Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused a devastating health crisis worldwide. In this review, we have discussed that prophylactic phytochemical quercetin supplementation in the form of foods or nutraceuticals may help manage the COVID-19 pandemic. The following evidence supports our argument. First, nuclear factor erythroid-derived 2-like 2 (NRF2) agonists abrogate replication of SARS-CoV-2 in lung cells, and quercetin is a potent NRF2 agonist. Second, quercetin exerts antiviral activity against several zoonotic coronaviruses, including SARS-CoV-2, mainly by inhibiting the entry of virions into host cells. Third, inflammatory pathways activated by nuclear factor kappa B, inflammasome, and interleukin-6 signals elicit cytokine release syndrome that promotes acute respiratory distress syndrome in patients with COVID-19, and quercetin inhibits these pro-inflammatory signals. Fourth, patients with COVID-19 develop thrombosis, and quercetin mitigates coagulation abnormalities by inhibiting plasma protein disulfide isomerase. This review provides a strong rationale for testing quercetin for the management of COVID-19.
Keywords: COVID-19, Quercetin, Immunomodulation, Cytokine response syndrome, NRF2, Antiviral
Graphical abstract
Highlights
-
•
Quercetin may inhibit SARS-CoV-2 entry into cells by altering viral envelope proteins.
-
•
Quercetin may inhibit SARS-CoV-2 replication by activating the NRF2 pathway.
-
•
Quercetin attenuates proinflammatory signals and cytokine release syndrome.
-
•
Quercetin may reduce coagulopathy by inhibiting protein disulphide isomerase.
1. Introduction
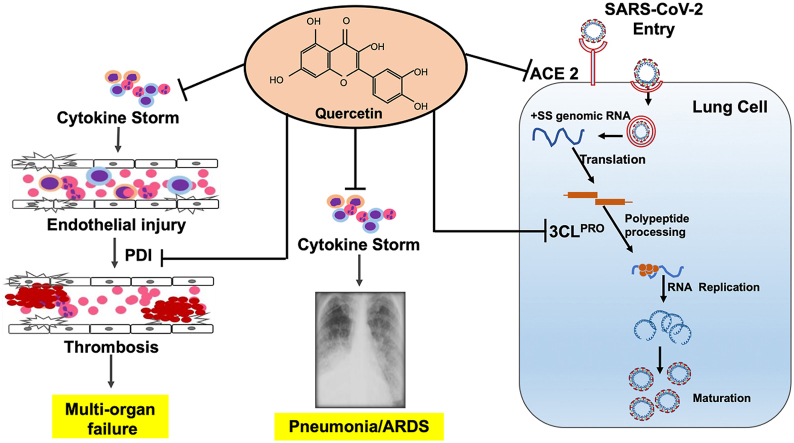
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused an unprecedented global health crisis and economic loss. As of August 7, 2021, over 201 million people were infected with SARS-CoV-2, and 4.2 million people died worldwide [1]. The common symptoms recorded among patients with non-severe COVID-19 include fever, fatigue, dry cough, sputum production, headache, and dyspnea associated with mild unilateral or bilateral pneumonia. Meanwhile, patients with severe COVID-19 disease are associated with pneumonia, acute respiratory distress syndrome (ARDS), metabolic acidosis, thromboembolism, multiple organ failure, and acute cardiac injury [2,3], which are common factors contributing to death. Although SARS-CoV-2 has affected every age group, from newborns to children to adults, severe morbidity and mortality are commonly observed in the elderly population aged >60 years and people with comorbidities, such as hypertension, diabetes, cardiovascular disease, and renal disease.
Currently, the treatment modalities for hospitalized patients with COVID-19 involve supportive care, such as supplemental oxygen therapy and mechanical ventilation, and there are no Food and Drug Administration (FDA)-approved drugs for COVID-19 treatment. Nonetheless, few FDA-approved drugs are being repurposed for COVID-19 management and the outcomes have been mixed. During the early phase of the pandemic, the antimalarial drug hydroxychloroquine and anti-human immunodeficiency virus (HIV) drug lopinavir/ritonavir were repurposed for the treatment of COVID-19; however, the trials were discontinued by the World Health Organization as there were no significant clinical benefits between the repurposed drug and standard of care. Remdesivir, a small molecule inhibitor of viral RNA-dependent RNA polymerase used initially against the Ebola virus, has been administered at the bedside for the management of patients with COVID-19, and the results were promising. Remdesivir did not lower the mortality of hospitalized patients with COVID-19; however, it shortened the recovery time of severely ill patients compared to patients who were receiving standard care of treatment [4]. A more recent success story was from the clinical trials of dexamethasone, a glucocorticoid drug, which dampens host inflammatory responses. Dexamethasone treatment significantly lowered 28-day mortality among mechanically ventilated patients with COVID-19 compared to the placebo [5]. Based on the knowledge gained from a mechanistic understanding of COVID-19 pathogenesis and big-data analysis using artificial intelligence, several approved drugs are being repositioned for COVID-19 treatment [6]. In addition, intense efforts are in progress to develop an effective vaccine. Fortunately, a number of vaccines have been effective in eliciting immune response against SARS-CoV-2. Mass vaccination is the most effective way of preventing transmission; however, it is premature to assume that the first-generation of the vaccine will be effective in reducing the infection and transmission of COVID-19. First, the efficacy of the currently available COVID-19 vaccines ranges from 50% to 90% [7]. Second, SARS-CoV-2 has been mutating. Whether the first-generation vaccine, especially those designed against the viral spike protein, will be effective against new variants of SARS-CoV-2, such as B.1.1.7 (United Kingdom), B.1.351 (South Africa), and B.1.617 (India) remains unclear. Third, there is an insufficient supply of vaccines and inadequate resources to vaccinate a large population in low-/middle-income countries. Therefore, it may take at least two to three years before a large percentage of the global population is vaccinated to achieve complete protection from SARS-CoV-2 infections. Fourth, the adverse events in first-generation vaccines, especially in people older than 80 years, have dampened the public's motivation for vaccination. Hence, it is imperative to continue searching for chemoprotective agent(s) to protect the general population and high-risk groups from SARS-CoV-2 infection.
Although no systematic randomized control trials have been conducted to elucidate the benefits of herbal medicine on patients with COVID-19, several studies emanated mainly from China have reported intake of herbal medicine by patients with COVID-19 with the standard of care [8,9]. Furthermore, researchers have carried out in silico analysis of natural product libraries to identify potential antiviral phytochemical(s) against SARS-CoV-2 [10]. Quercetin, a flavonoid present in many fruits and vegetables, has been identified as a promising candidate that exhibits a broad-spectrum antiviral activity. In silico data suggest that quercetin interacts with SARS-CoV-2 spike protein and main proteases and may be useful in inactivating or killing SARS-CoV-2 [11,12]. In this review, we have integrated the latest and established evidence on quercetin and postulated that it confers prophylactic and therapeutic benefits in the management of COVID-19. We have built a strong case for the plausible use of quercetin or its derivatives as chemoprotective nutraceutical agents for the protection of the general public from SARS-CoV-2 infection and management of patients with COVID-19.
2. Structure and forms of quercetin
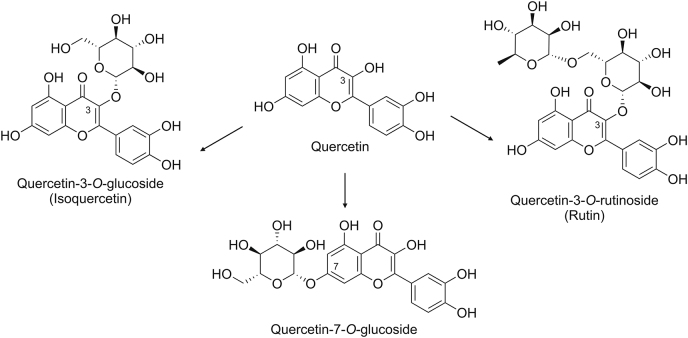
Quercetin is a pentahydroxyflavone with hydroxy groups at the 3-, 3′-, 4′-, 4-, and 7- positions (Fig. 1), and it belongs to the flavonol subclass of flavonoids. Quercetin is an aglycone; however, it is usually conjugated to simple sugars (i.e., glucose, xylose, rhamnose, arabinose, or galactose) or disaccharide (e.g., rutinose) at the 3-position in plants (Fig. 1). In onions, quercetin is attached to the glucose moiety and forms quercetin-3-O-glucoside (isoquercetin) [13], whereas in apples and tea, quercetin is conjugated to rutinose to form quercetin-3-O-rutinoside (rutin) [14]. Either aglycones or the O-glycoside form of quercetin can be used to assess its biological activity.
Fig. 1.
Structure of quercetin and its natural derivatives.
3. Anti-viral effects of quercetin against zoonotic coronaviruses, including SARS-CoV-2, and the underlying mechanisms
By integrating established evidence derived from experimental model systems using Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and the new knowledge related to the immunopathogenesis of COVID-19, we have highlighted three main antiviral mechanisms by which quercetin may inhibit SARS-CoV-2 infection.
3.1. Quercetin may repress SARS-CoV-2 replication by activating nuclear factor erythroid-derived 2-like 2 (NRF2)
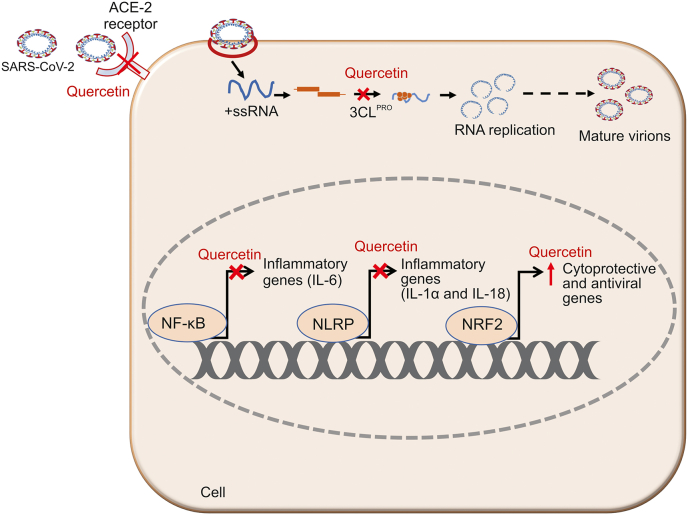
NRF2 transcriptionally upregulates a network of cytoprotective genes, including antioxidant genes, such as genes encoding for glutathione biosynthesis, heme oxygenase-1, and several other antioxidant proteins. In normal cells, NRF2 is held in the cytoplasm by an adaptor protein, Kelch-like ECH-associated protein 1 (KEAP1), which is in turn associated with Cullin3 containing E3 (Cul3-E3)-ubiquitin ligase. The KEAP1-Cul3-E3-ubiquitin ligase complex ubiquitinates and targets NRF2 for proteasomal degradation. During oxidative stress, reactive oxygen species or electrophiles modify critical cysteine residues in the BTB and kelch domain of KEAP1, which results in the dislocation of NRF2 from the KEAP1/Cul3-E3-ubiquitin ligase complex and translocation into the nucleus. Inside the nucleus, NRF2 binds to a conserved motif called “antioxidant responses element” and transactivates target gene expression. Most small-molecule inducers stabilize NRF2 by disrupting the NRF2-KEAP1 interaction [15]. Olagnier et al. [16] reported that the NRF2 agonist, 4-octyl-itaconate, inhibited SARS-CoV-2 replication in a variety of human lung epithelial cells, such as Calu3 (cancer cell line), Nuli (immortalized human airway epithelial cells), and primary human airway epithelial cells. To further confirm this observation, they used dimethylfumarate, another NRF2 agonist and an FDA-approved drug for the treatment of multiple sclerosis, and demonstrated that dimethylfumarate also terminated SARS-CoV-2 replication. This antiviral response against SARS-CoV-2 elicited by NRF2 activation was not restricted to only chemical inducers; activation of NRF2 by ablating KEAP1 using KEAP1 siRNA was also effective in limiting viral replication. The same researchers [16] analyzed the publicly available transcriptomic profile of lung biopsy samples of patients with COVID-19 and discovered that the NRF2 pathway was repressed. The study concluded that NRF2 activation elicits antiviral responses against SARS-CoV-2 and is independent of the chemical nature of pharmacological inducers. Several studies have reported quercetin to be a potent NRF2 agonist [16,17]. Quercetin treatment stabilizes NRF2 by modifying KEAP1, thereby inhibiting NRF2 ubiquitination and proteasomal degradation [18]. Dietary feeding of quercetin in mouse models activated the NRF2 pathway in the lungs, as indicated by the expression of NRF2 regulated genes [19], and protected from bleomycin-induced lung fibrosis. Quercetin supplementation also protected against other organ injuries, such as kidney injury, in an NRF2-dependent manner [20]. Considering this new evidence, we argue that quercetin supplementation may attenuate SARS-CoV-2 replication through the activation of NRF2 (Fig. 2).
Fig. 2.
Schematics depicting plausible underlying mechanisms of how quercetin may abrogate SARS-CoV-2 infection and mitigate pathogenesis of COVID-19. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ACE-2: angiotensin converting enzyme-2; 3CLpro: 3C-like proteases; NF-κB: nuclear factor kappa B; IL: interleukin; NLRP: nucleotide-binding oligomerization domain leucine rich repeat and pyrin domain-containing protein; NRF2: nuclear factor erythroid-derived 2-like 2.
3.2. Quercetin inhibits 3C-like proteases (3CLpro)
The 3CLpro (also referred to as the main protease) and papain-like protease (PLpro) are highly conserved among human coronaviruses and are attractive drug targets. Following coronavirus infection, 3CLpro and PLpro are translated and activated, which then cleave the polyproteins pp1a and pp1ab into various non-structural proteins, including RNA-dependent RNA polymerase, which is essential for viral replication. Computational and experimental studies have revealed that quercetin-3-β-D-glucoside inhibits MERS-CoV 3CLpro activity [21]. Nguyen et al. [22] demonstrated that quercetin inhibits the activity of recombinant SARS-CoV 3CLpro by up to 80%. More recently, Abian et al. [23] reported that quercetin inhibits SARS-CoV-2 3CLpro activity by destabilizing its structure. Studies on the structure-activity relationship revealed that quercetin inhibitory activity against 3CLpro is related to carbon 3′-substituted with hydroxide [24]. In summary, the accumulated evidence suggests that quercetin or its analogs inhibit 3CLpro activity, which is vital in the replication of coronaviruses, including SARS-CoV-2.
3.3. Quercetin interacts with viral and host cell surface proteins and may block coronavirus entry into host cells
Yi et al. [25] designed HIV-luciferase/SARS-CoV pseudotyped virus that expresses luciferase and used it as a tool for detecting the entry and replication of the virus in Vero cells. By measuring luciferase expression in Vero cells post-infection, the authors demonstrated that incubation of HIV-luciferase/SARS pseudotyped virus with quercetin inhibited its entry into Vero cells and concluded that quercetin might hinder the entry of SARS-CoV into host cells, possibly by altering surface proteins. There is a high degree of similarity (74%) in the protein sequences of the spike proteins between SARS-CoV and SARS-CoV-2 [26]. Molecular docking studies indicated that quercetin could potentially interact with the Asp38 residue of the angiotensin-converting enzyme 2 (ACE2) receptor and prevent the attachment of SARS-CoV-2 to the host cell membrane [27]. Hence, it is likely that quercetin may inhibit SARS-COV-2 infection by blocking its entry into lung cells by altering the viral spike protein or host ACE2.
4. Quercetin exerts broad-spectrum antiviral activity against common respiratory viruses by blocking viral infectivity
To further strengthen the concept of quercetin as a promising antiviral agent for managing COVID-19, we expanded our review on the antiviral effects of quercetin on common respiratory viruses. The evidence suggests that quercetin exerts antiviral activity primarily by preventing viral entry into target host cells by modifying the virion surface proteins.
4.1. Influenza A virus (IAV)
The antiviral activity of quercetin and its derivatives against IAV is driven by direct and indirect mechanisms. Glycoprotein hemagglutinin (HA) is the IAV envelope protein that helps with viral entry, by binding to the target host cell first and then helping with endosomal fusion. Quercetin binds to HA and inhibits IAV entry into host cells by disrupting HA-mediated membrane fusion events [28]. The quercetin-3-glucoside has also been reported to block IAV replication by inhibiting polymerase protein-2 [29]. Choi et al. [30] also reported the antiviral activity of quercetin-3-rhamnoside against IAV and subsequently demonstrated that prophylactic oral administration of quercetin-3-rhamnoside markedly decreased the viral titer in the lungs and significantly reduced the illness and mortality in IAV-infected mice [31].
4.2. Respiratory syncytial virus (RSV)
A large body of evidence has confirmed the antiviral effects of quercetin and its derivatives against RSV. The two glycoproteins, attachment (G) and fusion (F) present on the surface of RSV lipid envelope, mediate adhesion, fusion, and entry of virions into the host cells. Lopes et al. [32] reported the viricidal activity of quercetin pentaacetate against RSV and demonstrated that the compound interacts with RSV surface glycoprotein F, which prevents viral adhesion to the host cells. More recently, Machado et al. [33] reported that quercetin binds to RSV glycoprotein G and blocks RSV entry into the host cells. Together, these evidences suggest that quercetin blocks RSV infectivity.
4.3. Rhinovirus (RV)
RV infection is frequently associated with common cold and, more importantly, viral exacerbations in patients with asthma and chronic obstructive pulmonary disease (COPD). Quercetin 7-glucoside inhibits viral replication by affecting the early stages of human rhinovirus 2 (RV-2) infection in HeLa cells [34]. Ganesan et al. [35] reported that quercetin treatment suppressed RV replication in cultured airway epithelial cells by inhibiting endocytosis. The same study reported that oral quercetin supplementation in mice 2-h post RV infection markedly decreased viral load in the lungs, which was correlated with lower pro-inflammatory cytokine and interferon levels. Quercetin treatment also reduces airway hyperresponsiveness induced by RV-2 infection in a mouse model. In another study, Farazuddin et al. [36] demonstrated that supplementation of 0.1% quercetin mixed in the diet of a cigarette smoke-exposed mouse model of COPD reduced exacerbation of lung inflammation and expression of pro-inflammatory cytokines caused by RV infection.
5. Quercetin functions as an immunomodulator and may dampen the progression of ARDS in patients with COVID-19
5.1. Quercetin inhibits nuclear factor kappa B (NF-κB), inflammasome, and interleukin (IL)-6-driven cytokine release syndrome
The host immune response to SARS-CoV-2 is a critical determinant of the clinical symptoms, morbidity, and severity of COVID-19. Long et al. [37] analyzed a panel of circulating inflammatory markers in patients with or without COVID-19 symptoms and reported that asymptomatic patients were associated with markedly lower levels of cytokines, namely, TRAIL, M-CSF, IL-6, IL-2, IL-10, MCP-1, IL-8, IL-18, IFN-γ, and G-CSF, compared to symptomatic patients. Similarly, Lucas et al. [38] observed that moderately ill patients with COVID-19 showed lower inflammatory responses than severely ill patients, as indicated by lower circulating levels of inflammatory mediators (IL-6, IL-1α, IL1-β, TNF-α, IL-18, IFN-γ, and C-C motif chemokine ligand 1. Elevated levels of these inflammatory mediators during early infection are positively correlated with poor survival in patients with COVID-19 [38]. In contrast, viral RNA load levels are comparable between mildly and severely ill patients with COVID-19 during the first 10 days after infection. However, it steadily declines in moderately ill patients but not in severely ill patients [38]. The insights from the inflammatory cytokine profile data in patients with COVID-19 suggest that the cytokine response syndrome (CRS) is largely driven by activation of NF-κB, inflammasome, and IL-6 signals [[39], [40], [41], [42]].
A large body of evidence suggests that quercetin is effective in diminishing NF-κB, inflammasome, and IL-6 signaling. Quercetin pretreatment dampens NF-κB-mediated expression of cytokines, including IL-6, in various cell types [[43], [44], [45]]. In a mouse model of acute lung injury (ALI), quercetin administration significantly attenuated lipopolysaccharide (LPS)-induced pulmonary inflammation, as indicated by lower levels of neutrophil and lymphocyte infiltrates, decreased cytokine levels in the lungs, and improved survival [46] compared to the placebo group. Similarly, quercetin administration significantly suppresses ALI in an IAV-infected mouse model [47]. It also inhibits the activation of the NLRP3 inflammasome and secretion of IL-1α and IL-18 [48]. Quercetin pretreatment inhibits LPS-induced IL-6 secretion in neutrophils [49] and dendritic cells [50], as well as ameliorates IL-6 activated signal transducer and activator of transcriptions 3 (STAT3) signals [[51], [52], [53]]. As depicted in Fig. 2, the accumulated data suggest that quercetin is a promising immunomodulator that may dampen inflammation in patients with COVID-19 and prevent the development of ARDS.
5.2. Quercetin activates NRF2 pathway that mitigates cytokine release syndrome and attenuates acute lung injury/ARDS
Previously, using a mouse model of sepsis, we reported that deficiency of the NRF2 pathway promotes CRS and worsens survival, whereas activation of the NRF2 pathway dampens CRS and improves survival [[54], [55]]. Mechanistic evidence suggests that NRF2 downregulates inflammation by abolishing the transcriptional expression of pro-inflammatory cytokines regulated by NF-κB [[54], [55], [56]]. Hence, NRF2 inducers could be potent immunomodulators for the mitigation of SARS-CoV-2 pathogenesis (Fig. 2). It is conceivable that prophylactic supplementation of quercetin may protect against SARS-CoV-2 infection and help with the management of COVID-19 treatment through activation of the NRF2 pathway.
6. Quercetin inhibits protein disulfide isomerase (PDI) and may mitigate coagulation abnormalities associated with patients with COVID-19
It is postulated that in hospitalized patients with COVID-19, CRS induced by SARS-CoV-2 infection triggers systemic inflammatory responses, which cause vascular injury and abnormal coagulation, recapitulating the characteristic features of sepsis-associated disseminated intravascular coagulation [57]. Many studies have reported thrombotic events in hospitalized patients with COVID-19 [58]. The mortality rate is higher in COVID-19 patients with thrombotic events [59], and the circulating levels of D-dimer are positively correlated with the severity of illness [60]. This emerging evidence has led to a consensus that therapeutics to prevent the development of coagulation abnormalities may improve the outcomes of patients with COVID-19 [61]. Quercetin is a potent inhibitor of PDI [62], an enzyme implicated in platelet-mediated thrombin formation at the site of vascular injury. In a multicenter phase II trial, administration of isoquercetin with 1 g/day for 56 days to cancer patients caused a significant decrease in D-dimer, P-selectin, and platelet-dependent fibrin generation compared to placebo [63], indicating that isoquercetin supplementation protects against hypercoagulability in cancer patients. It is plausible that quercetin supplementation in high-risk patients with morbidities, such as diabetes or cardiovascular diseases, may help reduce coagulation abnormalities following SARS-CoV-2 infection.
7. Clinical trials of quercetin for disease intervention
Most of the clinical trials carried out on quercetin have been shown to be safe, and the FDA has categorized quercetin as “generally recognized as safe.” According to a database [64], 12 clinical trials on quercetin or its derivatives have been conducted in patients with COVID-19. The interim results from one of the randomized clinical trials (RCT) revealed that quercetin supplementation enhanced viral clearance and partially reduced the clinical symptoms post-treatment [65]. In a phase I clinical trial, 30 patients with hepatitis C virus infection were supplemented with various quercetin doses ranging from 250 mg/day to 5 g/day for 28 days in juice or water [66]. Quercetin was well-tolerated by the participants even at the highest dose of 5 g/day, and the median plasma concentration was 2.25 mg/L at week 4. Although there was no correlation between plasma quercetin levels and viral load, the viral load was decreased in at least a few patients [67]. In another pilot study, quercetin was found to decrease upper respiratory tract infection (URTI) among cyclists. The study found that intake of quercetin with 1 g/day for two weeks reduced self-reported URTI among cyclists. Later, in a 12-week RCT involving 1,002 participants, quercetin intake showed no significant effects on the incidence of URTI, although the study reported a reduction in the number of sick days and severity associated with URTI among cyclists aged >40 years [67]. More recently, a randomized safety trial was conducted in COPD patients and concluded that quercetin doses up to 2000 mg/day for 1 week were well tolerated by patients with no adverse events [68]. Isoquercitrin, a water-soluble form of quercetin, was found to improve endothelial function in high-risk adult participants with cardiovascular disease [69].
8. Conclusions
We have highlighted the plausible mechanisms underlying how quercetin may help in the management of COVID-19. The mechanisms include: 1) protection from SARS-CoV-2 infection by impeding viral entry into host cells by modifying viral spike protein or ACE2, repressing replication inside the cells by inhibiting 3CLpro, or activating the NRF2 pathway; 2) protection from the development of ARDS by diminishing CRS elicited by NF-κB, inflammasome, or IL-6 signals; and 3) mitigation of thrombotic events by inhibiting PDI. We have also discussed that frequent consumption of quercetin-rich foods (such as onion) could elevate plasma quercetin levels to the desired minimal effective concentrations for attaining the clinical benefits of quercetin. Based on the evidence discussed, further studies are warranted to evaluate whether consumption of quercetin-rich foods or nutraceuticals protects the high-risk population from SARS-CoV-2 infection and manages COVID-19.
CRediT author statement
Souparnika H. Manjunath: Writing - Original draft preparation, Reviewing, and Editing; Rajesh K. Thimmulappa: Conceptualization, Writing - Original draft preparation, Reviewing, Editing, and Supervision.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Rajesh Kumar Thimmulappa acknowledges the funding support from the Department of Biotechnology, Ramalingaswami Re-entry Fellowship (Grant No.: BT/RLF/Re-entry/37/2013) and the Department of Science and Technology grant (Grant No.: DST/INT/SOUTH AFRICA/P13/2016).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Coronavirus Resource Center https://coronavirus.jhu.edu Compilation prepared by Johns Hopkins University.
- 2.Grasselli G., Tonetti T., Protti A., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir. Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y., Wang F., Tang J., et al. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health. 2020;2:e667–e676. doi: 10.1016/S2589-7500(20)30192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng H., Peng Z., Luo W., et al. Efficacy and safety of COVID-19 vaccines in phase III trials: a meta-analysis. Vaccines (Basel) 2021;9:582. doi: 10.3390/vaccines9060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo L., Jiang J., Wang C., et al. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm. Sin. B. 2020;10:1192–1204. doi: 10.1016/j.apsb.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani J.S., Johnson J.B., Steel J.C., et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy E., Delvin E., Marcil V., et al. Can phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (COVID-19)? Am. J. Physiol. Endocrinol. Metab. 2020;319:E689–E708. doi: 10.1152/ajpendo.00298.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayakumar B.G., Ramesh D., Joji A., et al. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur. J. Pharmacol. 2020;886:173448. doi: 10.1016/j.ejphar.2020.173448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak J.-H., Seo J.M., Kim N.-H., et al. Variation of quercetin glycoside derivatives in three onion (Allium cepa L.) varieties. Saudi J. Biol. Sci. 2017;24:1387–1391. doi: 10.1016/j.sjbs.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Yao J., Han C., et al. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird L., Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell Biol. 2020;40 doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olagnier D., Farahani E., Thyrsted J., et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto N., Izumi H., Miyamoto R., et al. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Invest. Ophthalmol. Vis. Sci. 2011;52:1055–1063. doi: 10.1167/iovs.10-5777. [DOI] [PubMed] [Google Scholar]
- 18.Tanigawa S., Fujii M., Hou D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Boots A.W., Veith C., Albrecht C., et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm. Med. 2020;20:112. doi: 10.1186/s12890-020-1142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Ma B.-L., Xu C.-G., et al. Dihydroquercetin protects against renal fibrosis by activating the Nrf2 pathway. Phytomedicine. 2020;69:153185. doi: 10.1016/j.phymed.2020.153185. [DOI] [PubMed] [Google Scholar]
- 21.Jo S., Kim H., Kim S., et al. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T.T., Woo H.-J., Kang H.-K., et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., et al. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu Y.B., Jeong H.J., Kim J.H., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi L., Li Z., Yuan K., et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhowmik D., Nandi R., Prakash A., et al. Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu W., Li R., Li X., et al. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2015;8:6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nile S.H., Kim D.H., Nile A., et al. Probing the effect of quercetin 3-glucoside from Dianthus superbus L against influenza virus infection- in vitro and in silico biochemical and toxicological screening. Food Chem. Toxicol. 2020;135:110985. doi: 10.1016/j.fct.2019.110985. [DOI] [PubMed] [Google Scholar]
- 30.Choi H.J., Song J.H., Park K.S., et al. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Choi H.J., Song J.H., Kwon H.D. Quercetin 3-rhamnoside exerts antiinfluenza A virus activity in mice. Phytother Res. 2012;26:462–464. doi: 10.1002/ptr.3529. [DOI] [PubMed] [Google Scholar]
- 32.Lopes B.R.P., da Costa M.F., Genova Ribeiro A., et al. Quercetin pentaacetate inhibits in vitro human respiratory syncytial virus adhesion. Virus Res. 2020;276:197805. doi: 10.1016/j.virusres.2019.197805. [DOI] [PubMed] [Google Scholar]
- 33.Machado V.B., Maróstica de Sá J., Miranda Prado A.K., et al. Biophysical and flavonoid-binding studies of the G protein ectodomain of group A human respiratory syncytial virus. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J.H., Park K.S., Kwon D.H., et al. Anti-human rhinovirus 2 activity and mode of action of quercetin-7-glucoside from Lagerstroemia speciosa. J. Med. Food. 2013;16:274–279. doi: 10.1089/jmf.2012.2290. [DOI] [PubMed] [Google Scholar]
- 35.Ganesan S., Faris A.N., Comstock A.T., et al. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012;94:258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farazuddin M., Mishra R., Jing Y., et al. Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PloS One. 2018;13 doi: 10.1371/journal.pone.0199612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long Q.-X., Tang X.-J., Shi Q.-L., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 38.Lucas C., Wong P., Klein J., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang S., Tanaka T., Inoue H., et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U S A. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bovijn J., Lindgren C.M., Holmes M.V. Genetic variants mimicking therapeutic inhibition of IL-6 receptor signaling and risk of COVID-19. Lancet Rheumatol. 2020;2:e658–e659. doi: 10.1016/S2665-9913(20)30345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 42.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front. Immunol. 2020;11:1021. doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz P.A., Braune A., Hölzlwimmer G., et al. Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 2007;137:1208–1215. doi: 10.1093/jn/137.5.1208. [DOI] [PubMed] [Google Scholar]
- 44.Min Y.-D., Choi C.-H., Bark H., et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007;56:210–215. doi: 10.1007/s00011-007-6172-9. [DOI] [PubMed] [Google Scholar]
- 45.Cheng S.-C., Huang W.-C., Pang J.-H.S., et al. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 2019;20:2957. doi: 10.3390/ijms20122957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang R., Zhong T., Wu H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch. Med. Sci. 2015;11:427–432. doi: 10.5114/aoms.2015.50975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z., Zhao J., Li W., et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci. Rep. 2016;6:19095. doi: 10.1038/srep19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang W., Huang Y., Han N., et al. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016;54:592–596. doi: 10.1038/sc.2015.227. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Li X., Yue Y., et al. The inhibitory effect of quercetin on IL-6 production by LPS-stimulated neutrophils. Cell. Mol. Immunol. 2005;2:455–460. [PubMed] [Google Scholar]
- 50.Huang R.-Y., Yu Y.-L., Cheng W.-C., et al. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010;184:6815–6821. doi: 10.4049/jimmunol.0903991. [DOI] [PubMed] [Google Scholar]
- 51.Granato M., Gilardini Montani M.S., Zompetta C., et al. Quercetin interrupts the positive feedback loop between STAT3 and IL-6, promotes autophagy, and reduces ROS, preventing EBV-driven B cell immortalization. Biomolecules. 2019;9:482. doi: 10.3390/biom9090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michaud-Levesque J., Bousquet-Gagnon N., Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012;318:925–935. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Wung B.S., Hsu M.C., Wu C.C., et al. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 54.Thimmulappa R.K., Lee H., Rangasamy T., et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong X., Thimmulappa R., Craciun F., et al. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am. J. Respir. Crit. Care Med. 2011;184:928–938. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi E.H., Suzuki T., Funayama R., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iba T., Levy J.H., Levi M., et al. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zwicker J.I., Schlechter B.L., Stopa J.D., et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stopa J.D., Neuberg D., Puligandla M., et al. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ClinicalTrialgov https://clinicaltrials.gov Compilation prepared by National Library of Medicine.
- 65.Di Pierro F., Iqtadar S., Khan A., et al. Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial. Int. J. Gen. Med. 2021;14:2807–2816. doi: 10.2147/IJGM.S318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu N.T., Crespi C.M., Liu N.M., et al. A phase I dose escalation study demonstrates quercetin safety and explores potential for bioflavonoid antivirals in patients with chronic hepatitis C. Phytother. Res. 2016;30:160–168. doi: 10.1002/ptr.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nieman D.C., Henson D.A., Gross S.J., et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- 68.Han M.K., Barreto T.A., Martinez F.J., et al. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020;7 doi: 10.1136/bmjresp-2018-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bondonno N.P., Bondonno C.P., Ward N.C., et al. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020;123:182–189. doi: 10.1017/S0007114519002137. [DOI] [PubMed] [Google Scholar]