Abstract
Background
Non pharmaceutical interventions (NPI) including hand washing directives were implemented in China and worldwide to combat the COVID-19 pandemic, which are likely to have had impacted a broad spectrum of enteric pathogen infections.
Methods
Etiologically diagnostic data from 45 937 and 67 395 patients with acute diarrhea between 2012 and 2020, who were tested for seven viral pathogens and 13 bacteria respectively, were analyzed to assess the changes of enteric pathogen infections in China during the first COVID-19 pandemic year compared to pre-pandemic years.
Findings
Test positive rates of all enteric viruses decreased during 2020, compared to the average levels during 2012−2019, with a relative decrease of 71•75% for adenovirus, 58•76% for norovirus, 53•50% for rotavirus A, and 72•07% for the combination of other four uncommon viruses. In general, a larger reduction of positive rate in viruses was seen among adults than pediatric patients. A rebound of rotavirus A was seen after September 2020 in North China rather than South China. Test positive rates of bacteria decreased during 2020, compared to the average levels during 2012−2019, excepting for nontyphoidal Salmonella and Campylobacter coli with 66•53% and 90•48% increase respectively. This increase was larger for pediatric patients than for adult patients.
Interpretation
The activity of enteric pathogens changed profoundly alongside the NPIs implemented during the COVID-19 pandemic in China. Greater reductions of the test positive rates were found for almost all enteric viruses than for bacteria among acute diarrhea patients, with further large differences by age and geography. Lifting of NPIs will lead to resurgence of enteric pathogen infections, particularly in children whose immunity may not have been developed and/or waned.
Funding
China Mega-Project on Infectious Disease Prevention; National Natural Science Funds.
Keywords: Non pharmaceutical interventions, Acute diarrhea, COVID-19, China
Research in context
Evidence before this study
We searched PubMed on July 21, 2021 with the terms of (“COVID-19” or “SARS-COV-2”) and (“prevent” or “intervention” or “control” or “restriction”) and (“norovirus” or “rotavirus” or “adenovirus” or “astrovirus” or “sapovirus” or “diarrheagenic Escherichia coli” or “nontyphoidal Salmonella” or “Vibrio parahaemolyticus” or “Campylobacter jejuni” or “Campylobacter coli” or “Shigella” or “Aeromonas hydrophila” or “Plesiomonas shigelloides” or “Yersinia enterocolitica” or “Vibrio cholerae” or “Vibrio fluvialis” or “Vibrio mimicus” or “Yersinia pseudotuberculosis”) for articles published after Jan 1, 2020. Our search found a total of 301 research papers, among which six studies which focused on comparing enteropathogens activity during vs. prior to the COVID-19 pandemic. Among them, five studies revealed consistent decline of norovirus incidence during COVID-19 epidemic, and one focused on the decline of enterovirus and Shigella. These studies covered some of geographic regions of Australia, Philadelphia, England, Germany, the United States, and Israel. In China, however, where COVID-19 pandemic was brought under control quickly and effectively, the population-level effects on common enteric pathogens in the post-COVID-19 era was unknown.
Added value of this study
The current study, by using the nation-wide longitudinal data on the etiological surveillance of acute diarrhea related to twenty common enteropathogens in China during the past nine years from 2012 and 2020, explored the changed pattern in enteropathogens spectrum after COVID-19 pandemic. We found that the spread and seasonality of almost all these viruses were interrupted during 2020, particularly during weeks 5−15 when stringent and widespread NPIs were active. However, rebound was found for rotavirus A during Phase IV (after September 2020) in North China. In contrast to most other enteric pathogens, the activity of nontyphoidal Salmonella (NTS) was elevated to higher level than the historical levels consistently during COVID-19 year, especially when most NPIs were lifted and schools were reopened. This increase was larger for pediatric patients rather than adult patients, suggesting that they will be particularly susceptible to a rebound in infections as containment strategy restrictions are eased. Parents and school administrators should be alerted to this risk, and more frequent and meticulous hand hygiene and mask-wearing should be advocated during school hours.
Implications of all the available evidence
Understanding how the transmission dynamics of enteric pathogens coevolve with SARS-CoV-2 is an integral part of assessing the broad impact of COVID-19 pandemic and associated interventions on global health. These time-dependent findings might not be unique to China, which might enhance the understanding of the indirect impacts of COVID‐19 on enteric pathogen infections in other countries where COVID-19 is still circulating and may facilitate effective contingency plans for future infection control.
Introduction
Since its emergence in 2019, SARS-CoV-2 has spread rapidly and caused over 169 million cases and 3•5 million death of the coronavirus disease 2019 (COVID-19) globally by 1 June 2021 [1]. Although global numbers of both cases and deaths continued to decrease over the past weeks from the end of April, 2021, there are still increases in many global regions such as Africa and South-East Asia, and wide concern is mounting due to outbreaks and rebounds caused by SARS-CoV-2 variants, e.g., Alpha, Beta, Gamma and Delta [2]. Progress in controlling the pandemic has been slowed by the emergence of these variants that appear to be more transmissible and may escape control by both vaccine-induced and convalescent immune responses [3,4]. Thus, although SARS-CoV-2 vaccines are now available, non pharmaceutical interventions (NPI) continue to play an important role in reducing the transmission of COVID-19 globally [5,6]. NPIs including social distancing, increased hand hygiene, mask-wearing, surface decontamination, travel restrictions, and school closures effective for reducing SARS-CoV-2 transmission should also be effective for other viral pathogens. The effect of these strategies in reducing infectious diseases had been demonstrated in different countries or regions, but mainly for respiratory viral infections, such as influenza and respiratory syncytial virus [7], [8], [9], [10], [11], [12]. Pathogens with other transmission modes, e.g., gastrointestinal, sexually transmitted or even vector-borne diseases, could have been affected, due to the changes in human movement and associated behavioral patterns. For instance, reported cases of enteroviruses had been greatly reduced in England since COVID-19 control measures were introduced [13]. To plan preventive programs for common infectious diseases in the post-pandemic era, it is necessary to understand the broad impact of the nonpharmaceutical interventions on the pathogens other than SARS-CoV-2.
China was among the countries that had applied nationwide NPIs at the early epidemic. Since the fourth week of January, 2020, accompanying the block of Wuhan city [14,15], massive interventions including the isolation of the confirmed/suspected cases and quarantine of close contacts, even the strict and unprecedented measures like closure of school and entertainment venues, banning of mass gathering activities, have been implemented across China to contain the spread of COVID-19 [16]. At the individual level, the use of facemasks and hand washing at public areas had been encouraged widely in China. The effect of this on the transmission dynamics on other infections remained rarely investigated.
Here we focused on acute diarrhea disease, with the aim of exploring its national patterns of transmission during and after the first epidemic of COVID-19 in China. We extracted data from a National-based prospective surveillance of acute diarrhea in China that was run between January 2012 and December 2020. Using these data the intensity and timing of enteric pathogen activity, alongside the NPIs implemented during the COVID-19 pandemic were investigated.
Methods
Data collection and epidemiological description
A nationwide surveillance for acute diarrhea program was implemented by the Chinese Center for Disease Control and Prevention (China CDC) beginning in 2009, testing a spectrum of common contagious pathogens causing acute diarrhea, year round, in 31 provinces [17]. A case of acute diarrhea was defined as with presence of ≥3 passages of watery, loose, mucus-, or bloody-stools within a 24-h period. By the end of 2020, data from 134 sentinel hospitals located in 27 provinces were aggregated and used for this analysis. Seven viral pathogens were tested from the stool specimens, briefly, enzyme-linked immunosorbent assay was used to test rotavirus A antigen, and G and P genotyping was performed by reverse transcription-polymerase chain reaction (RT-PCR). Polymerase chain reaction (PCR) or RT-PCR were used to test norovirus, adenovirus, astrovirus, sapovirus, rotavirus B and rotavirus C. Thirteen bacterial pathogens were tested by performing isolation with or without enrichment procedures at the first step. For Yersinia enterocolitica (Y. enterocolitica), Yersinia pseudotuberculosis (Y. pseudotuberculosis), diarrheagenic Escherichia coli (DEC), Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli), the isolation was subsequently tested by PCR, and for nontyphoidal Salmonella (NTS), Vibrio parahaemolyticus (V. parahaemolyticus), Vibrio cholerae (V. cholerae), Vibrio fluvialis (V. fluvialis), Vibrio mimicus (V. mimicus), Aeromonas hydrophila (A. hydrophila), Plesiomonas shigelloides (P. shigelloides) and Shigella, the isolation was subsequently tested by biochemical and serological assays [18]. A detailed description of the assays were provided in the Supplementary Material (Appendix p 2). The National Health Commission of the People's Republic of China decided the study was part of continuing public health surveillance following national surveillance guidelines; parents/guardians of participants in this study were only required to provide brief verbal consent during their enrollment, which was recorded in each questionnaire by their physicians. This project and the above procedure for obtaining consent were approved by the ethical review committee of China CDC (2015-025).
To demonstrate the impact of NPIs on the circulation patterns of the pathogens, we defined four periods according to the timeline of major intervention events for containing the COVID-19 epidemic in China: Phase I was defined as from January 1–January 22 without massive NPI, Phase II as from January 23–April 7 when Wuhan city was lockdown, Phase III as from April 8–August 31 when nationwide NPIs was relaxed while school closure maintained, and Phase IV as from September 1–December 31 when schools were re-opened (Appendix p 4). To attain a controlled comparison with historical level, we define phases II–IV in 2020 as During-COVID-19, and the corresponding phases of the preceding eight years from 2012 to 2019 as Pre-COVID-19. Age standardized positive rate of each pathogen was contrasted between During-COVID-19 and Pre-COVID-19. The age-standard positive rate was calculated based on two age groups (children group of 0–17 years old, the adult group of ≥18 years old), and the percentage change were compared between During-COVID-19 and Pre-COVID-19, subgroup analysis was also made for data disaggregated by age, sex and geographic region. Percentage change of positive rate was calculated as: 100% × [(PRt1(k) − PRt0(k)]/PRt0(k) where the k indicated the phase we defined above, the PRt0(k) indicated the average positive rate during phase k in 2012−2019, and PRt1(k) indicated that of 2020. Due to small numbers of positive detection for astrovirus, sapovirus, rotavirus B and rotavirus C, these four viruses have been combined as “others” in the comparison between During-COVID-19 and Pre-COVID-19. Similarly, the eight bacteria including V. cholerae, V. fluvialis, V. mimicus, A. hydrophila, P. shigelloides, Shigella, Y. enterocolitica and Y. pseudotuberculosis also have been combined in the analysis. The age standard positive rate was ranked for each enteric pathogen for Pre-COVID-19 and During-COVID-19 respectively.
Statistical analysis
Monthly positive rates were plotted and fitted with generalized linear models (GLM) to quantify the impact of the COVID-19 related NPIs in different phases for five selected enteric pathogens, including three viruses (Norovirus, Rotavirus A, and Adenovirus) and two bacteria (DEC and NTS), stratified by age group and geographic regions. Those (15 viruses and bacteria) without positive detection in more than one phases were excluded in this analysis due to failure to model convergence for the beta-binomial distribution applied [19]. The periods were redefined for the monthly data, with phases II–IV corresponding to February–March, April–August and September–December respectively. The number of monthly positive cases were assumed to follows beta-binomial distribution. This distribution accounts for overdispersion and also consider the limited numbers of tested specimens when the data are grouped by region and age groups [20,21]. We observed smaller case number in the years of 2016 and 2017 when compared with other surveillance years (949 in 2016 and 1 616 in 2017 vs. average of 6 196 cases that were subject to all viruses tests, 4 995 in 2016 and 4 186 in 2017 vs. average of 8 317 cases that were subject to all bacteria test), therefore the data during 2016 and 2017 were excluded from GLM analysis, due to inadequate sampling size for the temporal trajectories of monthly positive rates. Dummy variables were introduced to the corresponding phases, e.g., coded A was set for all the months before February 2020 when nationwide NPIs started; coded B for Phase II from February to March 2020; coded C for Phase III from April to August 2020 when nationwide NPIs appropriately relaxed while school closure maintained; coded D for Phase IV from September to December 2020 when schools re-opened. Seasonality was accounted for using sinusoidal functions with both annual and semiannual cycles [22]. We reported exponentiated regression coefficients as seasonality-adjusted odds ratios (OR). Statistical significance was evaluated with two-sided and p-values at the level of α = 5%. Time series of annual positive rates were plotted for the subtypes of norovirus and DEC from 2012 to 2020. In order to attain a controlled comparison between periods with and without NPIs, we herein defined the start of each year as from Jan 23, the date of lockdown of Wuhan City in 2020. Coinfection pattern was determined for Pre-COVID-19 and During-COVID-19 epidemic, respectively. All statistical analysis was performed using R statistical software (version 4.0.3).
Role of the Funder/Sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Overall change of recruited patients
Etiologically diagnostic results and epidemiological data of 45 937 patients with acute diarrhea who were tested for all seven viral pathogens during 2012–2020 were used for analysis. The annual number of cases decreased from an average of 5 521 in 2012–2019 to 1 772 during 2020. The baseline characteristics of the patients were different between 2012–2019 and 2020 (Appendix p 5). Median age increased from 8 (IQR:1–43) to 13 (IQR:2–39) in 2020, the change in age group required adjusting test positive rates for age distribution. Compared with 2012–2019, we observed more patients were tested in Phase I (11•00% vs. 4•38%) and Phase IV (35•38% vs. 34•10%), fewer in Phase II (7•84% vs. 13•49%) and Phase III (45•77% vs. 48•04%) (p<0•0001). No differences in the case fatality rate were found between 2012–2019 and 2020 (0•05% vs. 0•06%, p=1•00), while the proportion of enrolled patients with fever (18•16% vs. 30•30%, p<0•0001) or dehydration (3•38% vs. 4•35%, p<0•0001) increased significantly (Appendix p 6).
Similarly, the data of 67 395 cases with acute diarrhea who were tested for all 13 bacteria in 2012–2020 were used for bacterial analysis. The annual number of cases decreased from an average of 8 076 in 2012–2019 to 2 791 during 2020 (Appendix p 5). Median age decreased from 22 (IQR:1–47) before to 13 (IQR:1–50) in 2020, with higher proportion of patients were enrolled tested in Phase I (5•98% vs. 4•04%) and Phase IV (36•94% vs. 32•67%), fewer in Phase II (7•52% vs. 13•02%) and Phase III (49•55% vs. 50•27%) (p<0•0001). No differences in the case fatality rate were found between 2012–2019 and 2020 (both as 0•04%, p=1•00), while the proportion of enrolled patients with fever (18•87% vs. 20•67%, p=0•018) or dehydration (3•44% vs. 6•56%, p<0•0001) increased significantly (Appendix p 6).
Both datasets had observed a slightly higher proportion of patients from Southern China over Northern China in 2020 (p<0•0001). No significant difference was observed for sex (Appendix p 5).
Overall change pattern of positive rates
The overall viral activity as measured by percentage of submitted specimens positive for any viruses decreased by 57•12% for Phase I (from 42•77% of during 2012‒2019 to 18•34% in 2020), by 74•47% for Phase II (from 34•12% to 8•71%), by 59•69% for Phase III (from 19•72% to 7•95%), and by 56•90% for Phase IV (from 32•69% to 14•09%) (all p<0•0001). When three phases were combined, each virus had exhibited a sharp decrease of positive rate During-COVID-19, compared with those of Pre-COVID-19 (Appendix p 7), with a relative decrease of 71•75% for adenovirus (from 3•54% to 1•00%), 58•76% for norovirus (from 11•42% to 4•71%), 53•50% for rotavirus A (from 9•87% to 4•59%), and 72•07% for the other uncommon viruses (combined rates of astrovirus, sapovirus, rotavirus B, and rotavirus C) (from 4•44% to 1•24%) (all p<0•0001) (Table 1). Ranking by the annual cumulative positive rate, a slight change was observed between Pre-COVID-19 and During-COVID-19 epidemic, and only sapovirus increased its order from the fifth to the fourth with an exchange with astrovirus, other viruses had no changes of their order (Appendix p 8).
Table 1.
Comparison of age-standardized positive rate (%) of pathogens between Pre-COVID-19 and During-COVID-19 in the mainland of China.
| Pathogens* |
Total† |
Phase II† |
Phase III† |
Phase IV† |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012‒2019 | 2020 | Change | p value | 2012‒2019 | 2020 | Change | p value | 2012‒2019 | 2020 | Change | p value | 2012‒2019 | 2020 | Change | p value | |
| Virus | ||||||||||||||||
| Norovirus | 11•42 | 4•71 | -58•76% | <0•0001 | 13•87 | 4•41 | -68•20% | 0•0039 | 8•58 | 3•11 | -63•75% | <0•0001 | 14•76 | 6•36 | -56•91% | <0•0001 |
| Rotavirus A | 9•87 | 4•59 | -53•50% | <0•0001 | 15•63 | 3•76 | -75•94% | 0•0011 | 5•12 | 3•39 | -33•79% | 0•022 | 13•41 | 5•85 | -56•38% | <0•0001 |
| Adenovirus | 3•54 | 1•00 | -71•75% | <0•0001 | 2•44 | 0•54 | -77•87% | 0•31 | 4•24 | 0•72 | -83•02% | <0•0001 | 3•17 | 1•39 | -56•15% | 0•0086 |
| Others | 4•44 | 1•24 | -72•07% | <0•0001 | 6•01 | 0•00 | -100•00% | 0•0052 | 3•75 | 1•44 | -61•60% | 0•00037 | 4•75 | 1•42 | -70•11% | <0•0001 |
| Bacteria | ||||||||||||||||
| DEC | 7•41 | 4•50 | -39•27% | <0•0001 | 3•15 | 1•52 | -51•75% | 0•22 | 9•48 | 6•65 | -29•85% | 0•00083 | 5•95 | 2•23 | -62•52% | <0•0001 |
| NTS | 4•90 | 8•16 | 66•53% | <0•0001 | 2•42 | 2•62 | 8•26% | 0•84 | 6•10 | 10•11 | 65•74% | <0•0001 | 4•15 | 6•75 | 62•65% | <0•0001 |
| V. parahaemolyticus | 1•80 | 1•77 | -1•67% | 0•47 | 0•13 | 0•00 | -100•00% | 1•00 | 2•33 | 2•14 | -8•15% | 0•14 | 1•61 | 1•66 | 3•11% | 0•77 |
| C. jejuni | 1•11 | 0•97 | -12•61% | 0•64 | 1•56 | 1•24 | -20•51% | 1•00 | 1•03 | 1•49 | 44•66% | 0•11 | 1•06 | 0•19 | -82•08% | 0•012 |
| C. coli | 0•21 | 0•40 | 90•48% | 0•041 | 0•21 | 0•55 | 161•90% | 1.00 | 0•20 | 0•52 | 160•00% | 0•0075 | 0•22 | 0•19 | -13•64% | 0•055 |
| Others | 2•82 | 1•57 | -44•33% | <0•0001 | 0•95 | 1•11 | 16•84% | 1•00 | 3•54 | 1•76 | -50•28% | 0•00024 | 2•46 | 1•46 | -40•65% | 1•00 |
* DEC, diarrheagenic Escherichia coli; NTS, nontyphoidal Salmonella
Total: Jan 23 to Dec 31; Phase I: Jan 1 to Jan 22; Phase II: Jan 23 to Apr 7; Phase III: Apr 8 to Aug 31; Phase IV: Sep 1 to Dec 31. The p-values were based on chi-square test. Blue (red) color indicates statistically significant decrease (increase) of test positive rate During-COVID-19 compared to Pre-COVID-19.
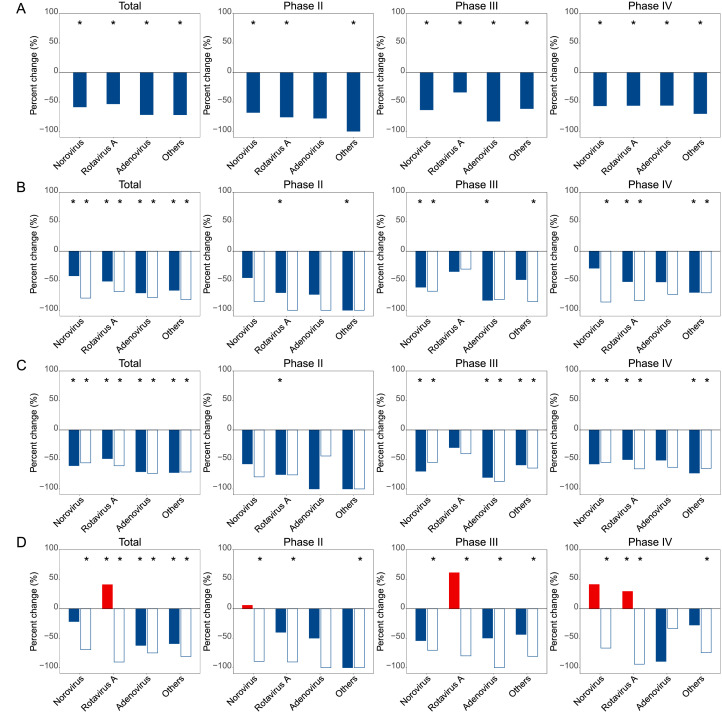
Persistent decreases of almost all viruses were displayed across phases II–IV. The most pronounced decrease was observed in Phase II, with the combined other viruses had the largest significant decrease of 100•00% (p=0•0052) (Table 1). During Phase III, the largest drop was observed for adenovirus (83•02%, p<0•0001) and during Phase IV, a comparable drop was seen for the three main viruses (Table 1, Fig. 1A). Despite of the overt decrease activity of norovirus, the genotype composition remained unchanged, with genogroup II (GII) as the dominant subtypes across the phases (Appendix p 9).
Fig. 1.
Percent change of viral test positive rate in the During-COVID-19 year 2020 compared to the average incidences in the Pre-COVID-19 years 2012–2019 for each of three predefined periods and stratified by age, sex and region. (A) Overall; (B) Children <18 years old (solid bars) vs. adults ≥18 years old (unfilled bars); (C) Male (solid bars) vs. female (unfilled bars); (D) North (solid bars) vs. South (unfilled bars). Red and blue bars indicate positive and negative percent changes, respectively. Statistically significant changes were marked with asterisks.
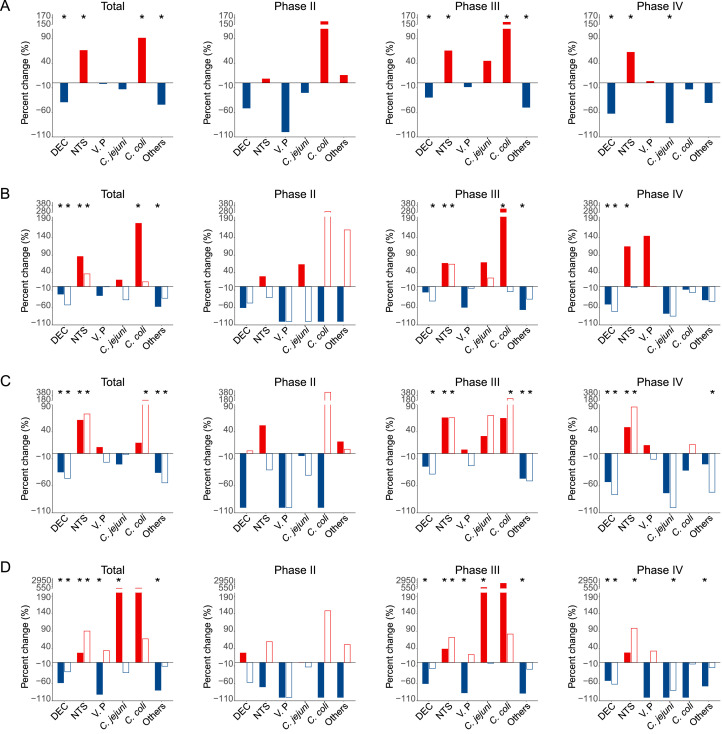
The overall bacterial activity as measured by percentage of submitted specimens positive for any of thirteen bacteria tested decreased by 37•56% (from 6•71% during 2012‒2019 to 4•19% in 2020, p=0•28) in Phase I, by 14•56% (from 8•24% during 2012‒2019 to 7•04% in 2020, p=0•66) in Phase II, by 0•05% (from 21•33% to 21•32%, p=0•85) in Phase III, and by 19•42% (from 14•78% to 11•91%, p=0•018) in Phase IV. Significant decrease of positive rate from Pre-COVID-19 to During-COVID-19 epidemic was noted for DEC (by 39•27% from 7•41% to 4•50%, p<0•0001) and other bacteria combined, however increase was observed for NTS (by 66•53% from 4•90% to 8•16%, p<0•0001), and C. coli (by 90•48% from 0•21% to 0•40%, p=0•041). Importantly the decrease of DEC and increase of NTS were seen during all three phases, with the greatest extent of increase seen in phases III (65•74%, p<0•0001) and IV (62•65%, p<0•0001) (Table 1, Fig. 2A). The ranking of bacteria was also altered, with NTS having taken over DEC to become the top listing bacterial enteric pathogen During-COVID-19. The commonly seen bacteria, V. parahaemolyticus, was detected with comparable levels for During-COVID-19 and Pre-COVID-19 epidemic, while C. jejuni, C. coli, and V. cholerae had increased their ranking, and shigella, A. hydrophila, and P. shigelloides had dropped in the order of bacteria (Appendix p 8).
Fig. 2.
Percent change of bacterial test positive rate in the During-COVID-19 year 2020 compared to the average incidences in the Pre-COVID-19 years 2012–2019 for each of three predefined periods and stratified by age, sex and region. (A) Overall; (B) Children <18 years old (solid bars) vs. adults ≥18 years old (unfilled bars); (C) Male (solid bars) vs. female (unfilled bars); (D) North (solid bars) vs. South (unfilled bars). Red and blue bars indicate positive and negative percent changes, respectively. Statistically significant changes were marked with asterisks.
Further subtypes of DEC revealed an altered genotype composition during COVID-19. Two of the three predominant genotypes, EAEC and ETEC were tested with reduced activity, while EPEC was increased instead, thus leading to their ranking change during COVID-19, i.e., EPEC > EAEC > ETEC (Appendix p 9).
Change pattern of positive rates by age, gender and geographic regions
Subgroup analysis revealed the extent of reduction for norovirus, adenovirus and rotavirus A differed according to age and geographic regions, but not for gender. More reductions were observed among adults rather than pediatric patients for all three viruses (Fig. 1B). Both norovirus and rotavirus A were observed with significant reduction in Southern China (Fig. 1D). For the other uncommon viruses, the reduction of their combined positive rate showed no significant difference regarding age, gender or geographic regions (Fig. 1B‒D). When three phases were separately analyzed, the change patterns were largely consistent between the two sexes and among age groups, displaying uniform decrease of activity during phases II‒ IV. Regional differences were observed, however, in that the activity of rotavirus A resurged in Phase IV in the north region of China, by contrast with a sustained decrease in the South China (Fig. 1B‒D).
The change patterns of bacterial activity also differed to some extent among age groups between Northern China and Southern China. For example, the increase of NTS was observed with higher extent in pediatric patients over adult patients, in females over males, or in southern over northern China (all p<0•050, Fig. 2B‒D). The reduction of DEC was observed with greater extent among adult than pediatric patients, in North China than South China, and among female patients than male (all p<0•050). The reduction of V. parahaemolyticus significant occurred in North China (Fig. 2B‒D). When phases were separately displayed, different phases showed different change patterns, and more rebound was observed during Phase III. A resurge was found in NTS and C. coli during Phase III among age group, with more pronounced change among children than adults (Fig. 2B). The increase of NTS activity occurred significantly in both regions during Phase III, but only among South China during Phase IV (Fig. 2D), which was mostly attributable to the resurgence for the adults in the Phase IV.
Coinfection pattern
The coinfection rate of two or more viruses decreased from 2•83% (1249/44 165) in Pre-COVID-19 to 0•85% (15/1 772) During-COVID-19. The most frequent coinfection type in Pre-COVID-19 period occurred between norovirus and rotavirus A (0•87%), followed by adenovirus - norovirus coinfection (0•41%) and adenovirus - rotavirus A coinfection (0•39%). The same the top 3 listing coinfection type was observed in During-COVID-19, although with their ranked reversed slightly, i.e., norovirus - rotavirus A (0•51%), adenovirus - rotavirus A (0•13%), and adenovirus - norovirus (0•06%).
The coinfection rate of two or more bacteria was comparable between Pre-COVID-19 and During-COVID-19 (0•93%, 598/64 604 vs. 0•90%, 25/2 791). The top listing coinfection type remained as NTS - DEC (in 0•27% of the Pre-COVID-19 and 0.38% of the During-COVID-19), which was followed by V. parahaemolyticus - DEC (0•13%) and V. parahaemolyticus - NTS (0•05%) in Pre-COVID-19, V. parahaemolyticus - NTS (0•11%) and C. jejuni - DEC (0•08%) in During-COVID-19 (Appendix p 10, Appendix p 11).
Effects of NPIs on seasonality and activity of enteric pathogens
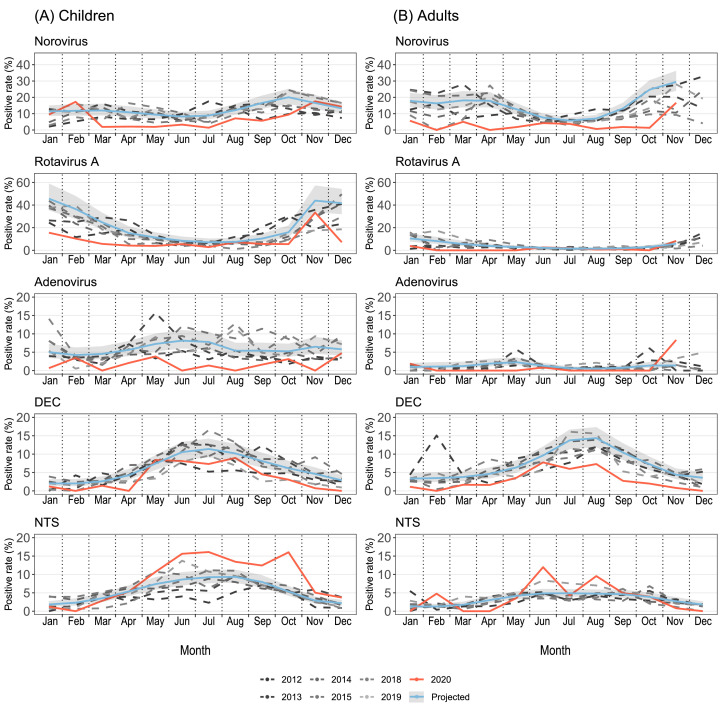
Generalized linear models (GLM) were fitted to temporal trajectories of monthly positive rates of the pathogens by age group (Fig. 3) and by region, South vs. North (Appendix p 12). The models based on the Pre-COVID-19 data (dashed carves) were projected to 2020 (blue curves and the 95% confidence bands) for the hypothetical scenario without the COVID-19 pandemic. Most pathogens had clear seasonality before the pandemic, featured by the peak of norovirus and rotavirus A in October‒February, peaking of DEC and NTS in June‒August. Under the effect of adopting NPIs, the peaks of most pathogens were suppressed or flattened except for NTS, for which the actual activity exceeded historical levels after the relaxation of NPIs (Fig. 3, Appendix p 12). The historical pattern of bimodal peaks of norovirus was clearly interrupted During-COVID-19. Accompanied by the relax or the lift of NPIs, a rebound was found in NTS for both age and region group, with a great extent in Southern than Northern China, and in children than adults. Rotavirus A resurged during Phase IV in Northern China but not in Southern China (Appendix p 12).
Fig. 3.
Observed and model-fitted monthly numbers of test positive samples by age groups. (A) Children <18 years old, (B) Adults ≥18 years old. Five pathogens were investigated: norovirus, rotavirus A, adenovirus, diarrheagenic Escherichia coli (DEC), nontyphoidal Salmonella (NTS). The data from 2016 and 2017 were excluded because of poor sampling. Different shades of dashed curves indicate observed monthly positive rates of enteric pathogens before 2020, and the observed monthly positive rates in 2020 were colored red. The model-projected trajectories in 2020 are shown in blue for the hypothetical scenario without the COVID-19 pandemic, and the 95% confidence bands were shaded grey.
Based on the GLMs, we re-assessed the difference in positive rate for these selected five pathogens between Pre-COVID-19 and During-COVID-19 for each phase separately (Table 2). As the GLMs were further adjusted for seasonality, Odds Ratios (ORs) could better capture the effectiveness of the NPIs than do the percent changes shown. The results between ORs and percentage change are largely consistent, with some subtle difference (Table 2, Fig. 1 and Fig. 2). OR of higher than 1 which indicated a resurge was observed for rotation A (OR: 1•93, 95% CI: 1•09‒3•39) exclusively in Phase IV among Northern China, in contrast with a sustained decrease among Southern China, in Phase IV (OR: 0•12, 95% CI: 0•04‒0•38). Decrease of norovirus was greater in adults than children, and in Southern than Northern China. The odds of positive samples for DEC were substantially reduced in either Phase III or Phase IV, for both age and region group. NTS showed strong early resurgence after the relax of NPIs on both age group and Southern China, but appeared less pronounced on Northern China, as the ORs were not significant.
Table 2.
GLM-estimated odds ratios (OR) for the odds of a positive test between Pre-COVID-19 and During-COVID-19. Statistically significant increases (decreases) were colored in red (blue). Children and adults were defined as 0–17 years and ≥18 years old, respectively.
|
Phase II† |
Phase III† |
Phase IV† |
|||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | ||
| Region | |||||||
| North | Viruses | ||||||
| Norovirus | 1•45 (0•51‒4•14) | 0•49 | 0•47 (0•19‒1•14) | 0•096 | 1•23 (0•62‒2•42) | 0•56 | |
| Rotavirus A | 0•55 (0•17‒1•74) | 0•31 | 1•62 (0•88‒3•00) | 0•12 | 1•93 (1•09‒3•39) | 0•023 | |
| Adenovirus | 0•82 (0•11‒6•26) | 0•85 | 0•53 (0•20‒1•40) | 0•20 | 0•16 (0•02‒1•16) | 0•070 | |
| Bacteria* | |||||||
| DEC | 0•68 (0•09‒5•16) | 0•71 | 0•39 (0•20‒0•78) | 0•0081 | 0•31 (0•10‒0•98) | 0•046 | |
| NTS | 0‡ | - | 1•51 (1•00‒2•30) | 0•052 | 1•64 (0•98‒2•75) | 0•061 | |
| South | Viruses | ||||||
| Norovirus | 0•12 (0•02‒0•88) | 0•037 | 0•27 (0•13‒0•58) | 0•00073 | 0•31 (0•17‒0•59) | 0•00031 | |
| Rotavirus A | 0•17 (0•04‒0•74) | 0•017 | 0•26 (0•09‒0•69) | 0•0075 | 0•12 (0•04‒0•38) | 0•00034 | |
| Adenovirus | 0‡ | - | 0‡ | - | 0•70 (0•29‒1•68) | 0•42 | |
| Bacteria | |||||||
| DEC | 0•31 (0•04‒2•24) | 0•24 | 0•86 (0•61‒1•21) | 0•39 | 0•31 (0•15‒0•64) | 0•0014 | |
| NTS | 1•10 (0•32‒3•77) | 0•88 | 1•85 (1•40‒2•43) | <0•0001 | 1•72 (1•16‒2•54) | 0•0070 | |
| Age groups | |||||||
| Children | Viruses | ||||||
| Norovirus | 0•60 (0•20‒1•83) | 0•37 | 0•40 (0•20‒0•83) | 0•013 | 0•73 (0•44‒1•21) | 0•22 | |
| Rotavirus A | 0•35 (0•13‒0•91) | 0•031 | 0•65 (0•33‒1•30) | 0•23 | 0•53 (0•29‒0•99) | 0•048 | |
| Adenovirus | 0•34 (0•05‒2•49) | 0•29 | 0•19 (0•06‒0•58) | 0•0034 | 0•44 (0•17‒1•10) | 0•079 | |
| Bacteria* | |||||||
| DEC | 0•48 (0•07‒3•53) | 0•47 | 0•70 (0•45‒1•10) | 0•122 | 0•44 (0•21‒0•93) | 0•031 | |
| NTS | 0•61 (0•12‒3•08) | 0•55 | 1•83 (1•38‒2•43) | <0•0001 | 2•31 (1•58‒3•37) | <0•0001 | |
| Adults | Viruses | ||||||
| Norovirus | 0•23 (0•03‒1•69) | 0•15 | 0•25 (0•10‒0•62) | 0•0026 | 0•19 (0•08‒0•45) | 0•00019 | |
| Rotavirus A | 0‡ | - | 0•81 (0•32‒2•04) | 0•66 | 0•43 (0•10‒1•82) | 0•26 | |
| Adenovirus | 0‡ | - | 0•23 (0•03‒1•69) | 0•15 | 0•42 (0•06‒3•19) | 0•41 | |
| Bacteria* | |||||||
| DEC | 0•40 (0•05‒2•96) | 0•37 | 0•63 (0•40‒0•99) | 0•045 | 0•22 (0•08‒0•59) | 0•0026 | |
| NTS | 0•83 (0•11‒6•15) | 0•86 | 1•57 (1•06‒2•34) | 0•025 | 0•97 (0•55‒1•71) | 0•92 | |
DEC, diarrheagenic Escherichia coli; NTS, nontyphoidal Salmonella
Phase II: Feb and Mar of 2020, 2020; Phase III: Apr 2020 to Aug 2020; Phase IV: Sep 2020 to Dec 2020
When OR=0, the 95% CI and p-value were not calculated.
Discussion
The current study explored the national surveillance data of acute diarrhea related to 20 common enteric pathogens in China during the last decade. Both the intensity and timing of enteric pathogen activity was changed alongside the implementation and subsequent relaxation of NPIs in response to the COVID-19 pandemic.
During the COVID-19 epidemic with intense NPI implementation in 2020, patients with acute diarrhea captured by the surveillance system decreased dramatically comparing with Phase I, when NPI had not yet been massively initiated. In a consistent manner, a study from the Emergency Department of Yale University Medical School reported that the emergency department visit counts decreased in five health care systems in five states during the first 4 months of 2020 [23]. This phenomenon might be ubiquitous and primarily due to the change of healthcare-seeking behavior of patients, since most of the diarrhea was clinically mild, and the patients might not seek medical care for fear of being exposed to COVID-19 or in response to the reduced access to the medical services [24,25].
The substantial decline of norovirus in 2020 from an historical baseline rate in Pre-COVID-19 was observed, which aligned with results from England, Germany, Australia and the United States [13,[26], [27], [28]]. Our results also extended the available finding by showing that rotavirus A, adenovirus, and DEC were likewise reduced for their activity in 2020. This effect was ubiquitous for most of the tested pathogens, logically supporting the role of NPIs (e.g., social isolation, hand hygiene, face masks, school closures, etc.) against the transmission of common enteropathogens [26], [27], [28]. Although with fecal-oral transmission route, the enteric pathogens were likewise susceptible to most of the NPIs that were aimed to contain the respiratory transmission. The most extraordinary effect might be exerted from the restrictions on social distancing, and the closure of public facilities, which had significantly reduced the risk of diarrhea from dining-out. This can be reflected by the greater extent of reduction of viral and most bacterial pathogens in the adults than the pediatric patients. Personal protective measures (wearing masks, hand hygiene, etc.) all effectively contained the spread of disease by reducing the exposure to the pathogen and reducing their load in the environment [29].
In an unexpected way, the incidence of NTS was increased above the historical level throughout the year of 2020, with particular higher level during the phases III and IV. Several explanations could be proposed. According to the Pre-COVID-19 data, higher frequency of fever was presented in patients with NTS infection than all other bacterial infection or viral infection (Appendix p 13), this might be related to a higher probability of seeking medical care than those with other enteric infection, thus indirectly resulting in its increased detection During-COVID-19. Use of broad-spectrum antibiotics during COVID-19 epidemic might be decreased, as has been described in South Korea [30]. Therefore the NTS might be more easily to be detected than the counterpart before the COVID-19. On the other hand, the resurgence of NTS and the several other enteric bacteria was observed. This might be rendered by the lift of NPIs, especially the reopening of public facility, school reopening, and relaxed social distance, together with the accumulation of susceptible population, all contributed to the increased transmission of enteric pathogens [31]. This phenomenon coincided with the recent finding from Australia, where the relax of COVID-19 NPIs was followed by a sudden increase in gastroenteritis outbreaks, peaking in November 2020 [32]. In contrast to bacteria pathogens, the activity of most viruses remained lower than historical levels consistently throughout the pandemic year, regardless of region and age group. Although the NPIs at public level was relaxed, some NPIs at the individual level such as mask wearing and hand hygiene were still in place, which could be highly effective against enteric virus which was likely to be acquired via multiple transmission routes. Especially when considering that infectious enteric virus persists not only on surfaces but also in aerosolization of virus-laden dust particles [33], it is logical to deduce that viral transmission in air can be reduced by these personal protective factors, thus resulting in the decreased detection. Based on these findings, we suggest that NPI is broadly effective in reducing the incidence of acute diarrhea - especially from viral causes. This indicates a potential risk of marked increases in disease activity when NPIs are relaxed. Parents, public health officials, and school officials should be made aware of this risk.
The diversity of age distribution might reflect a natural change in host immunity and/or dietary habit that is related to age. For example, norovirus and DEC, two pathogens commonly acquired by travelling or dining out, showed remarkably reduced activity in adults compared to children. This is in line with other reports stating that people with higher rates of social contact are more likely to contract infections [34,35]. In general during COVID-19 pandemic, northern China had higher magnitude of decrease in bacterial positive detection than southern China, with a reverse pattern for viruses between the two regions. The regional variation of the pathogen changes might possibly reflect the heterogeneity in the level of maintaining NPIs for preventing COVID-19 resurgence or might due to the more strict NPIs that were implemented in responses to the local COVID-19 outbreaks in several north regions during phases III−IV, such as in Mudanjiang, Beijing, Urumqi, Dalian, and Qingdao (Appendix p 4). The meteorological factors (temperature, humidity and wind speed, etc.) might have affected the incidence of enteric pathogen in a complicated way as we have specified in our previous studies [17].
Despite the reduced overall circulation of most viruses, the dominant genotypes of these viruses remained largely unchanged during the pandemic. For example, the norovirus GII still constituted the majority of their genera. The reversion of top listing DEC pathotype that were observed between EPEC and EAEC and ETEC are more likely driven by specific dynamics of population-level immunity profiles. Although the detection numbers of genotypes of norovirus and some of DEC were smaller during COVID-19 than Pre-COVID-19, the dominant genotypes remained unchanged, which was illustrative in planning for the future immunization campaign.
Our study has several limitations. Firstly, causality between enteric pathogen activity and NPI measures cannot be inferred from the surveillance data. Secondly, although the inclusion/exclusion criteria for the testing stayed the same during the pandemic, the total number of tests did decrease, and the profile of people who seek medical assistance for acute diarrhea might has shifted owing to the changed healthcare-seeking behavior during the COVID-19 pandemic, especially only the outpatients were enrolled in this surveillance. National health facility visits were estimated to decrease by about 23•9% in China from January to June 2020, indicating influence from the behaviors changes for patient and provider, suspension of health facilities or their non-emergency services, and massive mobility restriction, which were also influenced by the potential reduction in the risk of non-SARS-COV-2 diseases [36]. However, the reasons underlying the observed decrease in positive detection for enteric pathogens are complicated, which warrant further investigations to measure in a precise multi-factorial manner. Thirdly, monthly data redefined for GLM did not fully reflect the specific four periods according to the timeline of major intervention events for containing the COVID-19 epidemic in China. On the other hand, the current study had advantages that made use of large sample size established on the same inclusion/exclusion criteria, which allowed the adequate application of GLM to generate temporal trajectories of monthly positive rates, and estimate the effects of NPIs stratified by age and region. This study also shed light on more detailed analysis in specific regions, when specific policy changes to pathogen transmission could be temporally linked.
In conclusion, the NPIs implemented in the early pandemic greatly inhibited circulation of common enteric virus causative of acute diarrhea, and the impact was nearly universal across demographic groups and geographic regions. Still, a less strong effect was observed for enteric bacteria. It's highlighted that adults might be highly susceptible to the effect that was derived from the relief of the containment strategy thus could pose as a high priority for surveillance and control of acute diarrhea. At the personal level, behavior-based prevention measures, such as more frequent and meticulous hand hygiene and mask-wearing should be advocated as part of long-term prevention efforts beyond the COVID-19, in order to curb the possible rebounding or outbreaks in the post-COVID-19 era. Our findings might not be unique to China, and therefore might enhance the understanding of the indirect impacts of COVID-19 related NPIs on other infectious disease in other countries. Similar studies should be conducted worldwide to investigate the generalizability of these finding and to help with for more effective control of acute diarrhea in the post-pandemic era.
Contributors
L-PW, Z-JL, W-ZY, G-FG, YY, S-IH, L-QF and WL conceived, designed, and supervised the study. J-YH, S-XZ, L-JY, Q-BL, X-AZ, H-YZ, XR, C-HZ, Y-FW, S-HL, QX, B-GJ, C-LL, J-JC, C-JL cleaned and analyzed the data. WL, J-YH, S-XZ, YY, and L-QF wrote the draft of the manuscript and interpreted the findings. Z-JL, L-PW, YY, L-QF, WL, and S-IH commented on and revised drafts of the manuscript. L-QF and WL directly accessed and verified the underlying data. All authors read and approved the final report.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The Chinese Centers for Disease Control and Prevention (CDC) Etiology of Diarrhea Surveillance Study Team
-
1
Chinese Center for Disease Control and Prevention, Beijing, China: Wei-Zhong Yang; George F. Gao.
-
2
Division of Infectious Disease, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China: Zhong-Jie Li; Li-Ping Wang; Xiang Ren; Yi-Fei Wang; Sheng-Hong Lin; Cui-Hong Zhang; Meng-Jie Geng.
-
3
National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China: Xin Wang; Huai-Qi Jing.
-
4
National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China: Wen-Bo Xu; Ai-Li Cui.
-
5
National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Shanghai, China: Yu-Juan Shen; Yan-Yan Jiang.
-
6
Center of Disease Prevention and Control in Pudong New Area of Shanghai, Shanghai, China: Qiao Sun; Li-Peng Hao; Chu-Chu Ye.
-
7
State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, Beijing, China: Wei Liu; Xiao-Ai Zhang.
-
8
The Institute for Disease Prevention and Control of PLA, Beijing, China: Liu-Yu Huang; Yong Wang; Wen-Yi Zhang.
-
9
Wuhan University, Wuhan, China: Ying-Le Liu; Jian-Guo Wu; Qi Zhang.
-
10
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China: Wei-Yong Liu; Zi-Yong Sun.
-
11
Hubei Provincial Center for Disease Control and Prevention, Wuhan, China: Fa-Xian Zhan.
-
12
Jiangxi Provincial Center for Disease Control and Prevention, Nanchang, China: Ying Xiong.
-
13
Gansu Provincial Center for Disease Control and Prevention, Lanzhou, China: Lei Meng; De-Shan Yu.
-
14
Qinghai Provincial Center for Disease Control and Prevention, Xining, China: Chun-Xiang Wang; Sheng-Cang Zhao.
-
15
Inner Mongolia Autonomous Region Comprehensive Center for Disease Control and Prevention, Hohhot, China: Wen-Rui Wang; Xia Lei.
-
16
Lanzhou University, Lanzhou, China: Juan-Sheng Li.
-
17
Lanzhou Center for Disease Control and Prevention, Lanzhou, China: Yu-Hong Wang; Yan Zhang.
-
18
Baiyin Center for Disease Control and Prevention, Baiyin, China: Jun-Peng Yang; Yan-Bo Wang.
-
19
Tianshui Center for Disease Control and Prevention, Tianshui, China: Fu-Cai Quan; Zhi-Jun Xiong.
-
20
Wuwei Center for Disease Prevention and Control, Wuwei, China: Li-Ping Liang; Quan-E Chang.
-
21
Qingyang Center for Disease Control and Prevention, Qingyang, China: Yun Wang; Ping Wang.
-
22
Liaoning Provincial Center for Disease Control and Prevention, Shenyang, China: Zuo-Sen Yang; Ling-Ling Mao.
-
23
Tianjin Center for Disease Control and Prevention, Tianjin, China: Jia-Meng Li; Li-Kun Lv.
-
24
Heilongjiang Provincial Center for Disease Control and Prevention, Harbin, China: Jun Xu; Chang Shu.
-
25
Zhejiang University, Hangzhou, China: Xiao Chen; Yu Chen.
-
26
Zhejiang Center for Disease Control and Prevention, Hangzhou, China: Yan-Jun Zhang.
-
27
Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China: Lun-Biao Cui.
-
28
Fujian Center for Disease Control and Prevention, Fuzhou, China: Kui-Cheng Zheng.
-
29
Beilun People's Hospital, Ningbo, China: Xing-Guo Zhang.
-
30
Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China: Xi Zhang; Li-Hong Tu.
-
31
Shanghai Public Health Clinical Center, Shanghai, China: Zhi-Gang Yi; Wei Wang.
-
32
Yunnan Center for Disease Control and Prevention, Kunming, China: Shi-Wen Zhao; Xiao-Fang Zhou.
-
33
Sichuan University, Chengdu, China: Xiao-Fang Pei; Tian-Li Zheng.
-
34
Chongqing Medical University, Chongqing, China: Xiao-Ni Zhong.
-
35
Chongqing Center for Disease Control and Prevention, Chongqing, China: Qin Li; Hua Ling.
-
36
Guizhou Center for Disease Control and Prevention, Guiyang, China: Ding-Ming Wang; Shi-Jun Li.
-
37
Sichuan Province Center for Disease Control and Prevention, Chengdu, China: Shu-Sen He.
-
38
Sun Yat-sen University, Guangzhou, China: Meng-Feng Li; Jun Li; Xun Zhu.
-
39
Guangdong Provincial Center for Disease Control and Prevention, Guangzhou, China: Chang-Wen Ke; Hong Xiao.
-
40
Guangzhou Municipal Center for Disease Control and Prevention, Guangzhou, China: Biao Di; Ying Zhang.
-
41
Zhujiang Hospital, Southern Medical University, Guangzhou, China: Hong-Wei Zhou; Nan Yu.
-
42
Jinan University, Guangzhou, China: Hong-Jian Li; Fang Yang.
-
43
The Third People's Hospital of Shenzhen, Shenzhen, China: Fu-Xiang Wang; Jun Wang.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
The authors would like thank all the subjects, their families, and collaborating clinicians for their participation. This work was financially supported by grants from the China Mega-Project on Infectious Disease Prevention (No. 2018ZX10713001, 2018ZX10713002 and 2018ZX10201001 and 2017ZX10103004), the National Natural Science Funds (No. 81825019, 91846302). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100268.
Contributor Information
Zhong-Jie Li, Email: lizj@chinacdc.cn.
Yang Yang, Email: yangyang@ufl.edu.
Wei Liu, Email: liuwei@bmi.ac.cn.
Li-Qun Fang, Email: fang_lq@163.com.
Appendix. Supplementary materials
References
- 1.Coronavirus Resource Center. Johns Hopkins University. https://coronavirus.jhu.edu/map.html (accessed June 21, 2021 ).
- 2.World Health Organization. Weekly epidemiological update on COVID-19 - 15 June 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—15-june-2021 (accessed June 21, 2021).
- 3.Garcia-Beltran WF, Lam EC, St Denis K. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9):2372–2383. doi: 10.1016/j.cell.2021.03.013. .e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J, Peng P, Wang K. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell Mol Immunol. 2021;18(4):1061–1063. doi: 10.1038/s41423-021-00648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 pandemic lockdowns. https://en.wanweibaike.com/wiki-COVID-19%20lockdowns (accessed June 21, 2021 ).
- 6.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang QS, Wood T, Jelley L. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat commun. 2021;12(1):1001. doi: 10.1038/s41467-021-21157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117(48):30547–30553. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennon RP, Miller EL, Dong H, Rabago D, Zgierska AE. Association of the US COVID-19 pandemic and attenuated influenza detection. South Med J. 2021;114(6):343. doi: 10.14423/SMJ.0000000000001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennon RP, Miller EL, Snyder B, Van Scoy LJ. Self-reported influenza and influenza-like symptoms in U.S. adults age 18-64 between September 1, 2019 and April 15, 2020. J Clin Virol. 2021;134 doi: 10.1016/j.jcv.2020.104709. [DOI] [PubMed] [Google Scholar]
- 11.Yeoh DK, Foley DA, Minney-Smith CA. The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trenholme A, Webb R, Lawrence S. COVID-19 and infant hospitalizations for seasonal respiratory virus infections, New Zealand, 2020. Emerg Infect Dis. 2021;27(2):641–643. doi: 10.3201/eid2702.204041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas A, Sandmann FG, Allen DJ, Celma CC, Beard S, Larkin L. Impact of COVID-19 on national surveillance of norovirus in England and potential risk of increased disease activity in 2021. J Hosp Infect. 2021;112:124–126. doi: 10.1016/j.jhin.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Kraemer MUG, Yang CH, Gutierrez B. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368(6490):493–497. doi: 10.1126/science.abb4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian HY, Liu YH, Li YD. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368(6491):638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JM, Zhang L, Yan YQ. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. doi: 10.1136/bmj.n415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LP, Zhou SX, Wang X. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12(1):2464. doi: 10.1038/s41467-021-22551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing HQ, Huang LY, Duan ZJ. Sun Yat-Sen University Press; Guangzhou: 2016. Pathogen surveillance and detection techniques: diarrhea syndrome. (in Chinese) [Google Scholar]
- 19.Troubleshooting with glmmTMB. https://cran.r-project.org/web/packages/glmmTMB/vignettes/troubleshooting.html (accessed June 21, 2021 ).
- 20.Partridge E, McCleery E, Cheema R. Evaluation of seasonal respiratory virus activity before and after the statewide COVID-19 shelter-in-place order in Northern California. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.35281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najera-Zuloaga J, Lee DJ, Arostegui I. Comparison of beta-binomial regression model approaches to analyze health-related quality of life data. Stat Methods Med Res. 2018;27(10):2989–3009. doi: 10.1177/0962280217690413. [DOI] [PubMed] [Google Scholar]
- 22.Barnett A, Baker P, Dobson A. Analysing seasonal data. R Journal. 2012;4(1):5–10. [Google Scholar]
- 23.Jeffery MM, D'Onofrio G, Paek H. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020;180(10):1328–1333. doi: 10.1001/jamainternmed.2020.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pines JM. COVID-19, Medicare for all, and the uncertain future of emergency medicine. Ann Emerg Med. 2020;76(4):459–461. doi: 10.1016/j.annemergmed.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 26.Eigner U, Verstraeten T, Weil J. Decrease in norovirus infections in Germany following COVID-19 containment measures. J Infect. 2021;82(6):276–316. doi: 10.1016/j.jinf.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruggink LD, Garcia-Clapes A, Tran T, Druce JD, Thorley BR. Decreased incidence of enterovirus and norovirus infections during the COVID-19 pandemic, Victoria, Australia, 2020. Commun Dis Intell. 2021;(2018):45. doi: 10.33321/cdi.2021.45.5. [DOI] [PubMed] [Google Scholar]
- 28.Lennon RP, Griffin C, Miller EL, Dong H, Rabago D, Zgierska AE. Norovirus infections drop 49% in the United States with strict COVID-19 public health interventions. Acta Med Acad. 2020;49(3):278–280. doi: 10.5644/ama2006-124.317. [DOI] [PubMed] [Google Scholar]
- 29.Baker TL, Greiner JV. Guidelines for reopening a nation in a SARS-CoV-2 pandemic: A path forward. Medicina (Kaunas) 2021;57(5) doi: 10.3390/medicina57050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu S, Hwang Y, Ali ST. Decreased use of broad-spectrum antibiotics during COVID-19 epidemic in South Korea. J Infect Dis. 2021 doi: 10.1093/infdis/jiab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraay ANM, Han P, Kambhampati AK, Wikswo ME, Mirza SA, Lopman BA. Impact of non-pharmaceutical interventions (NPIs) for SARS-CoV-2 on norovirus outbreaks: an analysis of outbreaks reported by 9 US States. J Infect Dis. 2021 doi: 10.1093/infdis/jiab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NSW. Government. Communicable disease weekly report e weeks 48 and 49, 2020. Sydney: NSW Government; 2020. https://www.health.nsw.gov.au/Infectious/Reports/Pages/cdwr-2020.aspx. (accessed June 21, 2021 )
- 33.Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991;13(3):448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 34.Collender PA, Morris C, Glenn-Finer R. Mass Gatherings and diarrheal disease transmission among rural communities in coastal Ecuador. Am J Epidemiol. 2019;188(8):1475–1483. doi: 10.1093/aje/kwz102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenberg JN, Cevallos W, Ponce K. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc Natl Acad Sci U S A. 2006;103(51):19460–19465. doi: 10.1073/pnas.0609431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H, Dai XC, Wagenaar BH. The impact of the COVID-19 pandemic on health services utilization in China: Time-series analyses for 2016–2020. Lancet Reg Health West Pac. 2021;9 doi: 10.1016/j.lanwpc.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.