Abstract
Background
There is little information regarding the safety of intravenous tissue plasminogen activator (IV-tPA) in patients with stroke and COVID-19.
Methods
This multicenter study included consecutive stroke patients with and without COVID-19 treated with IV-tPA between February 18, 2019, to December 31, 2020, at 9 centers participating in the CASCADE initiative. Clinical outcomes included modified Rankin Scale (mRS) at hospital discharge, in-hospital mortality, the rate of hemorrhagic transformation. Using Bayesian multiple regression and after adjusting for variables with significant value in univariable analysis, we reported the posterior adjusted odds ratio (OR, with 95% Credible Intervals [CrI]) of the main outcomes.
Results
A total of 545 stroke patients, including 101 patients with COVID-19 were evaluated. Patients with COVID-19 had a more severe stroke at admission. In the study cohort, 85 (15.9%) patients had a hemorrhagic transformation, and 72 (13.1%) died in the hospital. After adjustment for confounding variables, discharge mRS score ≥2 (OR: 0.73, 95% CrI: 0.16, 3.05), in-hospital mortality (OR: 2.06, 95% CrI: 0.76, 5.53), and hemorrhagic transformation (OR: 1.514, 95% CrI: 0.66, 3.31) were similar in COVID-19 and non COVID-19 patients. High-sensitivity C reactive protein level was a predictor of hemorrhagic transformation in all cases (OR:1.01, 95%CI: 1.0026, 1.018), including those with COVID-19 (OR:1.024, 95%CI:1.002, 1.054).
Conclusion
IV-tPA treatment in patients with acute ischemic stroke and COVID-19 was not associated with an increased risk of disability, mortality, and hemorrhagic transformation compared to those without COVID-19. IV-tPA should continue to be considered as the standard of care in patients with hyper acute stroke and COVID-19.
Key Words: Stroke, COVID-19, Thrombolytic therapy, Longitudinal study, Safety, Outcomes, Disability, Stroke severity, death
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread across the globe, creating the coronavirus disease 2019 (COVID-19) pandemic. COVID-19 can be associated with a wide range of non-respiratory symptoms, including central nervous system manifestations,1 such as ischemic stroke.2 The pandemic has impacted stroke outcomes, stroke admission volumes, reperfusion therapy volume3 and altered stroke care workflow.4, 5, 6 In addition, although SARS-CoV-2 may be the cause for some strokes, it may be an incidental finding for some during the pandemic.7 In a systematic review of 61 studies from December 2019 to September 2020 (n=108,571 patients), the pooled frequency of stroke among COVID-19 patients was 1.4%.8 COVID-19 global studies observed a 1.1% to 1.5% rate of stroke across 119,967 COVID-19 hospitalizations.3 A recently published large observational cohort focusing on neurological manifestations of COVID-19 found an even higher stroke rate of 3%.9 In addition, stroke in those with COVID-19 was associated with a poor prognosis and higher mortality rate.8 As COVID-19 can be associated with bleeding tendencies,10 reperfusion treatments including intravenous tissue plasminogen activator (IV-tPA) in acute stroke can pose an unknown threat of higher risk of hemorrhagic transformation. In addition, there is a concern regarding increased thrombogenicity and fibrinolysis resistance11 which may lead to IV-tPA failure.
The current study was designed to assess the safety of IV-tPA in patients with acute ischemic stroke and COVID-19.
Methods
Study design
This was an observational multicenter cohort study on prospectively collected data to assess the safety and outcomes of IV-tPA in patients with acute ischemic stroke and COVID-19. This study is part of the CASCADE (Call to Action: SARS-CoV-2 and CerebrovAscular DisordErs) initiative.12 CASCADE is an international multicenter study focused on tracking the rate of stroke before, during, and after the pandemic in several centers worldwide. Due to the urgency in answering scientific questions, in CASCADE studies we provide our analytic codes in R for all centers to perform a similar analysis to support or rule out our findings in their local population.
Population
Consecutive acute ischemic stroke patients treated with IV-tPA at seven stroke centers in Iran, one center in Athens, Greece and one in Dresden, Germany from February 18, 2019 to December 31, 2020 were included. For each patient, demographic and clinical data including cerebrovascular risk factors, stroke etiology, and severity and presence of COVID infection were collected. All data from Iran were registered in the Safe Implementation of Treatments in Stroke (SITS) registry. Patients were categorized into those with COVID-19 versus those without COVID-19. Adult (>18 years old) age, clinical and neuroradiological confirmation of acute ischemic stroke and IV-tPA treatment were mandatory criteria for study inclusion. Patients treated with IV-tPA along with thrombectomy were included in the analysis, whereas patients undergoing mechanical thrombectomy alone were excluded. Patients with unknown COVID-19 status were excluded from the study. COVID-19 cases were defined as clinical or laboratory infection with SARS-CoV-2 within two weeks prior to or following admission.12 All cases who had clinical symptoms suggestive of COVID-19 were assessed using lung computerized tomography (CT) and SARS-CoV-2 polymerase chain reaction (PCR). All Iranian centers performed CT lungs on the first day of admission for all cases irrespective of their clinical symptoms. Those with suspected or definite CT lung findings were also assessed using SARS-CoV-2 PCR.
Outcomes and definitions
Primary outcomes included disability at discharge (mRS score ≥2), hemorrhagic transformation, and in-hospital mortality rates among all patients with and without COVID-19. Secondary outcomes included stroke severity, door-to-needle-time, and length of hospital stay. In a subgroup analysis, we assessed independent variables associated with the risk of disability at discharge, hemorrhagic transformation, and in-hospital mortality among cases with stroke and COVID-19.
The severity of stroke at admission and during the in-hospital stay was measured using the National Institutes of Health Stroke Scale (NIHSS). The modified Rankin Scale (mRS) was used to assess functional status and degree of disability at admission and at discharge. Disability was defined as mRS ≥2. Baseline assessments included: the mRS score, NIHSS score, vital signs, body weight, and laboratory tests. Past medical history of vascular risk factors (e.g. hypertension, diabetes mellitus, smoking status) was assessed through self-reported information or from the documented medical history. Follow-up CT heads were performed for all cases after 12 to 36 hours of receiving IV-tPA. We defined hemorrhagic transformation according to the European Cooperative Acute Stroke Study (ECASS) III trial.13 Hemorrhagic transformation was defined and categorized based on location and volume by neurologists and stroke fellows. Those with hemorrhagic transformation and worsening of symptoms (NIHSS increase of ≥4) were defined as symptomatic intracranial hemorrhage (sICH). In some centers, we did not have access to a follow-up NIHSS. In these centers, patients were considered symptomatic based on the clinical judgment of neurologists. Stroke etiology was classified according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment).14 COVID-19 was confirmed using the WHO definition using a combination of clinical history with confirmatory tests including SARS-Cov_2 polymerase chain reaction (PCR), antibody, or lung computerized tomography (CT) scan findings.15
Statistical analyses
Data was shown as number (%), mean ± standard deviation (SD), or median (interquartile range [IQR]) according to their distribution pattern. Using the simple Bayesian regression model, we compared the rate of hemorrhagic transformation, in-hospital mortality, and discharge mRS score between those with and without COVID-19. Variables with a significant value at α=0.1 in the univariate analysis were entered into the final analytic model. The adjusted posterior odds ratio was estimated [OR=exponential (Beta)] with 95% credible intervals (CrI) of outcome variables. We also used multiple Bayesian regression models to compare and track changes in NIHSS score at admission, 24 hours, and 7 days after admission. In the Bayesian analysis, we considered prior distributions according to the pattern of outcome variables (i.e. Bernoulli prior for in-hospital mortality, hemorrhagic transformation, and mRS score ≥2 and asymmetric Laplace prior for NIHSS score, door-to-needle time, and length of hospital stay). The adequacy and goodness of fit models were examined using R-hat, LOO, and posterior predictive plot. In the Bayesian analysis, the convergence of the model is defined as the R-hat <1.1. We defined the final model as the one with the lowest Watanabe–Akaike information criterion or LOO values.16 The Expectation-Maximization (EM) was used for the imputation of missing data. As the length of stay and in-hospital mortality interfere together, we used a competing risk model with a cumulative incidence and confidence intervals to show the effect, if any. The ROC curve was constructed by plotting the true positive rate (sensitivity) against the false positive rate (1 − specificity) at different threshold settings. Using a machine learning approach, we identified the receiver operating characteristic (ROC) to determine predictability and optimal cut points of independent variables on outcomes in the final model. All statistical analyses were conducted using ggplot2, rstan, brms, and ordinal packages in the R 4.0.5 environment.
Ethics
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Ethics Committee approval number: REC.1399.584), National & Kapodistrian University of Athens (Ethics Committee approval number: 259/18-5-2020) and Dresden, Germany (Ethics Committee approval number: BO-EK-122022021).
Results
During the study period, 569 acute ischemic stroke patients were treated with IV-tPA (mean age 66.82±13.5 years, male 243 [55.7%]). 24 patients were excluded due to a lack of valid information regarding COVID-19 status. The final study population included 101 patients with COVID-19 (18.5%) and 444 (81.5%) without COVID-19. Sixty-six (12.1%) patients underwent mechanical thrombectomy in Iranian and German centers along with receiving IV-tPA including 11 patients with COVID-19.
Demographic data and baseline characteristics are shown in Table 1 . At baseline, there were no significant differences in age, sex, and vascular risk factors between cases with and without COVID-19. We observed a significant difference among the following laboratory tests at the time of admission between the two groups: lymphocytes (lower in COVID-19 cases), polymorphnuclear neutrophils (higher in COVID-19 cases), high-sensitivity C-reactive protein (HS-CRP; higher in COVID-19 cases), erythrocyte sedimentation rate (ESR; higher in COVID-19 cases), aspartate aminotransferase (higher in COVID-19 case), and alanine aminotransferase (higher in COVID-19 case).
Table 1.
. Baseline demographic and study outcomes of patients with acute ischemic stroke treated with IV-tPA: A comparison between those with and without COVID-19.
| Variables | Acute ischemic stroke treated with IV-TPA |
Unadjusted 95% CrI for Beta |
|||
|---|---|---|---|---|---|
|
COVID-19 Positive n=101 (18.5%) |
COVID-19 Negative n=444 (81.5%) |
||||
| Age (year)* | 68.19± 13.3 | 68.34± 14.5 | [-0.02, 0.01] | ||
| Sex** | Male | 60 (59.41%) | 243 (54.85%) |
[-0.64, 0.25] |
|
| Female | 41 (40.59%) | 200 (45.15%) | |||
| Vascular risk factors | |||||
| Hypertension** | 58 (65.17%) | 300 (70.92%) | [-0.74, 0.2] | ||
| Diabetes** | 28 (33.33%) | 130 (32.58%) | [-0.5, 0.47] | ||
| Atrial fibrillation** | 15 (19.23%) | 94 (24.23%) | [-0.96, 0.25] | ||
| Hyperlipidemia** | 10 (12.35%) | 89 (22.94%) | [-1.5, -0.12] | ||
| Smoking (current or former smoker)** | 8 (10.13%) | 71 (18.35%) | [-1.54, 0.4] | ||
| Baseline assessments in the Emergency Room | |||||
| Total IV-tPA Dosage*** | 50 [50, 63.5] | 50 [50, 70] | [-0.03, 0.0001] | ||
| Door-to-needle time (Minutes)*** | 41 [24.5, 60] | 40 [25, 58] | [-4.24, 9.04] | ||
|
Blood pressure (mmHg) |
Systolic * | 154.23± 27.37 | 149.86± 28.71 | [-1.91, 10.84] | |
| Diastolic * | 90.15± 13.18 | 91.23± 19.81 | [-4.2, 2.79] | ||
| Mean arterial blood pressure* | 111.51± 15.97 | 110.78± 16.87 | [-2.71, 4.87] | ||
| NIHSS at admission*** | 13 [9, 19] | 11 [7, 17] | [0.15, 2.8] | ||
| Laboratory test at baseline | |||||
| Hemoglobin (g/dl)*** | 13.8 [12.73, 14.8] | 13.7 [12.3, 14.8] | [-0.32, 0.55] | ||
| Wight Blood Cell (1000/mm3)*** | 8.4 [6.73, 10.15] | 8.1 [6.5, 10] | [-0.04, 2.46] | ||
| Lymphocyte *** | 1.7 [1.23, 2.41] | 1.8 [1.4, 2.73] | [-9.67, -3.99] | ||
| Polymorphonuclear Neutrophils*** | 5.46 [3.62, 7.14] | 5.16 [3.8, 7.12] | [-16.91, -1.8] | ||
| Polymorphonuclear Neutrophils to Lymphocyte ratio | 3.11 [1.96, 4.54] | 2.57 [1.8, 4.2] | [-1.63, 5.7] | ||
| Platelet Count (1000/mm3)*** | 206 [151, 259.25] | 213 [175, 253] | [-22.76, 10.88] | ||
| Partial Thromboplastin Time*** | 31 [28.09, 34] | 29 [26, 31] | [-22.91, 10.03] | ||
| Erythrocyte Sedimentation Rate (mm/hr)*** | 21 [9.25, 34.75] | 12 [6, 25.5] | [0.83, 14.05] | ||
| High-Sensitivity C-reactive Protein (mg/dl)*** | 13 [5, 60.65] | 5 [1.5, 13.4] | [6.43, 39.05] | ||
| Glucose (mg/dl)*** | 129 [112.5, 155] | 127 [108.29, 161.44] | [-16.22, 18.76] | ||
| Creatinine (mg/dl)*** | 1.1 [0.9, 1.3] | 1.02 [0.9, 1.27] | [-0.09, 0.13] | ||
| Aspartate Aminotransferase (µmol/l)*** | 29 [19, 46] | 26 [19.86, 38] | [1.83, 25.29] | ||
| Alanine Aminotransferase (µmol/l)*** | 20.5 [13, 43.5] | 19 [13, 30.99] | [6.35, 46.39] | ||
| Lactate Dehydrogenase (µmol/l)*** | 469 [335.5, 674] | 333.27 [273, 438] | [58.09, 217.25] | ||
| Outcomes | |||||
|
Hemorrhagic transformation |
All cases (symptomatic or asymptomatic) |
22 (22.45%) | 63 (14.48%) | [1.03, 3.49] | |
| Symptomatic cases |
0 | 5 (1.7 %) | — | ||
| Symptomatic cases (clinical judgment) |
8 (8.16%) | 36 (8.28%) | [-0.4, 1.3] | ||
| Symptomatic cases either with NIHSS or clinical judgments | 8 (8.16%) | 29 (6.67%) | [-0.63, 1.3] | ||
|
Hemorrhagic transformation type |
HI1:Scattered small petechiae, no mass effect | 0 (0%) | 5 (1.51%) | [-0.02, 10.29] | |
| HI2: Confluent petechiae, no mass effect | 4 (4.08%) | 9 (2.71%) | [-0.69, 7.51] | ||
| PH1: Hematoma within infarcted tissue, occupying <30%, no substantive mass effect | 12 (12.24%) | 16 (4.82%) | [-0.9, 6.87] | ||
| PH2: Hematoma occupying 30% or more of the infarcted tissue, with obvious mass effect | 3 (3.06%) | 15 (4.52%) | [0.04, 8.29] | ||
| In-hospital mortality** | 26 (25.74%) | 46 (10.38%) | [0.6, 1.55] | ||
| 22 (47.8%) | 144 (48.5%) | [-0.16, 0.95] | |||
|
NIHSS |
NIHSS 24 hours*** | 10 [5, 17] | 7 [2, 13] | [-0.19, 4.04] | |
| NIHSS at 7th day*** | 6 [2, 10] | 3 [0, 8.75] | [-1.27, 2.79] | ||
| Length of hospital stay (day) | 8 [5, 17] | 7 [4, 13] | [3.72, 7.21] | ||
Abbreviations: NIHSS: National Institutes of Health Stroke Scale; SD: Standard deviation; IQR: interquartile; mRS: Modified Rankin score; CrI: Credible interval.
Numbers are presented as Mean +/- Standard deviations*, frequency (%)**, or Median (IQR)*** according to their distribution pattern. Values in bold indicate statistically significant results.
The most common etiology of stroke in all cases was large-artery atherosclerosis (31.5%). Among cases with COVID-19, the rate of large-artery atherosclerosis (47.3%) was significantly higher than those without COVID-19 (27.3%). We did not find any other differences between stroke causes in those with and without COVID-19, except a higher rate of stroke of undetermined etiology in those with COVID-19. After adjusting for vascular risk factors (age, sex, hypertension, diabetes, dyslipidemia and smoking), the odds of large-artery atherosclerosis was significantly higher in patients with COVID-19 as compared to other groups (odds ratio: 2.19, 95% CI: 1.25, 3.79; Supplemental Table 1).
Door-to-needle time and length-of-stay in hospital
There was no significant difference for the door-to-needle time between those with and without COVID-19: 41 (24.5, 60) versus 40 [25, 58] minutes, respectively. Hospital length-of-stay was significantly higher in those with COVID-19 versus those without: 8 [5, 17] versus 7 [4, 13] days, respectively. In competing risk analysis and after adjusting for age, sex, NIHSS score, mRS score at admission, and mortality, the length of hospital stay was higher in the COVID-19 group than patients without COVID-19 (hazard ratio 1.78, 95% CrI: 1.31, 2.25).
Stroke Severity
In the univariate analysis, those with COVID-19 had a more severe stroke based on their NIHSS at admission (Table 1). After adjustment for age, sex, history of hypertension, diabetes mellitus, mRS at admission, those with COVID-19 still had a more severe stroke at admission (B=2.11, 95% CrI: 0.85, 3.35). In the adjusted model for confounding variables, NIHSS score after 24 hours (Beta=0.46, 95%CrI: -0.78, 1.71), and after 7 days (data available for 106 alive cases; Beta=-0.05, 95% CrI: -0.33, 0.11) did not differ between those with and without COVID-19.
Hemorrhagic transformation
In total, hemorrhagic transformation (a combination of symptomatic or asymptomatic) was observed in 85 (15.9%) patients. 37 patients (6.9%) were classified as symptomatic hemorrhage. We did not have access to NIHSS at 24 hours in 25 cases (83.3%) from Iranian centers; however, they have been considered symptomatic according to expert opinion. Only 9 cases were classified symptomatic according to the ≥ 4 increase in NIHSS score at 24 hours. Twenty-three cases in total had PH2 bleeding (hematoma occupying 30% or more of the infarcted tissue with obvious mass effect), including 2 (2.2%) in those with COVID-19 and 17 (5.4 %) in patients without COVID-19.
In univariate analysis, the rate of hemorrhage was higher in those with COVID-19 vs. those without; however, we did not see any difference between the rate of symptomatic hemorrhage. Hemorrhagic transformation types were also similar between those with COVID-19 and non-COVID-19 except a higher rate of PH2 bleeding in those without COVID-19 (Table 1). We observed six cases with remote hemorrhage, including two in those with COVID-19.
The unadjusted risk of all hemorrhagic transformation (a combination of symptomatic or asymptomatic) increased, although not significantly, in those with COVID-19 (OR: 1.70; 95% CrI: 0.98, 2.92). However, the unadjusted odds of symptomatic hemorrhagic transformation did not differ between the two groups (OR=0.602, 95% CrI: 0.158, 2.14). In the multiple Bayesian models adjusted for confounding variables, there was no significant association between COVID-19 and risk of all hemorrhagic transformation (OR: 1.51; 95% CrI: 0.66, 3.30). Likewise, the adjusted odds of symptomatic hemorrhagic transformation was not different between the two groups (OR=1.07, 95% CrI: 0.29, 3. 6). The serum level of HS-CRP, ESR, lactate dehydrogenase, and mean arterial blood pressure at admission had a significant association with the risk of all hemorrhagic transformation. In symptomatic hemorrhage, we only found a significant association between HS-CRP level and the risk of bleeding (OR: 1.01, 95% CrI:1.001, 1.02) (Table 2 ).
Table 2.
Risk of hemorrhagic transformation in acute stroke with and without COVID-19 treated with IV-tPA: Results of Bayesian multiple regression model.
|
A: All cases with hemorrhage: Symptomatic and asymptomatic | |||||
|---|---|---|---|---|---|
| Variable | Hemorrhagic transformationn=85 | Without Hemorrhagic transformationn=449 | Posterior Odds ratio | Standard error | Adjusted 95% Crlfor odds ratio |
| Age (year)* | 65.5 [55, 71] | 69 [59, 78] | 1.001 | 1.01 | [0.9804, 1.024] |
| Sex (female/ male)** | 39/46 (45.9%) | 196/253 (43.7%) | 1.092 | 1.26 | [0.688, 1.687] |
| NIHSS at admission * | 14 [10, 19] | 11 [7, 17] | 1.078 | 1.02 | [1.0297, 1.129] |
| Mean arterial blood pressure * | 108.33 [96.6, 121.6] | 108 [98.33, 119.83] | 1.007 | 1.01 | [0.9874, 1.026] |
| High-sensitivity C-Reactive protein (mg/dl)* | 8.75 [3.36, 47.5] | 5.05 [1.8, 13.65] | 1.01 | 1 | [1.0026, 1.018] |
| COVID19 (yes/no)** | 22/63 (25.9%) | 76/372 (17%) | 1.514 | 1.5 | [0.6672, 3.31] |
| B: Cases with Symptomatic | |||||
| Age (year)* | 68.8± 13.6 | 68.3 ± 14.4 | 1.022 | 1.01 | [0.96, 1.026] |
| Sex (female/ male)** | 18/19 (48.6%) | 217/280 (43.7%) | 1.224 | 1.4 | [0.632, 2.369] |
| NIHSS at admission * | 14 [10, 17] | 11 [7, 17] | 1.072 | 1.04 | [0.999, 1.15] |
| high-Sensitivity C-reactive Protein (mg/dl)* | 8 [2, 68.5] | 5.9 [2, 14.2] | 1.011 | 1.01 | [1.002, 1.022] |
| Alanine Aminotransferase* | 22 [13, 43.31] | 19 [12.37, 30.94] | 0.995 | 1.01 | [0.974, 1.011] |
| COVID19 (yes/no)** | 8/29 (21.6%) | 90/406 (18.1%) | 1.077 | 1.94 | [0.295, 3.697] |
Abbreviations; SE: Standard error, CrI; Credible Interval, NIHSS: National Institute of Health Stroke Scale
Numbers are presented as median (IQR)* or frequency (%)** according to their distribution pattern
Values in bold indicate statistically significant results.
Stroke disability
At admission, 212 cases had premorbid disability (mRS score ≥2). 166 patients were discharged alive with mRS score ≥2. In the univariate analysis, the odds of mRS score ≥2 at discharge in COVID-19 patients did not differ from those without COVID-19 (OR: 0.856; 95% CrI: 0.39, 1.86). Likewise, after adjusting for confounding variables, we did not observe any difference in the rate of disability among patients with and without COVID-19 (OR: 0.5, 95% CrI: 0.09, 2.64) (Table 3 ). A similar finding was observed when we analyzed mRS as an ordinal variable (0-5) in the multivariate model (Data not shown; OR: 0.986, 95% CrI: 0.49, 1.76).
Table 3.
Risk of disability at discharge in acute stroke with and without COVID-19 treated with IV-tPA: Results of Bayesian multiple regression model.
| Variable | mRS≥ 2n=167 | mRS<2n=187 | Posterior Odds Ratio | Standard error | 95% Crlfor odds ratio |
|---|---|---|---|---|---|
| Age* | 72 [62, 81] | 65 [55.5, 78] | 1.04 | 1.01 | [1.012, 1.061] |
| Sex (male/ female)** | 84/83 (50.3%) | 109/69 (61.2%) | 1.269 | 1.49 | [0.585, 2.753] |
| mRS≥ 2 at admission** | 109 (65.7%) | 64 (36%) | 1.261 | 1.12 | [1.006, 1.594] |
| Hypertension** | 130 (83.2%) | 109 (64.5%) | 1.011 | 1.02 | [0.968, 1.052] |
| NIHSS at admission* | 12 [8, 18] | 8 [5, 13] | 1.151 | 1.04 | [1.074, 1.243] |
| high-Sensitivity C-reactive Protein (mg/dl)* | 5.6 [1.8, 12.5] | 3.47 [1, 10] | 1.066 | 1.03 | [1.02, 1.126] |
| Aspartate Aminotransferase (µmol/l)*** | 29.5 [20, 40] | 27 [19, 38.4] | 1.01 | 1.01 | [0.998, 1.028] |
| Hemoglobin (g/dl)* | 13.7 [12.26, 14.6] | 14 [12.8, 15.2] | 0.993 | 1.09 | [0.847, 1.168] |
| COVID19 (yes/no)** | 22 (13.2%) | 24 (13.6%) | 0.498 | 2.39 | [0.097, 2.64] |
Abbreviations: CrI; Credible Interval, NIHSS: National Institute of Neurological Disorders and Stroke Score. Numbers are presented as median (IQR)* or frequency (%)** according to their distribution pattern. The center effect is included as random in the Bayesian model. Values in bold indicate statistically significant results.
Missing data for mRS: 133 alive cases at discharge, including 23 with COVID-19.
The result significance was limited since we did not have access to mRS data of 133 alive cases at discharge (including 23 with COVID-19). In missing data analysis, we documented a similar result with no difference in stroke disability between patients with and without COVID-19 (Data not shown; OR: 1.03; 95% CrI: 0.68, 1.52).
In-hospital mortality
In total, 72 patients died in hospital. Among those with COVID-19 (n=101), 26 cases died in hospital, including 8 cases directly due to COVID-19 complications (12.9% of total mortality). The odds of mortality in the crude model was higher among those with COVID-19 (OR: 2.99; 95% CrI: 1.74, 5.13). However, after adjusting for confounding variables in the multiple Bayesian model, the increased risk of mortality in COVID-19 was not confirmed (OR: 3.60; 95% CrI: 0.83, 6.60). Among those with symptomatic hemorrhage (n=37), 17 patients (including 5 cases with COVID-19 and 12 without) died. We did not observe any difference in the unadjusted odds of mortality in those with symptomatic hemorrhage with COVID-19 and without COVID-19 (OR: 2.36, 95% CrI: 0.47, 11.8). We do not have access to enough data regarding cause of death among those with symptomatic hemorrhage. NIHSS score at admission and HS-CRP were associated with an increased risk of in-hospital mortality (Table 4 ). Finally, adjusted composite odds of disability at discharge (mRS score ≥2) and mortality did not differ among those with and without COVID-19 (OR: 1.33; 95% CrI: 0.7, 2.59).
Table 4.
Risk of in-hospital mortality in acute stroke patients with and without COVID-19 treated with IV-tPA: Results of Bayesian multiple regression model.
| Variables | Deathn=72 | Aliven=478 | Posterior Odds Ratio | Standard error | Adjusted 95% Crl |
|---|---|---|---|---|---|
| Age (year)* | 71 [61, 78] | 68 [58, 77] | 1.02 | 1.02 | [0.98, 1.064] |
| Sex (female/ male)** | 35/36 (49.3%) | 206/272 (43.1%) | 0.876 | 1.55 | [0.37, 2.09] |
| NIHSS at admission* | 15 [10, 19.5] | 13 [9, 18.5] | 1.099 | 1.04 | [1.021, 1.184] |
| Hypertension ** | 48/18 (72.7%) | 312/137 (62.5%) | 1.016 | 1.62 | [0.41, 2.68] |
| Glucose (mg/dl)* | 145 [120, 164.75] | 128 [110, 150] | 1.002 | 1 | [0.993, 1.012] |
| Lymphocyte * | 1.82 [1.14, 2.23] | 1.67 [1.24, 2.53] | 1.006 | 1.3 | [0.602, 1.716] |
| Polymorphonuclear Neutrophils | 3.58 [2.31, 5.36] | 2.84 [1.93, 4.34] | 1.042 | 1.1 | [0.865, 1.248] |
| High-sensitivity C-Reactive Protein (mg/l)* | 58.5 [9.58, 146.25] | 10 [5, 19.4] | 1.016 | 1.01 | [1.001, 1.033] |
| Lactate Dehydrogenase (µmol/l)* | 511.5 [334.25, 726] | 468 [329, 582] | 1.001 | 1 | [0.998, 1.004] |
| COVID19 (yes/no)* | 26/36 (41.9%) | 72/295 (19.6%) | 3.619 | 2.14 | [0.837, 6.67] |
Abbreviations: CrI: Credible Interval, NIHSS: National Institutes of Health Stroke Scale.
Numbers are presented as Median (IQR)* or frequency (%)** according to their distribution pattern
The effect of center is included as random in the Bayesian model.Values in bold indicate statistically significant results.
Stroke disability, hemorrhagic transformation and hospital death in patients with COVID-19
Among 101 cases with COVID-19, 28 (53.8%) had disability at discharge (mRS score ≥2), 22 (22.4%) had hemorrhagic transformation (including 8 cases with symptomatic hemorrhage (8.2%) and 26 (25.7%) patients died in hospital. Likewise, to all patients, in the Bayesian model adjusted for covariates, HS-CRP (OR: 1.04, 95%CrI; 1.009, 1.07) had a significant association with the risk of all hemorrhagic transformation in patients with COVID-19. We were not able to perform the same analysis in those with COVID-19 and symptomatic hemorrhage due to the low sample size (n=5). HS-CRP (OR, 1.02; 95% CrI: 1.003, 1.032) and polymorphonuclear neutrophils to lymphocyte ratio (OR: 1.10; 95% Ci: 1.003, 1.30) was also associated with in-hospital mortality. None of the selected variables had a significant association with disability at discharge among patients with stroke and COVID-19 (Supplemental Table 2).
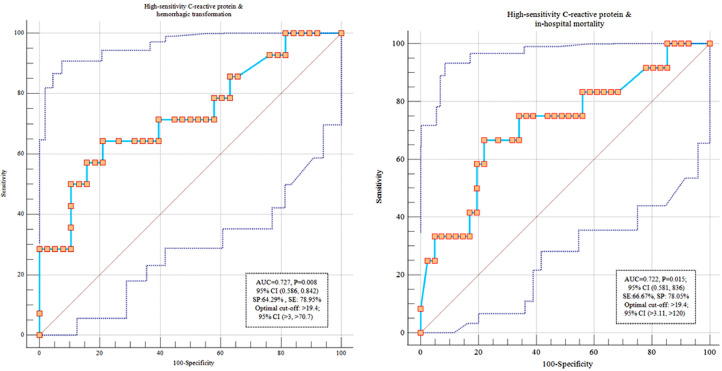
We depicted the ROC curve to assess diagnostic ability of HS-CRP to predict outcomes among individuals with COVID-19 and stroke. HS-CRP >19.4 was predictor of both stroke mortality and hemorrhagic transformation (area under the curve (AUC): 0.72; 95% CI: 0.58, 0.83) (Figure 1 ).
Fig. 1.
Diagnostic ability of High-sensitivity C reactive protein to predict mortality and hemorrhagic transformation among individuals with COVID-19.
Discussion
The current study has important clinical applications in the treatment of patients with acute stroke during the COVID-19 pandemic. In this longitudinal multi-center study of 545 patients with and without COVID-19, the odds of major clinical outcomes including in-hospital mortality, disability, composite death and disability and symptomatic hemorrhagic transformation did not differ between those with and without COVID-19. Length-of-stay in hospital was longer in those with COVID-19. HS-CRP level was confirmed to be a predictive factor for hemorrhagic transformation and death in all cases and in those with COVID-19. Patients with COVID-19 had a more severe stroke at admission and large-artery atherosclerosis was the most common cause of stroke among these patients.
COVID-19 incidence and mortality have a significant association with the burden of non-communicable diseases.17 , 18 SARS-CoV-2 can target the central nervous system1 and be associated or present with vascular complications, including stroke.2 Therefore, it is crucial to understand preventive and therapeutic measures for management of vascular diseases during the pandemic. Data regarding the safety and outcomes of IV-tPA in patients with acute ischemic stroke and COVID-19 is scarce. Early in the pandemic, there were many concerns regarding hemorrhagic manifestations of disseminated intravascular coagulation10; but further experience suggests hypercoagulopathy is a greater concern with increased thrombogenicity and fibrinolysis resistance (IV-tPA failure because of resistance or recurrent thrombosis).11 In the current study, large-artery atherosclerosis was a major cause of stroke in all cases, particularly in those with COVID-19, which is in contrast with other series where cryptogenic stroke was the most common mechanism of stroke among COVID-19 patients.19 , 20 This may indicate a possibility of increased thrombogenicity in those with COVID-19 and stroke.
In an early report of 15 patients with COVID-19 and subsequent stroke, although the general prognosis was dismal, one patient received IV-tPA and improved without hemorrhagic transformation.21 In an Egyptian case series of 10 patients with COVID-19 and ischemic stroke treated with IV-tPA, anticoagulants, and/or antiplatelet only one developed intracerebral hemorrhage after receiving anticoagulation.22 Finally, in a multi-center study in the US, 13 patients with stroke and COVID-19 received IV-tPA. None of these patients had systemic or symptomatic intracranial hemorrhages.23 In the current longitudinal study, we did not observe a significant difference in the risk of hemorrhagic transformation among patients with and without COVID-19. In cases with and without COVID-19, we did not also observe any significant differences between the risk of disability at hospital discharge, in-hospital death and composite death and disability. This is an important finding indicating the possible safety of IV-tPA in COVID-19 patients. The overall rate of symptomatic hemorrhagic transformation in Iranian centers was high (2.61% in Dresden, 4.76% in Athena, and 7.17% in Iran). This rate in Iran appears to be higher than our previous data (∼2% symptomatic prior to the COVID-19 pandemic) registered in SITS (paper under review). We do not have a good explanation for this finding, particularly it is difficult to explain why those without COVID-19 had a higher rate of hemorrhage during the pandemic. Access to thrombolytic therapy and patient care after treatment with IV-tPA has been consistently more challenging in low- and middle-income countries than high-income countries.24 In addition, it is also possible that the COVID-19 pandemic itself has affected patient care4 and diluted stroke expertise. Unfortunately, several members of the acute stroke team have been affected or passed away by COVID-19; in some centers, stroke units changed to COVID-19 care centers, and well-experienced nurses have been transferred to COVID-19 care centers.
In line with previous studies,25 , 26 inflammatory markers including HS-CRP were significantly associated with in-hospital mortality and hemorrhagic transformation. In addition, among cases with COVID-19, inflammatory markers, including HS-CRP had a significant association with hemorrhagic transformation and in-hospital mortality. Inflammation is a key driver of intravascular clotting, due to raised fibrinogen, raised platelets and importantly the increased exposure of tissue factor among other factors. Fibrinogen can amplify inflammation, and hypoxia activates pathways such as vascular endothelial growth factor (VEGF), also known as vascular permeability factor which might aggravate the local inflammation and increase the risk of hemorrhagic transformation27 ,.28 These combinations in patients with COVID-19 may explain the association between HS-CRP level and risk of hemorrhagic transformation. In the line of previous research in COVID-19, lymphopenia and Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19 the odds of mortality in those with COVID-19.
Our study has some limitations. In two centers, HS-CRP was recorded positive or negative with no information regarding the values. Symptomatic versus asymptomatic hemorrhagic transformation could not be analyzed and subgroup analysis in thrombectomy cases could not be performed due to the low sample size. Despite access to follow-up imaging of all cases, data regarding NIHSS score of some cases were not available. These patients were classified by experiences stroke neurologists to symptomatic vs. asymptomatic. Although mRS data at discharge was not available in 133 cases, in missing data analysis we did not find any significant difference in disability rate by COVID-19 status. In the current paper, we reported discharge mRS which is not the usual time point for the assessment of functional outcome. While heterogeneity of selected populations from multiple centres may be a limitation for our study, we reported one of the largest studies of cases with COVID-19 treated with IVtPA; this sample size would not be possible without collaboration of multi stroke centres. We are not able to comment regarding the rate of COVID-19 cases diagnosed as routine COVID-19 screening.
COVID-19 is associated with increased risk of acute ischemic stroke, and data regarding outcome after intravenous thrombolysis and thrombectomy in patients with COVID-19 is limited to case reports or small case series. This study outlines the safety of IV lysis in a large number of AIS with COVID-19 from several centers in three countries in Europe and the Middle East.
It will be helpful if the authors would separate patients with AIS who incidentally tested positive for COVID-19 versus those who were hospitalized with the viral infection and subsequently developed AIS, as the latter group is most likely to have worse outcome. what were the COVID-19 symptoms on presentation (fever, cough, etc.).
We are planning to report functional outcomes after 3 months and one year in our participants. In our next report from the CASCADE group, we will also categorize the type and frequency of large vessel occlusion and results of mechanical thrombectomy in our cases with COVID-19. To the best of our knowledge, this is the first longitudinal study of stroke with and without COVID-19 that assessed the safety of IV-tPA. In summary, IV-tPA appears to be safe in patients with acute ischemic stroke and COVID-19. Treatment with IV-tPA should not be delayed while the possibility of COVID-19 is ruled out. Approaches such as “Protected Code Stroke” in this setting may be a useful strategy.29 Length-of-stay in hospital was higher in our patients with COVID-19 in comparison with non-COVID-19 ones. Definitely, those with COVID-19 and stroke need more attention to prevent disease spread and reduce the chance of mortality and morbidity. HS-CRP can be used as a predictive factor to identify those at risk of hemorrhagic transformation. Using our multicenter database within the CASCADE initiative, we are planning to confirm this study's findings and evaluate the safety of mechanical thrombectomy in the near future. A higher rate of total symptomatic hemorrhage in all cases is an alarming finding indicating unfortunate changes in patient care after the pandemic. We will make the relevant scripts available in Github to facilitate and standardize data reporting for other centers.
Funding
None.
Declaration of Competing Interest
Authors reports no disclosures.
Acknowledgment
We would like to thank our patients and colleagues supporting us for this research despite all difficulties due to the pandemic.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2021.106121.
Appendix. Supplementary materials
References
- 1.Divani AA, Andalib S, Biller J, et al. Central Nervous System Manifestations Associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20(12):60. doi: 10.1007/s11910-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Divani AA, Andalib S, Di Napoli M, et al. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira RG, Abdalkader M, Qureshi MM, et al. Global impact of COVID-19 on stroke care. Int J Stroke. 2021 doi: 10.1177/1747493021991652. [DOI] [Google Scholar]
- 4.Ghoreishi A, Arsang-Jang S, Sabaa-Ayoun Z, et al. Stroke Care Trends During COVID-19 Pandemic in Zanjan Province, Iran. From the CASCADE Initiative: Statistical Analysis Plan and Preliminary Results. J Stroke Cerebrovasc Dis. 2020;29(12) doi: 10.1016/j.jstrokecerebrovasdis.2020.105321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TN, Haussen DC, Qureshi MM, et al. Decline in subarachnoid haemorrhage volumes associated with the first wave of the COVID-19 pandemic. Stroke Vasc Neurol. 2021. 10.1136/svn-2020-000695. [DOI] [PMC free article] [PubMed]
- 6.Nogueira RG, Qureshi MM, Abdalkader M, et al. Global Impact of COVID-19 on Stroke Care and Intravenous Thrombolysis. Neurology. 2021 doi: 10.1212/WNL.0000000000011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahjouei S, Anyaehie M, Koza E, et al. SARS-CoV-2 Is a Culprit for Some, but Not All Acute Ischemic Strokes: A Report from the Multinational COVID-19 Stroke Study Group. JCM. 2021;10(5):931. doi: 10.3390/jcm10050931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2021;16(2):137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SH-Y Chou, Beghi E, Helbok R, et al. Global Incidence of Neurological Manifestations Among Patients Hospitalized with COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020;12(7):648–653. doi: 10.1136/neurintsurg-2020-016220. [DOI] [PubMed] [Google Scholar]
- 12.Abootalebi S, Aertker BM, Andalibi MS, et al. Call to Action: SARS-CoV-2 and CerebrovAscular DisordErs (CASCADE) J Stroke Cerebrovasc Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020. 104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.WHO COVID-19 Case definition. World Health Organization. February 6, 2021 https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance-Case-Definition-2020.2 Accessed. [Google Scholar]
- 16.Vehtari A, Gelman A, Gabry J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. 2017;27(5):1413–1432. doi: 10.1007/s11222-016-9696-4. [DOI] [Google Scholar]
- 17.Azarpazhooh MR, Amiri A, Morovatdar N, et al. Correlations between COVID-19 and burden of dementia: An ecological study and review of literature. J Neurol Sci. 2020;416 doi: 10.1016/j.jns.2020.117013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azarpazhooh MR, Morovatdar N, Avan A, et al. COVID-19 Pandemic and Burden of Non-Communicable Diseases: An Ecological Study on Data of 185 Countries. J Stroke Cerebrovasc Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos-Araque ME, Siegler JE, Ribo M, et al. Stroke etiologies in patients with COVID-19: the SVIN COVID-19 multinational registry. BMC Neurol. 2021;21(1):43. doi: 10.1186/s12883-021-02075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabayan B, Moghadami M, Assarzadegan F, et al. COVID-19 Respiratory Illness and Subsequent Cerebrovascular Events, the Initial Iranian Experience. J Stroke Cerebrovasc Dis. 2021;30(1) doi: 10.1016/j.jstrokecerebrovasdis.2020.105454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagamine M, Chow DS, Chang PD, Boden-Albala B, Yu W, Soun JE. Impact of COVID-19 on Acute Stroke Presentation at a Comprehensive Stroke Center. Front Neurol.2020;11:850; 10.3389/fneur.2020.00850. [DOI] [PMC free article] [PubMed]
- 23.Carneiro T, Dashkoff J, Leung LY, et al. Intravenous tPA for Acute Ischemic Stroke in Patients with COVID-19. J Stroke Cerebrovasc Dis. 2020;29(11) doi: 10.1016/j.jstrokecerebrovasdis.2020.105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikkhah K, Avan A, Shoeibi A, Azarpazhooh A, Ghandehari K, Foerch C, et al. Gaps and hurdles deter against following stroke guidelines for thrombolytic therapy in Iran: exploring the problem. J Stroke Cerebrovasc Dis. 2015;24(2):408–415. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Mobarra N, Morovatdar N, Di Napoli M, et al. The Association between Inflammatory Markers in the Acute Phase of Stroke and Long-Term Stroke Outcomes: Evidence from a Population-Based Study of Stroke. Neuroepidemiology. 2019;53(1–2):20–26. doi: 10.1159/000494685. [DOI] [PubMed] [Google Scholar]
- 26.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32(1):133–138. doi: 10.1161/01.STR.32.1.133. [DOI] [PubMed] [Google Scholar]
- 27.Napoli C, Tritto I, Mansueto G, Coscioni E, Ambrosio G. Immunosenescence exacerbates the COVID-19. Arch Gerontol Geriatr. 2020;90 doi: 10.1016/j.archger.2020.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napoli C, Tritto I, Benincasa G, Mansueto G, Ambrosio G. Cardiovascular involvement during COVID-19 and clinical implications in elderly patients. A review. Ann Med Surg (Lond. 2020;57:236–243. doi: 10.1016/j.amsu.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected Code Stroke: Hyperacute Stroke Management During the Coronavirus Disease 2019 (COVID-19) Pandemic. Stroke. 2020;51(6):1891–1895. doi: 10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.