-CoV-2 antibodies (IgG, IgA and neutralizing antibodies) were measured again. At a third time point (t3), 2 weeks after the second dose of vaccine, all participants were again tested for anti-SARS-CoV-2 antibodies (IgG, IgA and neutralizing antibodies), ANAs, and their T-cell response (IGRA).
Study population and vaccination programme
Participants were recruited from healthcare workers of a German university hospital, who were prioritized to receive a vaccination against SARS-CoV-2 by the German SARS-CoV-2 vaccination roll-out campaign. More detailed information about the vaccination programme can be found in the Supplementary Material.
For analytical purposes, data on age, sex and the specific vaccine received were collected from each participant. Other data, such as on immunosuppressive medication received by the participants, were disclosed voluntarily by some participants but not collected systematically.
All participants provided written informed consent. The study was approved by the University of Kiel institutional review board (AZ: D642/20). The study was conducted in accordance with the Declaration of Helsinki [12].
Anti-SARS-CoV-2 antibodies
Anti-S1 antibodies of the classes IgA and IgG, and anti-NCP IgG, were measured via ELISA using kits by EUROIMMUNE (Lübeck, Germany) according to the manufacturer's instructions. Anti-S1 was chosen as the target antigen, as it is a main part of the immunogenic antigen induced via vaccination with BNT162b2 or mRNA-1273. Anti-NCP was chosen to detect possible unrecognized infections with SARS-CoV-2, as antibodies against NCP are not to be expected after vaccination. Further information on the interpretation of these assays can be found in the Supplement Material.
Neutralizing antibodies (ELISA)
Neutralizing antibodies were examined via the SARS-CoV-2-NeutraLISA surrogate neutralization assay (EUROIMMUN, Lübeck, Germany), according to the manufacturer's instructions. Further information on this assay can be found in the Supplementary Material.
Interferon-γ release assay (IGRA)
The T-cell response to the vaccination against SARS-CoV-2 was examined using an interferon-γ release assay (IGRA; EUROIMMUN, Lübeck, Germany), according to the manufacturer's instructions. Further information on the IGRA is provided in the Supplementary Material.
Antinuclear antibodies (ANAs)
ANAs were examined as a broad screening for autoantibodies against nuclear structures at t1 and t3. Indirect immunofluorescence microscopy (IIF) on the substrate HEp-20-10 (EUROIMMUN, Lübeck, Germany) was carried out according to the manufacturer's instructions. More detailed information on the examination of ANA is provided in the Supplementary Material.
Statistical analysis
For reporting averaged results, the median (measure of dispersion: median absolute deviation (MAD)) was used if not otherwise specified, as the dataset contained many outliers and skewed distributions.
Due to the abovementioned distribution of parts of the data, tests for statistically significant differences in continuous variables between two groups were performed mainly via the Mann–Whitney U test (unless otherwise specified). Tests for correlations between continuous variables were performed by calculating Spearman's ρ. Statistical significance was assumed for p-values <0.05. All statistical analyses were performed using the open-source software for statistical computing and graphics R (version 4.0.4) with the integrated development environment RStudio (Version 1.1.463) [14].
Results
Study population and sample characteristics
Overall, 531 participants were recruited. Of these, 389 (73.3%) were female. The mean age at the time of receipt of the first vaccine dose was 42.2 years (SD ± 12, range 18–67). There was no statistically significant difference in age between the sexes (t-test t 1.6954, df 269.45, p 0.09116). Supplementary Material Fig. S1 shows the distribution of age groups in both sexes; 481 participants (90.6%) received BNT162b2 and 50 (9.4%) mRNA-1273, as determined by the availability of the vaccines.
Most but not all samples were collected at the time scheduled by the study protocol. The median absolute deviations (MADs) of the day of sample collection for the different time points were: t1 ±1.5 days, range –6 to 27 days (of these, only those collected up until day 6 post-vaccination were included in calculations of the baseline serological status, as no vaccine-induced antibodies were to be expected within this time frame); t2 ±0, range 9–37, and t3 ±0, range 39–77).
Four hundred and twenty-six participants (80.3%) donated samples at all three time points; 82 (15.4%) did so at only two time points, and 23 (4.3%) at only one time point.
All medians (with median absolute deviation and respective group sizes) of all measured values at all time points can be gathered from Table 1 .
Table 1.
Average values (reported via medians ± median absolute deviation) for all measured markers at all time points, including the respective group sizes, for the whole cohort and the following subgroups: women, men, participants previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), participants having received BNT162b2, and participants having received mRNA-1273
| Group | t1 (0–6 days after first dose) |
t2 (14 days after first dose) |
t3 (14 days after second dose) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ (mIU/mL) | Anti-S1 IgA (OD ratio) | Anti-S1 IgG (BAU/mL) | Neutralizing antibodies (%) | Anti-S1 IgA (OD ratio) | Anti-S1 IgG (BAU/mL) | Neutralizing Ab (%) | IFN-γ (mIU/mL) | Anti-S1 IgA (OD ratio) | Anti-S1 IgG (BAU/mL) | Neutralizing antibodies (%) | |
| Whole cohort | 14.3 ± 21.2 | 0.3 ± 0.2 | <1 | 5 ± 5.9 | 6.7 ± 4.9 | 195.5 ± 172.7 | 39 ± 23.7 | 1897.2 ± 886.7 | 12 ± 0 | 3744 ± 2571.4 | 100 ± 0 |
| Women | 12.6 ± 18.7 | 0.3 ± 0.2 | <1 | 6 ± 4.4 | 6.3 ± 4.9 | 190.4 ± 167 | 38 ± 23.7 | 1859.3 ± 938.8 | 12 ± 0 | 3830.4 ± 2585.7 | 100 ± 0 |
| Men | 21.7 ± 32.1 | 0.3 ± 0.3 | <1 | 4 ± 4.4 | 8.2 ± 5.3 | 219.8 ± 187.9 | 43 ± 25.2 | 1901.6 ± 883.2 | 12 ± 0 | 3564.8 ± 2495.5 | 100 ± 0 |
| Previously infected | 1875.4 ± 906.5 | 3.7 ± 4.5 | 110 ± 39.4 | 40 ± 5.9 | 12 ± 0 | 3753.6 ± 3415.9 | 100 ± 0 | 2203.6 ± 401.7 | 12 ± 0 | 3323.2 ± 517.1 | 100 ± 0 |
| BNT162b2 | 12.7 ± 18.8 | 0.3 ± 0.2 | <1 | 6 ± 4.4 | 6.2 ± 4.8 | 183.4 ± 155.4 | 37 ± 23.7 | 1807.2 ± 1016.9 | 12 ± 0 | 3654.4 ± 2571.4 | 100 ± 0 |
| mRNA-1273 | 18.4 ± 23.4 | 0.5 ± 0.2 | <1 | 3 ± 5.9 | 11.0 ± 1.4 | 512 ± 415.1 | 60.5 ± 25.2 | 2471 ± 35.4 | 12 ± 0 | 4926.4 ± 3598.6 | 100 ± 0 |
Temporal course of the immune responses to the vaccination against SARS-CoV-2
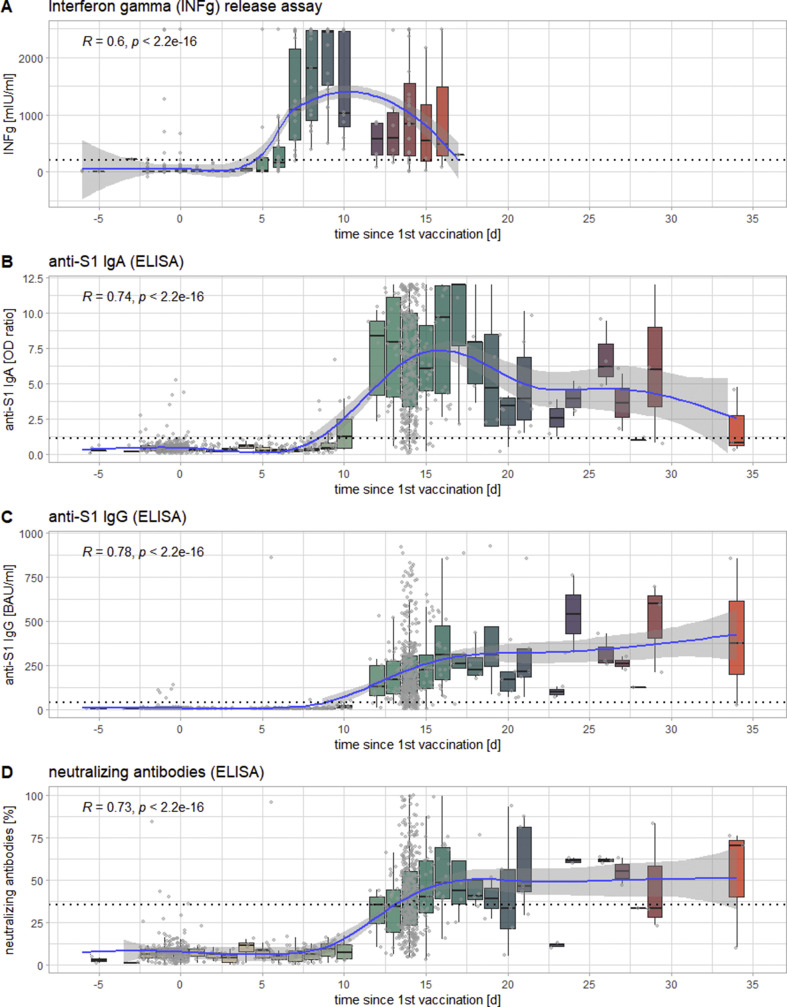
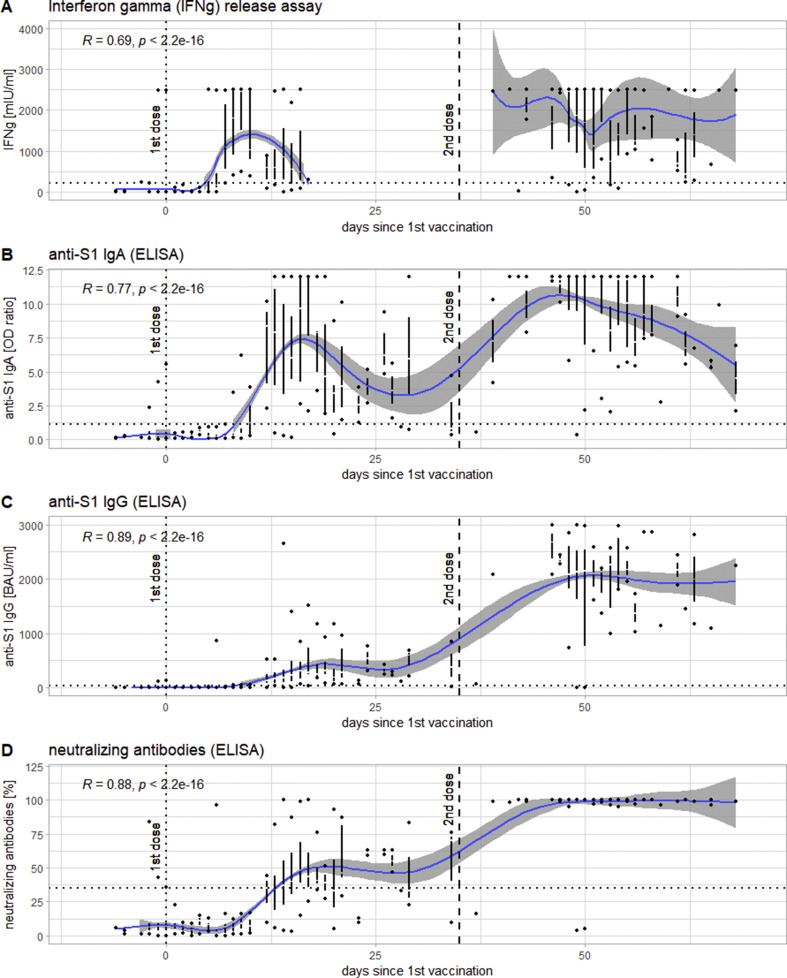
The first component of the immune response to become detectable was the T-cell response as measured via IGRA, the medians of which first crossed the threshold for positivity (200 mIU/mL IFN-γ) in samples collected on day 7 after vaccination, at 1083 ± 914 mIU/mL. It was followed by anti-S1 IgA (crossing the threshold of 1.1 (OD ratio) at day 10 with 1.23 ± 1.3 (OD ratio)) and anti-S1 IgG (crossing the threshold of 35.2 BAU/mL at day 12 at 128.6 ± 150.4 BAU/mL) as well as neutralizing antibodies (also crossing the threshold of 35% at day 12 with 35 ± 11.9%) (see Fig. 1 ). At t2, the responses reached a plateau, with IFN-γ and IgA showing a tendency to decrease again after peaks in samples collected 9 and 17 days post-vaccination, respectively. At t3, all measured responses exhibited significantly higher values (p < 0.0001) than at t2. All time points being considered, all responses showed highly significant correlations of medium to strong effect sizes with the number of days after vaccination (see Fig. 2 ).
Fig. 1.
Timeline of the inter-individually measured kinetics of the different markers of the immune response to the first dose of the vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presented as a function of days after the administration of the first dose: T-cell response as measured via interferon-γ release assay (IGRA) (panel A), anti-S1 IgA (panel B), anti-S1 IgG (panel C), and neutralizing antibodies (panel D). Each box-plot represents 1 day from administration of the first dose, the jittered dots represent each individual measurement. The blue line indicates the smoothed means, with a grey confidence band of 95%. In the upper left-hand corner of each panel is Spearman's ρ for the correlation of x (days since first dose) and y (levels of the respective marker) of each panel, as well as its p-value. The dotted line indicates the respective cut-offs for positivity/reactivity of the different markers.
Fig. 2.
Levels of the different serological markers in the course of the administration of both doses of the vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presented as a function of days since the first dose of the vaccine: interferon-γ release assay (IGRA) (panel A), anti-S1 IgA (panel B), anti-S1 IgG (panel c), and neutralizing antibodies (panel D). The results for each day are represented as a Tufte boxplots (the line representing the interquartile range (IQR), the gap in it the median, the dots above and below the ends of the whiskers). The blue line indicates the smoothed means with a 95% confidence band around it. The dotted line indicates the time of the first dose, the dashed line that of the second dose of the vaccine. In the upper right-hand corner is Spearman's ρ for the correlation between x and y for the whole period of time examined.
Of note, at t2, 22 participants did not yet develop an anti-S1 IgG response, and 190 did not develop levels of neutralizing antibodies considered to be reactive. At t3, the same is true for only three participants (see ‘Influence of Immunosuppression’ below).
Influence of different factors on the immune response to the vaccination against SARS-CoV-2
Influence of age
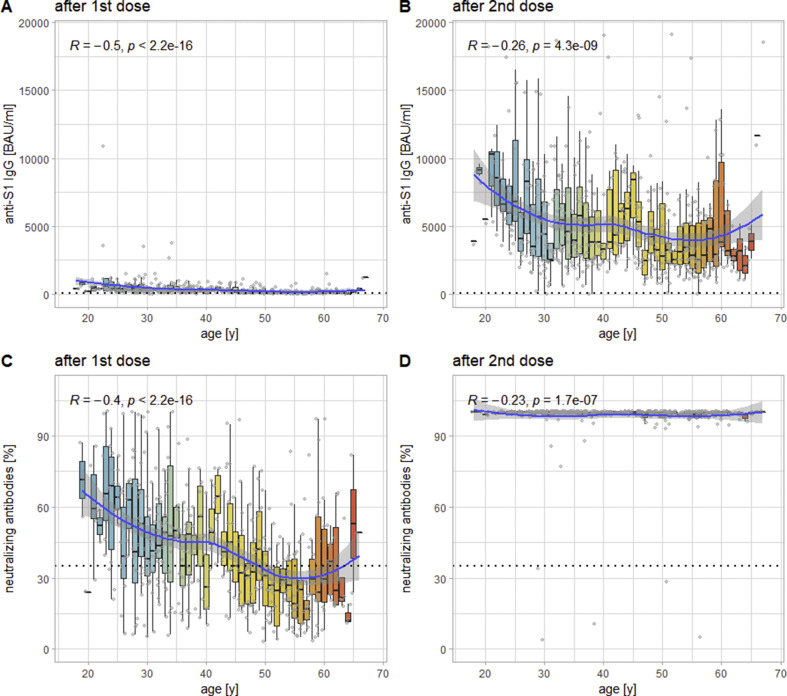
There is a highly significant inverse correlation of medium effect size between values and age at t2 for both anti-S1 IgG (R = –0.5, p < 0.0001) and neutralizing antibodies (R = –0.4, p < 0.0001). At t3, the same correlation can be observed with only small effect sizes for both anti-S1 IgG (R = –0.26, p < 0.0001) and neutralizing antibodies (R = –0.23, p < 0.0001; see Fig. 3 ). For anti-S1 IgA, this correlation can only be shown at t2 (R = –0.32, p < 0.0001). For IFN-γ via IGRA (which was not performed at t2) an inverse correlation with age of only negligible effect size can be observed at t3 (R = –0.13, p 0.004).
Fig. 3.
Levels of anti-S1 IgG (panel A) and neutralizing antibodies (panel B) as a function of recipients' age at t2 (14 days after the first dose of vaccine); panels C and D show the same analysis for t3 (14 days after the second dose of the vaccine). Each box-plot represents 1 year, each (jittered) point one individual measurement. The blue line indicates the smoothed means with a 95% confidence band. The dashed lines indicate the respective cut-offs for positivity. In the upper left-hand corner is Spearman's ρ for the correlation between recipients' ages and levels of the different markers.
Influence of prior infection with SARS-CoV-2
We could identify five participants with a self-reported, anamnestic history of coronavirus disease 2019 (COVID-19). These individuals already showed detectable immune responses against SARS-CoV-2 (including anti-NCP IgG) at t1 (anti-S1 IgG 110 ± 39.4 BAU/mL; anti-S1 IgA 3.7 ± 4.5 (OD ratio); neutralizing antibodies 40 ± 5.9%; IFN-γ (IGRA) 1875.4 ± 906.5 mIU/mL), which increased dramatically in size at t2 (anti-S1 IgG 3753.6 ± 3415.9 BAU/mL; anti-S1 IgA 12.0 ± 0 (OD ratio); neutralizing antibodies 100 ± 0%), reaching levels observed only after the second dose of the vaccination in previously uninfected individuals. For anti-S1 IgA, IgG and neutralizing antibodies, this difference between previously infected and uninfected individuals at t2 is statistically significant (p ≤ 0.0001). At t3, their responses (especially anti-S1 IgG 3323.2 ± 517.1 BAU/mL) did not increase further in size.
Influence of immunosuppressive medication
Eleven participants disclosed receiving immunosuppressive medication. While eight of these (receiving dimethylfumarate, methotrexate, adalimumab or certolizumab) did not show reduced immune responses compared to the rest of the cohort, three participants (receiving rituximab, fingolimod and mycophenolate plus belatacept, respectively) showed no immune response at any time point. Of note, information about immunosuppressive medication received by the vaccinees was not collected systematically but relied on the voluntary disclosure of this fact by participants during the course of the study.
Influence of the vaccine received (mRNA-1273 versus BNT162b2)
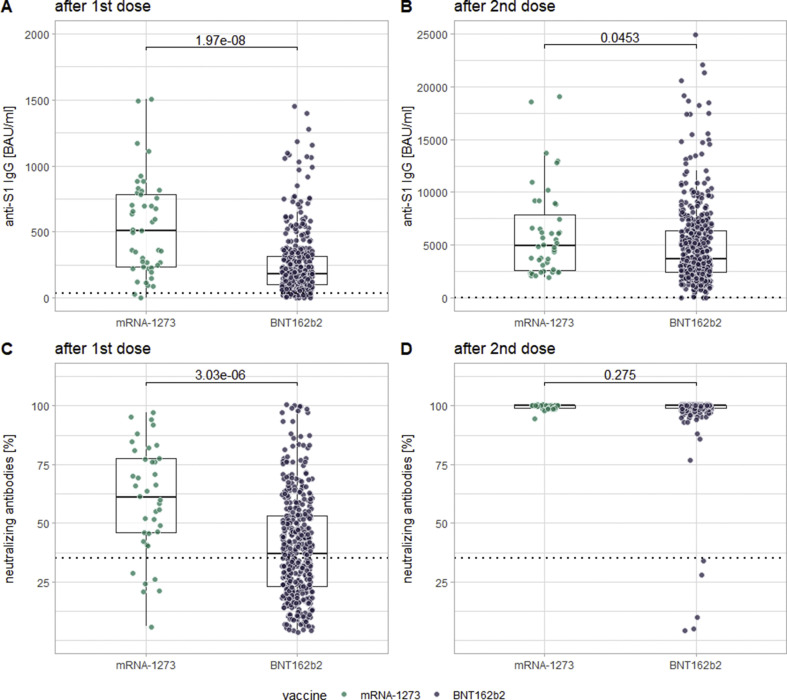
At t2, significantly higher values (p < 0.0001) could be measured for anti-S1 IgG (512 ± 415.1 versus 183.4 ± 155.4 BAU/mL), anti-S1 IgA (11.0 ± 1.4 versus 6.2 ± 4.8 (OD ratio)) and neutralizing antibodies (60.5 ± 25.2 versus 37 ± 23.7) for patients receiving mRNA-1273 compared to BNT162b2. At t3, this difference could still be shown for anti-S1 IgG (p 0.0453) and for IFN-γ via IGRA (which was not performed at t2; p 0.0051). Importantly, these differences cannot be explained by age differences between recipients of different vaccines as statistical analyses revealed none (t-test t –1.245, df 58.821, p 0.2181). See Fig. 4 for this comparison for anti-S1 IgG and neutralizing antibodies.
Fig. 4.
Comparison of levels of anti-SARS-CoV-2 IgG (panels A and B) and neutralizing antibodies (panels C and D) in between recipients of the two different examined mRNA-vaccines, both after the first dose (panels A and C) and after the second dose (panels B and D) of the respective vaccines.
Influence of sex
At no time point was there any relevant difference in any of the measured immune responses between the two sexes (no participant having self-declared a non-binary sexual identity).
Anti-nuclear antibodies (ANAs)
The detailed results of the examination of ANAs can be found in the Supplementary Material. In brief, no short-term induction of ANAs after vaccination against SARS-CoV-2 could be seen.
Discussion
In general, the results of this study show that adults develop strong and specific immune responses to both examined anti-SARS-CoV-2 mRNA vaccines, at both the T- and the B-cell levels. Almost all participants developed high levels of all examined markers, while there was no evidence of a vaccine-associated induction of antinuclear antibodies.
While the T-cell response (IFN-γ via IGRA) became detectable 7 days after the first dose of the vaccine on average, the B-cell response (anti-S1 IgA and IgG) did so at 10 or 12 days post-vaccination, respectively. Others have found timelines very similar to ours in intra-individual measurements [15]. This timeline observed by us can be correlated with the clinical observation that the cumulative incidences of unvaccinated and vaccinated individuals start to diverge 12 days [6,8] to 14 days [16] after the first dose. In agreement with our data, this divergence is likely explained by the emergence of measurable levels of anti-SARS-CoV-2 IgG at exactly this point. This assumption is further supported by the fact that one of our participants tested positive for SARS-CoV-2 via PCR 10 days after having received the first dose of BNT162b2. One day prior to this, they had donated a sample already showing a strong T-cell response but at that time no anti-S1 IgG, which suggests that the T-cell response alone, as measured via IGRA, is not sufficient to convey immunity against SARS-CoV-2. Furthermore, the fact that vaccine-induced clinical protection against SARS-CoV-2 becomes detectable at the same time as anti-S1 IgG suggests that relatively low levels of anti-S1 IgG and neutralizing antibodies might already convey at least some degree of clinical protection (median anti-S1 IgG titre between days 12 and 35 after the first dose of the vaccine 193.3 ± 167.9 BAU/mL; median level of inhibition for the same time frame 38 ± 23.7%). This assumption is supported by findings that even low levels of neutralizing antibodies convey protection against SARS-CoV-2 [17].
The levels of all examined markers increased significantly after the second dose of the vaccine. This increase, and the fact that a considerable share of participants did not develop levels of anti-S1 IgG and/or neutralizing antibodies considered to be reactive after the first dose of the vaccine, are strong arguments in favour of administering both doses of the vaccine to all recipients, especially older ones. While older vaccinees have a general tendency to develop lower anti-S1 IgG titres and levels of neutralizing antibodies than younger vaccinees, this discrepancy is much more pronounced after the first dose of the vaccine than after the second one. Most likely, this is due to immune responses being delayed but also less pronounced in older individuals, a phenomenon generally summarized under the term immunosenescence [18]. A delay in immune responses of older recipients would explain why the age dependency of the markers examined by us is greater after the first dose of the vaccine, while a generally weaker response would explain why this dependency persists after the second dose. The same mechanisms may also underlie the heightened risk in older patients of developing a severe course of COVID-19 [[19], [20], [21]]. Therefore, while all age groups profit from the second dose, in regard to the levels of markers examined by us, older vaccinees do so even more; other studies have shown that this trend continues beyond the age groups we examined [22]. Nevertheless, there is as yet no evidence to show that different antibody levels translate to different levels of clinical protection against SARS-CoV-2.
Further, three participants did not develop any immune response at any time point, most likely as a consequence of immunosuppressive medication. Although the mechanisms of immunosuppression were very different between these three, both B- and T-cell responses were absent for all of them. Although it can't be proved serologically, it must to date be assumed that a missing seroconversion translates to missing clinical protection against SARS-CoV-2. Other studies have also shown an impaired immune response to vaccination with BNT162b2, especially in patients with a liver or kidney transplant [[23], [24], [25]]. To counter possible adverse effects of a false sense of security it is therefore critical to identify non-responders to the vaccination, as is usual for other vaccines as well. Other forms of immunosuppressive medication did not affect immune responses to the vaccination against SARS-CoV-2, as has been shown previously [26].
In our cohort, a vaccination with mRNA-1273 elicited somewhat stronger immune responses than BNT162b2. It is doubtful, however, whether this translates to better clinical protection, as the differences (especially in neutralizing antibodies at t3), while statistically significant, are very small. Clinical data seem to suggest a very comparable efficacy for both vaccines [9].
A further observation of our study is that individuals with a history of COVID-19 develop much higher antibody levels already after the first dose of vaccine compared to unexposed recipients. While this has been shown before [[27], [28], [29], [30]], we were also able to show that these vaccinees do not benefit further from a second dose of the vaccine, which could potentially be employed more efficiently if administered to another unexposed vaccinee.
An interesting side note of the examination of ANAs in our cohort is the unusually high prevalence of the pattern AC-2. Considering the fact that AC-2 and its target antigen DFS70 are negatively associated with the presence of autoimmune diseases [31], this observation serves to show that non-specific findings increase in frequency when tests are ordered without preselection. To avoid unnecessary diagnostics and therapy, a correct interpretation of these findings is all the more important.
Our study has several limitations. Information on immunosuppressive medication, while being reported voluntarily by several participants, was not collected systematically. Neither was information collected about possible medical conditions, especially autoimmune ones, or other medication received by the participants. The impact of these factors may therefore have been underestimated, or not recognized at all. The proportion of participants receiving mRNA-1273 was considerably smaller, possibly underestimating the differences between the two vaccines. The temporal course of the immune response after vaccination was gained through inter-individually gathered data. Ideally, intra-individual measurements in short (ideally daily) intervals after the vaccination would be performed. Also, the interval between the second dose of the vaccine and the last collection of blood from was relatively short at 2 weeks. However, another follow-up measurement with a longer interval is in the planning at the time of writing. Lastly, our participants having been recruited from a population of employed individuals of working age, there may have been a bias towards more healthy individuals in our cohort.
In conclusion, we were able to show that mRNA-1273 and BNT162b2 elicit strong and specific immune responses that were significantly enhanced after the second dose. Important influencing factors were age, prior infection, immunosuppression, and the vaccine received. In light of recent evidence showing the antibody responses to the vaccines to be sustained [32,33], and the vaccines to be clinically effective [5,6,[8], [9], [10]], our findings are encouraging indications that the available vaccines against SARS-CoV-2 may contribute decisively the containment of the COVID-19 pandemic.
Author contributions
RM, DP, SS, K-PW, BS, FL and RJ designed the study. RM, JD, SE, JR and SG contributed to the recruitment of participants. The described vaccinations were performed under the direction of SG. The assays were performed by RM, DP, KS, VH, DZ and CK. Data analysis was performed by RM, KS, VH and DZ. The manuscript was written by RM, with support from all authors. All authors have read and approved the final version of the manuscript.
Transparency declaration
KS, VH, DZ and CK are currently employees of the EUROIMMUN AG (Lübeck, Germany). All other co-authors have no conflicts of interest to declare. No external funding was received for this study.
Acknowledgements
The authors want to express their gratitude towards all colleagues and co-workers who have made this study possible. Special thanks go to: Mrs Ingrid Ascha, Mrs Franziska Peters, Mrs Dorothea Bachmann, Mrs Sarah Schultz, Mrs Silke Lipke-Reinke, Mrs Astrid Messall, Mrs Kathrin Johannsen, Mrs Michaela Seidel, Mrs Alexandra Jurat, Mrs Grazyna Wardzinski, Mrs Sarah Kuckertz, Mrs Sabine Arp, Mrs Jana Eicke-Metzenthin, and Mrs Gesa Schreyer, without whom this project would not have been possible.
Editor: R. Chemaly
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freudenthal R., Horowitz M., Jadhav S., Moncrieff J. Lack of safeguards in response to restrictive public health measures. Lancet. 2020;396:e70. doi: 10.1016/S0140-6736(20)32118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauchemez S., Kiem C.T., Paireau J., Rolland P., Fontanet A. Lockdown impact on COVID-19 epidemics in regions across metropolitan France. Lancet. 2020;396:1068–1069. doi: 10.1016/S0140-6736(20)32034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holy E.W., Jakob P., Manka R., Stähli B.E., Siegrist P.T., Ruschitzka F., et al. Impact of a nationwide COVID-19 lockdown on acute coronary syndrome referrals. Cardiol J. 2020;27:633–635. doi: 10.5603/CJ.a2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel W., Nivet M., Warner J., Podolsky D.K. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med. 2021;384:1962–1963. doi: 10.1056/NEJMc2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team . 2020. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria.https://www.R-project.org Available from: [Google Scholar]
- 15.Wisnewski A.V., Campillo Luna J., Redlich C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PloS One. 2021;16 doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azamgarhi T., Hodgkinson M., Shah A., Skinner J.A., Hauptmannova I., Briggs T.W.R., et al. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021 17;12:3698. doi: 10.1038/s41467-021-23927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 18.Lanzer K.G., Johnson L.L., Woodland D.L., Blackman M.A. Impact of ageing on the response and repertoire of influenza virus-specific CD4 T cells. Immun Ageing A. 2014;11:9. doi: 10.1186/1742-4933-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacher P., Rosati E., Esser D., Martini G.R., Saggau C., Schiminsky E., et al. Low-avidity CD4+ T cell responses to SARS-CoV-2 in unexposed individuals and humans with severe COVID-19. Immunity. 2020;53:1258–1271. doi: 10.1016/j.immuni.2020.11.016. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol. 2020;11:571416. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markewitz R., Torge A., Wandinger K.-P., Pauli D., Franke A., Bujanda L., et al. Clinical correlates of anti-SARS-CoV-2 antibody profiles in Spanish COVID-19 patients from a high incidence region. Sci Rep. 2021;11:4363. doi: 10.1038/s41598-021-83969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73:ciab381. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozen-Zvi B., Yahav D., Agur T., Zingerman B., Ben-Zvi H., Atamna A., et al. Antibody response to mRNA SARS-CoV-2 vaccine among kidney transplant recipients—prospective cohort study. Clin Microbiol Infect. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korth J., Jahn M., Dorsch O., Anastasiou O.E., Sorge-Hädicke B., Eisenberger U., et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13:756. doi: 10.3390/v13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisen U.M., Berner D.K., Tran F., Sümbül M., Vullriede L., Ciripoi M., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1–6. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley T., Grundberg E., Selvarangan R., LeMaster C., Fraley E., Banerjee D., et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadat S., Tehrani Z.R., Logue J., Newman M., Frieman M.B., Harris A.D., et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahler M., Andrade L.E., Casiano C.A., Malyavantham K., Fritzler M.J. Anti-DFS70 antibodies: an update on our current understanding and their clinical usefulness. Expert Rev Clin Immunol. 2019;15:241–250. doi: 10.1080/1744666X.2019.1562903. [DOI] [PubMed] [Google Scholar]
- 32.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.