Abstract
A 60-year-old man presented with dyspnea four days after the second dose of the coronavirus disease (COVID-19) vaccine. Imaging revealed extensive ground-glass opacification. Blood tests were notable for elevated KL-6 levels. Bronchoalveolar lavage fluid analysis showed increased lymphocyte-dominant inflammatory cells and decreased CD4/CD8 ratio. These findings were consistent with the diagnosis of drug-induced interstitial lung disease (DIILD). To the best of our knowledge, this has never been reported in previous literature. Treatment with glucocorticoids relieved his symptoms. This paper highlights that although extremely rare, COVID-19 vaccine could cause DIILD, and early diagnosis and treatment are crucial to improve patient outcomes.
Keywords: COVID-19 vaccine, Adverse events, Drug-induced interstitial lung disease
1. Introduction
Coronavirus disease (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to significant harm and challenges worldwide [1]. Although treatment with remdesivir and dexamethasone has shown efficacy [2], [3], the number of reported cases of COVID-19 continues to rise. Due to 95% efficacy rate [4] and ability to reduce disease severity and mortality [5], BNT162b2 mRNA COVID-19 vaccine are being used worldwide. Serious adverse events related to this vaccine include shoulder injury related to vaccine administration (SIRVA), right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia [4]. However, no vaccination-associated deaths have been observed [4]. Here, we report the first case of drug-induced interstitial lung disease (DIILD) following administration of the second dose of COVID-19 vaccine.
2. Case report
A 60-year-old man with a history of asthma-chronic obstructive lung disease (COPD) overlap syndrome and hypertension presented to our hospital with dyspnea four days after inoculation with the second dose of COVID-19 vaccine. His comorbid conditions had been treated with antihypertensives and combined inhaled corticosteroid/long-acting beta-agonist (ICS/LABA), but his medications had not been changed in the past six months. He also had a history of complete right bundle branch block and mild mitral regurgitation at the time of his medical checkup and was under observation. He had no history of allergies. He had been smoking 40 cigarettes per day for 20 years and had reportedly quit 3 years prior.
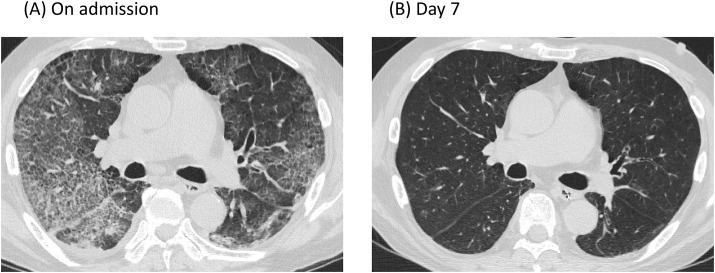
Approximately 24 days prior to admission, he received the first dose of the COVID-19 vaccine with no obvious adverse reactions. Four days prior to admission, he received the second dose of the same vaccine. However, two days later, he presented with dyspnea on exertion and was referred to our outpatient clinic for further examination. The patient was conscious, but was febrile (37.7 °C) and had decreased oxygen saturation (80% on room air). The patient was immediately treated using a 5L oxygen mask. Chest radiography showed infiltrative shadows in bilateral lung fields. Chest computed tomography (CT) (Fig. 1 A) revealed extensive ground-glass opacification (GGO) with right upper lobe predominance, mild interlobar septal wall thickening, and diffuse bronchial wall thickening. Blood analyses (Table 1 ) showed elevated C-reactive protein (CRP = 10.87 mg/dL), lactate dehydrogenase (LD = 299 U/L), Krebs von den Lungen-6 (KL-6 = 800 U/mL), surfactant protein-D (SP-D = 155 ng/mL), and surfactant protein-A (SP-A = 68.5 ng/mL). Procalcitonin levels (0.06 ng/mL) were normal. No significant organisms were detected in the sputum. Urine pneumococcal antigen test, Legionella antigen test, and quantitative test for SARS-CoV-2 antigen by nasopharyngeal swab sample on admission were negative. In addition, markers of various autoimmune diseases were negative. Brain natriuretic peptide (BNP) levels were elevated to 553.1 pg/mL. Electrocardiography (ECG) on admission revealed no difference compared to the results of a previous ECG taken two months ago. Cardiac enzymes are within normal limits.
Fig. 1.
Chest computed tomography (CT) image, 1A) A CT scan on admission. CT image on admission revealed extensive ground-glass opacification (GGO) with right upper lobe predominance, mild interlobar septal wall thickening, and diffuse bronchial wall thickening. 1B) A CT scan seven days after admission (after extubation), CT image revealed a prominent improvement of GGO bilaterally.
Table 1.
Blood analyses at admission.
| WBC | 12,400 | /μL | IgG | 825 | mg/dL |
|---|---|---|---|---|---|
| Neutrophil | 88.3 | % | IgA | 396 | mg/dL |
| Eosinophil | 2.0 | % | IgM | 128 | mg/dL |
| Basophil | 0.2 | % | IgE | 286 | IU/mL |
| Monocyte | 3.9 | % | RF | <15 | IU/mL |
| Lymphocyte | 5.6 | % | KL-6 | 800 | U/mL |
| RBC | 4.07 × 106 | /μL | SP-A | 68.5 | ng/mL |
| Hb | 13.6 | g/dL | SP-D | 155 | ng/mL |
| MCV | 97.8 | fL | BNP | 553.1 | pg/mL |
| HCT | 39.8 | % | ACE | 15.6 | U/L |
| PLT | 28.7 × 104 | /μL | Antinuclear antibody | 40 | times |
| Anti CCP antibody | 1.1 | U/mL | |||
| TP | 7.1 | g/dL | Anti MDA5 antibody | <5 | times |
| Alb | 3.5 | g/dL | Anti SS-A antibody | <1.0 | U/mL |
| T-Bil | 0.5 | mg/dL | Anti SS-B antibody | 1.2 | U/mL |
| AST | 33 | U/L | Anti Scl-70 antibody | <1.0 | U/mL |
| ALT | 25 | U/L | Anti Jo-1 antibody | <1.0 | U/mL |
| LD | 299 | U/L | PR3-ANCA | <1.0 | U/mL |
| γ-GT | 366 | U/L | MPO-ANCA | <1.0 | U/mL |
| ALP | 127 | U/L | BNP | 553.1 | pg/mL |
| CK | 118 | U/L | |||
| BUN | 15 | mg/dL | Influenza antigen | (−) | |
| Cre | 0.84 | mg/dL | Mycoplasma antigen | (−) | |
| Na | 144 | mEq/L | CMV C10C11 | 0/0 | |
| K | 2.8 | mEq/L | Tuberculosis specific IFNγ | (−) | |
| Cl | 102 | mEq/L | β-D Glucan | <6.0 | |
| Ca | 8.8 | mg/dL | Urine pneumococcal antigen | (−) | |
| CRP | 10.87 | mg/dL | Urine legionella antigen | (−) | |
| Procalcitonin | 0.06 | ng/mL | |||
| Glu | 139 | mg/dL | Arterial blood gas | 6L mask | |
| PT | 12.5 | S | pH | 7.542 | |
| PT-INR | 0.97 | pCO2 | 29.6 | mmHg | |
| APTT | 23.5 | S | pO2 | 57.3 | mmHg |
| D-dimer | 3.0 | μg/mL | HCO3− | 24.9 | mEq/L |
| hs Troponin I | 17.8 | pg/mL |
Thereafter, his respiratory condition gradually worsened, which required mechanical ventilation. Since bacterial infection could not be ruled out, antimicrobial therapy with sulbactam/ampicillin (12 g/day) and azithromycin (250 mg/day) was initiated. Though the patient was treated for bronchial asthma-COPD, he had been extremely stable for several years. Besides, he had no other drugs as triggers, no underlying pulmonary disease background except for mild emphysema and no chronic heart failure. Therefore, we considered vaccine-induced pneumonia as the most likely diagnosis. Then, steroid pulse therapy (methylprednisolone 1g for 3 days) was administered for potential vaccine-induced pneumonia.
SARS-CoV-2 reverse transcription – polymerase chain reaction (RT-PCR) tests of nasopharyngeal swab samples taken on the second and third day of hospitalization were negative, and the patient was released from isolation. Bronchoscopy with bronchoalveolar lavage was performed. Although alveolar hemorrhage could not be ruled out on gross examination alone, iron staining did not reveal hemosiderin phagocytosis suggesting the absence of diffuse alveolar hemorrhage. Bronchoalveolar fluid (BALF) analysis revealed 32 cells comprising of neutrophils (21.9%), lymphocytes (31.3%), macrophage (46.9%), and eosinophils (0%). The CD4+/CD8+ ratio (1.26) was slightly low. These findings were consistent with drug-induced lung disease, despite completion of two days of steroid pulse therapy. Furthermore, considering the possibility of infection, the BALF of the patient was examined using the FilmArray™ Respiratory Panel. However, no significant bacteria or viruses were detected (Table 2 ). Drug lymphocyte stimulation test (DLST) for BNT162b2 mRNA COVID-19 vaccine under steroid administration revealed a stimulation index (S.I.%) of 174%, which was found to be negative (positive: more than 180%). After steroid pulse therapy, 1 mg/kg of prednisolone was administered. On the 7th day, the patient was extubated. Blood analysis on the same day indicated an improving trend with KL-6 (602 U/mL), SP-D (101 ng/mL), and SP-A (62.6 ng/mL). Chest CT (Fig. 1B) also showed marked improvement. The patient is currently on a low steroid and is improving well with no further exacerbations.
Table 2.
FilmArray™ Respiratory panel.
| Adenovirus | Not detected |
|---|---|
| Coronavirus 229E | Not detected |
| Coronavirus HKU1 | Not detected |
| Coronavirus NL63 | Not detected |
| Coronavirus OC43 | Not detected |
| Human metapneumovirus | Not detected |
| Human rhinovirus/enterovirus | Not detected |
| Influenza A | Not detected |
| Influenza B | Not detected |
| Parainfluenza virus 1 | Not detected |
| Parainfluenza virus 2 | Not detected |
| Parainfluenza virus 3 | Not detected |
| Parainfluenza virus 4 | Not detected |
| Respiratory syncytial virus | Not detected |
| Bordetella pertussis | Not detected |
| Chlamydophila pneumoniae | Not detected |
| Mycoplasma pneumoniae | Not detected |
3. Discussion
This is a suspected case of vaccination-induced interstitial lung disease. As the patient was being treated for bronchial asthma-COPD as a clear allergic predisposition, had a sudden worsening of her medical condition that had been extremely stable for several years, and had no other drugs as triggers, no underlying pulmonary disease background except for mild emphysema and no chronic heart failure, we considered vaccine-induced pneumonia as the most likely diagnosis. We also differentiated the causes of respiratory failure, such as bacterial and viral pneumonia, COVID-19, collagen disease, and acute heart failure. We performed the laboratory tests shown in Table 1. We could not identify the causative organism of pneumonia within the available tests (e.g., multiple SARS-CoV-2 tests and FilmArray™ Respiratory Panel (Table 2) were all negative). Furthermore, there was no suspicion of collagen disease on physical examination or blood analyses. A history of mitral regurgitation, elevated BNP levels, and the findings of moderate mitral regurgitation and mitral valve deviation on transthoracic echocardiography cannot exclude the possibility that heart failure was involved in this episode. However, no obvious cause of acute heart failure was determined.
The absence of peripheral sparing on chest CT, elevated KL-6, SP-A, SP-D levels on blood tests, and lymphocyte predominance on BALF analysis were consistent with the diagnosis of DIILD. DIILD is a recognized subtype of diffuse parenchymal lung disease according to the American Thoracic Society/European Respiratory Society classification [6,7]. However, it is difficult to differentiate it from other interstitial pneumonia owing to the lack of specific findings. Among the classification of idiopathic pulmonary fibrosis based on the guideline [8], hypersensitivity pneumonia was speculated from analyses of BALF and extremely well steroid reactivity. Eosinophilic pneumonia could be a differential diagnosis; however, this was not consistent with the BALF and blood analyses (i.e., eosinophils were not elevated).
The causative agents of DIILD include cancer drugs, disease-modifying anti-rheumatic drugs, antibiotics, non-steroidal anti-inflammatory agents, psychiatric medications, and anti-arrhythmic agents [7]. Since there were no changes in his medications since the past 6 months and he did not take any supplements or herbal remedies, other potential causes of DIILD. Moreover, DIILD has been reported to develop after administration of influenza vaccine [9], [10] and immunotherapy with BCG for bladder cancer [11]. Hence, COVID-19 vaccination was suspected as the etiology of DIILD due to the worsening of symptoms four days after inoculation. Age, pre-existing lung disease, smoking, drug dose, and male sex have been cited as risk factors for DIILD [7]. Watanabe et al. have implicated pre-existing lung disease or post-pulmonary surgery as risk factors for influenza vaccine-induced DIILD [9]. In this case, the patient had an underlying asthma-COPD overlap syndrome.
DIILD is characterized by bilateral GGO with or without consolidation distributed on the basal, peripheral, and/or bilateral areas in multiple lobes [7]. This is consistent with the present case. The onset of DIILD depends on the causative agent. Watanabe et al. reported that among seven patients with DIILD caused by influenza vaccine, the onset was 1–2 days in four patients and 6–10 days in three patients [9]. The onset of DIILD in this patient may have been relatively early (approximately 2 days) because this was his second vaccination dose, and the patient was already sensitized. It has been suggested that DIILD may be caused by direct volume-dependent cytotoxic lung injury or by T cell-mediated lung injury [12]. DLST and leukocyte migration test (LMT) are often used to detect drug-sensitized T cells [7]. However, owing to their low sensitivity, a negative result cannot rule out the disease [13], [14]. In this case, the negative result was probably because the DLST was performed after steroid pulse therapy.
Although the evidence for steroids in drug-induced interstitial pneumonia is limited, steroid administration and discontinuation of the suspected drug may improve prognosis. The mortality rate of DIILD varies depending on the drug and the study. The mortality rates were high ranging from 14% to 51.3% in oncologic cases and 0–41% in non-oncologic cases. Although this case was successfully treated with steroids, it might be fatal in case of the delayed diagnosis or for older people and people with pre-existing medical conditions. In conclusion, we report a suspected case of vaccination-related DIILD. Although the frequency of DIILD is considered to be very low, this paper highlights that DIILD could potentially develop with the COVID-19 vaccines, similar to those reported with influenza vaccines. It is extremely important to be aware of the possibility of DIILD in order to provide an early diagnosis and treatment.
Funding and disclosures
No funding is supported for this report.
Authorship statement
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. Furthermore, each author certifies that this material has not been and will not be submitted to or published in any other publication before its appearance in the Journal of Infection and Chemotherapy.
Authorship contributions
AY, KI, YM, SS, SM, KS examined the patient. YU analyzed the samples. YU and NH participated in the discussion for the treatment and gave the important suggestion. AY and KI drafted the manuscript. All authors critically revised the paper for important intellectual content, and all authors approved the final version of the manuscript.
Informed consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Declaration of competing interest
The authors have declared that no conflict of interest exists.
Acknowledgement
We would like to thank all the medical professionals who cared for the patient and all the members of COVID-19 vaccination team. We would also like to thank Editage (www.editage.com) for English language editing.
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 dash-board by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. (https://coronavirus.jhu.edu/map.html).
- 2.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard Care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. J Am Med Assoc. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis W.D., Costabel U., Hansell D.M., et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skeoch S., Weatherley N., Swift A.J., et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghu G., Collard H.R., Egan J.J., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe S., Waseda Y., Takato H., et al. Influenza vaccine-induced interstitial lung disease. Eur Respir J. 2013;41:474–477. doi: 10.1183/09031936.00146912. [DOI] [PubMed] [Google Scholar]
- 10.Numata T., Hida N., Yazaki K., et al. Seasonal influenza vaccine-induced pneumonitis presenting with multiple pulmonary nodules. Intern Med. 2018;57:707–711. doi: 10.2169/internalmedicine.9399-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan L., Testa G., Yung T. Diffuse alveolar damage in BCGosis: a rare complication of intravesical bacillus Calmette-Guérin therapy for transitional cell carcinoma. Pathology. 1999;31:55–56. doi: 10.1080/003130299105566. [DOI] [PubMed] [Google Scholar]
- 12.Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13:39. doi: 10.1186/1465-9921-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camus P., Fanton A., Bonniaud P., et al. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 14.Hirata S., Hattori N., Kumagai K., et al. Lymphocyte transformation test is not helpful for the diagnosis of methotrexate-induced pneumonitis in patients with rheumatoid arthritis. Clin Chim Acta. 2009;407:25–29. doi: 10.1016/j.cca.2009.06.024. [DOI] [PubMed] [Google Scholar]