Abstract
Myocarditis occurs with a variety of infectious agents including viruses, bacteria, protozoa and parasites. We present a rare case of myocarditis secondary to Toxoplasma gondii in a 23-year-old immunocompetent male presenting with acute chest pain. Workup revealed evidence of biventricular myocarditis on cardiac magnetic resonance imaging, elevated Toxoplasma serologies with rising titers over time. The patient was treated with sulfadiazine and pyrimethamine for eighteen days with resolution of symptoms. This case highlights alternative diagnostic and treatment modalities for Toxoplasma myocarditis in immunocompetent hosts.
Keywords: Toxoplasmosis, Myocarditis, Immunocompetent, Cardiac magnetic resonance imaging
Introduction
Myocarditis is an inflammatory disease of the heart muscle. This entity may result from a variety of infectious etiologies, many of which have distinct clinical presentations. The pathogenesis of infectious myocarditis is thought to be related to both direct pathogen myocyte invasion as well as secondary inflammation and immune system activation [1]. Prevalence varies based on the specific infectious etiology, patient population, and epidemiologic risk factors. The most common worldwide infectious etiologies are viral, with Adenovirus and Enteroviruses (including Coxsackievirus B) among the most prevalent [2]. In immunodeficient populations the opportunistic protozoa Toxoplasma is a common culprit of infectious myocarditis, with Toxoplasma found in up to 12% of patients in one necropsy series of patients with acquired immunodeficiency syndrome [3]. However, it is rare in immunocompetent hosts. Around one-third of the global population is infected by Toxoplasma, though it may be underdiagnosed in the United States, especially in the southern states where it is more common [4]. Contributory to this is that Toxoplasma gondii infections are often asymptomatic, with a minority of patients manifesting symptoms such as fever, headache, myalgias, lymphadenopathy, and transaminase elevation [4], [5]. Systemic complications are rare. Here, we present a case of Toxoplasma myocarditis in an immunocompetent host.
Case
A 23-year-old healthy Caucasian male presented to the emergency department (ED) with acute intermittent, severe substernal chest pain radiating to both forearms. He had experienced two similar episodes during the prior week which were of lesser severity and had resolved spontaneously. The day of admission, his symptoms returned with increasing severity, prompting presentation to the ED.
The patient’s past medical history included generalized anxiety disorder and seasonal allergies. He denied any personal or family history of cardiac disease. He worked as a landscaper and denied recent illnesses or sick contacts. He consumed alcohol infrequently, chewed tobacco, and did not smoke or use illicit drugs. He had recently returned from a three-day camping trip to Pigeon Forge, Tennessee one day prior to the first episode. He denied any history of international travel or Tuberculosis risk factors. Animal exposures included a pet dog and chickens.
Sublingual nitroglycerin administered en route to the ED improved his pain with complete resolution of symptoms by one hour. Vital signs were temperature 98.5°F, pulse 68, blood pressure 128/75, and SpO2 98% and physical examination was without pertinent findings. Initial differential included acute coronary syndrome, stress-induced cardiomyopathy, myocarditis, pericarditis, and pulmonary embolism.
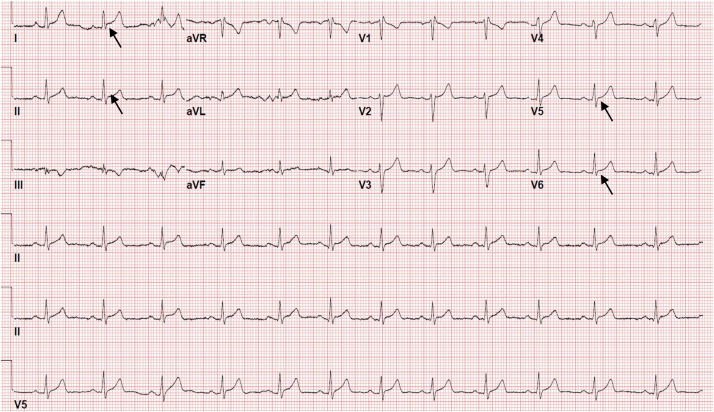
Initial electrocardiogram (ECG) revealed only subtle lateral ST elevations (Fig. 1). Laboratory work-up revealed an elevated serum troponin of 8.51 ng/mL with peak of 12.8 ng/mL (normal< 0.30 ng/mL), mildly elevated transaminases of aspartate aminotransferase and alanine aminotransferase at 108 and 91, respectively, with otherwise unremarkable complete blood count, basic metabolic panel, erythrocyte sedimentation rate, C-reactive protein, D-dimer, urine drug screen, and chest x-ray. COVID-19 real-time reverse-transcription polymerase chain reaction (PCR) was negative. Urgent coronary angiography showed normal coronary artery anatomy with no acute obstruction. Left ventriculogram showed no wall motion abnormality or changes suggestive of stress cardiomyopathy. Transthoracic echocardiography was normal with left ventricular ejection fraction 60–65% and no structural heart disease or pericardial effusion.
Fig. 1.
Initial electrocardiogram (ECG) demonstrating normal sinus rhythm with rate of 75 beats per minute. Subtle lateral ST segment elevation in lead I, II, V5, and V6 (arrow). No prior ECG was available.
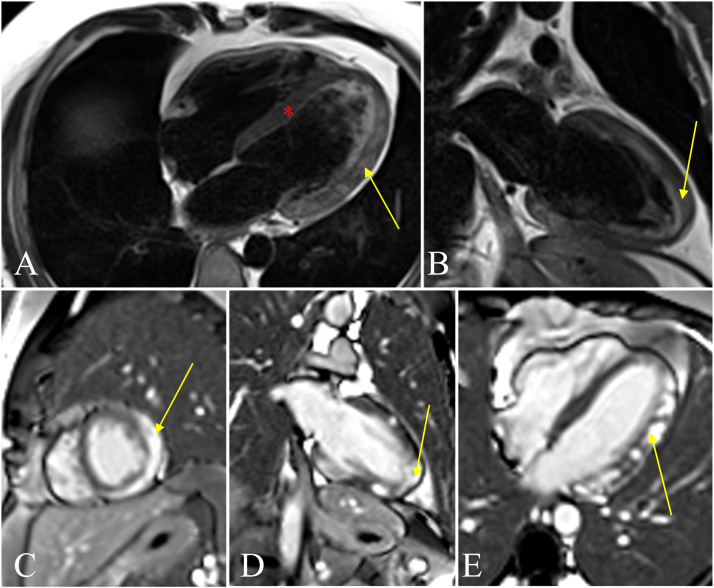
Cardiac magnetic resonance imaging (cMRI) showed evidence of biventricular myocarditis and failure, with LVEF of 45% and RVEF of 41% (Fig. 2). Comprehensive infectious work-up (Table 1) subsequently revealed elevated Toxoplasma gondii IgM and IgG titers (IgM > 160 AU/mL, IgG 36.7 IU/mL).
Fig. 2.
Markedly abnormal cardiac magnetic resonance imaging consistent with an acute phase of biventricular myocarditis. Fig. A and B represent T2 sequence with associated edema (yellow arrows) and normal septum (red asterisk). Figures C-E represent late gadolinium enhancement (LGE). Fig. C indicates LGE of the mid-myocardium (yellow arrow). Fig. D indicates transmural apex involvement (yellow arrow). Fig. E indicates patchy enhancement of the myocardium (yellow arrow) as well as the right ventricle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Infectious workup.
| Laboratory test | Result | Reference value |
|---|---|---|
| Adenovirus | Not Detected | Not Detected |
| Anaplasmosis DNA PCR | Not Detected | Not Detected |
| Aspergillus Antigen | Not Detected | Not Detected |
| Babesia DNA PCR | Not Detected | Not Detected |
| Blastomycoses Quant. Antigen | Not Detected | 0.2–14.7 ng/mL |
| Borrelia DNA PCI | Not Detected | Not Detected |
| Coxsackie Antibody | Not Detected | Not Detected |
| Cytomegalovirus DNA PCR | < 200 IU/mL | < 200 IU/mL |
| EBV DNA PCR | < 200 copies/mL | < 200 copies/mL |
| Ehrlichia DNA PCR | Not Detected | Not Detected |
| Fungitell (1–3)-B-D-Glucan | Not Detected | Not Detected |
| Hepatitis B Core Ab, Total | Nonreactive | Nonreactive |
| Hepatitis B Surface Ab | < 10 MIU/mL | < 10 MIU/mL |
| Hepatitis B Surface Ag | Nonreactive | Nonreactive |
| Hepatitis C Ab | Negative | Negative |
| Histoplasma Quant. Antigen | Not Detected | 0.4–19 ng/mL |
| HIV-1/HIV-2 Ag/Ab | Nonreactive | Nonreactive |
| Human Metapneumovirus | Not Detected | Not Detected |
| Influenza A | Not Detected | Not Detected |
| Influenza B | Not Detected | Not Detected |
| Legionella Urine Ag | Negative | Negative |
| Mycoplasma pneumoniae Ab IgGa | 2.4a | < 0.90 |
| Mycoplasma pneumoniae Ab IgM | 378 U/mL | < 779 U/mL |
| Parvovirus B19 IgGa | 7.2a | < 0.9 |
| Parvovirus B19 IgMa | 0.1a | < 0.9 |
| Rhinovirus | Not Detected | Not Detected |
| RSV A/B | Not Detected | Not Detected |
| SARS-COV-2 IgG | Negative | Negative |
| Toxoplasma IgG (initial) | 36.7 IU/mL | < 7.2 IU/mL |
| Toxoplasma IgG (repeat 5 days) | 97.6 IU/mL | < 7.2 IU/mL |
| Toxoplasma IgG (repeat 5 weeks) | 117 IU/mL | < 7.2 IU/mL |
| Toxoplasma IgM Ab (initial) | > 160 AU/mL | < 8.0 AU/mL |
| Toxoplasma IgM Ab (repeat 5 days) | > 160 AU/mL | < 8.0 AU/mL |
| Toxoplasma IgM Ab (repeat 5 weeks) | > 160 AU/mL | < 8.0 AU/mL |
Elevated Toxoplasma IgM and IgG. Repeat titers showed continued elevation of IgM and uptrending IgG.
Parvovirus B19 IgM, Parvovirus B19 IgG, and Mycoplasma IgG elevation was thought related to remote viral exposure rather than an active infection.
The patient was initiated on a planned four-week course of pyrimethamine, sulfadiazine and leucovorin plus guideline-directed medical therapy for ventricular systolic dysfunction. He had ophthalmologic evaluation which ruled out ocular involvement. HIV testing, which had been performed and was negative during the initial work-up, was repeated to rule out the possibility of false negative result and was again negative.
As myocardial biopsy was ultimately deferred given clinical improvement with antibiotics, serial toxoplasma serologies were followed to support diagnosis (Table 1): at 5 days, IgM was> 160 AU/mL and IgG 97.6 IU/mL, and at 5 weeks, IgM was> 160 AU/mL and IgG 117 IU/mL.
During follow-up evaluation with cardiology and infectious disease, he only complained of occasional intermittent fatigue with otherwise complete return to baseline without limitation in activity level. Eighteen days into the planned four-week antimicrobial course, he developed significant gastrointestinal intolerance and antimicrobial therapy was stopped. It was not restarted given his symptomatic improvement and return to clinical baseline. Repeat echocardiography performed five weeks after discharge showed normal cardiac function. The patient was instructed to have repeat cardiac MRI in 6 months to ensure sustained resolution of myocarditis.
Discussion
Toxoplasma should be considered as an etiology of acute myocarditis even in immunocompetent patient populations. While Toxoplasmosis in the immunocompromised patient is extensively described in literature, Toxoplasma myocarditis is exceedingly rare in the setting of a competent immune system with only limited case reports available [6], [7]. The prognosis in untreated immunocompetent patients is unclear. However, infection in untreated immunocompromised individuals carries high morbidity and mortality [4]. Given the availability of curative treatment, it is important to highlight and discuss the utility of detection and management of this rare finding.
The identification of a specific infectious etiology is limited due to non-specific prodromes and similar pretest probabilities among a number of potential etiologies, in the absence of a distinct epidemiologic risk factor. Diagnostic criteria for myocarditis such as the Dallas criteria require evidence of an inflammatory infiltrate and associated myocyte necrosis or degeneration on endomyocardial biopsy (EMB) not otherwise explained by an ischemic event. These criteria are highly specific; however, sensitivity is as low as 43% owing to the possibility of sampling error during EMB [8]. In one postmortem analysis of proven myocarditis, right ventricular biopsy samples were positive in only 17% of cases and left ventricular samples in only 20% [9].
Though biopsy remains the gold standard for diagnosing myocarditis, current guidelines do not support its use in the absence of new-onset heart failure associated with hemodynamic compromise, new arrhythmia, or failure to respond to medical therapy [10]. Newer diagnostics such as the Lake Louise criteria are based on advancements in cMRI accessibility, do not require invasive tissue sampling, and improve on the Dallas criteria with both high specificity and sensitivity of up to 81% and 71%, respectively [11]. Furthermore, cMRI adds long-term prognostication, differentiating ischemic from non-ischemic cardiomyopathy based on contrast enhancement patterns and identifying reversibility of myocardial injury [12].
Using Lake Louise criteria and Toxoplasma serology trends, our patient was diagnosed with biventricular myocarditis secondary to Toxoplasma gondii. Toxoplasma gondii transmission in adults is traditionally associated with cat exposure but also commonly occurs via ingestion of either oocysts in contaminated vegetables or water or of cysts in undercooked meat. In our case, exposure was suspected to be due to the patient’s occupation as a landscaper.
The pathogenesis of Toxoplasma myocarditis is likely secondary both to direct myocyte invasion by Toxoplasma tachyzoites, as well as additional cellular damage by an immune and inflammatory reaction to the parasites themselves [1]. The use of serologic testing with both IgM and IgG titers is the primary method of diagnosis. While IgM appears within the first few days after exposure, IgG begins to rise within 1–2 weeks post-exposure, peaks at 1–2 months, and then declines but may remain present for life [13]. In cases where myocarditis is suspected and EMB is not available, addition of serum PCR performed via reference laboratory (e.g. University of Washington Medical Center, Seattle, WA or Mayo Clinic Laboratories, Rochester, MN) has also been used in diagnosis and can increase diagnostic sensitivity in cases presenting prior to seroconversion [14].
Evidence for treatment of Toxoplasma myocarditis in immunocompetent patients is limited to individual case studies. A recent systematic review by Zhou and colleagues examining cardiac toxoplasmosis between 1990 and 2020 identified sixty cases. Among 7 immunocompetent patients, 3 received no antimicrobial treatment, 2 were treated with trimethoprim-sulfamethoxazole, one was treated with sulfadiazine and pyrimethamine, and one was treated with pyrimethamine and spiramicin. Most literature favors a 2–4 week course of antimicrobial therapy, though the optimal duration is unknown. There may also be a role for adjunctive steroid therapy and pacemaker placement for patients who present with arrhythmias and conduction defects [5].
Conclusions
To date, Toxoplasma myocarditis among immunocompetent patients remains an extremely rare clinical entity. In patients presenting with evidence of myocarditis without preexisting cardiac risk factors, infectious myocarditis should remain high on the differential, and toxoplasma serology should be considered. Non-invasive modalities for diagnosis and workup of Toxoplasma myocarditis should include the use of cardiac MRI as well as serial serologies. While a guideline-based treatment approach remains lacking among immunocompetent patients, with some evidence for resolution of myocarditis with conservative measures alone, it would seem prudent to follow established treatment paradigms for immunocompromised patients until further studies are conducted.
Ethical approval
Patient consent was obtained for the preparation of this manuscript. All identifying information has been omitted and IRB review was not necessary for this case report.
CRediT authorship contribution statement
Katherine Mustafa, DO: Conceptualization, Writing – original draft, Writing – review & editing—Final draft preparation. Jonathan Hillyard, DO: Writing – review & editing. Elizabeth Nowak, DO: Writing – review & editing. Jacek Slowikowski, MD: Supervision. Ijeoma Okogbue, MD: Supervision. Dorothy Garner, MD: Supervision.
Author statement
All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Conflict of interest
None.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- 1.Magnani J.W., Dec G.W. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113(6):876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 2.Bowles N.E., Ni J., Kearney D.L., Pauschinger M., Schultheiss H.-P., McCarthy R. Detection of viruses in myocardial tissues by polymerase chain reaction. J Am Coll Cardiol. 2003;42(3):466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 3.Hofman P., Drici M.D., Gibelin P., Michiels J.F., Thyss A. Prevalence of toxoplasma myocarditis in patients with the acquired immunodeficiency syndrome. Heart. 1993;70(4):376–381. doi: 10.1136/hrt.70.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z., Ortiz Lopez H., Pérez G.E., Burgos L.M., Farina J.M., Saldarriaga C. Toxoplasmosis and the heart. Curr Probl Cardiol. 2021;46(3) doi: 10.1016/j.cpcardiol.2020.100741. [DOI] [PubMed] [Google Scholar]
- 5.Hidron A., Vogenthaler N., Santos-Preciado J.I., Rodriguez-Morales A.J., Franco-Paredes C., Rassi A. Cardiac involvement with parasitic infections. Clin Microbiol Rev. 2010;23(2):324–349. doi: 10.1128/CMR.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosser M.S., Simpson S.Q. Acute toxoplasma gondii infection manifesting as myocarditis in an immune competent host. Chest. 2007;132(4):727A. [Google Scholar]
- 7.Pergola G., Cascone A., Russo M. Acute pericarditis and myocarditis by Toxoplasma gondii in an immunocompetent young man: a case report. Infez Med. 2010;18(1):48–52. (Mar) [PubMed] [Google Scholar]
- 8.Chow L.H., Radio S.J., Sears T.D., Mcmanus B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14(4):915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 9.Hauck A.J., Kearney D.L., Edwards W.D. Evaluation of [ostmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 10.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U. The role of endomyocardial biopsy in the management of cardiovascular disease. Circulation. 2007;116(19):2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 11.Lurz P., Eitel I., Adam J., Steiner J., Grothoff M., Desch S. Diagnostic performance of cmr imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5(5):513–524. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Aquaro G.D., Ghebru Habtemicael Y., Camastra G., Monti L., Dellegrottaglie S., Moro C. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74(20):2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 13.Montoya J.G. Laboratory diagnosis of toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002;185(s1):73–82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 14.Lévêque M.F., Chiffré D., Galtier C., Albaba S., Ravel C., Lachaud L. Molecular diagnosis of toxoplasmosis at the onset of symptomatic primary infection: a straightforward alternative to serological examinations. Int J Infect Dis. 2019;79:131–133. doi: 10.1016/j.ijid.2018.11.368. [DOI] [PubMed] [Google Scholar]