Summary
Nitrile (C≡N bond) activation for direct organic synthesis has been less explored so far due to a high redox potential of nitrile and its low dissociation energy of C−CN bond. Herein, we demonstrate a direct reductive coupling of nitriles and 1,2-phenylenediamines to yield various benzimidazoles in excellent yields (95%–99%) by using rhodium phosphide (Rh2P) catalyst supported on lignin-derived carbon (LC) using H2 (or hydrazine hydrate) as a hydrogen source. The high catalytic performance of Rh2P/LC is attributed to enhanced charge transfer to Rh and strong P−Rh interactions. Our isotope trace experiment confirms the presence of H/D exchange between H2 and the inert –CD3 group of CD3CN via an intramolecular D-shift. Reusability of Rh2P/LC is further demonstrated by a seven-time recycling without evident loss of activity. This research thus highlights a great potential in organic transformation with nitrile as a synthetic building block.
Subject areas: chemistry, inorganic chemistry, catalysis, organic chemistry, organic synthesis
Graphical abstract

Highlights
-
•
Nitrile was developed as synthetic building block for organic synthesis
-
•
Reductive coupling of nitriles to 1,2-phenylenediamines yielded benzimidazoles
-
•
Strong P−Rh interaction and charge transfer to Rh enhanced Rh2P activity
-
•
H/D exchange between H2 and –CD3 in CD3CN occurred via intramolecular D-shift
Chemistry; Inorganic chemistry; Catalysis; Organic chemistry; Organic synthesis
Introduction
Benzimidazole and its derivatives are pharmaceutically important heterocyclic compounds with a broad range of biological activities and pharmacological properties such as anti-viral, anti-fungal, anti-bacterial, anti-ulcer, anti-inflammatory, anti-hypertensive, anti-histaminic, anti-cancer, anti-tumor, and anti-HIV features (Chakrabarti et al., 2019; Keri et al., 2015). Their synthetic methods have received extensive attention due to their pharmaceutical importance. Benzimidazoles are traditionally prepared according to Ladenburg ring closure method (Scheme 1A) by direct condensation of 1,2-phenylenediamines (1) with carboxylic acids and their derivatives such as acids, acyl chlorides, anhydrides, aldehydes, amides and nitriles in the presence of strong acid at high reaction temperature. Among these carboxylic acid derivatives, due to a facile access to nitriles and their high availability as commodity chemicals, direct condensation of 1 with nitriles should have great potential in synthetic chemistry of benzimidazoles and in the productions of benzimidazole-related agrochemicals and pharmaceuticals (Dalziel et al., 2018; Le Questel et al., 2000; Tamura et al., 2013).
Scheme 1.
Methods for benzimidazole syntheses
(A) Traditional condensation.
(B) Present reductive coupling.
(C) Comparison of condensation and coupling method.
Unfortunately, research on direct condensation of 1 and nitriles is very limited. According to the reported results (Hölljes and Wagner, 1944), 27% yield of 2-methylbenzimidazole (3a) was obtained from 1,2-phenylenediamine (1a) and acetonitrile (2a) in the presence of an equivalent of anhydrous hydrogen chloride (HCl) in a sealed tube at 200°C for six hours (Scheme 1C). The corresponding condensation reaction mechanism suggests an initial formation of highly reactive ammonoacyl chloride (4, Scheme 2A) from nitrile (2) and HCl under anhydrous conditions. The in situ formed 4 smoothly promotes subsequent ring closure to yield benzimidazoles (3). Therefore, nitrile activation, via additively uniting with HCl (4, Scheme 2A) at 200°C, is a prerequisite for the condensation reaction. However, nitrile (C≡N bond) activation has been less explored so far when compared with C=C, C=O, C=N and O−N=O bonds owing to the high redox potential of nitriles and the low dissociation energy of C−CN bond.
Scheme 2.
Proposed reaction mechanism for 3 formation
(A) HCl-induced condensation.
(B) transition-metal-promoted reductive coupling.
Most recently, nitrile hydrogenation was developed for atom-economic synthesis of amine with transition-metal-based catalysts (Bagal and Bhanage, 2015; Werkmeister et al., 2014; Liu et al., 2018a; Nandi et al., 2017) (Table S1). However, a crucial selectivity issue arises from an inevitable formation of mixtures of primary (7), secondary (9) and tertiary amines (10) with alkylimines (6) and dialkylimines (8) as the proposed reactive intermediates (Scheme 2B). High reactivity of these reaction intermediates (6 and 8) competitively induces a series of parallel and consecutive reactions, resulting in a challenge of selectivity control and product separation. Generally, nitrile hydrogenation can be performed under relatively mild reaction conditions (20–140°C). We thus think that the presence of 1 in the nitrile-hydrogenation system should be able to trap the in situ formed two reactive imines intermediates (6 and 8) to give N-(o-aminopheny)-imine intermediate (11, Scheme 2B). A subsequent cyclization of 11 and successive dehydrogenation of the resulting ring-closing product (12, Scheme 2B) should yield 3 under the reaction conditions (Scheme 2B). Therefore, a transition-metal-promoted reductive coupling of nitriles and 1,2-phenylenediamines was investigated in this research for green and atom-economic synthesis of benzimidazoles (Scheme 2B).
As shown in Scheme 2B, the reductive coupling process should be initialized from catalytic hydrogenation of nitriles. Both heterogeneous and homogeneous catalysts were reported for nitriles hydrogenation. Precious metal-complex-based homogeneous catalysts evidently show excellent catalytic performance on the hydrogenation (Bagal and Bhanage, 2015; Werkmeister et al., 2014; Chakraborty and Berke, 2014; Hou et al., 2020; Islam et al., 2010; Liu et al., 2018b; Chakraborty and Milstein, 2017). Although the developed heterogeneous catalysts are very limited and generally suffer from low activity, low selectivity, and low tolerance to functional group when compared with homogeneous catalysts (Wang et al., 2019; Huang and Sachtler, 1999a, 1999b; Zhang et al., 2019; Monguchi et al., 2017; Li et al., 2012; Carothers and Jones, 1925; Braos-García et al., 2010). Efforts to explore efficient catalytic systems with excellent recyclability have always been going on for industrial purpose.
Rhodium-phosphine complexes are highly efficient for nitrile hydrogenation among various investigated homogeneous catalysts. While, rhodium phosphide (Rh2P) crystal shows surface Rh atoms surrounded by two coordinated P atoms, which is sterically and structurally similar to the Rh-P interactions in bisphosphine ligand-modified Rh complexes. Moreover, integrating P atoms into the lattices of Rh metal can tune its internal electronic structure, thus improving the intrinsic catalytic activity of the resulting Rh2P catalyst (Shi and Zhang, 2016; Zhuang et al., 2016). Currently, Rh2P is developed as an excellent heterogeneous catalyst for hydrogenation, hydrodeoxygenation, hydroformylation, hydrodesulfurization, and hydrodenitrogenation (Luo et al., 2020; Griffin et al., 2017; Alvarado Rupflin et al., 2017; Hayes et al., 2010).
Herein, we demonstrated the first example of a reductive coupling of nitriles and 1,2-phenylenediamines to 2-alkylbenzimidazoles (Scheme 1B) by using Rh2P catalyst (Rh2P/LC) supported on lignin-derived hierarchically porous carbon (LC, Figure 1). The lignin was selected as precursor of the catalyst support due to its unique structure of three-dimensional and porous framework, rich in carbon-oxygen-based functional groups on the framework, scalable and renewable nature as a carbon-based feedstock (Zhu et al., 2019; Chatterjee and Saito, 2015). The developed Rh2P/LC demonstrates high efficiency by coupling serial tandem reactions of nitrile hydrogenation, 11 cyclization and 12 dehydration in one-pot (Scheme 2B). In contrast to traditional condensation method (Feng et al., 2016; Wade et al., 2015; Shiraishi et al., 2010; Wang et al., 1997; Selvam et al., 2009) (Table S2), Rh2P/LC promoted reductive coupling of 1a and 2a can readily perform at 140°C with >99% yield of 3a by using H2 or hydrazine hydrate (N2H4⋅H2O) as hydrogen sources (Scheme 1C).
Figure 1.
Rh2P/LC-promoted reductive coupling 1a with 2a
Results and discussion
Catalyst preparation and characterization
In this research, LC was used as catalyst support, which was obtained by calcination a mixture of enzymatic hydrolysis lignin (EHL) and potassium bicarbonate (KHCO3) at 800°C under atmospheric N2, followed by thoroughly leaching with aqueous HCl solution. While, Rh2P/LC400 catalyst was prepared by co-loading RhCl3 and bis(diphenylphosphino)ethane (dppe) ligand on the resulting LC surface, followed by a pyrolysis at 400°C under atmospheric H2/N2. The subscript 400 in Rh2P/LC400 indicates the final calcination temperature for catalyst preparation. The introduced dppe ligand provides P source for the formation of Rh2P species on the LC support during calcination procedure. For comparison, Rh catalyst (Rh/LC400) supported on LC was prepared without addition of the dppe ligand to investigate the electronic effect of the introduced P on the catalytic performance of the resulting Rh2P catalyst. Moreover, to understand the influence of metallic site on the reductive coupling, Pd (Pd/LC400) and Ru (Ru/LC400) catalysts were synthesized with same method to Rh2P/LC400; however, the expected metal phosphides of Pd-P and Ru-P samples were undetected on the resulting catalyst surfaces.
The morphology of the obtained samples was initially characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). EHL shows a bulky, solid, and compact architecture with irregular shapes and rough surface based on the SEM analysis (Figure S1A). While, the resulting LC exhibits a sponge-like structure with readily accessible, highly crosslinked and randomly opened macropores (Figures S1B and S1C). After Rh2P loading, the obtained Rh2P/LC400 possesses similar SEM micrograph to that of LC (Figure S1D). The TEM of Rh2P/LC400 exhibits ultrathin carbon nanosheet-assembled three-dimensional (3D) network with a crumpled, wrinkled, and rippled structure (Figure 2A). Additionally, Rh, P, and C elements are homogeneously and highly dispersed on the detected area of LC surface as shown by the TEM energy-dispersive X-ray (EDX) images of Rh2P/LC400 (Figure 2M–2P). The estimated average nanoparticle size of Rh2P was 4.3 nm (Figure 2B) with a detectable crystal fringe spacing of 0.280 nm, corresponding to the (200) crystal plane of Rh2P (Su et al., 2020) (Figure 2C).
Figure 2.
TEM and TEM-EDX characterizations
(A–C) TEM of Rh2P/LC400.
(D–F) TEM of Rh/LC400.
(G–I) TEM of Pd/LC400.
(J–L) TEM of Ru/LC400.
(M–P) TEM-EDX mapping of Rh2P/LC400. Inserts of (B), (E), (H), (K) show the size distribution histogram by statistical analysis of 200 corresponding nanoparticles.
In the case of Rh/LC400, its TEM images reveal a mean Rh nanoparticle size of 7.4 nm (Figures 2D and 2E) with lattice fringe spacing of 0.220 nm (Figure 2F), which can be indexed to the (111) plane of the Rh (Su et al., 2020). The TEM images of Pd/LC400 indicate a significantly increased average size to 18.3 nm for Pd nanoparticle on the LC support (Figures 2G and 2H). The observed lattice fringe spacing was detected as 0.224 nm, belonging to the (111) plane of Pd (Sun et al., 2020) (Figure 2I). Finally, for Ru/LC400 sample, TEM images show an average nanoparticle size of 3.3 nm (Figures 2J and 2K) with the lattice fringe spacing around 0.214 nm, corresponding to the (002) plane of Ru (Wang et al., 2020) (Figure 2L). Therefore, the average nanoparticle size decreased in the order of Pd > Rh > Rh2P > Ru among the investigated samples. Moreover, Rh2P/LC400 exhibits much smaller size of Rh nanoparticle with more homogeneous and more uniform dispersion if compared with Rh/LC400 (Figures 2A–2F). The observed porous architecture of Rh2P/LC400 should be favorable for mass transfer and diffusion in the investigated hydrogenative coupling reaction.
The textural properties of the developed samples were investigated with N2 sorption isotherm (Figure 3A), Table 1 lists the resulting results. LC support exhibits a steep rise at low P/P0 zone (P/P0 < 0.1) with a very weak hysteresis loop from middle to high P/P0 zone (Figure 3A). Therefore, LC has a micropore-prevailing and hierarchically micro-mesoporous morphology (Deng et al., 2015) (Figure S2) showing a specific Brunauer-Emmet-Teller (BET) surface area around 1,664 m2 g−1. After loading with transition metal, the resulting metallic catalyst exhibits a significantly reduced specific surface area (Table 1). For example, Rh2P/LC400 exhibits a specific surface area of 724 m2 g−1 (Table 1).
Figure 3.
N2 sorption and XRD characterizations
(A) N2 adsorption-desorption isotherm.
(B) XRD patterns.
Table 1.
Textural parameters of the investigated samples
| Sample | SBETa [m2 g−1] | Smicrob [m2 g−1] | Smesoc [m2 g−1] | Dmicro/Dmesod [nm] | Vtotale [cm3 g−1] | Vmicro/Vmesof [cm3 g−1] |
|---|---|---|---|---|---|---|
| LC | 1664 | 1320 | 344 | 0.78/2.27 | 1.20 | 0.55/0.65 |
| Rh2P/LC400 | 724 | 176 | 548 | 1.02/4.02 | 0.45 | 0.08/0.37 |
| Rh2P/LC600 | 505 | 178 | 327 | 1.03/4.01 | 0.62 | 0.08/0.54 |
| Rh2P/LC800 | 696 | 232 | 464 | 1.01/4.00 | 0.71 | 0.11/0.60 |
| Rh/LC400 | 1156 | 484 | 672 | 0.85/3.11 | 0.69 | 0.21/0.48 |
| Pd/LC400 | 630 | 135 | 495 | 0.85/3.08 | 0.41 | 0.12/0.29 |
| Ru/LC400 | 567 | 274 | 293 | 0.85/3.10 | 0.37 | 0.10/0.27 |
| recovered Rh2P/LC400 | 426 | 195 | 231 | 0.60/2.05 | 0.33 | 0.10/0.23 |
| recovered Rh/LC400 | 797 | 325 | 472 | 1.01/3.99 | 0.57 | 0.16/0.41 |
SBET, specific surface area.
Smicro, the specific surface area of micropore.
Smeso, the specific surface area of mesopore.
Dmicro/Dmeso, the average diameters of micropore (Dmicro) and mesopore (Dmeso).
Vtotal, the total specific pore volume.
Vmicro/Vmeso, the specific pore volume of micropore (Vmicro) and mesopore (Vmeso).
All of the obtained samples were then performed with X-ray diffraction (XRD) analysis. Rh2P/LC400 shows a broad diffraction peak at 2θ = 21.0° (Figure 3B), which is indexed to the diffraction peak from amorphous carbon. In addition, five characteristic peaks at 2θ = 32.5, 46.8, 58.0, 68.2, and 77.8° are respectively assigned to the (200), (220), (222), (400), and (420) planes of Rh2P (JCPDF file no. 02-1299), suggesting the presence of Rh2P crystal on the LC surface (Duan et al., 2017). In the case of Rh/LC400, three representative diffraction peaks at 2θ values of 41.1, 47.8 and 69.9° (Figure 3B) are individually indexed to the (111), (200), and (220) planes of metallic Rh (JCPDF file no. 05-0685) (Kundu et al., 2018), which indicates successful loading of metallic Rh on the LC surface.
Pd/LC400 displays three characteristic peaks at 2θ = 40.2, 46.7, and 68.2° (Figure 3B), which are respectively assigned to the (111), (200), and (220) planes of metallic Pd (JCPDF file no. 46-1043). Therefore, Pd(0) crystals, rather than palladium phosphide, are suggested to be deposited on the LC surface for the obtained Pd/LC400 (Li et al., 2016). Notably, Ru/LC400 only displays a weak characteristic peak at 2θ = 44.2° (Figure 3B), corresponding to the crystalline phases of Ru (JCPDF file no. 06-0063). This observation can presumably be attributed to the highly dispersed and homogeneous Ru species with small particle size on the LC surface (Zou et al., 2019).
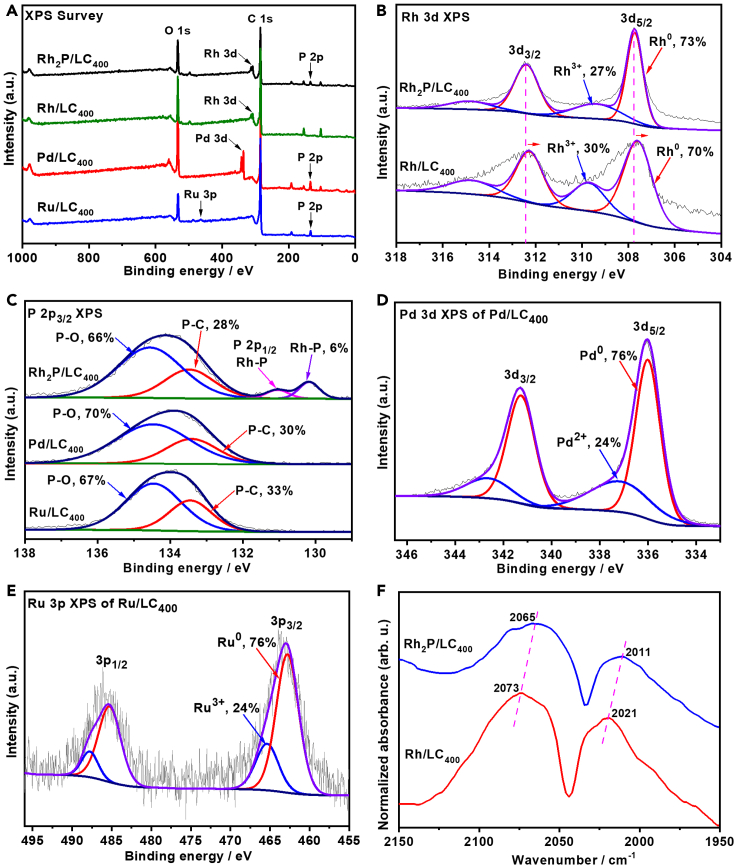
Surface elements and their chemical states of the obtained samples were further examined by X-ray photoelectron spectroscopy (XPS). For Rh2P/LC400, the presence of Rh, P, C, and O elements is confirmed in the XPS survey (Figure 4A and Tables S3–S7). The high-resolution Rh 3d XPS of Rh2P/LC400 can be generally deconvolved into two sets of doublet peaks (Figure 4B). The set of strong doublet peaks, located at binding energies of 312.4 eV (indexed to Rh 3d3/2) and 307.7 eV (indexed to Rh 3d5/2), can be ascribed to metallic Rh, corresponding to Rh2P species (Luo et al., 2020). The other set of weak doublet peaks at 314.8 eV (indexed to Rh 3d3/2) and 309.8 eV (indexed to Rh 3d5/2) are ascribed to the Rh(III) oxidation state (Luo et al., 2020). Rh2P (73%) is formed as the predominant species on the Rh2P/LC400 surface based on the integration areas of these two doublets, accordingly demonstrating successful formation of Rh2P catalyst from Rh-dppe complex under the synthetic conditions. High-resolution P 2p3/2 XPS of Rh2P/LC400 (Figure 4C) can be deconvoluted into three characteristic peaks at the binding energy of 134.5 eV (indexed to P−O), 133.3 eV (indexed to P−C), and 130.1 eV (indexed to Rh−P). The presence of P−O species presumably originates from oxidation of the surface P upon exposure to air, whereas the existence of P−C indicates the formation of doped P atom into the LC matrix (Chen et al., 2016). Finally, the formation of Rh2P species can be demonstrated by the presence of Rh−P species from the P 2p3/2 XPS of Rh2P/LC400. Therefore, our XPS analysis of Rh2P/LC400 is in accordance with its TEM and XRD results, confirming the major Rh2P species on the Rh2P/LC400 surface.
Figure 4.
XPS and CO-probed FT-IR characterizations
(A) XPS scan survey of Rh2P/LC400, Rh/LC400, Pd/LC400 and Ru/LC400.
(B) Rh 3d XPS of Rh2P/LC400 and Rh/LC400.
(C) P 2p3/2 XPS of Rh2P/LC400, Pd/LC400 and Ru/LC400.
(D) Pd 3d XPS of Pd/LC400.
(E) Ru 3p XPS of Ru/LC400.
(F) CO-probed FT-IR spectra for Rh2P/LC400 and Rh/LC400.
In the case of Rh/LC400, its high-resolution Rh 3d XPS can be deconvolved into two sets of doublet peaks (Figure 4B). The set of strong doublet peaks, located at binding energies of 312.3 eV (indexed to Rh 3d3/2) and 307.6 (indexed to Rh 3d5/2), can be ascribed to metallic Rh as a prevailing species (70%), whereas the set of weak doublet peaks at 314.7 eV (indexed to Rh 3d3/2) and 309.7eV (indexed to Rh 3d5/2) are ascribed to the Rh(III) oxides. Notably, Rh2P/LC400 shows evident shifts to higher binding energies in the Rh 3d XPS if compared with those of Rh/LC400 (Figure 4B). The observed positive shifts in the Rh 3d XPS indicate enhanced charge transfer to Rh as well as stronger interactions between P and Rh in the Rh2P/LC400 sample (Su et al., 2020). The Rh-P interaction in Rh2P can modify the surface charge states and electron cloud density on the Rh site, which should be beneficial to H2 activation.
For Pd/LC400 sample, its high-resolution Pd 3d XPS are deconvolved into two sets of doublet peaks (Figure 4D). The doublet peaks with strong intensity, located at a binding energy of 341.2 eV (indexed to Pd 3d3/2) and 336.0 eV (indexed to Pd 3d5/2), can be assigned to metallic Pd as a prevailing species (76%). The set of weak doublet peaks at 342.2 eV (indexed to Pd 3d3/2) and 337.0 eV (indexed to Pd 3d5/2) are ascribed to the Pd(II) oxides (Li et al., 2016). The high-resolution P 2p3/2 XPS of Pd/LC400 (Figure 4C) can be deconvoluted into two peaks, which are ascribed to P−O (134.5 eV) and P−C (133.3 eV) species.
In the case of Ru/LC400, the most intensive photoemission line of Ru 3d is strongly overlapped with C 1s line from the LC support. The surface Ru species were thus examined with Ru 3p XPS. The high-resolution Ru 3p XPS were fit into two sets of doublet peaks (Figure 4E). The strong doublet peaks with binding energy of 484.0 eV (indexed to Ru 3p1/2) and 461.5 eV (indexed to Ru 3p3/2), can be assigned to metallic Ru as a predominant species (76%) (Li et al., 2020a). The set of weak doublet peaks at 486.5 eV (indexed to Ru 3p1/2) and 464.0 eV (indexed to Ru 3p3/2) are ascribed to Ru(III) oxides (Zhao et al., 2020). The high-resolution P 2p3/2 XPS peaks of Ru/LC400 is very close to that of Pd/LC400 as described above (Figure 4C).
Notably, palladium phosphide and ruthenium phosphide species were unobserved on the surfaces of Pd/LC400 and Ru/LC400, respectively, based on TEM, XRD, and XPS analysis. Therefore, the XPS analysis further suggests that only Rh2P/LC400 sample contains Rh2P species on the LC surface although the synthetic procedure is the same for Rh2P/LC400, Pd/LC400 and Ru/LC400 samples. In the cases of Pd/LC400 and Ru/LC400 samples, the expected metal phosphide species are unobserved on the LC surface.
Hydrogenative coupling reaction
The hydrogenative coupling of 1,2-phenylenediamine (1a) and acetonitrile (2a) for 2-methyl-1H-benzo[d]imidazole (3a) synthesis with H2 as hydrogen source was investigated as a model reaction to optimize reaction conditions with the obtained catalysts (Figures 5A and 5B). 3a was unobserved under catalyst free conditions, indicating the absence of catalytic active sites for the coupling reaction. Rh/LC400 gave 80% yield of 3a, which is very close to the catalytic activity of Ru/LC400 (83% yield, Figure 5B). A significantly increased 3a yield to 93% was obtained by Pd/LC400. However, among the investigated catalysts, Rh2P/LC400 was the most efficient one by producing quantitative yield (>99%) of 3a.
Figure 5.
Catalyst screen for hydrogenative coupling
(A) Hydrogenative coupling of 1a with CH3CN to 3a.
(B) Comparison of catalyst activity based on one run of each catalyst.
(C) 3a formation rate over various catalysts. aPerformed with catalyst (20 mg), 1a (0.3 mmol), PH2 (1.0 MPa), CH3CN (3.0 mL); bbased on an equimolar amount of the Rh site in the Rh2P/LC400 [Rh (8.0 × 10−3 mmol) and P (3.2 × 10−2 mmol)]; ccatalyst (10 mg); dN2H4⋅H2O (1.0 mmol), PN2 (1.0 MPa), t (6 h).
The Rh2P/LC400 was obtained by pyrolysis of a mixture of RhCl3, dppe and LC under H2/N2 at 400°C. Therefore, to probe the catalytic site of Rh2P/LC400, a mixture of RhCl3−PPh3 (PPh3, triphenyl phosphine) and RhCl3−dppe were respectively investigated as homogeneous catalysts for the coupling (Figure 5B). However, negligible 3a yields (2–10%) were obtained with the above homogeneous system. Moreover, the reductive coupling was further carried out with Rh-based classical hydrogenation catalysts such as Rh(PPh3)3Cl and Rh(dppe)2Cl, which, again, yielded very limited amount of 3a (5–8% yield, Figure 5B). It is thus believed that Rh2P species, instead of Rh-P based complexes, is the true active site on the Rh2P/LC400 surface for the reductive coupling reaction.
To understand the influence of pyrolysis temperature on the catalytic performance of the catalyst, the reductive coupling reaction were performed with a low loading level of Rh2P/LC catalysts obtained at 400°C-pyrolysis (denoted as Rh2P/LC400), 600°C-pyrolysis (denoted as Rh2P/LC600), and 800°C-pyrolysis (denoted as Rh2P/LC800). Evidently, Rh2P/LC400 is the most effective one for the reaction (Figure 5B). Characterization of Rh2P/LC600 and Rh2P/LC800 are almost the same with Rh2P/LC400 (Figures S1–S3). However, a significantly increased mean nanoparticle size of Rh2P was observed with 8.6 nm for Rh2P/LC600 and 12.6 nm for Rh2P/LC800, respectively, which may presumably lead to the reduced activity. Finally, in addition to H2, N2H4⋅H2O was an alternative excellent hydrogen source for the reductive coupling reaction in the presence of Rh2P/LC400 catalyst by producing quantitative yield of 3a (>99%, Figure 5B).
The catalytic activities of various catalysts were further quantitatively compared based on formation rate of 3a. In this research, 3a formation rate in the hydrogenative coupling was obtained under a low 3a yield around 10–15%, given as the amount of formed 3a per amount of metal sites per hour for the investigated catalyst. Figure 5C demonstrates a comparable activity between Rh/LC400, Ru/LC400, and Pd/LC400. Although Rh2P/LC400 was proved to be the most active catalyst among various investigated samples, increase of reaction temperature slightly enhanced 3a formation rate. Moreover, Rh2P/LC400−N2H4⋅H2O system shows a doubled activity when compared with the Rh2P/LC400−H2 system under investigated conditions. Therefore, Rh2P/LC400 exhibits superior catalytic activity over Rh/LC400, presumably due to the electronic effect of Rh on the hydrogenative coupling.
2a thus functions as both reagent and solvent with an excess amount for the hydrogenative coupling under the above reaction conditions. In fact, the hydrogenative coupling can be well performed in tetrahydrofuran (THF) solvent. The influence of 2a concentration in THF on the coupling revealed that 3a yields increased with 2a concentration up to 92% with an optimal 2a concentration of 1.0 mmol mL−1 in THF, corresponding to 10 molar equivalents of 2a to 1a (Figure S4). Notably a maximal 3a yield of 96% was obtained in THF with the 2a/1a molar ratio of 30. The effect of reaction solvent on the coupling reaction demonstrated that both 2a and THF were effective for 3a formation (Table S8).
Electronic effect of Rh2P
The electronic state of Rh species on Rh2P/LC400 and Rh/LC400 was then investigated and compared by using Fourier transform infrared spectroscopy (FT-IR) with carbon monoxide (CO) as a probe molecule. The FT-IR spectra of CO-adsorbed Rh2P/LC400 revealed two different CO coordination modes (Figure 4F). The band at 2065 cm−1 is indexed to typically terminal monocarbonyl species of Rh−CO, corresponding to the terminal CO adsorbed on metallic Rh of the Rh2P, whereas the band at 2011 cm−1 is ascribed to the dicarbonyl species of Rh(CO)2 (Alvarado Rupflin et al., 2017; Yates et al., 1979). In contrast, the corresponding CO coordination modes over Rh/LC400 are observed at 2073 cm−1 for Rh−CO and at 2021 cm−1 for Rh(CO)2, respectively. Evidently, Rh2P/LC400 shows blue-shifts in the CO-probed FT-IR spectra if compared with Rh/LC400 (Figure 4F). The observed blue-shift indicates that Rh species on the Rh2P/LC400 has weaker electron-donating capability than in the case of the Rh/LC400, which suggests a decrease of electron density on the Rh site in the Rh2P/LC400 due to P doping (Komanoya et al., 2017; Nakajima et al., 2013). Moreover, P doping leads to a decreased adsorption intensity of Rh2P/LC400 in the CO-probed FT-IR spectra when compared with those of Rh/LC400. Our Rh 3d XPS analysis for Rh2P/LC400 indicates an enhanced charge transfer to Rh as well as strong interactions between P and Rh in the sample (Figure 4B). Generally, the results from CO-probed FT-IR are in line with our Rh 3d XPS analysis, suggesting a decreased electron density on Rh species in the Rh2P/LC400. Therefore, the electronic state of Rh2P/LC400 may presumably affect adsorption and activation of H2, leading to an efficient reductive coupling.
Isotope trace investigation
Hydrogenative couplings of 1a with CH3CN and CD3CN were then respectively performed under H2 with Rh2P/LC400 catalyst. The proton nuclear magnetic resonance (1H NMR) and mass spectrometry (MS) were applied to monitor the H/D exchange in the coupling process (Scheme 3A). Figure 6A shows the resulting 1H NMR of the 3a formed from 1a−CH3CN, the peak of the –NH– from 3a was unobserved. While the signals at 7.48 ppm (dd, JHH = 3.2, 6.0 Hz, aromatic –CH–), 7.15 ppm (dd, JHH = 3.2, 6.0 Hz, aromatic –CH–), 2.53 ppm (s, –CH3) were well separated in the 1H NMR of 3a. The resulting relative integration intensity reflected a 2(CH):2(CH):3(CH3) ratio of proton, thus confirming the chemical structure of 2-methyl-1H-benzo[d]imidazole for the 3a. In the case of 3a formed from 1a−CD3CN, the observed aromatic –CH– signals were almost the same with those from 1a−CH3CN; however, the resulting signals at methyl-group region were quite complicated (Figure 6B). In addition to a singlet at 2.52 ppm (–CH3), a 1:1:1 triplet (JHD = 1.7 Hz) was observed at 2.50 ppm (–CH2D), and a 1:2:3:2:1 quintet (JHD = 1.7, 3.4 Hz) was detected at 2.49 ppm (–CHD2) (Anet and O'Leary, 1989; Denny et al., 1994; Wheelhouse and Stevens, 1993; Horn and Everett, 1971). The above 1H NMR analysis indicates the formation of mono-protonated, di-protonated and tri-protonated methyl (−CDnH3-n) group in 3a with a total of 66% proton incorporation at the inert methyl carbon atoms during the 1a−CD3CN coupling. The molar ratio of –CH3, –CH2D and –CHD2 groups were 13:23:10 based on the corresponding integration areas (Figure 6B).
Scheme 3.
Isotope trace investigation
(A) Hydrogenative coupling of 1a−CH3CN and 1a−CD3CN. Performed with 1a (0.3 mmol), Rh2P/LC400 (20 mg), PH2 (1.0 MPa), CH3CN, or CD3CN (3.0 mL).
(B) Dissociative mechanism in C3H6–H2 reaction.
(C) Intramolecular H-shift mechanism in C3H6–H2 reaction.
(D) Proposed H/D exchange in CD3CN–H2 reaction.
Figure 6.
Comparison of 1a−CH3CN and 1a−CD3CN in hydrogenative coupling
(A) 1H NMR spectra of 3a from 1a–CH3CN.
(B) 1H NMR spectra of 3a from 1a–CD3CN.
(C) MS of 3a from 1a–CH3CN and 1a–CD3CN.
Figure 6C further compares the MS of 3a formed from CH3CN and CD3CN. For the hydrogenative coupling of 1a−CH3CN, the most abundant parent molecular ions for the resulting 3a were observed at m/z = 132, suggesting formation of the expected 2-methyl-1H-benzo[d]imidazole. In contrast, the 3a, formed in 1a−CD3CN, contained at least three compounds of 2-methyl-1H-benzo[d]imidazole (m/z = 132), 2-(methyl-d1)-1H-benzo[d]imidazole (m/z = 133) and 2-(methyl-d2)-1H-benzo[d]imidazole (m/z = 134) with the –CH2D group-containing 3a as the most predominant molecule. Our 1H NMR and MS analyses thus clearly indicate the appearance of proton at the methyl group (−CDnH3-n) of the formed 3a via the hydrogenative coupling of 1a−CD3CN, suggesting the presence of H/D exchange between the inert –CD3 group of CD3CN and H2 during the CD3CN-hydrogenation process.
Previously, propene–deuterium (C3H6–D2) reaction was extensively investigated with various transition metal catalysts and equally exchangeable of all the hydrogens in C3H6 were observed (Hirota and Hironaka, 1965; Naito and Tanimoto, 1999). The suggested reaction mechanism involved a rate-determining step of a D2 dissociation and subsequent incorporation of D atom into the dissociatively adsorbed intermediates such as n-propenyl, sec-propenyl and allylic species (Scheme 3B). Moreover, an intramolecular double-bond migration mechanism in propene between C1 and C3 carbon was suggested by a bridged hydrogen during the deuteration (Scheme 3C). In the case of reported CH3CN–D2 reaction, a series of deuterated amines such as (CD3CD2)2NH and (CH3CD2)NH(CD2CD3) were detected by an isotope exchange of D2 with the H atoms in –CH3 group of CH3CN (Huang and Sachtler, 1999a, 1999b). Therefore, in our case, an intramolecular D-shift may presumably promote H/D exchange between the –CD3 group of CD3CN and H2 during the hydrogenation (Scheme 3D).
Controlled experiments and catalyst reusability
Rh2P/LC400 catalyst was then selected for reductive coupling with both H2 and N2H4⋅H2O as hydrogen sources to probe the influence of reaction temperature and reaction time on the reaction. Reaction temperature significantly promotes the coupling reaction (Figure 7A). Negligible 3a yields were observed below 80°C. While 3a yields steeply increased with reaction temperature above 80°C with quantitative 3a yield (>99%) obtained at 140°C. Regarding the influence of reaction time on the coupling (Figure 7B), N2H4⋅H2O can remarkably reduce reaction time to 6 h by giving >99% yield of 3a. However, a significantly prolonged reaction time to 24 h was observed for H2 to yield quantitative 3a. Finally, the effect of H2 pressure on the reaction revealed that the coupling cannot occur in the absence of H2, 3a yield steeply increased with H2 pressure with an optimal H2 pressure of 1.0 MPa (Figure 7C).
Figure 7.
Reaction optimization and catalyst reusability
(A) Reaction temperature.
(B) Reaction time.
(C) Initial H2 pressure.
(D) Reusability of Rh2P/LC400. The recovered Rh2P/LC400 was collected by vacuum filtration, thoroughly washed with ethyl alcohol, dried under the vacuum, added into the autoclave, and performed with the previous reaction conditions. Performed with: (A) Rh2P/LC400 (20 mg), 1a (0.3 mmol), CH3CN (3.0 mL), PH2 (1.0 MPa) or [PN2 (1.0 MPa) and N2H4⋅H2O (1.0 mmol)], t (24 h); (B) T (140 °C); (C) t (24 h), T (140°C); (D) Rh2P/LC400 (10 mg), 1a (0.2 mmol).
The recyclability of Rh2P/LC400 was subsequently examined with the hydrogenative coupling for 3a synthesis. The recovered Rh2P/LC400 was collected by vacuum filtration, thoroughly washed with ethyl alcohol, dried under the vacuum, added into the autoclave, and performed with the previous reaction conditions. Noticeable decline in catalytic activity of RhP2/LC400 was unobserved during the consecutive seven-time recycling (Figure 7D). Our XRD, XPS, and TEM analyses of the recovered RhP2/LC400 indicated its high durability (Figure S5). However, the recovered RhP2/LC400 exhibited an evidently decreased specific surface area when compared with the fresh one (Table 1 and Figure S2), presumably owing to a strong adsorption of 3a on the RhP2/LC400 surface. In addition to Rh2P/LC400, Rh/LC400 also showed excellent recyclability and durability during the consecutive seven-time recycling (Figures S6–S8). The above recycling experiments thus demonstrated outstanding stability of lignin-derived Rh catalysts.
Scope of the hydrogenative coupling
Therefore, we have demonstrated hydrogenative coupling of 1a−CH3CN for efficient synthesis of 3a (Scheme 4). Without addition of 1a, a direct hydrogenation of CH3CN afforded trace amount of triethylamine (1.4% yield) with TOF of 1.1 molEt3N molRh−1 h−1 (Scheme 4). To obtain complementary information of the hydrogenative coupling for mechanism investigation, in addition to aromatic 1,2-diamine 1a, primary monoamines such as aniline (13) and benzylamine (14) were respectively introduced into the CH3CN−hydrogenation system to quickly condense with the in situ formed ethanimine intermediate (6, R1 = CH3, Scheme 2B). In the case of 13, both N-ethylaniline (13a) and N,N-diethylaniline (13b) were observed under the hydrogenation conditions with secondary amine (13a) as the major product (Scheme 4). This result is presumably attributed to weaker nucleophilicity and steric hindrance of the resulting aromatic amine 13a, which inhibits its further reductive amination with CH3CN. As expected, tertiary amine of N-benzyl-N-ethylethanamine (14a, Scheme 4) was obtained in a quantitative yield from 14 by consecutive ethylation. Finally, various aliphatic diamines such as ethylenediamine (15), 1,2-cyclohexanediamine (16), and 1,4-butanediamine (17) were further probed with the CH3CN−hydrogenation system. In the cases of 15 and 16, cyclization products of hydro-1H-imidazoles were unobserved, the CH3CN−hydrogenation system directly followed ethylation pathway under the investigated conditions by yielding a mixture of stepwisely ethylated amines products (Scheme 4). For long chain aliphatic diamine 17, similar results were observed with various ethyl-substituted amines as products. Therefore, CH3CN functions as ethylation reagent for aromatic monoamine (13), aliphatic monoamine (14), and aliphatic diamine (15–17) via the reductive substitution reaction. Our research thus demonstrated that Rh2P/LC400 is a versatile and efficient catalyst by hydrogenative coupling of CH3CN with aromatic 1,2-diamine to afford benzimidazole (Scheme 4). While, hydrogenative coupling of CH3CN with monoamine and aliphatic diamine led to formation ethyl-substituted amine. Finally, a direct hydrogenation of CH3CN with Rh2P/LC400 yielded trace amount of triethylamine.
Scheme 4.
Effect of amines on the hydrogenative coupling
Performed with amine (0.3 mmol), Rh2P/LC400 (20 mg), PH2 (1.0 MPa), CH3CN (3.0 mL).
The coupling scope was then evaluated by using Rh2P/LC400 for a variety of substituted 1a with CH3CN under the optimal conditions (Figure 8). Various substituted 1a were readily coupled with CH3CN to give the corresponding benzimidazoles (3a−f) in excellent yields. The substituted 1a bearing electron-donating groups (CH3, OCH3; 1b−d) and electron-withdrawing groups (F, Cl; 1e−f) were all selectively and quantitatively transformed into the corresponding 3 with the current catalytic system. Notably, in the case of 4-chloro-1,2-phenylenediamine (1f), dehalogenation products were unobserved. In addition to CH3CN, various nitriles such as n-butylnitrile (2b), phenylacetonitrile (2c), benzonitrile (2d), and 2-furonitrile (2e) were also applicable to the hydrogenative coupling system by affording excellent yields of the corresponding 2-alkylbenzimidazoles (3g−k). THF solvent was used when the reductive couplings were performed with 2c−e. The above results thus demonstrated that the developed Rh2P/LC400 catalyst enables highly selective and efficient synthesis of various 2-alkylbenzimidazoles by the hydrogenative coupling of the corresponding nitriles and aromatic 1,2-diamines (Figures S10–S20). In addition to 2-alkylbenzimidazoles, 2-unsubstituted benzimidazoles were recently reported in 35–86% yields with a wide tolerance of functional groups by a direct coupling of 1 with dimethyl sulfoxide (DMSO), in which DMSO functions as methyne source, oxidant, and solvent (Zhu et al., 2020). These two synthetic methods thus highlight a powerful and important extension to benzimidazoles in a sustainable and green way.
Figure 8.
Scope of diamines and nitriles for hydrogenative coupling
aPerformed with Rh2P/LC400 (20 mg), 1 (0.3 mmol), 2 (3.0 mL), PH2 (1.0 MPa). b2 (3.0 mmol) in THF (3.0 mL) was used instead of 2 (3.0 mL).
Proposed reaction mechanism
Finally, Scheme 2B shows proposed hydrogenative coupling mechanism with Rh2P/LC400 catalyst for 3 formation. Hydrogenation (with H2) and transfer hydrogenation (with N2H4) of nitrile 2 lead to formation imine intermediate 6 and primary amine 7. Subsequent reaction of 1 with 6 results in 11 with elimination of NH3. Cyclization of 11 followed by catalytic dehydrogenation of intermediate 12 give 3 with aromatization as a driven force. Moreover, 7 reacts with 6 leading to the formation of secondary imine 8 with exclusion of NH3. A successive reaction of 8 with 1 also yields 11 with release of 7. Notably, an equivalent amount of H2 to nitrile molecule is required to initialize the reaction. However, an equivalent H2 is finally released from dehydrogenative aromatization step for 3 formation. Therefore, “hydrogen-borrowing” (HB) mechanism is involved in the coupling reaction. The reductive coupling reaction is thus initialized from nitrile-activation by hydrogenation.
Controlled experiments were then performed for mechanism investigation (Scheme 5). Our coupling scope experiments have demonstrated that hydrogenative coupling of 2b with 1a led to 2-propyl-1H-benzo[d]imidazole (3g) formation in excellent yield. According to above proposed mechanism (Scheme 2B), the reductive coupling process should be initialized from catalytic hydrogenation of 2b with butan-1-imine (6a) and N-butylbutan-1-imine (8a) as the intermediates (Scheme 5). Due to high reactivity of 6a, 8a (Figure S9) was instead directly prepared by condensation of n-butyl aldehyde (18) and n-butylamine (19). As expected, treatment 8a with 1a in the presence of Rh2P/LC400 quantitatively afforded 3g under N2 atmosphere in THF, thus demonstrating intermediate nature of 8a. Rh2P/LC400 mainly promoted catalytic dehydrogenation process for aromatization under such conditions. Our controlled experiments thus suggested the mechanism as shown in Scheme 2B.
Scheme 5.
Controlled experiments for mechanism investigation
In summary, a direct reductive coupling of nitriles and 1,2-phenylenediamines was readily achieved with Rh2P catalyst to give various benzimidazoles in excellent yields (95%–99%). Both H2 and N2H4⋅H2O are effective hydrogen sources for the reductive coupling reaction. The high efficiency of Rh2P in the reaction is presumably attributed to the enhanced charge transfer to Rh, as well as strong interactions between P and Rh. Isotope trace experiments confirmed the presence of H/D exchange in reductive coupling between H2 and the inert –CD3 group of CD3CN via an intramolecular D-shift. The Rh2P catalyst can be easily recycled at least for seven times without any significant deactivations. This efficient and convenient method should have great impact on the synthesis of benzimidazole-based heterocyclic compounds.
Limitations of the study
The developed reductive coupling reaction works well with various 1,2-diamines and nitriles to give the corresponding benzimidazoles. However, the hydrogenative coupling of 3,4-diaminobenzonitrile with CH3CN led to formation a serial of products with full conversion of 3,4-diaminobenzonitrile, suggesting a complicated competition between hydrogenation, hydrogenolysis, ethylation and consecutive ethylation. Direct co-coupling of 3,4-diaminobenzonitrile was unobserved in THF solvent in the absence of CH3CN. The detailed reason is still not clear.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| RhCl3⋅xH2O | Aladdin Co. Ltd. | CAS 20765-98-4 |
| PdCl2 | Aladdin Co. Ltd. | CAS 7647-10-1 |
| RuCl3⋅xH2O | Aladdin Co. Ltd. | CAS 14898-67-0 |
| N2H4⋅H2O | Aladdin Co. Ltd. | CAS 7803-57-8 |
| Aniline | Aladdin Co. Ltd. | CAS 62-53-3 |
| Butyronitrile | Aladdin Co. Ltd. | CAS 109-74-0 |
| Phenylacetonitrile | Aladdin Co. Ltd. | CAS 140-29-4 |
| Benzonitrile | Aladdin Co. Ltd. | CAS 100-47-0 |
| 2-Furonitrile | Aladdin Co. Ltd. | CAS 617-90-3 |
| Benzylamine | Aladdin Co. Ltd. | CAS 100-46-9 |
| Ethylenediamine | Aladdin Co. Ltd. | CAS 107-15-3 |
| 1,2-Diaminocyclohexane | Aladdin Co. Ltd. | CAS 694-83-7 |
| 1,4-Diaminobutane | Aladdin Co. Ltd. | CAS 110-60-1 |
| Butylamine | Aladdin Co. Ltd. | CAS 109-73-9 |
| Butyraldehyde | Aladdin Co. Ltd. | CAS 123-72-8 |
| Acetonitrile | Guangzhou Kutai Trade Co. Ltd | CAS 75-05-8 |
| Tetrahydrofuran | Guangzhou Kutai Trade Co. Ltd | CAS 109-99-9 |
| High purity gases H2(≥99.9%) | Guangzhou Yinglai Gas Co. Ltd. | CAS 1333-74-0 |
| High purity gases N2(≥99.9%) | Guangzhou Yinglai Gas Co. Ltd. | CAS 7727-37-9 |
| 1,2-Phenylenediamine | Macklin Biochemical Science Co. Ltd. | CAS 95-54-5 |
| 3,4-Diaminotoluene | Bide Pharmatech Co. Ltd. | CAS 496-72-0 |
| 4,5-Dimethylbenzene-1,2-diamine | Bide Pharmatech Co. Ltd. | CAS 3171-45-7 |
| 4-Methoxy-o-phenylenediamine | Bide Pharmatech Co. Ltd. | CAS 102-51-2 |
| 4-Fluorobenzene-1,2-diamine | Bide Pharmatech Co. Ltd. | CAS 367-31-7 |
| 4-Chlorobenzene-1,2-diamine | Bide Pharmatech Co. Ltd. | CAS 95-83-0 |
| Other | ||
| Optima 2000 DV inductively coupled plasma atomic emission spectrometer (ICP-AES) | PerkinElmer, USA | https://www.perkinelmer.com.cn |
| Vertex 70 FI-TR spectrometer | Bruker | https://bruker.com |
| ULTRA 55 Scanning Electron Microscope (SEM) | Zeiss | http://www.lingrn.com |
| TECNAL-12 Transmission Electron Microscope (TEM) | FEI | https://www.thermofisher.cn |
| TriStar II 3flex adsorption analyzer | Micromeritics | https://www.micromeritics.com |
| D8 ADVANCE X-ray Powder Diffractometer (XRD) | Bruker | https://bruker.com |
| K-Alpha X-Ray photoelectron spectrometer (XPS) | Thermo scientific | https://www.thermofisher.cn |
| Fuli 9790 (Type II) Gas Chromatography (GC) | Fuli Instruments | http://www.cnfuli.com.cn |
| GC-2010 Plus Gas Chromatography-Mass Spectrometer (GC-MS) | Shimadzu Corporation of Japan | https://www.shimadzu.com/ |
| AV III 300 (300 MHz) spectrometer | Bruker | https://bruker.com |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jinzhu Chen (chenjz@jnu.edu.cn).
Materials availability
This work did not generate new unique reagents. All stable reagents generated in this study are available from the lead contact without restriction.
Method details
Materials synthesis
Preparation of Rh(PPh3)3Cl and Rh(dppe)2Cl: Rh(PPh3)3Cl and Rh(dppe)2Cl were prepared according to literature methods (Osborn and Wilkinson, 1967; Kunin et al., 1985).
Preparation of LC: LC was prepared according to the literature method with slight modification (Chen et al., 2020), enzymatic hydrolysis lignin (EHL, 3.0 g) and potassium bicarbonate (6.0 g) were finely grinded and mixed uniformly in a mortar. The resulting mixture was then transferred into an alundum boat and calcined under nitrogen atmosphere at 800°C for 3 h with a heating rate of 2°C min−1. The obtained black solid was collected, grinded into black powder, and further leached with aqueous HCl solution (100 mL, 2 mol L−1) for 12 h with vigorous stirring at room temperature. LC was then obtained as black solid (about 300 mg) by solution filtration, thoroughly washed with water (1 L), and dried at 80°C under the vacuum overnight.
Preparation of Rh2P/LC400: RhCl3⋅xH2O (25 mg, 0.095 mmol) and 1,2-bis(diphenylphosphino)ethane (dppe, 150 mg, 0.38 mmol) were dissolved in tetrahydrofuran (THF, 80 mL) in a round bottom flask. LC (150 mg) was subsequently added into the above solution, the resulting mixture was vigorously stirred at 60°C for 12 h. THF solvent in the mixture was then removed by rotary evaporator under reduced pressure. The obtained mixture was transferred into a quartz boat and calcined under atmospheric mixture of H2/N2 (VH2:VN2 = 8%) for 2 h at 400°C with a heating rate of 5°C min−1 to give Rh2P/LC400 (Rh, 4.8 wt.%). The Rh content was obtained by inductively coupled plasma-atomic emission spectrometry (ICP-AES) analysis.
Preparation of Rh/LC400: Rh/LC400 (Rh, 5.5 wt.%) was prepared with the same procedure used for Rh2P/LC400 without addition of dppe.
Preparation of Pd/LC400: Pd/LC400 (Pd, 5.2 wt.%) was prepared with the same procedure used for Rh2P/LC400 except that RhCl3⋅xH2O (25 mg) and THF solvent were respectively replaced by PdCl2 (25 mg) and acetonitrile solvent.
Preparation of Ru/LC400: Ru/LC400 (Ru, 5.1 wt.%) was prepared with the same procedure used for Rh2P/LC400 except that RhCl3⋅xH2O (25 mg) was replaced by RuCl3⋅xH2O (25 mg).
General procedure
Reductive coupling: Typically, Rh2P/LC400 (20 mg), 1,2-phenylenediamine (1a, 32 mg, 0.3 mmol) and acetonitrile (2a, 3.0 mL) were added into a Teflon-lined autoclave reactor (25 mL). Then, H2 (0.5 MPa) was slowly loaded into the reactor to remove the air inside for three times. Finally, H2 (1.0 MPa) was slowly charged into the reactor at ambient temperature. The reductive coupling was performed for 24 h at 140°C. After the reaction, the obtained mixture was analyzed by Gas Chromatography.
Calculation of1aconversion and3ayield: After the reaction, the mixture was filtered to remove catalyst, the resulting filtrate was then transferred into a volumetric flask, diluted to the volume with acetonitrile, and finally analyzed by GC. 1a conversion and 3a yield were then obtained by the corresponding formulas:
Characterization of products 3a-3k (NMR data)
2-Methyl-1H-benzo[d]imidazole 3a

Yield: 39.3 mg (99%). 1H NMR (300 MHz, CD3CN, 25°C) δ 7.48 (dd, J = 6.0, 3.2 Hz, 2H), 7.15 (dd, J = 6.0, 3.2 Hz, 2H), 2.52 (s, 3H). 13C {1H} NMR (75 MHz, CDCl3, 25°C) δ = 151.28, 138.77, 122.32, 114.63, 15.13 ppm. The spectral data is consistent with the literature data (, 2020b).
2,5-Dimethyl-1H-benzo[d]imidazole. 3b

Yield: 43.5 mg (99%). 1H NMR (300 MHz, DMSO-d6, 25°C) δ 7.35 (d, J = 8.1 Hz, 1H), 7.25 (s, 1H), 6.95 (d, J = 8.1 Hz, 1H), 2.47 (s, 3H), 2.38 (s, 3H). 13C {1H} NMR (75 MHz, DMSO, 25°C) δ 150.81, 138.19, 136.79, 130.35, 122.65, 114.01, 113.63, 21.30, 14.40. The spectral data is consistent with the literature data (Gan et al., 2018).
2,5,6-Trimethyl-1H-benzo[d]imidazole. 3c

Yield: 47.6 mg (99%). 1H NMR (300 MHz, DMSO-d6, 25°C) δ 7.20 (s, 2H), 2.43 (s, 3H), 2.27 (s, 6H). 13C {1H} NMR (75 MHz, DMSO-d6, 25°C) δ 150.16, 129.06, 19.88, 14.57. The spectral data is consistent with the literature data (Gan et al., 2018).
5-Methoxy-2-methyl-1H-benzo[d]imidazole. 3d

Yield: 47.7 mg (98%). 1H NMR (300 MHz, DMSO-d6, 25°C) δ 7.33 (d, J = 8.6 Hz, 1H), 6.96 (s, 1H), 6.74 (d, J = 10.7 Hz, 1H), 3.75 (s, 3H), 2.44 (s, 3H). 13C {1H} NMR (75 MHz, DMSO-d6, 25°C) δ 155.08, 150.80, 110.08, 55.41, 14.64. The spectral data is consistent with the literature data (Wan et al., 2019).
5-Fluoro-2-methyl-1H-benzo[d]imidazole. 3e

Yield: 44.1 mg (98%). 1H NMR (300 MHz, DMSO-d6, 25°C) δ 7.45 (dd, J = 8.6, 4.9 Hz, 1H), 7.27 (d, J = 9.6 Hz, 1H), 6.98 (t, J = 9.3 Hz, 1H), 2.47 (s, 3H). 13C {1H} NMR (75 MHz, DMSO-d6, 25°C) δ 159.69, 156.59, 152.81, 109.00, 108.67, 40.80, 14.65. The spectral data is consistent with the literature data (Yamini et al., 2021).
5-Chloro-2-methyl-1H-benzo[d]imidazole. 3f

Yield: 47.5 mg (95%). 1H NMR (300 MHz, DMSO-d6, 25°C) δ 7.50 (s, 1H), 7.45 (d, J = 8.5 Hz, 1H), 7.14 (d, J = 8.5 Hz, 1H), 2.48 (s, 3H). 13C {1H} NMR (75 MHz, DMSO-d6, 25°C) δ 153.00, 125.48, 121.25, 115.13, 114.22, 14.63. The spectral data is consistent with the literature data (Gan et al., 2018).
2-Propyl-1H-benzo[d]imidazole. 3g

Yield: 45.6 mg (95%). 1H NMR (300 MHz, DMSO-d6, 25°C) δ 12.18 (s, 1H), 7.45 (s, 2H), 7.09 (d, J = 5.9 Hz, 2H), 2.78 (t, J = 7.4 Hz, 2H), 1.75 (dd, J = 14.8, 7.4 Hz, 2H), 0.94 (t, J = 7.4 Hz, 3H). 13C {1H} NMR (75 MHz, DMSO-d6, 25°C) δ 155.05, 121.04, 30.56, 21.03, 13.71. The spectral data is consistent with the literature data (An et al., 2021).
5-Chloro-2-propyl-1H-benzo[d]imidazole. 3h

Yield: 52.4 mg (90%). 1H NMR (300 MHz, DMSO-d6) δ 12.36 (s, 1H), 7.73–7.27 (m, 2H), 7.12 (s, 1H), 2.77 (t, J = 7.2 Hz, 2H), 1.77 (q, J = 7.3 Hz, 2H), 0.93 (t, J = 7.2 Hz, 3H). 13C {1H} NMR (75 MHz, DMSO-d6, 25°C) δ 156.65, 125.20, 121.24, 115.26, 30.90, 20.89, 13.18. The spectral data is consistent with the literature data (Sriramoju et al., 2018).
2-Benzyl-1H-benzo[d]imidazole. 3i

Yield: 57.4 mg (92%). 1H NMR (300 MHz, DMSO-d6) δ 12.25 (s, 1H), 7.47 (dd, J = 5.8, 3.2 Hz, 2H), 7.39–7.26 (m, 4H), 7.23 (dd, J = 3.4, 2.4 Hz, 1H), 7.12 (dd, J = 5.9, 3.1 Hz, 2H), 4.17 (s, 2H). 13C{1H} NMR (75 MHz, DMSO-d6) δ 153.68, 139.48, 137.10, 128.83, 126.58, 121.43, 114.71, 35.08. The spectral data is consistent with the literature data (An et al., 2021).
2-Phenyl-1H-benzo[d]imidazole. 3j

Yield: 53.5 mg (92%). 1H NMR (300 MHz, 25°C, DMSO-d6) δ 12.95 (s, 1H), 8.20 (d, J = 8.2 Hz, 2H), 7.89–7.38 (m, 5H), 7.35–7.01 (m, 2H). 13C{1H} NMR (75 MHz, 25°C, DMSO-d6) δ 151.25, 130.19, 129.87, 128.99, 126.46, 122.14. The spectral data is consistent with the literature data (Lin et al., 2021).
2-(2-furanyl)-1H-benzo[d]imidazole. 3k

Yield: 49.7 mg (90%). 1H NMR (300 MHz, 25°C, DMSO-d6) δ 12.93 (s, 1H), 7.91 (d, J = 18.7 Hz, 1H), 7.54 (s, 2H), 7.18 (d, J = 16.8 Hz, 3H), 6.69 (d, J = 19.4 Hz, 1H). 13C{1H} NMR (101 MHz, 25°C, DMSO-d6) δ 145.45, 144.61, 143.55, 134.19, 122.46, 121.81, 118.72, 112.30, 111.34, 110.44. The spectral data is consistent with the literature data (Lin et al., 2021).
Acknowledgments
This research is financially supported by National Natural Science Foundation of China (Grant No. 22075104, U1810111, 21676089), Science and Technology Planning Project of Guangdong Province-China (2015A010106010), and Youth Science and Technology Innovation Talent of Guangdong TeZhi Plan (Grant No. 2019TQ05L111).
Author contributions
J.C. designed and directed the investigations. J.C., T.L., and Y.X. composed the manuscript. J.Z. developed the catalytic method. R.Y. studied the substrate scope. All the authors were involved the analysis of results and discussions of the project.
Declaration of interests
The authors declare no competing interests.
Published: September 24, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103045.
Contributor Information
Jinzhu Chen, Email: chenjz@jnu.edu.cn.
Tao Li, Email: tli@ecust.edu.cn.
Yisheng Xu, Email: yshxu@ecust.edu.cn.
Supplemental information
Data and code availability
Original code was not used in this manuscript. All relevant data supporting the findings of this study are available within the paper and its Supplemental Information files. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
References
- Alvarado Rupflin L., Mormul J., Lejkowski M., Titlbach S., Papp R., Gläser R., Dimitrakopoulou M., Huang X., Trunschke A., Willinger M.G. Platinum group metal phosphides as heterogeneous catalysts for the gas-phase hydroformylation of small olefins. ACS Catal. 2017;7:3584–3590. [Google Scholar]
- An W.-K., Zheng S.-J., Zhang H.-X., Shang T.-T., Wang H.-R., Xu X.-J., Jin Q., Qin Y., Ren Y., Jiang S. s-Tetrazine-functionalized hyper-crosslinked polymers for efficient photocatalytic synthesis of benzimidazoles. Green. Chem. 2021;23:1292–1299. [Google Scholar]
- Anet F.A.L., O'Leary D.J. H-D coupling constants and deuterium isotope effects on the proton chemical shifts in partially deuteriated methanes. Tetrahedron Lett. 1989;30:2755–2758. [Google Scholar]
- Bagal D.B., Bhanage B.M. Recent advances in transition metal-catalyzed hydrogenation of nitriles. Adv. Synth. Catal. 2015;357:883–900. [Google Scholar]
- Braos-García P., García-Sancho C., Infantes-Molina A., Rodríguez-Castellón E., Jiménez-López A. Bimetallic Ru/Ni supported catalysts for the gas phase hydrogenation of acetonitrile. Appl. Catal. A-gen. 2010;381:132–144. [Google Scholar]
- Carothers W.H., Jones G.A. The preparation of some primary amines by the catalytic reduction of nitriles. J. Am. Chem. Soc. 1925;47:3051–3057. [Google Scholar]
- Chakrabarti K., Maji M., Kundu S. Cooperative iridium complex catalyzed synthesis of quinoxalines, benzimidazoles and quinazolines in water. Green. Chem. 2019;21:1999–2004. [Google Scholar]
- Chakraborty S., Berke H. Homogeneous hydrogenation of nitriles catalyzed by molybdenum and tungsten amides. ACS Catal. 2014;4:2191–2194. [Google Scholar]
- Chakraborty S., Milstein D. Selective hydrogenation of nitriles to secondary imines catalyzed by an iron pincer complex. ACS Catal. 2017;7:3968–3972. [Google Scholar]
- Chatterjee S., Saito T. Lignin-derived advanced carbon materials. ChemSusChem. 2015;8:3941–3958. doi: 10.1002/cssc.201500692. [DOI] [PubMed] [Google Scholar]
- Chen M., Wu Q., Lin C., Zhang J., Zhao J., Chen J., Xu Y. Chemical fixation of CO2 using highly dispersed Cu on hierarchically porous N-doped carbon. ACS Appl. Mater. Inter. 2020;12:40236–40247. doi: 10.1021/acsami.0c08001. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Y., Zhang Y., Jiang W.-J., Zhang X., Dai Z., Wan L.-J., Hu J.-S. Pomegranate-like N,P-doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution. ACS Nano. 2016;10:8851–8860. doi: 10.1021/acsnano.6b04725. [DOI] [PubMed] [Google Scholar]
- Dalziel M.E., Deichert J.A., Carrera D.E., Beaudry D., Han C., Zhang H., Angelaud R. Magnesium ethoxide promoted conversion of nitriles to amidines and its application in 5,6-dihydroimidazobenzoxazepine synthesis. Org. Lett. 2018;20:2624–2627. doi: 10.1021/acs.orglett.8b00824. [DOI] [PubMed] [Google Scholar]
- Deng J., Xiong T., Xu F., Li M., Han C., Gong Y., Wang H., Wang Y. Inspired by bread leavening: one-pot synthesis of hierarchically porous carbon for supercapacitors. Green. Chem. 2015;17:4053–4060. [Google Scholar]
- Denny B.J., Wheelhouse R.T., Stevens M.F.G., Tsang L.L.H., Slack J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33:9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- Duan H., Li D., Tang Y., He Y., Ji S., Wang R., Lv H., Lopes P.P., Paulikas A.P., Li H. High-performance Rh2P electrocatalyst for efficient water splitting. J. Am. Chem. Soc. 2017;139:5494–5502. doi: 10.1021/jacs.7b01376. [DOI] [PubMed] [Google Scholar]
- Feng F., Ye J., Cheng Z., Xu X., Zhang Q., Ma L., Lu C., Li X. Cu-Pd/γ-Al2O3 catalyzed the coupling of multi-step reactions: direct synthesis of benzimidazole derivatives. RSC Adv. 2016;6:72750–72755. [Google Scholar]
- Gan Z., Tian Q., Shang S., Luo W., Dai Z., Wang H., Li D., Wang X., Yuan J. Imidazolium chloride-catalyzed synthesis of benzimidazoles and 2-substituted benzimidazoles from o-phenylenediamines and DMF derivatives. Tetrahedron. 2018;74:7450–7456. [Google Scholar]
- Griffin M.B., Baddour F.G., Habas S.E., Nash C.P., Ruddy D.A., Schaidle J.A. An investigation into support cooperativity for the deoxygenation of guaiacol over nanoparticle Ni and Rh2P. Catal. Sci. Technol. 2017;7:2954–2966. [Google Scholar]
- Hayes J.R., Bowker R.H., Gaudette A.F., Smith M.C., Moak C.E., Nam C.Y., Pratum T.K., Bussell M.E. Hydrodesulfurization properties of rhodium phosphide: comparison with rhodium metal and sulfide catalysts. J. Catal. 2010;276:249–258. [Google Scholar]
- Hirota K., Hironaka Y. The chemisorbed state of propene on metals in reference to the catalytic hydrogenation. J. Catal. 1965;4:602–607. [Google Scholar]
- Hölljes E.L., Wagner E.C. Some reactions of nitriles as acid anammonides1. J. Org. Chem. 1944;9:31–49. [Google Scholar]
- Horn R.R., Everett G.W. Proton and deuteron nuclear magnetic resonance isotope shifts in partially deuterated tris(2,4-pentanedionato)vanadium(III) J. Am. Chem. Soc. 1971;93:7173–7178. [Google Scholar]
- Hou S.-F., Chen J.-Y., Xue M., Jia M., Zhai X., Liao R.-Z., Tung C.-H., Wang W. Cooperative molybdenum-thiolate reactivity for transfer hydrogenation of nitriles. ACS Catal. 2020;10:380–390. [Google Scholar]
- Huang Y., Sachtler W.M.H. Concerted reaction mechanism in deuteration and H/D exchange of nitriles over transition metals. J. Catal. 1999;184:247–261. [Google Scholar]
- Huang Y., Sachtler W.M.H. On the mechanism of catalytic hydrogenation of nitriles to amines over supported metal catalysts. Appl. Catal. A Gen. 1999;182:365–378. [Google Scholar]
- Islam M., Mondal P., Singha Roy A., Tuhina K. Catalytic hydrogenation of various organic substrates using a reusable plymer-anchored palladium(II) complex. J. Mater. Sci. 2010;45:2484–2493. [Google Scholar]
- Keri R.S., Hiremathad A., Budagumpi S., Nagaraja B.M. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 2015;86:19–65. doi: 10.1111/cbdd.12462. [DOI] [PubMed] [Google Scholar]
- Komanoya T., Kinemura T., Kita Y., Kamata K., Hara M. Electronic effect of ruthenium nanoparticles on efficient reductive amination of carbonyl compounds. J. Am. Chem. Soc. 2017;139:11493–11499. doi: 10.1021/jacs.7b04481. [DOI] [PubMed] [Google Scholar]
- Kundu M.K., Mishra R., Bhowmik T., Barman S. Rhodium metal–rhodium oxide (Rh–Rh2O3) nanostructures with Pt-like or better activity towards hydrogen evolution and oxidation reactions (HER, HOR) in acid and base: correlating its HOR/HER activity with hydrogen binding energy and oxophilicity of the catalyst. J. Mater. Chem. A. 2018;6:23531–23541. [Google Scholar]
- Kunin A.J., Nanni E.J., Eisenberg R. Chemical reduction of bis[bis(diphenylphosphino)ethane]rhodium(1+), [Rh(dppe)2]+. characterization of Rh(dppe)20 and Rh(dppe)2- Inorg. Chem. 1985;24:1852–1856. [Google Scholar]
- Le Questel J.-Y., Berthelot M., Laurence C. Hydrogen-bond acceptor properties of nitriles: a combined crystallographic and ab initio theoretical investigation. J. Phys. Org. Chem. 2000;13:347–358. [Google Scholar]
- Li C., Yang M., Liu Z., Zhang Z., Zhu T., Chen X., Dong Y., Cheng H. Ru-Ni/Al2O3 bimetallic catalysts with high catalytic activity for N-propylcarbazole hydrogenation. Catal. Sci. Technol. 2020;10:2268–2276. [Google Scholar]
- Li J., Tian Q., Jiang S., Zhang Y., Wu Y. Electrocatalytic performances of phosphorus doped carbon supported Pd towards formic acid oxidation. Electrochim. Acta. 2016;213:21–30. [Google Scholar]
- Li Y., Gong Y., Xu X., Zhang P., Li H., Wang Y. A practical and benign synthesis of amines through Pd@mpg-C3N4 catalyzed reduction of nitriles. Catal. Commun. 2012;28:9–12. [Google Scholar]
- Li Z., Ye Z., Chen L., Cui J., Chen J. Hierarchically nanoporous titanium-based coordination polymers for photocatalytic synthesis of benzimidazole. ACS Appl. Nano Mater. 2020;3:10720–10731. [Google Scholar]
- Lin C., Wan W., Wei X., Chen J. H2 activation with Co nanoparticles encapsulated in N-doped carbon nanotubes for green synthesis of benzimidazoles. ChemSusChem. 2021;14:709–720. doi: 10.1002/cssc.202002344. [DOI] [PubMed] [Google Scholar]
- Liu L., Liu Y., Ai Y., Li J., Zhou J., Fan Z., Bao H., Jiang R., Hu Z., Wang J. Pd-CuFe catalyst for transfer hydrogenation of nitriles: controllable selectivity to primary amines and secondary amines. iScience. 2018;8:61–73. doi: 10.1016/j.isci.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Sahoo B., Junge K., Beller M. Cobalt complexes as an emerging class of catalysts for homogeneous hydrogenations. Acc. Chem. Res. 2018;51:1858–1869. doi: 10.1021/acs.accounts.8b00262. [DOI] [PubMed] [Google Scholar]
- Luo F., Guo L., Xie Y., Xu J., Cai W., Qu K., Yang Z. Robust hydrogen evolution reaction activity catalyzed by ultrasmall Rh-Rh2P nanoparticles. J. Mater. Chem. A. 2020;8:12378–12384. [Google Scholar]
- Monguchi Y., Mizuno M., Ichikawa T., Fujita Y., Murakami E., Hattori T., Maegawa T., Sawama Y., Sajiki H. Catalyst-dependent selective hydrogenation of nitriles: selective synthesis of tertiary and secondary amines. J. Org. Chem. 2017;82:10939–10944. doi: 10.1021/acs.joc.7b01823. [DOI] [PubMed] [Google Scholar]
- Naito S., Tanimoto M. Mechanistic study of the hydrogen exchange and hydrogenation of propene over alumina supported rhodium and ruthenium carbonyl cluster complexes. J. Mol. Catal. A Chem. 1999;141:205–214. [Google Scholar]
- Nakajima K., Noma R., Kitano M., Hara M. Titania as an early transition metal oxide with a high density of Lewis acid sites workable in water. J. Phys. Chem. C. 2013;117:16028–16033. [Google Scholar]
- Nandi S., Patel P., Jakhar A., Khan N.H., Biradar A.V., Kureshy R.I., Bajaj H.C. Cucurbit[6]uril-stabilized palladium nanoparticles as a highly active catalyst for chemoselective hydrogenation of various reducible groups in aqueous media. ChemistrySelect. 2017;2:9911–9919. [Google Scholar]
- Osborn J.A., Wilkinson G. Tris(triphenylphosphine)halorhodium(I) Inorg. Synth. 1967;10:67–71. [Google Scholar]
- Selvam K., Krishnakumar B., Velmurugan R., Swaminathan M. A simple one pot nano titania mediated green synthesis of 2-alkylbenzimidazoles and indazole from aromatic azides under UV and solar light. Catal. Commun. 2009;11:280–284. [Google Scholar]
- Shi Y., Zhang B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016;45:1529–1541. doi: 10.1039/c5cs00434a. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y., Sugano Y., Tanaka S., Hirai T. One-pot synthesis of benzimidazoles by simultaneous photocatalytic and catalytic reactions on Pt@TiO2 nanoparticles. Angew. Chem. 2010;122:1700–1704. doi: 10.1002/anie.200906573. [DOI] [PubMed] [Google Scholar]
- Sriramoju V., Kurva S., Madabhushi S. Oxone-mediated annulation of 2-aminobenzamides and 1,2-diaminobenzenes with sec-amines via imine-N-oxides: new syntheses of 2,3-dihydroquinazolin-4(1H)-ones and 1H-benzimidazoles. New J. Chem. 2018;42:3188–3191. [Google Scholar]
- Su J., Zhao H., Fu W., Tian W., Yang X., Zhang H., Ling F., Wang Y. Fine rhodium phosphides nanoparticles embedded in N, P dual-doped carbon film: new efficient electrocatalysts for ambient nitrogen fixation. Appl. Catal. B Environ. 2020;265:118589–118596. [Google Scholar]
- Sun W., Wu S., Lu Y., Wang Y., Cao Q., Fang W. Effective control of particle size and electron density of Pd/C and Sn-Pd/C nanocatalysts for vanillin production via base-free oxidation. ACS Catal. 2020;10:7699–7709. [Google Scholar]
- Tamura M., Honda M., Noro K., Nakagawa Y., Tomishige K. Heterogeneous CeO2-catalyzed selective synthesis of cyclic carbamates from CO2 and aminoalcohols in acetonitrile solvent. J. Catal. 2013;305:191–203. [Google Scholar]
- Wade A.R., Pawar H.R., Biware M.V., Chikate R.C. Synergism in semiconducting nanocomposites: visible light photocatalysis towards the formation of C–S and C–N bonds. Green. Chem. 2015;17:3879–3888. [Google Scholar]
- Wan Y., Zhang Z., Ma N., Bi J., Zhang G. Acylamino-directed specific sequential difunctionalizations of anilides via metal-free relay reactions for p-oxygen and o-nitrogen incorporation. J. Org. Chem. 2019;84:780–791. doi: 10.1021/acs.joc.8b02636. [DOI] [PubMed] [Google Scholar]
- Wang H., Partch R.E., Li Y. Synthesis of 2-alkylbenzimidazoles via TiO2-mediated photocatalysis. J. Org. Chem. 1997;62:5222–5225. [Google Scholar]
- Wang K., Jiang P., Yang M., Ma P., Qin J., Huang X., Ma L., Li R. The metal-free nitrogen-doped carbon nanosheets: a catalyst for directly synthesis imines under mild conditions. Green. Chem. 2019;21:2448–2461. [Google Scholar]
- Wang Y., Zhu Q., Xie T., Peng Y., Liu S., Wang J. Promoted alkaline hydrogen evolution reaction performance of Ru/C by introducing TiO2 nanoparticle. ChemElectroChem. 2020;7:1182–1186. [Google Scholar]
- Werkmeister S., Junge K., Beller M. Catalytic hydrogenation of carboxylic acid esters, amides, and nitriles with homogeneous catalysts. Org. Process. Res. Dev. 2014;18:289–302. [Google Scholar]
- Wheelhouse R.T., Stevens M.F.G. Decomposition of the antitumour drug temozolomide in deuteriated phosphate buffer: methyl group transfer is accompanied by deuterium exchange. J. Chem. Soc. Chem. Commun. 1993:1177–1178. [Google Scholar]
- Yamini, Sharma S., Das P. Rhodium catalyzed 2-alkyl-benzimidazoles synthesis from benzene-1,2-diamines and tertiary alkylamines as alkylating agents. Appl. Organomet. Chem. 2021;35:e6278. [Google Scholar]
- Yates J.T., Duncan T.M., Worley S.D., Vaughan R.W. Infrared spectra of chemisorbed CO on Rh. J. Chem. Phys. 1979;70:1219–1224. [Google Scholar]
- Zhang Y., Yang H., Chi Q., Zhang Z. Nitrogen-doped carbon supported nickel nanoparticles: a robust catalyst to bridge the hydrogenation of nitriles and the reductive amination of carbonyl compounds. ChemSusChem. 2019;12:1246–1255. doi: 10.1002/cssc.201802459. [DOI] [PubMed] [Google Scholar]
- Zhao J.P., Hernández W.Y., Zhou W.J., Yang Y., Vovk E.I., Wu M., Naghavi N., Capron M., Ordomsky V. Nanocell type Ru@quinone core-shell catalyst for selective oxidation of alcohols to carbonyl compounds. Appl. Catal. A-gen. 2020;602:117693–117700. [Google Scholar]
- Zhu X., Zhang F., Kuang D., Deng G., Yang Y., Yu J., Liang Y. K2S as sulfur source and DMSO as carbon source for the synthesis of 2-unsubstituted benzothiazoles. Org. Lett. 2020;22:3789–3793. doi: 10.1021/acs.orglett.0c00994. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li Z., Chen J. Applications of lignin-derived catalysts for green synthesis. Green. Energy Environ. 2019;4:210–244. [Google Scholar]
- Zhuang M., Ou X., Dou Y., Zhang L., Zhang Q., Wu R., Ding Y., Shao M., Luo Z. Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N,P-doped graphene for hydrogen generation. Nano Lett. 2016;16:4691–4698. doi: 10.1021/acs.nanolett.6b02203. [DOI] [PubMed] [Google Scholar]
- Zou J., Wu M., Ning S., Huang L., Kang X., Chen S. Ru@Pt core-shell nanoparticles: impact of the atomic ordering of Ru metal core on the electrocatalytic activity of Pt shell. ACS Sustain. Chem. Eng. 2019;7:9007–9016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original code was not used in this manuscript. All relevant data supporting the findings of this study are available within the paper and its Supplemental Information files. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.