The messenger ribonucleic acid (mRNA) - based BNT162b2 (Pfizer, Inc) and mRNA-1273 vaccines (Moderna, Inc) were demonstrated in large randomized clinical trials to be highly effective in preventing severe illness from COVID-19 and to have acceptable safety profiles [1], [2]. Given the short timeline between development and widespread utilization of this novel vaccine technology, however, careful investigation of possible unexpected adverse effects during real-world use is essential. In this report, we describe 2 cases of myocardial inflammation temporally associated with receipt of a COVID-19 mRNA vaccine in patients without evidence of concurrent or prior COVID-19 infection.
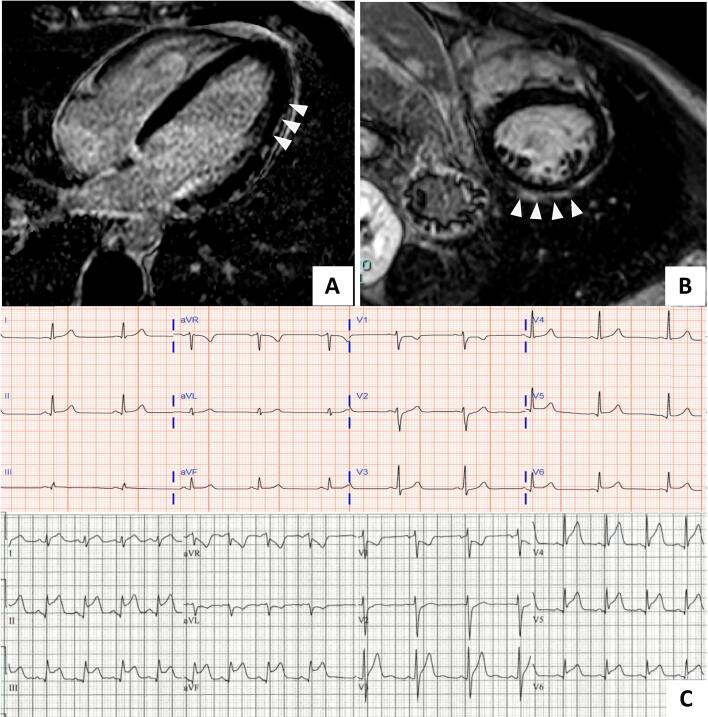
We describe 2 patients with acute myocarditis occurring within 72 h after the administration of mRNA-based COVID-19 vaccine. Clinical presentations, in-hospital events, treatments, and outcomes are presented in the Table 1. Cardiac magnetic resonance imaging findings and electrocardiograms of both patients with myocarditis are shown in Fig. 1. Both patients were young males without significant medical history, and presented 2–3 days after receiving the second vaccine dose. No patient had a history of prior or current COVID-19 infection, and all had negative RT-PCR nasopharyngeal swab testing for COVID-19 during their hospital course. With prompt recognition and treatment, all patients recovered quickly and were discharged from the hospital. The second patient followed up at 3 months after discharge and was completely asymptomatic without any other side effects.
Table 1.
Patient clinical characteristics, in-hospital events and outcomes.
| Patient 1 | Patient 2 | |
|---|---|---|
| Age (years) | 28 | 21 |
| Sex | Male | Male |
| Ethnicity | Caucasian | Caucasian |
| Vaccine/Dose | mRNA-1273 (Moderna, Inc)/2nd dose | BNT162b2 (Pfizer, Inc)/2nd dose |
| Time from vaccine administration to hospital presentation | 3 days | 2 days |
| Presenting complaint | Chest pain | Substernal chest pain |
| Other symptoms | Fever, headache, neck pain, myalgia | Fever, chills, headache |
| Medical history | None | None |
| Vitals on presentation | T: 98.6F HR: 75 beats/min BP:118/60 mmHg O2 sat: 98% on room air |
T: 98.9F HR: 83 beats/min BP: 131/83 mmHg O2 sat: 96% on room air |
| Physical examination | Unremarkable | Unremarkable |
| Electrocardiogram | Infero-lateral ST elevation with no reciprocal changes | Diffuse ST elevation |
| Peak troponin I (ng/mL) | 7.75 | 17.0 |
| Other pertinent labs | ESR: 15 mm/hr CRP: 6 mg/dL |
ESR: 20 mm/hr CRP: 3.8 mg/dL D-dimer: 509 ng/mL RF: <10 ANA: 1:80 |
| Coronary angiogram | Normal | Not performed |
| Initial echocardiogram | LVEF 55%, mid inferolateral wall hypokinesis, normal RV systolic function, no pericardial effusion | LVEF 25%, mildly dilated RV and reduced systolic function, moderate mitral regurgitation, no pericardial effusion |
| Repeat echocardiogram | LVEF 55–60%, normal wall motion, normal RV systolic function, no pericardial effusion | LVEF 50–55%, normal wall motion. RV mildly dilated with mildly reduced function, mild mitral regurgitation |
| Cardiac MRI Performed | Yes | Yes |
| SARS-CoV-2 RT PCR | Negative | Negative |
| SARS-CoV-2 IgG antibody | Negative | Not performed |
| Other tests | Respiratory multiplex negative, Coxsackie virus serology negative |
Respiratory multiplex negative |
| Other clinical events | None | Brief episode of supraventricular tachycardia |
| Treatment | Conservative care | Solumedrol 1000 mg once daily for 3 days, colchicine 0.6 mg twice daily, losartan 25 mg daily and metoprolol succinate 25 mg daily |
| Length of stay | 3 days | 3 days |
| Clinical condition at the time of discharge | Asymptomatic | Asymptomatic |
Abbreviations: ANA: anti-nuclear antibody, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, LVEF: Left ventricular ejection fraction, MRI: magnetic resonance imaging, RF – rheumatoid factor, RV: right ventricle, RT-PCR: reverse transcriptase-polymerase chain reaction.
Normal laboratory range: BUN - < 20 mg/dL; CRP - <1.0 mg/dL; D-dimer - < 500 ng/mL; ESR – 0–15 mm/hr; Troponin - < 0.03 ng/mL.
Fig. 1.
Cardiac magnetic resonance (CMR) images and electrocardiograms at the time of diagnosis. Panel A and B: CMR image from Patient 1 and 2 respectively showing delayed hyperenhancement in the lateral epicardium (A, arrowheads) and in the pericardium and epicardial surface of the anterior and lateral wall (B, arrowheads) consistent with myocarditis by the Lake Louise criteria. Panel C shows electrocardiogram from patient 1 (top) showing diffuse ST segment elevation and patient 2 (bottom) showing sinus rhythm with marked sinus arrhythmia and diffuse ST segment elevation (II, III, aVF, V5 and V6) and ST segment depressions in aVR and V1. CMR – cardiac magnetic resonance imaging.
This case series contributes to a limited body of literature describing acute myocardial and/or pericardial inflammatory illness occurring in close temporal association with the receipt of a mRNA COVID-19 vaccine. The possibility of a connection between COVID-19 vaccination and cardiac inflammatory illness was first suggested by a prelimary report from the the Israeli Health Ministry [3] which described a small number of cases of myopericarditis among young adults after receiving the BNT162b2 vaccine. Subsequently, 3 other case reports [4], [5], [6] and 3 case series [7], [8], [9] reporting myocarditis following COVID-19 vaccination have been published. Consistent with those recent reports, in our series all affected patients were males, and their illness was characterized by an uncomplicated hospital course and uneventful recovery with conventional treatment for myopericarditis.
In the published randomized clinical trials of these two COVID-19 mRNA vaccines, cardiovascular adverse events were exceedingly rare. Those that were reported included paroxysmal ventricular arrhythmias, arteriosclerosis and cardiac arrest, but there were no occurrences of myocarditis or pericarditis [1], [2]. Myopericarditis has been reported in association with other vaccines, and the temporal relationship between the onset of symptoms and administration of the vaccine in our patients would suggest a possible causal association. Although mehanisms such as molecular mimicry between the viral spike protein and a cardiac protein or alternatively aberrant activation of the innate and acquired immune system leading to a non-specific inflammatory response have been suggested these potential mechanisms remain speculative.
It is important to consider these case reports within the broader context of the COVID-19 pandemic and the worldwide vaccination effort. COVID-19 has caused tremendous morbidity and mortality throughout the world, and the rapid development of safe and effective vaccines has provided hope that the pandemic can eventually be brought under control. Out of the more than 142 million Americans that have been vaccinated, fewer than 1000 cases of potential vaccine-associated myocarditis or pericarditis have been reported to Vaccine Adverse Event Reporting System (VAERS). An investigation by the US Centers for Disease Control (CDC) and their Advisory Committee on Immunization Practices (ACIP) is ongoing [10]. Even if a causal relationship between mRNA COVID-19 vaccination and myopericarditis is established, the incidence appears to be extremely low, the outcomes seem to be favorable, and the benefit of vaccination far outweighs any potential risk.
We report 2 cases of myocarditis occurring in close temporal relation to receipt of an mRNA COVID-19 vaccine. Clinicians should be alert to the possibility of myocarditis in patients presenting with compatible symptoms after vaccination, and appropriate diagnostic and therapeutic steps should be undertaken. Further basic and epidemiologic research is required to determine if a causal relationship exists and, if so, to elucidate the immunological basis of this aberrant inflammatory response.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Israel examining heart inflammation cases in people who received Pfizer COVID shot | Reuters, n.d. https://www.reuters.com/world/middle-east/israel-examining-heart-inflammation-cases-people-who-received-pfizer-covid-shot-2021-04-25/ (accessed May 26, 2021).
- 4.Bautista García J., Peña Ortega P., Bonilla Fernández J.A., Cárdenes León A., Ramírez Burgos L., Caballero Dorta E. Miocarditis aguda tras administración de vacuna BNT162b2 contra la COVID-19. Rev. Española Cardiol. 2021;74(9):812–814. doi: 10.1016/j.recesp.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthukumar A., Narasimhan M., Li Q.-Z., Mahimainathan L., Hitto I., Fuda F., Batra K., Jiang X., Zhu C., Schoggins J., Cutrell J.B., Croft C.L., Khera A., Drazner M.H., Grodin J.L., Greenberg B.M., Mammen P.P.A., Morrison S.J., de Lemos J.A. In Depth Evaluation of a Case of Presumed Myocarditis Following the Second Dose of COVID-19 mRNA Vaccine. Circulation. 2021 doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Rep. 2021;16(8):2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson K.F., Ammirati E., Adler E.D., Cooper L.T., Hong K.N., Saponara G., Couri D., Cereda A., Procopio A., Cavalotti C., Oliva F., Sanna T., Ciconte V.A., Onyango G., Holmes D.R., Borgeson D.D. Myocarditis after BNT162b2 and mRNA-1273 Vaccination. Circulation. 2021;144(6):506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., Damluji A.A., de Lemos J.A., Desai S.S., Emaminia A., Flanagan M.C., Khera A., Maghsoudi A., Mekonnen G., Muthukumar A., Saeed I.M., Sherwood M.W., Sinha S.S., O’Connor C.M., deFilippi C.R. Myocarditis Temporally Associated with COVID-19 Vaccination. Circulation. 2021;144(6):502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammirati E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F., Schroeder J.W., Morici N., Giannattasio C., Frigerio M., Metra M., Camici P.G., Oliva F. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int. J. Cardiol. Heart Vasc. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC investigating rare myocarditis in teens, young adults; COVID-19 vaccine still advised for all who are eligible | American Heart Association, n.d. https://newsroom.heart.org/news/cdc-investigating-rare-myocarditis-in-teens-young-adults-covid-19-vaccine-still-advised-for-all-who-are-eligible (accessed June 18, 2021).