Abstract
Objectives:
To assess anti-Ro52 and anti-Ro60 serologic profiles as markers of clinically relevant phenotypic subsets of patients with Sjögren’s syndrome (SS).
Methods:
From a cohort of 839 consecutive patients with suspected or established SS seen in our multidisciplinary Sjögren’s syndrome center, we compared the association of key phenotypic features in 390 patients who fulfilled SS classification criteria and in the parent cohort, stratifed by the presence of both anti-Ro60 and anti-Ro52, anti-Ro60 alone, and anti-Ro52 alone.
Results:
The SS cohort included 227 (58%) with anti-Ro60 and anti-Ro52, 65 (17%) with anti-Ro60 alone, 58 (15%) with anti-Ro52 alone, and 40 (10%) with neither antibody. Those with both anti-Ro60 and anti-Ro52 had a significantly increased prevalence of abnormal ocular surface staining, focal lymphocytic sialadenitis with focus score≥1, ANA≥1:320, anti-SSB/La, rheumatoid factor, and IgG≥15.6 g/L (p<0.0016 for all). The groups with isolated anti-Ro52 and anti-Ro60 were equivalent to each other in their phenotypic associations, except for rheumatoid factor, which was higher in the Ro52 alone group. The associations of these Ro antibody serologic profiles were similar in the parent cohort, except for additional associations with salivary gland enlargement and parotid gland ultrasound score.
Conclusion:
SS patients with both anti-Ro60 and Ro52 antibodies are distinguished by a higher prevalence of markers of B-cell hyperactivity and glandular inflammation. Antibody reactivity to both Ro60 and Ro52 may thus serve as an important inclusion criterion for SS patients in clinical trials where the therapeutic agent targets pathways mediating these pathogenic abnormalities.
Keywords: Sjögren’s syndrome, biomarkers, antibodies
Anti-SSA/Ro antibodies, the serologic marker of Sjögren’s syndrome (SS), comprise reactivity to two proteins, Ro52 and Ro60, encoded by separate genes and found in distinct cellular compartments(1). The reactivity of Ro60 is largely dependent on conformational epitopes(2), whereas that of Ro52 is dependent on linear epitopes(3). Traditionally, anti-Ro60 was detected by immunodiffusion or immunoprecipitation, while anti-Ro52 detection required ELISA and/or Western blot(4). The separate detection of anti-Ro52 and anti-Ro60 has now been enabled by the use of recombinant or native Ro52 and Ro60 antigens in bead immunoassays(5). However, the phenotypic correlates of anti-Ro52 and anti-Ro60 profiles have not been well-defined. We assessed the association of specific profiles of anti-Ro52 and anti-Ro60 with key SS phenotypic features in order to determine their utility as markers of distinct patient subsets, including the potential identification of patients most likely to respond to immunomodulatory or immunosuppressive disease-modifying therapies.
PATIENTS AND METHODS
Patient and public involvement
We studied 839 consecutive patients referred to the Johns Hopkins Sjögren`s Syndrome Center from 6/2009–12/2016. These patients either had an established diagnosis of SS or sufficient clinical or laboratory features to warrant concern for this diagnosis. Each patient provided written informed consent to provide serum and allow data collection for this observational study, approved by the Johns Hopkins Institutional Review Board. The patients in the cohort with SS fulfilled the 2016 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria(6).
Data collection
Key SS phenotypic features were identified from a computer database, maintained prospectively, and where needed, from retrospective chart review. Ocular Staining Score (OSS) was graded by the examining ophthalmologist according to the Oxford or Sjögren’s International Collaborative Clinical Alliance (SICCA) scheme(7, 8). We converted Oxford to van Bijsterveld staining scores to allow classification according to the 2016 ACR/EULAR criteria. Oxford conjunctival and corneal scores of 0 or 1+, 2+, 3+, and 4+ or 5+ were classified, respectively as van Bijsterveld scores of 0, 1+, 2+ and 3+. Abnormal OSS was defined by a SICCA score≥5 or van Bijsterveld≥4 in at least one eye. Abnormal salivary gland scintigraphy was defined by decreased uptake with or without decreased excretion fraction in at least two of four salivary glands. Impaired discharge only or impaired uptake in one gland was not defined as abnormal. Salivary gland ultrasound findings were graded according to the following scheme, adapted from Theander et al(9): Grade 0, homogeneous background parenchymal echogenicity with expected number of echogenic lines; Grade 1, subjective heterogeneity of the parenchyma without discrete ovoid hypoechoic foci; Grade 2 and 3, discrete hypoechoic foci and/or cysts occupying <50% or ≥50% gland volume. Systemic involvement was defined by fulfillment of one or more non-glandular domains of the European League Against Rheumatism (EULAR) SS Disease Activity Index (ESSDAI) (10).
Anti-Ro52 and anti-Ro60 detection
Serum from each patient was tested for anti-Ro52, anti-Ro60, and anti-SSB/La in the Johns Hopkins Rheumatic Diseases Research Core Center. Anti-Ro52 and anti-SSB/La were assayed using commercially available ELISA kits, per the manufacturer’s protocol (QUANTA Lite, Inova Diagnostics, San Diego, CA). Anti-Ro60 was determined by immunoprecipitation of 35S-methionine-labeled Ro60 generated by in vitro transcription and translation, as previously described(11). All other blood testing was performed by commercial or hospital laboratories.
Statistical analysis
Differences in categorical variables between groups were compared using the Pearson Chi-squared or Fisher exact test. The Student’s t-test and one-way ANOVA test were used to compare continuous variables, except where the distributions were highly skewed, in which case non-parametric Mann-Whitney U and Kruskal-Wallis tests were used. All statistical tests were two-sided. Significance for statisticial comparisons was also adjusted for multiple comparisons using the Bonferroni correction for 32 primary first level comparisons, such that p values less than 0.05/32=0.0016 were assumed to indicate significant differences. When primary tests were significant, for groups with more than two levels, pairwise comparisons were conducted using the same criterion for significance, a p-value less than 0.0016. All statistical analyses were performed using JMP Pro 13 software (SAS Institute, Inc, Cary, NC).
RESULTS
The 839 patients with suspected or established SS had a mean age of 52.2±13.4 years; 90% were women and 85% Caucasian. A second systemic rheumatic disease was present in 112 patients (13%). SS was present in 390 patients (46%). The sociodemographic and key phenotypic features of the entire cohort and those who fulfilled classification criteria for SS are shown in Supplementary Table S1. As expected, the SS patients had a higher prevalence of objective markers of lacrimal and salivary gland dysfunction (Schirmer test, abnormal OSS and unstimulated whole saliva flow rate), focal lymphocytic sialadenitis with a focus score≥1 (FLS/FS≥1) on minor salivary gland biopsy, and serologic abnormalities typical of SS, including anti-SSA/Ro and anti-SSB/La antibodies, rheumatoid factor (RF), high titer antinuclear antibodies (ANA) and hyperglobulinemia (IgG>15.6 g/L). Notably, anti-SSA/Ro and anti-SSB/La were present, respectively, in 90% and 39% of the SS patients.
The associations of key SS phenotypic features with specific SSA/Ro antibody profile groups were analyzed for the 390 patients with SS (Tables 1 and 2) and for the parent cohort of 839 patients with suspected or established SS (Supplementary Tables S2 and S3). Specifically, we analyzed whether these phenotypic features differed among anti-SSA/Ro-positive patients with different profiles of anti-Ro60 and anti-Ro52 reactivity.
Table 1:
Demographic and key phenotypic characteristics 390 patients with known Sjögren syndrome, stratified by anti-Ro60 and anti-52 antibody profiles (categorical variables)
| Feature | Antibody profiles | P value | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group A: Anti-Ro60+anti-Ro52 n=227, (%) | Group B: Anti-Ro60 alone n=65, (%) | Group C: Anti-Ro52 alone n=58, (%) | Group D: Anti-Ro negative n=40, (%) | Overall A-C | A vs B | A vs C | B vs C | |
| Women | 208 (92) | 60 (92) | 53 (91) | 36 (90) | 0.98 | |||
| Caucasian | 187 (82) | 55 (85) | 45 (78) | 37 (93) | 0.58 | |||
| Dry eye symptoms | 208 (92) | 55 (85) | 54 (93) | 39 (98) | 0.18 | |||
| Dry mouth symptoms | 202 (89) | 57 (88) | 52 (90) | 35 (88) | 0.94 | |||
| Salivary gland enlargement | 67 (30) | 6 (9) | 14 (24) | 12 (30) | 0.004 | |||
| Schirmer ≤ 5 mm/5 min in at least one eye | 160/213 (75) | 42/61 (69) | 43/55 (78) | 31/40 (78) | 0.48 | |||
| Abnormal ocular surface staining* | 97/150 (65) | 14/39 (36) | 18/40 (45) | 16/28 (57) | 0.0015 | 0.0011 | 0.0004 | 0.2181 |
| UWSF ≤ 0.5 mL/5 min | 49/86 (57) | 14/25 (56) | 12/16 (75) | 11/18 (61) | 0.38 | |||
| FLS with FS ≥ 1 | 98/129 (76) | 17/53 (32) | 25/42 (60) | 40/40 (100) | <0.0001 | <0.0001 | <0.0001 | 0.0151 |
| Hypoechoic foci on parotid gland ultrasonography | 42/62 (68) | 4/8 (50) | 4/13 (31) | 1/11 (9) | 0.04 | |||
| Abnormal salivary gland scintigraphy | 14/68 (21) | 17/31 (55) | 8/19 (42) | 9/15 (60) | 0.002 | |||
| ANA ≥ 1:320 | 182/226 (81) | 17/65 (26) | 25/58 (43) | 9/39 (23) | <0.0001 | <0.0001 | <0.0001 | 0.0223 |
| SSB/La antibodies | 132 (58) | 8 (12) | 10 (17) | 0 | <0.0001 | <0.0001 | <0.0001 | 0.5529 |
| Rheumatoid factor | 130/227 (57) | 6/63 (10) | 20/58 (34) | 1/40 (3) | <0.0001 | <0.0001 | <0.0001 | 0.0008 |
| IgG ≥ 15.6 g/L | 103/221 (47) | 10/63 (16) | 10/56 (18) | 0/39 (0) | <0.0001 | <0.0001 | <0.0001 | 0.8402 |
| C4 < 0.12 g/L | 23/219 (11) | 1/62 (2) | 3/56 (5) | ¾0 (8) | 0.05 | |||
| WBC ≤ 4.0×109/L | 51/224 (23) | 8/64 (13) | 10/57 (18) | 6/40 (20) | 0.17 | |||
| Monoclonal gammopathy | 31/217 (14) | 4/59 (7) | 7/53 (13) | 7/35 (20) | 0.31 | |||
| Associated systemic rheumatic disease | 40/227 (18) | 9 (14) | 10 (17) | 7 (18) | 0.77 | |||
| Systemic involvement† | 201 (89) | 62 (95) | 50 (86) | 33 (83) | 0.20 | |||
| Lymphoma†† | 12 (5) | 1 (2) | 3 (5) | 0 (0) | 0.43 | |||
Abbreviations: ANA, antinuclear antibody; C4, complement 4 protein; FLS, focal lymphocytic sialadenitis; FS, focus score; IgG, Immunoglobulin G; OSS: ocular staining score; RF, rheumatoid factor; USWF, unstimulated whole saliva flow rate; WBC, white blood cells
Abnormal ocular surface staining included any abnormal ocular staining score (OSS) and/or van Bijsterveld score in a patient.
Fulfillment of one or more non-glandular domains of the ESSDAI[13]
Marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) in 14 patients; the diagnosis of SS preceded that of lymphoma in 12 and was concomitant with that of lymphoma in the remaining four.
Table 2:
Demographic and key phenotypic characteristics 390 patients with known Sjögren syndrome, stratified by anti-Ro60 and anti-52 antibody profiles (continuous variables)
| Feature | Antibody profiles | P value | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group A: Anti-Ro60+ anti-Ro52 n=227 | Group B: Anti-Ro60 alone n=65 | Group C: Anti-Ro52 alone n=58 | Group D: Anti-Ro negative n=40 | Overall A-C | A vs B | A vs C | B vs C | |
| Age, years, mean±SD | 52.0±14.8 | 51.7±12.9 | 53.8±12.1 | 55.0±14.5 | 0.63 | |||
| Disease duration, years (mean±SD) | 9.5±8.8 | 8.2±1.1 | 9.6±8.3 | 11.6±11.6 | 0.54 | |||
| Schirmer, mm/5 min (median (min-max) average of eyes) | 4 (0–35) | 5.5 (0–35) | 5 (0–35) | 4.5 (0.5–35) | 0.20 | |||
| SICCA OSS, (median (min-max) maximum score of eyes | 7 (0–12) | 3 (0–11) | 6 (0–11) | 6 (0–11) | 0.03 | |||
| van Bijsterveld score, (median (min-max) maximum score of eyes | 4 (0–8) | 0 (0–6) | 0 (0–7) | 1 (0–5) | 0.0017 | |||
| UWSF, mL/5 min, median (min-max) | 0.49 (0–4.4) | 0.45 (0–3.5) | 0.37 (0–5.2) | 0.38 (0–1.5) | 0.65 | |||
| Focus score, median (min-max) | 1.8 (0–12) | 0 (0–3.3) | 1 (0–6) | 1.3 (1–5.7) | <0.0001 | <0.0001 | <0.0001 | 0.009 |
| IgG g/L, median (min-max) | 15.1 (3.5–44.6) | 11.0 (2.8–43.7) | 12.3 (3.9–26.8) | 9.96 (4.0–14.7) | <0.0001 | <0.0001 | <0.0001 | 0.76 |
| WBC (109/L), median (min-max) | 5.1 (1.7–17.6) | 6.0 (1.9–15.2) | 5.5 (2.4–12.1) | 6.2 (3.7–10.1) | 0.0018 | |||
| Parotid gland ultrasound grade, (Maximum score for both), median (min-max) | 3 (0–3) | 2 (0–3) | 0 (0–2) | 0 (0–0) | 0.005 | |||
| ESSDAI score, median (min-max) | 6 (0–37) | 7 (0–26) | 6 (0–25) | 4 (0–22) | 0.75 | |||
Abbreviations: ESSDAI: EULAR Sjögren’s Syndrome Disease Activity Index; IgG, Immunoglobulin G; OSS: ocular staining score; USWF, unstimulated whole saliva flow rate; WBC, white blood cells
Among the 390 SS patients, there were 227 (58%) with anti-Ro60 and anti-Ro52, 65 (17%) with anti-Ro60 alone and 58 (15%) with anti-Ro52 alone. These three groups did not differ with respect to sociodemographic features or sicca symptom prevalence (Tables 1 and 2). The group with both anti-Ro60 and anti-Ro52 had signficantly stronger associations with key phenotypic features, relative to the other two. In an overall comparison of the three groups, there were significant differences (p<0.0016) differences with respect to the prevalence of abnormal OSS, FLS/FS≥1, ANA≥:320, anti-SSB/La, rheumatoid factor, and serum IgG>15.6 g/L. These significant differences reflected a higher prevalence among the group with both anti-Ro60 and anti-Ro52 antibodies compared to the other two groups. In pair-wise comparisons, these differences were statistically significant (p<0.0016) when the group with both anti-Ro60 and anti-Ro52 was tested against those with anti-Ro52 or anti-Ro60 alone.
In overall comparisons, the three groups with anti-SSA/Ro also differed significantly (p<0.0016) with respect to two continuous variables, focus score and IgG levels (Table 2). In addition, the van Bijsterveld OSS, white blood count, and parotid gland ultrasound scores shared the same trend, but did not reach the pre-established level of significance. Again, each of these parameters reflected more severe disease involvement in the group with both anti-Ro60 and anti-Ro52 than in the other two groups. In pair-wise comparisons, the differences in focus score and IgG levels were also statistically significant (p<0.0016) when the group with both anti-Ro60 and anti-Ro52 was tested against those with anti-Ro52 or anti-Ro60 alone.
Systemic involvement did not differ between the anti-SSA/Ro serologic profile groups in terms of prevalence or severity (Tables 1 and 2). Lymphoma prevalence was also comparable between the groups.
In a comparison of the groups with anti-Ro52 or anti-Ro60 alone, the group with anti-Ro52 alone had a higher frequency of RF (p=0.0008).
We repeated the same analysis in the parent cohort of 839 patients with established SS or with one or more phenotypic features suggestive of the diagnosis (Supplementary Tables S2 and S3; Figure). This analysis allowed an examination of phenotypic correlates of the three different anti-SSA/Ro serologic profiles without prior filtering by the classification criteria. These criteria mandate the presence of relatively severe ocular and/or oral dryness and may thus exclude patients from SS classification who have a mild phenotype or early disease. There were 311 patients (37%) with both anti-Ro60 and anti-Ro52, 108 (13%) with anti-Ro60 alone, and 95 (11%) with anti-Ro52 alone. SS classification was fulfilled by 227/311 (73%) subjects with both anti-Ro60 and anti-Ro52, 65/108 (60%) of those with anti-Ro60 alone, and 58/95 (61%) of those with anti-Ro52 alone (p=0.0128). In an overall comparison of the three groups with anti-SSA/Ro antibodies, significant differences in phenotypic feature prevalence were seen for salivary gland enlargement, abnormal OSS, FLS/FS≥1, ANA≥1:320, anti-SSB/La, rheumatoid factor, and IgG≥15.6 g/L (Figure and Supplementary Table S2). These were the same as for the 390 SS patients, except for the additional finding of salivary gland enlargement. For each of these phenotypic features, the prevalence was highest in the group with both anti-Ro60 and anti-Ro52. In pair-wise comparisons, these differences were statistically significant (p<0.0016) when the group with both anti-Ro60 and anti-Ro52 was tested against those with anti-Ro52 or anti-Ro60 alone, except for three phenotypic features (salivary gland enlargement, abnormal OSS, and FLS/FS≥1). The three groups with anti-SSA/Ro also differed significantly in overall comparisons with respect to van Bijsterveld OSS, focus score, parotid gland ultrasound score, and IgG levels (Supplementary Table S3). Again, each of these parameters reflected more severe disease involvement in the group with both anti-Ro60 and anti-Ro52 than in the other two groups. In pair-wise comparisons, some but not all differences met the significance level of p<0.0016.
Figure.
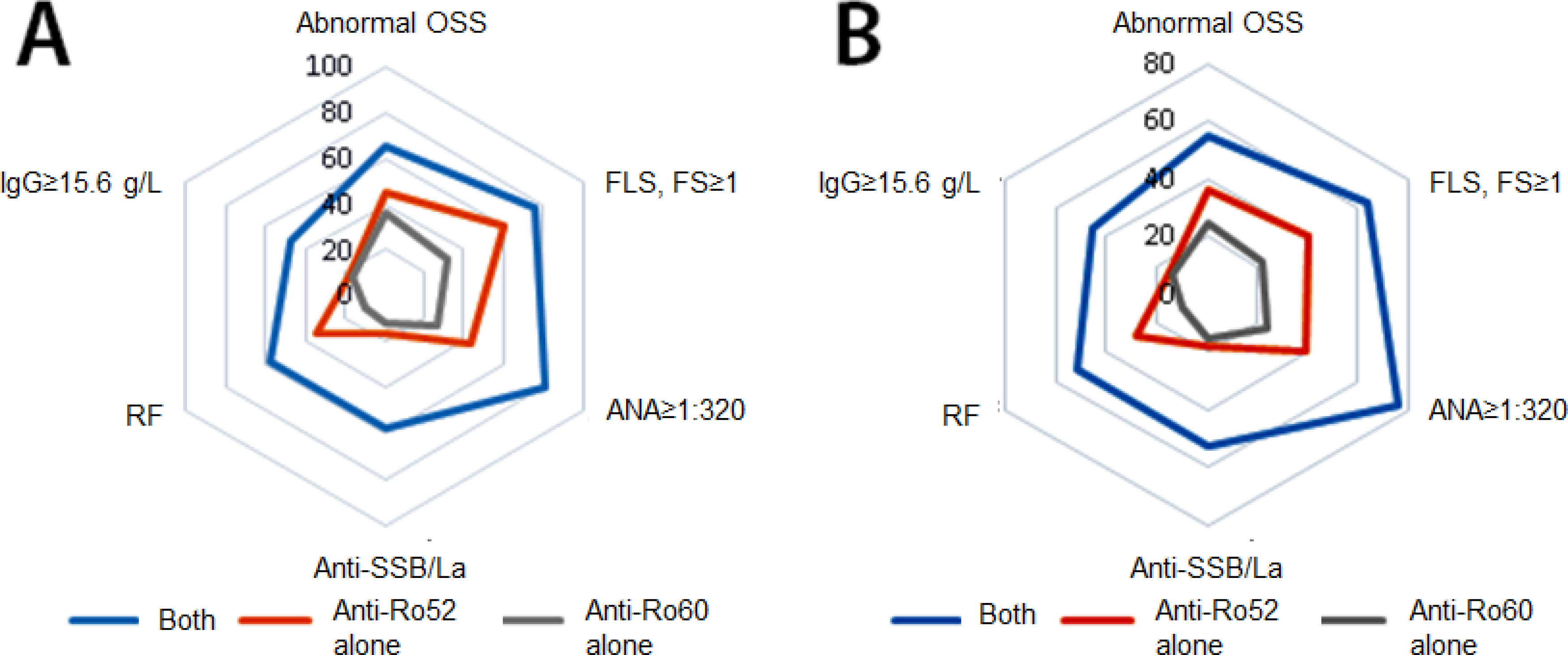
Prevalence of key phenotypic features in relation to three anti-SSA/Ro serologic profiles (radar plots), shown for the 390 patients with Sjögren’s syndrome (panel A) and the parent cohort of 839 patients with suspected or established Sjögren’s syndrome (panel B). Overall comparisons of the three serologic profile groups showed significant differences with respect to each of these phenotypic features.
DISCUSSION
Among patients with SS, those with both anti-Ro60 and anti-Ro52 antibodies have the strongest associations with key SS phenotypic features when compared to those with only anti-Ro60 or anti-Ro52. These associates are noteworthy for being markers of B-cell hyperactivity and glandular disease and include measures of ocular dryness and minor salivary gland inflammation in addition to serologic abnormalities (ANA≥1:320, RF, IgG≥15.6 g/L, and SSB/La).
The current study expands on observations by other investigators regarding the clinical significance of anti-Ro52 and anti-Ro60 serologic profiles in rheumatic disease. In a study of 508 consecutive patient sera that tested positive for anti-Ro52 and/or anti-Ro60 in a hospital laboratory using a line immunoblot assay, combined reactivity to both antigens was the most common profile, and coexisted significantly more often with anti-SSB/La and anti-DNA than isolated anti-Ro52 or anti-Ro60(12). Among 136 patients in this series with both anti-Ro52 and anti-Ro60, 57 had SS and there was a higher prevalence of sicca manifestations, salivary gland enlargement, and interstitial nephritis when compared to patients with anti-Ro52 or anti-Ro60 alone. In a study of 13032 sera tested for ANA, Robbins et al identified 399 adults with anti-Ro52 and/or anti-Ro60 using a multiplex flow immunoassay with recombinant antigens(13). Those with both anti-Ro52 and anti-Ro60 had a statistically significant higher frequency of RF and anti-SSB/La. SS was identified as the diagnosis in 76 of the 399 patients, of whom 51 (67%) had both anti-Ro52 and anti-Ro60, 12 (16%) had anti-Ro52 alone, and 13 (17%) had anti-Ro60 alone. The SS patients with both anti-Ro60 and anti-Ro52 had a higher frequency of anti-SSB/La and hypergammaglobulinemia in comparison with those with isolated anti-Ro52 or isolated anti-Ro60. Anemia and renal insufficiency were more prevalent in the anti-Ro52 alone SS patients.
In the current study, the patient groups with isolated anti-Ro52 or anti-Ro60 were similar to each other, with the exception of a higher frequency of RF in the anti-Ro52 group. Isolated anti-Ro52 is associated with autoimmune hepatitis, myositis, and interstitial lung disease(1, 12, 14, 15), but we did not observe an increase of these extraglandular manifestations among our patients. Similarly, anti-Ro52 is associated with malignancy(14, 15), but the rate of malignancy, including lymphoma, did not vary across the SSA/Ro-positive serologic groups in our study (data not shown).
The distinctive phenotypic features of the patient group with both anti-Ro60 and anti-Ro52 is a reflection in part of the strong association of this serologic profile with high expression of interferon-inducible genes (interferon-gene signature) (16). SS patients with this signature have a higher prevalence of autoantibodies, including dual anti-Ro60 and anti-Ro52 reactivity and rheumatoid factor. Additionally, they have hyperglobulinemia and more prevalent glandular enlargement, rashes, and hematologic manifestations. Lee et al have recently distinguished low versus high levels of anti-Ro60 reactivity in sera analyzed with a lineblot immunoassay(17). Patients with high anti-Ro60 levels were significantly more likely to have dual reactivity to Ro60 and Ro52, as well as co-existing anti-SSB/La, hyperglobulinemia, and rheumatoid factor. The low anti-Ro60 antibody subset had less somatic hypermutation and less tendency to undergo isotype switching, arguing for their derivation from a less mature immune response. The authors postulated that the low anti-Ro60 response may originate from a T-cell independent extrafollicular pathway in contrast to the high anti-Ro60 response, generated from the classic follicular pathway.
Our cohort of SS patients had a 90% prevalence of anti-SSA/Ro antibodies. This relatively high prevalence may reflect in part our use of the validated methodology of Daniels et al to assess the labial gland histopathology and calculate the focus score (18). With calibrated measurement of the total glandular surface area and a count of the total number of lymphocytic foci, this method more stringently differentiates SS patients from those with non-autoimmune sicca than methods that rely on visual estimates of the number of foci per 4 square millimeter of tissue. Those SS patients who lack SSA and SSB antibodies (“seronegative”) have a lower frequency of the known correlates of anti-SSA/Ro antibodies (hyperglobulinemia, vasculitis, leucopenia, lymphoma) but have also been reported to have more severe and widespread pain(19–21).
Our results support the utility of reactivity to both Ro52 and Ro60 in a patient with SS as an important marker of a disease subset characterized by B-cell hyperactivity and glandular inflammation. This disease subset may thus be particularly suitable for clinical trials of drugs targeting pathways responsible for these immunologic phenomena. This is particularly relevant given the recent failure of many clinical trials in SS, which may relate in part to the inclusion of patients without signs of robust B-cell hyperactivity or glandular inflammation. The concept that dual antibody positivity for Ro60 and Ro52 is relevant to treatment outcome clearly requires validation in large phase III trials, and this could be done easily by stratifying treatment response in relation to baseline anti-Ro serologic profiles. Use of anti-Ro60+anti-Ro52 as an inclusion criterion would not necessarily hinder recruitment for these trials, given its presence in the majority of SS patients.
Strengths of our study include the large size of our cohort and the use of assays optimal for the specific detection of anti-Ro52 and anti-Ro60. Limitations included incomplete data relative to key SS phenotypic features of each patient, such as sialometry, Schirmer testing, OSS, and labial gland biopsy. In addition, we utilized an immunoprecipitation assay for anti-Ro60 antibodies that is not routinely used in clinical laboratories. However, we confirmed the findings of the current study by performing the same analysis in a separate cohort of 194 patients seen at our Sjögren’s Center (69 fulfilling SS classification criteria), each of whom had anti-Ro52 and anti-Ro60 antibody testing by a new chemiluminescent assay (Inova Bioflash, San Diego, CA, USA) adopted by our hospital’s clinical laboratory in 2017. There was >95% concordance in test results when assessed in 63 patients who had testing by both methods on the same serum sample.
In summary, the presence of both anti-Ro60 and anti-Ro52 antibodies in a patient with suspected or known SS is a biomarker for B-cell hyperactivity and glandular inflammation. This subset may be most suitable for inclusion in clinical trials where the therapeutic agent targets these derangements.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS:
Anti-SSA/Ro reactivity is comprised of antibodies to both anti-Ro52 and anti-Ro60 or either antigen alone.
Dual reactivity to Ro60 and Ro52 is strongly associated with markers of B-cell hyperactivity and glandular inflammation in SS.
The serologic profile, anti-Ro60 and anti-Ro60, may thus mark a SS subset best suited for clinical trials of disease-modifying therapies that target these abnormalities.
Acknowledgements:
The authors acknowledge and thank the contribution of the patients who participated in this study. We also thank Dr. Laura Gutierrez and Mr. David Hines for performing the anti-Ro and anti-La antibody testing.
This work was supported by National Institutes of Health [grant number RO1-DE12354–15A1] and the Jerome L. Greene Foundation. The Johns Hopkins Rheumatic Disease Research Core Center, where the assays were performed, is supported by the National Institutes of Health [grant number P30-AR-053503].
Footnotes
Conflicts of interest: Dr. Grader-Beck reports grants from Lilly and Novartis, outside the submitted work.
Dr. Akpek reports grants from WL Gore & Associates, personal fees from Novaliq, Clementia, Regeneron, EpiTech, Sylenitis, Aurinia, and Dompe, and other from CorneaGen., outside the submitted work.
Dr. Kim reports personal fees from ALK and Genentech, outside the submitted work.
Dr. Baer reports personal fees from Bristol-Myers Squibb, AbbVie, Sanofi-Aventis, and Mitsubishi Tanabe Pharma America, outside the submitted work.
Ethics: This human study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine and all patients provided written informed consent for the participation in the study.
Data availability statement: All data relevant to the study are included in the article.
References
- 1.Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol 2012;2012:606195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boire G, Lopez-Longo FJ, Lapointe S, Menard HA. Sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum 1991; Jun;34(6):722–30. [DOI] [PubMed] [Google Scholar]
- 3.Scofield RH, Farris AD, Horsfall AC, Harley JB. Fine specificity of the autoimmune response to the Ro/SSA and La/SSB ribonucleoproteins. Arthritis Rheum 1999; Feb;42(2):199–209. [DOI] [PubMed] [Google Scholar]
- 4.Peene I, Meheus L, De Keyser S, Humbel R, Veys EM, De Keyser F. Anti-Ro52 reactivity is an independent and additional serum marker in connective tissue disease. Ann Rheum Dis 2002; Oct;61(10):929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Infantino M, Bentow C, Seaman A, Benucci M, Atzeni F, Sarzi-Puttini P, et al. Highlights on novel technologies for the detection of antibodies to Ro60, Ro52, and SS-B. Clin Dev Immunol 2013;2013:978202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017; Jan;76(1):9–16. [DOI] [PubMed] [Google Scholar]
- 7.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003; Oct;22(7):640–50. [DOI] [PubMed] [Google Scholar]
- 8.Whitcher JP, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren’s Syndrome International Registry. Am J Ophthalmol 2010; Mar;149(3):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theander E, Mandl T. Primary Sjogren’s syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res (Hoboken) 2014; Jul;66(7):1102–7. [DOI] [PubMed] [Google Scholar]
- 10.Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, et al. EULAR Sjogren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open 2015; Feb 20;1(1):e000022,2014–000022. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaraju K, Cox A, Casciola-Rosen L, Rosen A. Novel fragments of the Sjogren’s syndrome autoantigens alpha-fodrin and type 3 muscarinic acetylcholine receptor generated during cytotoxic lymphocyte granule-induced cell death. Arthritis Rheum 2001; Oct;44(10):2376–86. [DOI] [PubMed] [Google Scholar]
- 12.Zampeli E, Mavrommati M, Moutsopoulos HM, Skopouli FN. Anti-Ro52 and/or anti-Ro60 immune reactivity: autoantibody and disease associations. Clin Exp Rheumatol 2020; Feb 18;. [PubMed] [Google Scholar]
- 13.Robbins A, Hentzien M, Toquet S, Didier K, Servettaz A, Pham BN, et al. Diagnostic Utility of Separate Anti-Ro60 and Anti-Ro52/TRIM21 Antibody Detection in Autoimmune Diseases. Front Immunol 2019; Mar 12;10:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menendez A, Gomez J, Escanlar E, Caminal-Montero L, Mozo L. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection. Autoimmunity 2013; Feb;46(1):32–9. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira JP, Almeida I, Marinho A, Cerveira C, Vasconcelos C. Anti-ro52 antibodies and interstitial lung disease in connective tissue diseases excluding scleroderma. ISRN Rheumatol 2012;2012:415272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brkic Z, Maria NI, van Helden-Meeuwsen CG, van de Merwe JP, van Daele PL, Dalm VA, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren’s syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis 2013; May;72(5):728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AYS, Beroukas D, Brown L, Lucchesi C, Kaur A, Gyedu L, et al. Identification of a unique anti-Ro60 subset with restricted serological and molecular profiles. Clin Exp Immunol 2021; Jan;203(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels TE, Cox D, Shiboski CH, Schiodt M, Wu A, Lanfranchi H, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjogren’s syndrome among 1,726 registry participants. Arthritis Rheum 2011; Jul;63(7):2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quartuccio L, Baldini C, Bartoloni E, Priori R, Carubbi F, Corazza L, et al. Anti-SSA/SSB-negative Sjogren’s syndrome shows a lower prevalence of lymphoproliferative manifestations, and a lower risk of lymphoma evolution. Autoimmun Rev 2015; Nov;14(11):1019–22. [DOI] [PubMed] [Google Scholar]
- 20.Segal BM, Pogatchnik B, Henn L, Rudser K, Sivils KM. Pain severity and neuropathic pain symptoms in primary Sjogren’s syndrome: a comparison study of seropositive and seronegative Sjogren’s syndrome patients. Arthritis Care Res (Hoboken) 2013; Aug;65(8):1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ter Borg EJ, Kelder JC. Lower prevalence of extra-glandular manifestations and anti-SSB antibodies in patients with primary Sjogren’s syndrome and widespread pain: evidence for a relatively benign subset. Clin Exp Rheumatol 2014; May-Jun;32(3):349–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


