Highlights
-
•
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm of the epithelial tissue, which has unique mode of geographic and epidemiologic distributions. Approximately 70% of newly diagnosed NPC cases are classified as locoregionally advanced disease and radiotherapy (RT) plays an essential role.
-
•
With the improved local and regional control, distant metastasis changes into the main failure pattern in locoregionally advanced NPC after intensity-modulated radiotherapy (IMRT). Individuals with N3 NPC are at higher risk for distant metastasis.
-
•
A retrospective analysis of 143 patients with N3 NPC has been conducted. In our research, induction chemotherapy followed by IMRT and adjuvant chemotherapy achieved satisfactory long-term survival outcomes and high locoregional control.
-
•
Neck lymph node necrosis and late T stage served as predictors of poor prognosis for patients. Patients with neck lymph node necrosis and T4N3 diseases have extremely poor outcome and more aggressive treatment options should be further studied.
Abstract
Objectives
To evaluate long-term outcomes of induction chemotherapy (IC) followed by intensity-modulated radiotherapy (IMRT) and adjuvant chemotherapy (AC) in nasopharyngeal carcinoma (NPC) patients with N3 disease.
Materials and methods
From September 2005 to August 2016, 143 patients confirmed NPC with the 8th AJCC/UICC staging criteria N3 were reviewed. All patients received IC followed by IMRT and AC.
Results
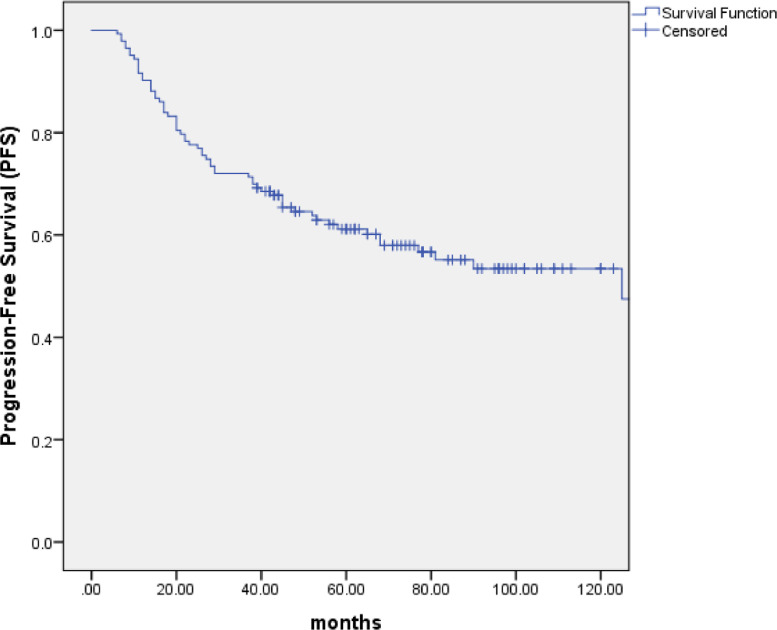
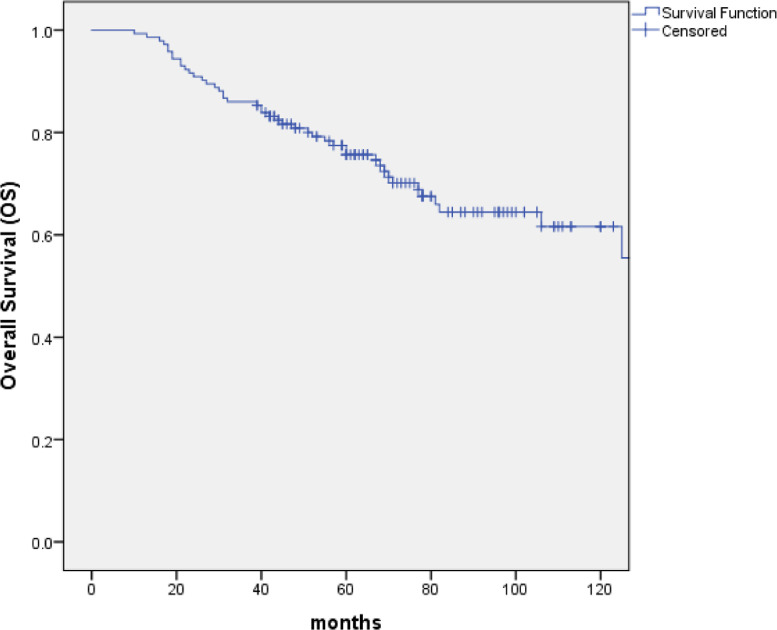
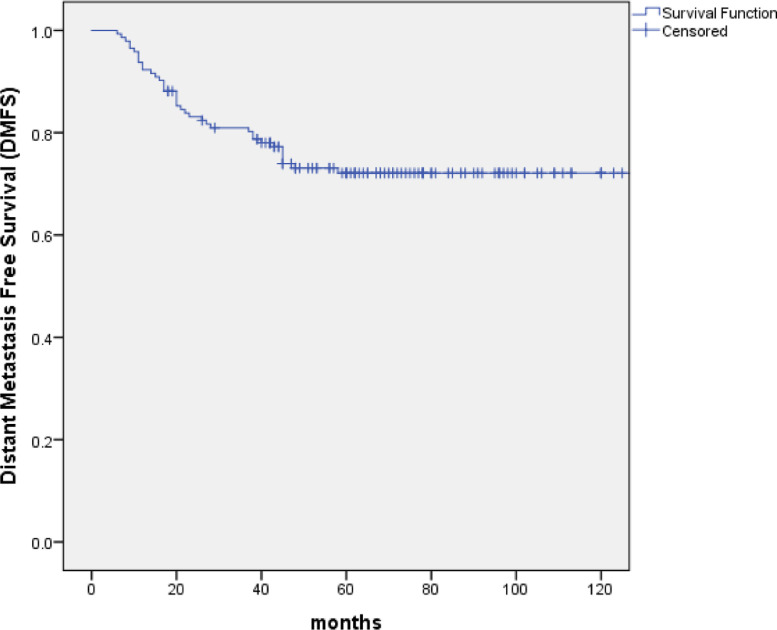
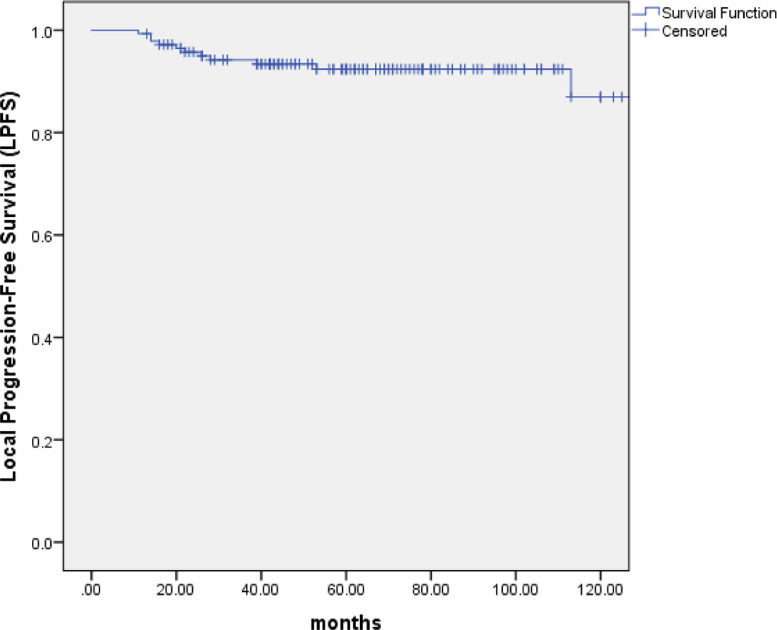
After a median follow-up of 67 months, the 5-year and 10-year overall survival (OS), progression-free survival (PFS), distant metastasis free survival (DMFS), local progression-free survival (LPFS) and regional progression-free survival (RPFS) were 75.7% and 61.6%, 61.2% and 53.4%, 73.1% and 72.1%, 92.4% and 87%, 88.9% and 81.8%, respectively. Multivariate analyses indicated that T stage (P = 0.001) appeared to be prognostic factors for OS. T stage (P = 0.001 and P = 0.002) and neck lymph node necrosis (P = 0.015 and P = 0.045) were independent predictors of PFS and DMFS. The acute toxicities were mainly grade 1/2 hematologic toxicities in patients treated with IC+IMRT+AC, and severe toxicities were uncommon.
Conclusions
IC followed by IMRT and AC achieved satisfactory long-term survival outcomes in NPC patients with N3 disease. Neck lymph node necrosis and late T stage served as predictors of poor prognosis for patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm of the epithelial tissue, which has unique epidemiologic and geographic distributions. Approximately 70% of newly diagnosed NPC cases are classified as locoregionally advanced disease. Radiotherapy (RT) plays an essential role in the treatment of NPC [1]. Data from several studies suggest that the addition of chemotherapy to RT significantly improves overall survival and progression-free survival compared to RT alone in advanced NPC. In the era of 2-dimensional radiotherapy (2DRT), concurrent chemoradiotherapy (CCRT) with/without adjuvant chemotherapy (AC) has been considered as the standard treatment due to the Intergroup 0099 trial and several subsequent studies [2], [3], [4]. However, in the Intergroup 0099 trial, up to 37% patients did not complete protocol CCRT because of high incidence of acute toxicity.
The past twenty years have seen increasingly rapid advances in the field of radiation technology, and intensity-modulated radiotherapy (IMRT) has become the standard RT technique of NPC due to its superiority in dosimetry. The role of CCRT for advanced NPC was not confirmed in the IMRT era to our knowledge. With the improved localregionally control, distant metastasis changes into the main failure in advanced NPC after IMRT [5,6], especially in the individuals with N3 NPC. How to control distant metastases is a continuing concern within more effective systemic therapy in the IMRT era. Induction chemotherapy (IC) may reduce tumor burden and eliminate potential micro-metastases. AC can reduce the subsequent occurrence of distant metastases. The combined analyses of NPC-9901 and NPC-9902 trials [7] demonstrated that additional AC contributed to the improvement of distant control. Du et al. [8] reported that induction–adjuvant chemotherapy obtained encouraging outcomes with well compliance in locoregionally advanced NPC. Sequential chemotherapy combined with RT might be an attractive alternative. The addition of IC and AC to RT might reduce distant metastasis in patients with N3 disease. Thus, a retrospective analysis of 143 patients with N3 NPC has been conducted. Moreover, this study aimed to analyze the long-term outcome of IC followed by IMRT and AC, and to identify the clinical features to further develop the stratification of N3 disease.
Materials and methods
Patients
From September 2005 to August 2016, 143 histologically diagnosed non-metastatic NPC patients with the 8th AJCC/UICC staging criteria N3 were enrolled in this study. All patients received sequential chemoradiotherapy (IC + IMRT + AC). Initial assessment consisted of medical history and physical examination, blood routine and biochemistry tests, enhanced magnetic resonance imaging (MRI) of the nasopharynx, enhanced MRI/CT of the neck, and nasopharyngoscopy. Other assessment included positron emission tomography-CT (PET-CT), or replaced by chest CT, abdominal ultrasound/CT and bone emission CT. Dental extraction, if deemed necessary, was performed before RT. All patients provided informed written consent before treatment.
Radiotherapy
All patients were treated with IMRT. The details of the tumor volume delineation have been described previously [9]. The prescribed dose given to primary tumor was 66 Gy in 30 fractions for T1 or T2 disease and 70.4 Gy in 32 fractions for T3 or T4 lesion (PTV-NX: GTV-NX +5 mm). A total dose of 66 Gy was given to the planned target volume of the lymph nodes (PTV-LN: GTV-LN +3 mm) in 30–32 fractions. The PTV-60 covering the high-risk CTV and a 5-mm margin was prescribed 60 Gy/30–32 F. The PTV-54 covering the low-risk CTV and a 5-mm margin was prescribed 54 Gy/30–32 F. Radiotherapy was given once daily, 5 fractions per week.
Chemotherapy
All patients received IC and AC with PF, TPF, TP or GP regimen. Generally, the IC/AC regimens for 2 cycles were delivered: PF (DDP 25 mg/m2 d1–3 + 5-FU 500 mg/m2 /d with 120-h infusion), TPF (docetaxel 60 mg/m2 d1+DDP 25 mg/m2 d1–3 + 5-FU 500 mg/m2 /d with 120-h infusion), TP (docetaxel 60 mg/m2 d1+DDP 25 mg/m2 d1–3) or GP (gemcitabine 1.0 g/m2 d1, d8+DDP 25 mg/m2 d1–3). The regimens were repeated every 21 days for IC and AC phase. AC stated at 28 days after the end of RT.
Complete blood count, and blood biochemical parameters were tested before each chemotherapy cycle. Chemotherapy would be postponed if the hematologic parameters of patients were disqualified. The dose of the following cycle would be reduced by 20% in case of grade 4 hematological toxicity.
Assessment and follow-up
Chemotherapy-related toxicities were graded by the National Cancer Institute Common toxicity criteria (NCI-CTC) version 5.0. Radiotherapy-related toxicities were assessed according to the Radiation Therapy Oncology Group (RTOG). Short-term response outcomes were evaluated as complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Short-term responses to treatment were assessed after 2 cycles of IC, IMRT and 3 months after IMRT. After treatment completion, follow-ups occurred every 3 months for the first 2 years, every 6 months from the third through the fifth year and annually thereafter. Physical examination, nasopharyngoscopy and imaging assessments were detailed in the previous study [9].
Statistical analysis
SPSS 23.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis in this study. The estimated overall survival (OS), progression-free survival (PFS), local progression-free survival (LPFS), regional progression-free survival (RPFS), and distant metastasis-free survival (DMFS) were calculated by the Kaplan–Meier method. Factors (P < 0.01) were included in a multifactor Cox model to determine the independent prognostic factors. The duration of survival was measured from the time of treatment until death or the date of the last follow up visit for patients alive. A 2-sided P < 0.05 was considered statistically significant.
Results
Patient characteristics
From September 2005 to August 2016, 143 non-metastatic NPC patients with the 8th AJCC/UICC staging criteria N3 were enrolled in this study. The median age was 48 years (range, 20–73 years). The study included 106 male (74.1%) and 37 female (25.9%). The numbers of patients with stage T1, T2, T3 and T4 disease were 27 (18.9%), 61 (42.7%), 36 (25.2%) and 19 (13.3%), respectively. The characteristics of patients and detailed features of neck lymph node included size, necrosis and side were shown in Table 1.
Table 1.
Characteristics of patients.
| Characteristic | No. of patients | Percent(%) |
|---|---|---|
| Age (years) | ||
| Median 48 Range 20–73 | ||
| ≤48 | 74 | 51.7 |
| >48 | 69 | 48.3 |
| Gender | ||
| Male | 106 | 74.1 |
| Female | 37 | 25.9 |
| KPS Score | ||
| 90 | 73 | 51 |
| 80 | 56 | 39.2 |
| 70 | 14 | 9.8 |
| T Stage | ||
| 1 | 27 | 18.9 |
| 2 | 61 | 42.7 |
| 3 | 36 | 25.2 |
| 4 | 19 | 13.3 |
| Neck Lymph Node Size (cm) | ||
| Median 6 Range 1–10 | ||
| ≤6 | 78 | 54.5 |
| >6 | 65 | 45.5 |
| Neck Lymph Node Necrosis | ||
| with | 72 | 50.3 |
| without | 71 | 49.7 |
| Neck Lymph Node Side | ||
| Ipsilateral | 63 | 44.1 |
| Contralateral | 3 | 2.1 |
| Bilateral | 77 | 53.8 |
Treatment and compliance
All patients received IC and AC with PF, TPF, TP or GP regimen. For the cases with IC, 95.1% patients completed two cycles and 3 patients only completed one cycle because of severe bone marrow suppression, liver function damage and severe vomiting. Thirty-four patients (23.8%) did not receive AC after IMRT, of whom the main reasons were the diseases evaluated as CR (nasopharynx 91.2% and lymph node 67.6%) at the end of RT. Others reasons were bone marrow suppression and refusal of chemotherapy by patients. All 143 patients completed radical radiotherapy (IMRT). The overall response rate (CR and PR) of nasopharynx and lymph node to IC and 3 months after the completion of the treatment were 82.7% and 83.1%, 98.6% and 98.6%, respectively. Treatment summary of patients were listed in Table 2.
Table 2.
Treatment summary of patients.
| Treatment summary | No. of patients | Percent(%) |
|---|---|---|
| IC+IMRT+AC | ||
| PF | 19 | 13.3 |
| TPF | 87 | 60.9 |
| TP | 4 | 2.8 |
| GP | 33 | 23.1 |
| Chemotherapy cycles (IC) | ||
| 1 | 3 | 2.1 |
| 2 | 136 | 95.1 |
| 3 | 4 | 2.8 |
| Chemotherapy cycles (AC) | ||
| 0 | 34 | 23.8 |
| 1 | 29 | 20.3 |
| 2 | 80 | 55.9 |
Survival and prognostic analysis
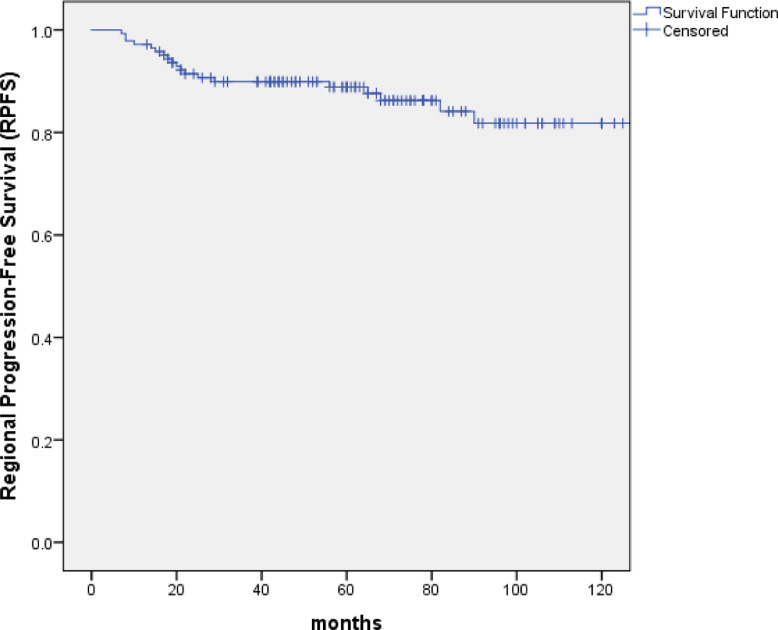
With the median follow-up of 67 months (range: 10 to 154 months), 99 patients (69.2%) were alive, of whom 82 were disease free, 8 were distant metastasis alone, 7 were recurrence in regional lymph nodes and 2 were recurrence in nasopharynx and regional lymph nodes. At the last follow-up visit, a total of 44 patients died: 23 patients died of distant metastasis, 4 of distant metastases accompanied by recurrence in the regional lymph nodes, 1 of distant metastases accompanied by recurrence in nasopharynx, 2 of distant metastases accompanied by recurrence in nasopharynx and regional lymph nodes, 2 of recurrence in nasopharynx and regional lymph nodes, 3 of recurrence in nasopharynx, 2 of recurrence in regional lymph nodes, 1 of non-neoplastic disease, 2 of second tumor and 4 of unknown reasons. The 5-year and 10-year OS, PFS and DMFS rates were 75.7% and 61.6%, 61.2% and 53.4%, 73.1% and 72.1%, respectively. (Figs. 1–3) The 5-year and 10-year LPFS and RPFS rates were 92.4% and 87%, 88.9% and 81.8%, respectively. (Figs. 4 and 5)
Fig. 2.
Kaplan–Meier estimate of progression-free survival (PFS) curve for all the patients.
Fig. 1.
Kaplan–Meier estimate of overall survival (OS) curve for all the patients.
Fig. 3.
Kaplan–Meier estimate of distant metastasis free survival (DMFS) curve for all the patients.
Fig. 4.
Kaplan–Meier estimate of local progression-free survival (LPFS) curve for all the patients.
Fig. 5.
Kaplan–Meier estimate of regional progression-free survival (RPFS) curve for all the patients.
Impact of prognostic factors on OS, PFS, DMFS, RPFS and LPFS were evaluated using univariate and multivariate analyses, including age, gender, KPS, T stage, neck lymph node size, neck lymph node necrosis and neck lymph node side. Univariate analyse revealed that age (P = 0.013) and T stage (P = 0.000) were the two factors that significantly influenced OS. T stage (P = 0.001) and neck lymph node necrosis (P = 0.024) were the two factors to significantly impact PFS. T stage (P = 0.001) was the factor that significantly influenced DMFS. (Tables 3) The 5-year and 10-year RPFS of T4 compared with those of T1–3 were 82.5% vs. 93.8%, 82.5% vs. 88.0%, respectively. The 5-year and 10-year LPFS with or without neck lymph node necrosis were 84.9% vs. 92.8%, 73.5% vs. 90.3%, respectively .
Table 3.
Univariate analysis of prognostic factors.
| Characteristic | OS | PFS | DMFS | |||
|---|---|---|---|---|---|---|
| P value | HR(95%CI) | P value | HR(95%CI) | P value | HR(95%CI) | |
| Age (≤48/>48) | 0.013 | 2.173 (1.174–4.019) |
0.070 | 1.600 (0.963–2.659) |
0.502 | 1.244 (0.658–2.352) |
| Gender (Male/Female) | 0.090 | 0.497 (0.221–1.115) |
0.148 | 0.627 (0.333–1.179) |
0.142 | 0.520 (0.217–1.245) |
| KPS (70 and 80/90) | 0.155 | 0.643 (0.350–1.182) |
0.494 | 0.839 (0.506–1.389) |
0.617 | 0.850 (0.449–1.607) |
| T stage (T1–3/T4) | 0.000 | 3.501(1.828–6.708) | 0.001 | 2.821(1.568–5.074) | 0.001 | 3.286 (1.626–6.641) |
| Neck Lymph Node Size (≤6/>6) |
0.245 | 0.697 (0.380–1.281) |
0.560 | 0.860 (0.517–1.429) |
0.342 | 0.729 (0.380–1.398) |
| Neck Lymph Node Necrosis (without/with) | 0.053 | 1.835 (0.993–3.392) |
0.024 | 1.817 (1.082–3.051) |
0.077 | 1.812 (0.937–3.504) |
| Neck Lymph Node Side (Contralateral and Bilateral /Ipsilateral) | 0.748 | 0.906 (0.498–1.649) |
0.413 | 0.807 (0.482–1.349) |
0.649 | 1.159 (0.613–2.192) |
Multivariate analyses indicated that T stage (P = 0.001) appeared to be prognostic factors for OS. T stage (P = 0.001 and P = 0.002) and neck lymph node necrosis (P = 0.015 and P = 0.045) were independent predictors of PFS and DMFS. (Tables 4)
Table 4.
Independent prognostic factors by multivariate analyses.
| Endpoint | Factor | P value | HR(95%CI) |
|---|---|---|---|
| OS | Age (≤48/>48) | 0.105 | 1.700 (0.896–3.226) |
| Gender (Male/Female) | 0.264 | 0.625 (0.273–1.427) | |
| T stage (T1–3/T4) | 0.001 | 3.165 (1.614–6.206) | |
| Neck Lymph Node Necrosis (without/with) | 0.060 | 1.819 (0.975–3.393) | |
| PFS | Age (≤48/>48) | 0.246 | 1.358 (0.809–2.280) |
| T stage (T1–3/T4) | 0.000 | 2.920 (1.599–5.333) | |
| Neck Lymph Node Necrosis (without/with) | 0.015 | 1.925 (1.138–3.253) | |
| DMFS | T stage (T1–3/T4) | 0.000 | 3.525 (1.738–7.151) |
| Neck Lymph Node Necrosis (without/with) | 0.046 | 1.964 (1.012–3.809) |
Toxicities
There were no treatment-related deaths. The acute toxicities were mainly grade 1/2 hematologic toxicities in patients treated with IC+IMRT+AC, and severe toxicities were uncommon. During the RT phase, mucositis and weight loss were the most common acute treatment toxicities in our study. Among all the patients, 16 (11.2%), 94 (65.7%), 31 (21.7%), and 2 (1.4%) had grade 1, 2, 3, and 4 mucositis, respectively. Seventy-six patients needed intravenous nutritional support for mucosal reaction, and the median duration was 3 days (range: 2–21days). None of these patients required tube feeding support. A total of 137 patients suffered weight loss, while the median weight loss was 8.2% in all patients.
Overall, most late injuries were assessed as grades 0–1. Four patients had cranial nerve palsy, and the possibility of recurrent disease was excluded by a series of MRI scans and physical examination. Only one patient suffered osteomyelitis 51 months after the end of RT. Five patients had the second primary tumor, of whom three were lung cancer. No cases of radiation-induced temporal necrosis were observed.
Discussion
In this study, we retrospectively evaluated long-term outcomes of IC followed by IMRT and AC in NPC patients with N3 disease. With a median follow-up of 67 months, the 5-year and 10-year OS, PFS and DMFS were 75.7% and 61.6%, 61.2% and 53.4%, 73.1% and 72.1%, respectively. The 5-year and 10-year LPFS and RPFS rates were 92.4% and 87%, 88.9% and 81.8%, respectively. IC followed by IMRT and AC achieved satisfactory survival outcomes in NPC patients with N3 disease, and severe toxicities were uncommon.
CCRT with or without AC has been deemed the standard treatment for locally advanced NPC for years [2,[10], [11], [12]]. However, due to the high incidence of acute toxicity, the tolerance of concurrent chemotherapy was unsatisfied. Recently, IC has been frequently used in clinical practice for locoregionally advanced NPC, especially in endemic areas with plenty of patients waiting for RT. Liu et al. [13] showed that compared with CCRT, IC-RT achieved similar long-term survivals but significantly reduced severe acute toxicities (grade 3–4). Individuals with N3 NPC are at higher risk for distant metastasis. Therefore, more effective systemic treatments are needed to reduce distant metastasis. Sequential chemotherapy combined with RT might be an attractive alternative. The addition of IC and AC to RT might reduce distant metastasis in patients with N3 disease. A retrospective study [14] from Zhejiang Cancer Hospital analyzed 110 patients with N3 NPC. All patients received IMRT and various chemotherapy. 95 patients received IC, 103 patients received CCRT and 53 patients received AC. The 5-year OS of the patients were 70.53%. Although the CCRT was not used in our research, the 5-year OS was 75.7% higher than 70.53%.
Late T stage served as a predictor of poor prognosis for patients. Similarly, Wu et al. [15] retrospectively evaluated the 10-year survival outcomes for 614 patients with NPC receiving IMRT. The 10-year local relapse-free survival rates for T1, T2 and T3 were 94.2%, 92.5% and 91.4% (P > 0.05), respectively, and significantly higher than that of T4 disease (79.3%, P < 0.05 for all rates). Lin et al. [16] conducted survival impacts of different T-classification in N3 NPC patients. They found that patients with T4, compared with those of T1–3 have worse OS, DFS, LRFFS and DMFFS. In our study, patients with T4, compared with those of T1–3 have worse RPFS (5-year rates, 82.5% vs. 93.8%, 10-year rates, 82.5% vs. 88.0%, P = 0.099). Compared with 79.3% in Wu's research, the 10-year RPFS of T4 stage was 82.5% in our research. Even in the T4 stage, IC followed by IMRT and AC achieved satisfactory long-term local control.
Neck lymph node necrosis served as another predictor of poor prognosis for NPC patients. It was found that cervical lymph node necrosis was an independent negative prognostic factor for NPC patients in Lan's study [17]. Additionally, Feng et al. [18] suggested that N stage should be further upgraded in patients with cervical lymph node necrosis. Furthermore, 757 patients confirmed NPC were retrospectively examined to assess the impact of tumor necrosis on treatment sensitivity and long-term survival. Multivariate analyses indicated that necrosis of the total tumor was an independent predictor of OS, FFS, DMFS, and LRRFS [19]. In N3 disease of NPC patients in the present research, the incidence rates of neck lymph node necrosis reached up to 50.3%. Patients with neck lymph node necrosis had worse LPFS (5-year rates, 84.9% vs. 92.8%, 10-year rates, 73.5% vs. 90.3%, P = 0.091). Multivariate analyses indicated that neck lymph node necrosis (P = 0.015 and P = 0.045) were independent predictors of PFS and DMFS.
IMRT was related to less severe physician-assessed toxicities compared with 2D or 3DRT [20,21]. In a retrospective study of 3328 NPC patients from 6 public hospitals in Hong Kong over a 10-year period, a small number of patients had late adverse reactions as follows: hearing loss requiring hearing aids (7.1%), cranial nerve palsies (5.1%), dysphagia requiring tube feeding for a long period (3%), and symptomatic temporal lobe necrosis (0.9%) [22]. In our cohort, no treatment-related deaths were observed. The acute toxicities were mainly grade 1/2 hematologic toxicities. Most late injuries were assessed as grades 0 to 1. Four patients had cranial nerve palsy and one patient suffered osteomyelitis 51 months after the end of RT. No cases of radiation-induced temporal necrosis were observed.
Conclusion
IC followed by IMRT and AC achieved satisfactory long-term survival outcomes and high locoregional control. Individuals with N3 NPC are at higher risk for distant metastasis. Neck lymph node necrosis and late T stage served as predictors of poor prognosis for patients. Patients with neck lymph node necrosis and T4N3 diseases have extremely poor outcome and more aggressive treatment options should be further studied.
Author Contributions Statement
XN, FX, and XH are responsible for the conception and design of the study. XN and XH collected and assembled the data. FX and PL did data analysis and interpretation. CH and XH provided administrative support. All authors contributed to manuscript writing and approved the final manuscript.
Declaration of Competing Interest
No conflicts of interest to disclose.
Funding Sources
This work was supported by the Shanghai Anticancer Association EYAS PROJECT (grant no: SACA-CY20C06), the Shanghai Sailing Program (grant no: 21YF1408400) and institutional grant of Fudan University Shanghai Cancer Center (grant no: YJQN202023).
References
- 1.Chen Y.P., Chan A., Le Q.T. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sarraf M., LeBlanc M., Giri P.G. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998;16(4):1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 3.Wee J., Tan E.H., Tai B.C. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J. Clin. Oncol. 2005;23(27):6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 4.Lee A.W., Tung S.Y., Chua D.T. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J. Natl. Cancer Inst. 2010;102(15):1188–1198. doi: 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 5.Ou X., Zhou X., Shi Q. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget. 2015;6(35):38381–38397. doi: 10.18632/oncotarget.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng W.T., Corry J., Langendijk J.A. Current management of stage IV nasopharyngeal carcinoma without distant metastasis. Cancer Treat. Rev. 2020;85 doi: 10.1016/j.ctrv.2020.101995. [DOI] [PubMed] [Google Scholar]
- 7.Lee A.W., Tung S.Y., Ngan R.K. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur. J. Cancer. 2011;47(5):656–666. doi: 10.1016/j.ejca.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Du C., Ying H., Zhou J. Experience with combination of docetaxel, cisplatin plus 5-fluorouracil chemotherapy, and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Int J. Clin. Oncol. 2013;18(3):464–471. doi: 10.1007/s10147-012-0403-y. [DOI] [PubMed] [Google Scholar]
- 9.Wu M., He X., Hu C. Intensity-modulated radiotherapy combined with sequential cisplatin and fluorouracil chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Medicine. 2018;97(50):e13361. doi: 10.1097/MD.0000000000013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchard P., Lee A., Marguet S. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee A.W., Lau W.H., Tung S.Y. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J. Clin. Oncol. 2005;23(28):6966–6975. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 12.Lin J.C., Jan J.S., Hsu C.Y. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J. Clin. Oncol. 2003;21(4):631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y.C., Wang W.Y., Twu C.W. Comparison long-term outcome of definitive radiotherapy plus different chemotherapy schedules in patients with advanced nasopharyngeal carcinoma. Sci. Rep. 2018;8(1):470. doi: 10.1038/s41598-017-18713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J., Liu T., Sun Q. Clinical and prognostic analyses of 110 patients with N3 nasopharyngeal carcinoma. Medicine. 2018;97(49):e13483. doi: 10.1097/MD.0000000000013483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L.R., Liu Y.T., Jiang N. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol. 2017;69:26–32. doi: 10.1016/j.oraloncology.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Lin T.Y., Lan M.Y., Tsou H.H. Survival impacts of different nodal characteristics and T-classification in N3 nasopharyngeal carcinoma patients. Oral Oncol. 2020;108 doi: 10.1016/j.oraloncology.2020.104820. [DOI] [PubMed] [Google Scholar]
- 17.Lan M., Huang Y., Chen C.Y. Prognostic value of cervical nodal necrosis in nasopharyngeal carcinoma: analysis of 1800 patients with positive cervical nodal metastasis at MR imaging. Radiology. 2015;276(2):536–544. doi: 10.1148/radiol.15141251. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y., Cao C., Hu Q. Prognostic value and staging classification of lymph nodal necrosis in nasopharyngeal carcinoma after intensity-modulated radiotherapy. Cancer Res. Treat. 2019;51(3):1222–1230. doi: 10.4143/crt.2018.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S.B., Chen L.S., Yang X.L. Influence of tumor necrosis on treatment sensitivity and long-term survival in nasopharyngeal carcinoma. Radiother. Oncol. 2021;155:219–225. doi: 10.1016/j.radonc.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Huang T.L., Chien C.Y., Tsai W.L. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck. 2016;(38 Suppl 1):E1026–E1032. doi: 10.1002/hed.24150. [DOI] [PubMed] [Google Scholar]
- 21.Chan J.W., Parvathaneni U., Yom S.S. Reducing radiation-related morbidity in the treatment of nasopharyngeal carcinoma. Future Oncol. 2017;13(5):425–431. doi: 10.2217/fon-2016-0410. [DOI] [PubMed] [Google Scholar]
- 22.Au K.H., Ngan R., Ng A. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study) Oral Oncol. 2018;77:16–21. doi: 10.1016/j.oraloncology.2017.12.004. [DOI] [PubMed] [Google Scholar]