Abstract
Patient: Female, 11-year-old
Final Diagnosis: Pediatrics multisystem inflammatory syndrome
Symptoms: Cough • fever • shortness of breath
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Unusual clinical course
Background:
Early in the COVID-19 pandemic, children who were infected with severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) with vascular inflammation were described as having a vasculitis similar to Kawasaki’s disease. There are now consensus clinical guidelines that have described the presentation and diagnosis of multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection. This report aims to describe a case of MIS-C in an 11-year-old Saudi Arabian girl who presented with coronary artery aneurysm and cardiac involvement.
Case Report:
We describe an 11-year-old Saudi girl who was asymptomatic for 3 weeks after contracting SARS-CoV-2. Three weeks after suffering a mild flulike illness, she developed a high fever, cough, and severe clinical deterioration within 12 h of admission, including shock, rash, pleural effusion, high inflammatory markers, and a coronary aneurysm. As per current practice, the diagnosis was confirmed as multisystem inflammatory syndrome based on a SARS-CoV-2 test with reverse transcription polymerase chain reaction (RT-PCR) from 2 nasopharyngeal aspirates. Her condition was successfully treated with antibiotics, inotropes, IVIG, aspirin, and Tocilizumab, in addition to high-flow oxygen therapy. Eventually, she was able to return home after fully recovering.
Conclusions:
The findings in this report suggest that children with MIS-C due to SARS-CoV-2 infection can have a good prognosis, even when they suffer from coronary artery and cardiac involvement. The increasing number of emerging SARS-CoV-2 variants that affect children supports the importance of RT-PCR for the COVID-19 diagnostic test for children with multisystem or cardiovascular inflammation, which may guide the most appropriate clinical management of the variants of MIS-C.
Keywords: COVID-19; Severe Acute Respiratory Syndrome Coronavirus 2; Pediatric Multisystem Inflammatory Disease, COVID-19 Related; Coronary Aneurysm; Case Reports; COVID-19 Diagnostic Testing
Background
Since the middle of spring 2020, a new cluster of cases of multisystem inflammatory syndrome (MIS-C) in children has been identified. The MIS-C variant of SARS-CoV-2 infection is characterized by acute febrile illness, stomach pain, gastrointestinal symptoms, shock, and cardiac involvement. There were many similar cases reported initially in Europe, and later in the USA, indicating the possibility of SARS-CoV2 infection, especially in northern Italy and France. However, diagnosis requires either RT-PCR, serological testing, or contact with a COVID-19 patient [1–3]. The incidence of the multisystem inflammatory syndrome in the USA person was 316 (95% CI, 278–357) persons per 1 million SARS-CoV-2 infections [4]. Although the prevalence of MIS-C is unknown, more than 4018 cases have been reported so far in the USA by the Centers for Disease Control and Prevention (CDC) [3]. A case definition of MIS-C has been published by the World Health Organization (WHO) and the Disease Control and Prevention Centers (CDC) [5,6].
In the study of 95 confirmed MIS-C patients in New York, the pro-B-type natriuretic peptide (proBNP) level was elevated in 74 out of 82 (90%). Sixty-three out of 89 patients (71%) showed increased troponins with echocardiographic features indicative of perimyocarditis, heart function defects, and shock, but with normal coronaries. Around 62% of the cases required vasopressors, with predominant clinical features of gastrointestinal and mucocutaneous involvement [7]. The prospective Brazilian multicenter study by Fernanda Lima-Setta et al documented 56 cases of MIS-C, and more than half of the affected patients had gastrointestinal symptoms, nearly 60% had rash, and more than 60% were in shock. Furthermore, approximately two-thirds of MIS-C case studies show echocardiographic features such as pericardial effusion, depressed left ventricular function, and coronary artery ectasia of about 27% [8]. A case series experiment was conducted at the Tertiary Care Hospital in southern Turkey with 52 children diagnosed with MIS-C. The median age of the patients was 9 (5–13) years. There was significant left ventricular dysfunction of 17.3%, along with fever (92.3%), nausea (76.9%), rash (48.1%), and vomiting (48.1%). Neither coronary artery disease nor myocarditis has been diagnosed [9].
There have been other studies showing a median age of 7 years, with a significant number of patients having comorbidities and elevated D-dimers, as in previous cohort studies, as well as fever, abdominal pain, vomiting, mucocutaneous involvement, and elevated inflammation markers. Lymphopenia and thrombocytopenia were both found in 24 (53%) and 17 (38%) patients, respectively. Among 25 patients (56%) who were diagnosed with cardiac involvement, 31% had coronary artery involvement and 18% had myocarditis [10]. The SARS-CoV-2 infection associated with this novel syndrome, MIS-C, has been associated with a number of cardiovascular complications, including heart failure, pericardial effusions, myocarditis, and rhythm irregularities. A severe case may present with cardiogenic shock or distribute shock, requiring fluid resuscitation and inotropic support [11–14]. Among 10 patients in Saudi Arabia, 30% experienced cardiovascular involvement, shock, left ventricular dys-function, and pericardial effusion, but coronary artery ectasia and aneurysms were not observed [15]. A similar echocardio-graphic finding was noted in more than half of MIS-C cases with coronary artery ectasia rather than aneurysm [16]. Further, a few published case reports indicated no coronary lesion [17–19]. We believe this to be the first reported case of coronary artery aneurysm associated with MIS-C in Saudi Arabia. The purpose of this report is therefore to describe a case of MIS-C in an 11-year-old Saudi Arabian girl who presented with a coronary artery aneurysm and cardiac involvement.
Case Report
An 11-year girl with a high-grade fever and a productive cough was admitted to the pediatric ward on 25 October 2020. Three weeks prior to her presentation, she had become infected with SARS-CoV-2. Initially, the symptoms of her illness appeared similar to that of mild flu. At home, she was able to cope with her illness. In terms of systemic review, the symptoms were unremarkable aside from mild abdominal pain, throat pain, and a lack of appetite without taste distortion. She had no unusual history of drugs, allergies, medical history, or surgical procedures. Her family lives in the eastern region of Saudi Arabia. She is a first-degree relative of her parents. Although her mother and 3 siblings are healthy, the family was in crisis after her father died from complications caused by SARS-CoV-2.
She had a fever of 39.5°C, was looking unwell, with congested tonsils, but no conjunctivitis or lymphadenopathy and no skin rash. The patient was fully oriented, did not experience respiratory symptoms, and her hemodynamic state was stable. An examination of the abdomen revealed no anomalies. In the initial laboratory tests, 2 nasal aspirates were positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Tests of the blood found elevated levels of inflammatory markers, notably C-reactive protein (CRP) 127 and ferritin 1313.9. However, white blood cell totals were normal at 5.29. A differential count revealed that the neutrophil count was normal, lymphocytopenia was present, and the D-dimer was 4.02, with normal prothrombin time (PT), partial thromboplastin time (aPTT), and international normalized ratio (INR). An X-ray of the chest (Figure 1) and a renal and liver profile (Table 1) revealed no abnormalities.
Figure 1.
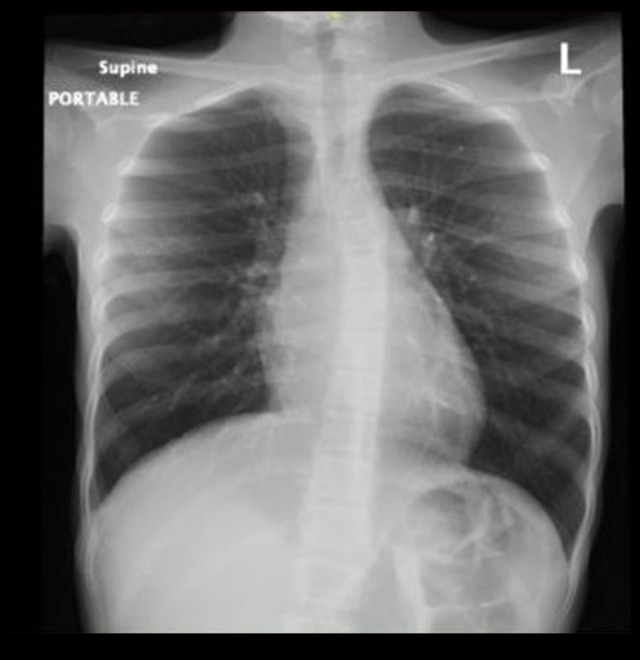
Chest X-ray on admission. Both lung fields are over-aerated. No parenchymal consolidation or ground-glass opacities in either lung field. Normal chest X-ray finding.
Table 1.
The results of laboratory testing for the first 172 h of hospitalization. Neutrophil counts were normal, lymphocytopenia and thrombocytopenia were present, and inflammatory markers, such as CRP, Procalcitonin, Ferritin, and high D-dimer, peaked at 96 h after the illness began. Normal prothrombin time (PT), partial thromboplastin time (aPTT), and international normalized ratio (INR).
| Lab test | Admission | 48 hrs | 96 hrs | 120 hrs. | 148 hrs | 172 hrs | Reference |
|---|---|---|---|---|---|---|---|
| WBC | 5.29 | 10.60 | 15.30 | 13.00 | 7.12 | 6.13 | 4–12×109/L |
| Neutrophil | 4.08 | 8.78 | 8.87 | 4.80 | 3.01 | 0.92 | 1.10–7.2×109/L |
| Lymphocyte | 0.55 | 0.40 | 0.64 | 1.14 | 1.28 | 3.17 | 1.30–7.20×109/L |
| Hb | 10.2 | 9.2 | 8.7 | 6.6 | 8.5 | 8.3 | 113–150 gm/L |
| Platelet | 199 | 108 | 114 | 99 | 129 | 143 | 150–400×109/L |
| CRP | 127.5 | 139.1 | 186.7 | 225.4 | 46.1 | 23.1 | (≤1.20 mg/L) |
| Procalcitonin | 2.13 | 12.7 | 24.8 | 36.1 | 21.9 | 11.6 | ≤0.05 ug/L (ng/mL) |
| ESR | 25 | 45 | 49 | 64 | 42 | 31 | 0–20 |
| Ferritin | 1313.9 | 4538.62 | 4543.0 | 1120.0 | 437.4 | 125.4 | 4.6–204 ug/L |
| LDH | 276 | 231 | 218 | X | 244 | X | 125–220 u/L |
| D-dimer | 4.02 | 3.21 | 4.59 | 3.1 | 2.4 | 1.7 | 0.00–0.50 mg/L |
| aPTT | 34.9 | 31.3 | 31.2 | 29.4 | 24.9 | 22.1 | 26.2–37.0 sec |
| PT | 11.6 | 9.5 | 10.9 | 14.4 | 9.8 | 9.9 | 7.6–10.4 sec |
| INR | 1.1 | 1.1 | 1.6 | 1.1 | X | 1.1 | 0.8–1.2 |
| Fibrinogen | 3.3 | X | 0.5 | 1.5 | X | 1.5 | 1.50–4.10 gm/L |
| Troponin | X | 48.3 | X | 29.3 | 23.4 | 17.9 | ≤15.6 pg/mL |
| Sodium | 130 | 130 | 132 | 134 | 133 | 135 | 138–145 mmol/L |
| Potassium | 3.4 | 3.6 | 3 | 3.8 | 3.5 | 4.1 | 3.4–4.7 mmol/L |
| Urea | 2.3 | 2 | 3 | 4.2 | 3.5 | 2.5 | 2.5–6.0 mmol/L |
| Creatinine | 52 | 39 | 40 | 46 | 40 | 45 | 27–62 umol/L |
| AST | 47 | 30 | 21 | 22 | X | 13 | 5–34 U/L |
| ALT | 33 | 41 | 32 | 24 | 27 | 25 | 5–55 U/L |
| Calcium | 2.05 | 1.84 | 1.74 | 1.89 | 2.03 | 2.14 | 2.20–2.70 mmol/L |
| Magnesium | 0.82 | 0.73 | 0.78 | 0.86 | 0.83 | 0.87 | 0.70–0.86 mmol/L |
WBC – white blood cell; CRP – C-reactive protein; LDH – lactate dehydrogenase; AST – aspartate transaminase; ALT – alanine aminotransferase.
Initially, she was admitted to the general pediatric ward for observation, but her condition deteriorated 12 h after admission.
A progressive cough was accompanied by a spiked temperature of 39.8°C, hypotension, tachycardia, and an erythematous maculopapular rash with no itching, mostly on the face, trunk, and neck. She did not have lymphadenopathy or nonpurulent conjunctivitis. After receiving fluid boluses, she was transferred to the pediatric intensive care unit. Due to persistent high-grade fever, the patient underwent additional testing, including blood culture, urine culture, and chest X-ray. At 12 h after admission, a chest X-ray demonstrated bilateral basal pneumonic patches (Figure 2).
Figure 2.
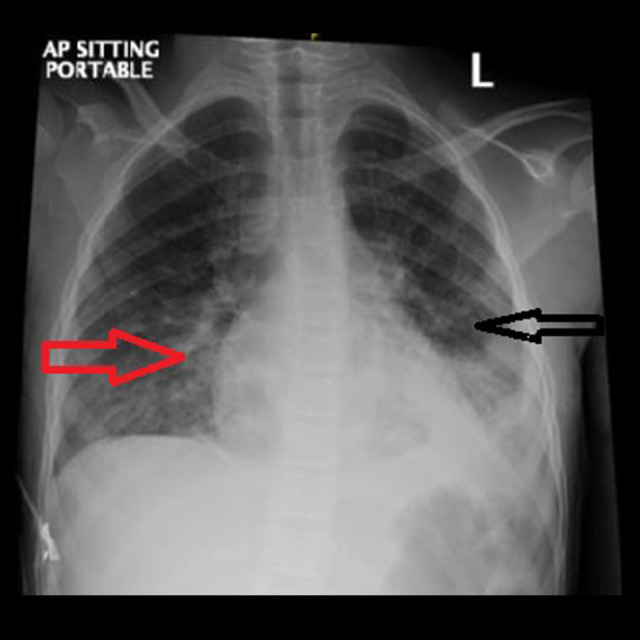
Chest X-ray 12 h after admission, showing moderatesize patchy area of consolidation in the left lower lobe. Also, there is an area of glass-ground opacity and consolidation noted in the right lower zone medially, suggestive of bronchopneumonia.
Azithromycin and Ceftriaxone were started after consultation with the infectious disease team at the time of admission to the PICU. Additionally, norepinephrine 0.3 microg/kg/ min was administered to help maintain blood pressure during this time. At 48 h after admission, the inflammatory markers D-dimer 5.21, ferritin 4538.6, procalcitonin 12.7, and CRP 139.1 had increased along with higher oxygen consumption via the non-rebreathing face mask. Dexamethasone, A low molecular weight heparin, and tocilizumab were further recommended as per institutional guidelines for SARS-CoV-2 infections, along with a cardiology referral for a high suspicion of Kawasaki-like vasculitis.
In spite of 2 doses of tocilizumab, her condition remained critical at 96 h after admission, with a persistent fever of 39.8°C, respiratory distress, and bilateral pleural effusions, as well as need for a high-flow nasal cannula oxygen (25 L/min). Moreover, the levels of inflammatory markers (Table 1) remained high, with CRP 186.7, ferritin 4543, and D-dimer 4.59. In the meantime, echocardiographic findings revealed mildly diminished left ventricular function, dilated main left coronary artery 4.3 mm (Z score=3.9), and left descending coronary artery 3.4 mm (Z score=3.1). Due to the sustained high-grade fever, high inflammatory markers, rash, shock, and positive SARS-CoV-2 RT-PCR tests, the diagnosis of MIS-C was established. The possibility of other differential diagnoses was ruled out since all cultures were negative.
The antibiotics were switched to Pipracillin+Tazobactam 100 mg/kg/dose every 8 h after 96 h of illness. In addition to aspirin, intravenous immunoglobulin was introduced. The patient was treated with intravenous immunoglobulin (IVIG) infusions of 2 g/kg, given slowly over 12 h, and given a high dose of aspirin (80-100 mg/kg/day). After a single dose of IVIG and a few doses of aspirin, her condition dramatically improved with no spike of fever within the next 48 h of illness. Subsequently, inflammatory markers decreased. The daily aspirin dose was reduced. She developed bilateral pleural effusion (Figure 3) during her stay in the Pediatric ICU, which was completely resolved on the follow-up chest X-ray taken on the 14th day of illness. Within 10 days of starting dexamethasone, the dose is gradually tapered off. There was no additional intervention needed in the PICU. Following a restful week at the Pediatric High-Dependent Unit, she was eventually discharged home from the general pediatric unit on her 17th day of illness after full recovery and normal electrocardiogram results. She was advised to follow up with her pediatric cardiologists after 2 weeks. Cardiologists conducted a follow-up exam on December 3, 2020, and determined that the patient’s cardiac function and coronary arteries were normal, so she was advised not to use aspirin anymore.
Figure 3.
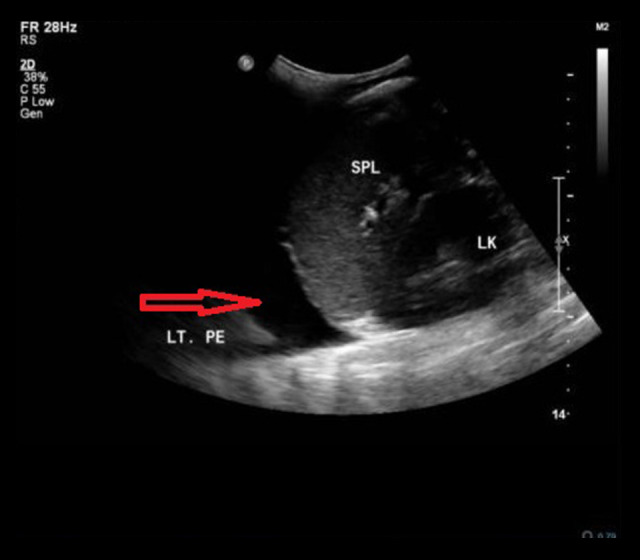
Left subdiaphragmatic longitudinal view ultrasonography, showing a moderate left pleural effusion with collapsed left lung.
Discussion
Researchers have found that MIS-C is a serious variant of SARSCoV-2, which is different from both KD and severe COVID-19 [20]. Classically, manifestation of MIS-C typically occurs 3–4 weeks after SARS-CoV-2 infection. Positive antibodies to SARS-CoV-2 were prevalent in many cases, but the RT-PCR test was negative at the time of the diagnosis of MIS-C. It consists of 6 main components: persisting fever, pediatric age group, high laboratory marker of inflammation, signs or symptoms of organ damage, lack of other diagnoses, and clinically confirmed COVID-19 infection or exposure [21]. According to the published literature, our patient fulfilled the MISC case definition [5–6,22], manifesting unremitting fever >38°C with rash, shock, and cardiac involvement in the form of myocardial dysfunction, or coronary ectasia, as well as abdominal pain [7,23–25]. In addition, there is laboratory evidence of elevated inflammatory markers, CRP, and procalcitonin [20,26,27] and negative blood culture.
The further point of relevance is an epidemiological link with a SARS-CoV-2-positive case because she had contact with a family member who acquired the virus, as confirmed by a reverse transcription polymerase chain reaction (RT-PCR) manufacturer, Cepheid, kit type Xpert Xpress SARS-CoV2 test, approved by the Saudi Food and Drug Authority in October 2020.
This is the first case report of multisystem inflammatory syndrome in children (MIS-C) in Saudi Arabia involving coronary artery aneurysm, left ventricular dysfunction, hyperinflammation, and multiorgan damage associated with SARS-CoV-2 infection. Rapid clinical deterioration has been shown in most studies as a cardinal feature, associated with shock, poor capillary refilling times, and skin rashes, and necessitating fluid bolus administration followed by inotrope support, as in our case [15–17,24] In fact, shock is either cardiogenic or distributive in nature [28,29]. de Lama Caro-Paton published a case series in a pediatric intensive care unit in Madrid, Spain, with 12 patients with MIS-C ages 5–14 years old. All patients presented with shock and received volume resuscitation, 75% required vasoactive/inotropic support, 67% had a high hs-cT-nI, and 33% demonstrated left ventricular dysfunction [29].
Kawasaki disease (KD), Behcet’s syndrome, Takayasu disease, connective tissue disorders, HIV, and fungal and syphilitic infections are among the prominent causes of coronary artery ectasia or aneurysm in adults [30]. However, KD, polyarteritis nodosa, and other vasculitis are more commonly responsible for coronary artery ectasia and aneurysms in children [13], but MIS-C in association with SARS-CoV-2 has only recently been observed as a common cause of coronary artery involvement. This has not been documented in Saudi Arabia in published case reports.
In a systematic review published by Rodriguez-Gonzalez et al, 688 MIS-C cases were identified as having cardiovascular events. Cardiogenic shock was present in 53.20% (48.70–57.80%), pro-BNP increased was present in 86.80% (83.60–89.40%), and ECG abnormalities were present in 27.6% (23.9–31.6%). The incidence of myocardial dysfunction was 52.20% (48.40–56.00%), coronary artery ectasia/aneurysm was 15.40% (12.40–17.80%), and 53.4% of patients required PICU (Pediatric Intensive Care Unit) care [31]. There have been a number of cohort studies and meta-analyses that reached similar conclusions [12,32,33,35]. Although coronary artery aneurysm rates in MIS-C are unknown, patients with MIS-C but without KD features have been reported to have coronary artery aneurysms [34,36]. The American College of Cardiology called attention to the risks of cardiac involvement in patients infected with Covid-19 in Feb 2020 [37]. According to existing evidence, worldwide published articles reported major cardiac manifestations, including ventricular dysfunction, coronary artery ectasia, arrhythmia, conduction abnormalities, and vasodilatory shock requiring fluid resuscitation, along with ino-tropic support [38].
Nonetheless, the pathophysiology of cardiac lesions caused by MIS-C in children has not been fully described. Various theories suggest that SARS-CoV-2 spike proteins, infecting cardiomyocytes through the angiotensin converting enzyme 2 (ACE2) receptor, caused myocardial damage and deregulated immune function, and the subsequent cytokine storm leads to multiorgan failure and severe cardiotoxicity [31]. Further, due to prolonged hypoxia and ischemia, microvascular thrombosis, coronary artery disease, right heart strain, and stress cardiomyopathy, a consequence of oxygen demand and supply imbalance, all contribute to cardiac muscle damage [29]. Medications like lopinavir/ritonavir, hydroxychloroquine, and tocilizumab, whose safety margins are less clear, could worsen cardiac dysfunction in pediatric patients with COVID-19 infection [31]. As reported by biopsy, a COVID-19 viral particle localized in the myocardium [39] leads to severe necrotizing myocarditis [40]. High levels of troponin I (Trop I), myoglobin (myo), and n-terminal brain natriuretic peptide (NTBNP) generally indicate severe, fulminant myocarditis [39]. In terms of intensive care unit admission, ventilation, and death, abnormally elevated cardiac markers provide valuable prognostic information [13]. There is a significant role for genetic susceptibilities in the pathogenesis of MIS-C in both immuno-logical and non-immune ways [41]. Cardiovascular magnetic resonance imaging (MRI) on T2-STIR sequences and native-T1 mapping without late gadolinium enhancement clarified post-infectious myocarditis in children [42].
At 12 h after admission, her condition was rapidly deteriorating, with severe lung consolidation, which required administration of a high-flow nasal cannula. This led to the physician misdiagnosing her illness as severe COVID-19, rather than MIS-C. As a consequence, despite adequate antibiotic coverage and steroid treatment, as well as 2 doses of tocilizumab, she remained ill and febrile. Moreover, her overall health rapidly deteriorated, as she developed persistent fever, worsening respiratory distress, and progressive elevation of inflammatory markers. As a rule, respiratory manifestations such as breathlessness, coughing, increased oxygen demand, and progressive lung consolidation do not occur in MIS-C [43]. As other papers have documented, the clinical presentation of MIS-C has sometimes resembled that of severe COVID-19 disease [44–47].
At 96 h after hospital admission, her CRP was 186.7, ferritin was 4543, and D-dimer was 4.59. Meanwhile, echocardiography reported mildly depressed left ventricular function, dilated main left coronary artery 4.3 mm (Z score=3.9), and left descending coronary artery 3.4 mm (Z score= 3.1). Our patient’s ECHO finding was consistent with a minor coronary aneurysm. A normal coronary artery Z score is less than 2, ectasia is Z score 2-2.5, and a minor aneurysm is Z score greater than 2.5 to less than 5 [48]. In fact, coronary artery involvement was described in the majority of studies [47,49–54]. Skin rash, which appeared in the early course of illness of our patient, is another cardinal sign of MIS-C. Our findings agree with previous findings that 36–81% of MIS-C patients had rash [45,47,55–61]. Laboratory findings of our case are in keeping with similar publications on leukopenia and thrombocytopenia [20,62,63] high ferritin, abnormal D-dimer, and elevated troponin [20,46,64,65]. In contrast to other findings, acute kidney injury (AKI) [64,66–69], prolonged coagulation profile, abnormal fibrinogen, and abnormal liver function[70–73] were not observed in our case.
According to a recent literature review, the vast majority of multisystem inflammatory syndrome patients recovered from treatment with intensive care, which typically included respiratory support, inotropes, intravenous immunoglobulin (IVIG), and steroids [74–76], as well as Anakinra or tocilizumab for immunomodulation [24,77]. A second dose of immunoglobulin and pulse methylprednisolone may be considered prior to immunomodulatory adjuvants [74]. Our case provides a noteworthy example of the failure of both tocilizumab and steroids to control the symptoms rather than IVIG, since most references suggest IVIG combined with steroids and Anakinra as the second-line treatment option, and then tocilizumab as the third. In Saudi Arabia there were no guidelines on MIS-C management, so most patients were treated according to COVID-19 procedures, which included antiviral drugs, interleukin-6 (IL-6) inhibitors, and low molecular weight heparin [70]. Anakinra was unavailable to all the patients in the Saudi Arabian case series, which had a fatality rate of 20% [16]. It is currently included in hospital guidelines, to the best of our knowledge.
The fever gradually subsided and inflammatory markers began to fall after adding IVIG in addition to steroids after 96 h of hospitalization. The treatment was augmented with high-dose aspirin and standard-dose IVIG 2.4 gm/kg/dose. We observed coronary artery dilation in 17% of cases of MIS-C, either observed shortly after hospitalization or at follow-up, which is mainly short-term cases either observed during hospital recovery or at follow-up [13]. Additionally, a giant coronary aneurysm with a Z score >10 was reported, which required prolonged anticoagulant treatment and cardiac monitoring [76]. No appropriate treatment scheme for pediatric multisystem inflammatory syndrome has yet been identified, but some guidelines have been established [27,77]. Antiplatelet-dose aspirin may be prescribed for patients who have coronary artery aneurysms, and anticoagulants may be prescribed for coagulopathic patients (high D-dimer level) or patients with large or giant aneurysms [16]. The conclusion on the optimal treatment of MIS-C will be determined by improved understanding of the disease and randomized multicenter double-blinded controlled studies.
Conclusions
The MIS-C illness is a completely distinct condition following SARS-CoV-2 infection. There is very little risk of mortality, even if there are some long-term effects. Most patients get better quickly. The most effective clinical management requires a multidisciplinary approach that includes critical care, infectious disease, immunology, cardiology, and rheumatology. Various therapies have been proven to be successful, including intravenous immunoglobulins, steroids, and other immunomodulatory drugs. Long-term follow up is recommended for those with significant cardiac damage.
Acknowledgments
The authors acknowledge the support of the Head of the Department, Dr. Maher Al Hathlani, and Dr. Ahmed Alamoudi, Pediatric ICU consultant, Pediatric Cardiology consultant Dr. Al Dijani, and all the staff involved in the management of the case.
Footnotes
Department and Institution Where Work Was Done
Department of Pediatrics, Imam Abdulrahman Bin Faisal Hospital, Ministry of National Guard, Dammam, Saudi Arabia
Conflict of Interest
None declared.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021;73(4):e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–22. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Multisystem inflammatory disease in children (MIS-C) May, 2021. Available at: https://www.cdc.gov/mis-c.
- 4.Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Network Open. 2021;4(6):1–13. doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children and proposed management. Children. 2020;7(69):2–14. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–87. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Multisystem inflammatory syndrome in children and adolescents with COVID-19. 2020 [Google Scholar]
- 8.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–58. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lima-setta F, Magalhães-Barbosa MC De, Rodrigues-santos G, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARSCoV-2 pandemic in Brazil: A multicenter, prospective cohort study. J Pediatr (Rio J) 2021;97(3):354–61. doi: 10.1016/j.jped.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolunay O, Çelik Ü, Arslan İ, et al. Multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19: A case series experience in a tertiary care hospital of Southern Turkey. J Trop Pediatr. 2021;67(2):1–26. doi: 10.1093/tropej/fmab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamishi S, Movahedi Z, Mohammadi M, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: A first report from Iran. Epidemiol Infect. 2020;148:e196. doi: 10.1017/S095026882000196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valverde I, Singh Y, Sanchez-de-Toledo J, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 13.Malviya A, Mishra A. Childhood multisystem inflammatory syndrome: An emerging disease with prominent cardiovascular involvement – a scoping review. SN Compr Clin Med. 2021;3(1):48–59. doi: 10.1007/s42399-020-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: Implications for the cardiovascular system. Circulation. 2020;2019:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 15.Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143(1):78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 16.Almoosa ZA, Al Ameer HH, AlKadhem SM, et al. Multisystem inflammatory syndrome in children, the real disease of COVID-19 in pediatrics – a multi-center case series from Al-Ahsa, Saudi Arabia. Cureus. 2020;6(10):e11064. doi: 10.7759/cureus.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asseri AA, AlHelali I, Elbastawisi E, et al. Multi-system inflammatory syndrome in children during the coronavirus disease 2019 in Saudi Arabia: Clinical perspective from a case series. Medicine. 2021;100(22):e25919. doi: 10.1097/MD.0000000000025919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Ameer HH, AlKadhem SM, Busaleh F, et al. Multisystem inflammatory syndrome in children temporally related to COVID-19: A case report from Saudi Arabia. Cureus. 2020;12(9):8–12. doi: 10.7759/cureus.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alyousef AM, Alshamrani MA, Alruwaili AS, et al. Multi-system inflammatory syndrome in children (MIS-C) reported in COVID-19 positive 2.5-year old boy. International Journal of Innovative Research in Medical Science. 2020;5(9):377–81. [Google Scholar]
- 20.Sleiman RA, Okash WA, Alruwaili AS, et al. Multisystem Inflammatory Syndrome Associated with COVID-19 in pediatrics: A case report in Saudi Arabia. Dr Sulaiman Al Habib Medical Journal. 2020;3(1):12. [Google Scholar]
- 21.Zhao Y, Yin L, Patel J, et al. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J Med Virol. 2021;93(7):4358–69. doi: 10.1002/jmv.26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multisystem inflammatory syndrome in children (MIS-C) >fact sheets> Yale Medicine. Accessed January 9, 2021 https://www.yalemedicine.org/conditions/multisystem-inflammatory-syndrome-in-children-mis-c. [Google Scholar]
- 24.Torres JP, Izquierdo G, Acuña M, et al. Multisystem inflammatory syndrome in children (MIS-C): Report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis. 2020;100:75–81. doi: 10.1016/j.ijid.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kest H, Kaushik A, DeBruin W, et al. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr. 2020;2020:8875987. doi: 10.1155/2020/8875987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrensia S, Henrina J, Wijaya E, et al. Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2: A new challenge amid the pandemic. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00602-8. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children : A systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Rheumatology MIS-C and COVID-19 Related Hyperinflammation Task Force Clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS-C) Associated with SARS-CoV-2 and hyperinflammation in COVID-19. American College of Rheumatology. 2020:1–5. (Version 1): [Google Scholar]
- 29.Mart A, Garc A, Cabrero-Hern M. Shock and myocardial injury in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection: What we know. Case series and review of the literature. J Intensive Care Med. 2021;36(4):392–403. doi: 10.1177/0885066620969350. [DOI] [PubMed] [Google Scholar]
- 30.Revzin M, Raza S, Srivastava NC, et al. Multisystem imaging manifestations of COVID-19, Part 2: From cardiac complications to pediatric manifestations. Radiographics. 2020;40(7):1866–92. doi: 10.1148/rg.2020200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saling LJ, Raptis DA, Parekh K, et al. Abnormalities of the coronary arteries in children: Looking beyond the origins. Radiographics. 2017;37(6):1665–78. doi: 10.1148/rg.2017170018. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. 2020;8(21):5250–83. doi: 10.12998/wjcc.v8.i21.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newburger J, Vanderpluym C, Ferranti S De, Friedman KG. Detailed assess-ment of left ventricular function in multisystem inflammatory syndrome in children using strain analysis. CJC Open. 2021;3(7):880–87. doi: 10.1016/j.cjco.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogana Shanmugam V, Psaltis PJ, Wong DTL, et al. Outcomes after primary percutaneous coronary intervention for ST-elevation myocardial infarction caused by ectatic infarct related arteries. Heart Lung Circ. 2017;26(10):1059–68. doi: 10.1016/j.hlc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Yasuhara J, Watanabe K, Takagi H, et al. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(5):837–48. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 1. 2020;72(11):1791–805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanna G, Serrau G, Bassareo PP, et al. Children’s heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur J Pediatr. 2020;179(7):1079–87. doi: 10.1007/s00431-020-03699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–32. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of corona-virus in COVID- 1 9 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–15. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurence C, Haini M, Thiruchelvam T, et al. Endomyocardial biopsy in a pediatric patient with cardiac manifestations of COVID-19. Circ Heart Fail. 2020;13(11):e007384. doi: 10.1161/CIRCHEARTFAILURE.120.007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hui J, Ying Z, Liu X, et al. First case of COVID 19 complicated with fulminant myocarditis: A case report and insights. Infection. 2020;48(5):773–77. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobbs CV, Khaitan A, Kirmse BM, Borkowsky W. COVID-19 in children: A Review and parallels to other hyperinflammatory syndromes. Frontiers in Pediatrics. 2020;8:593455. doi: 10.3389/fped.2020.593455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297(3):E283–88. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiff DD, Mannion ML, Samuy N, et al. Distinguishing active pediatric COVID-19 pneumonia from MIS-C. Pediat Rheumatol Online J. 2021;19(1):21. doi: 10.1186/s12969-021-00508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–36. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 46.Miller J, Cantor A, Zachariah P, Ahn D. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: A single center experience of 44 cases. Gastroenterology. 2020;159:1571–74.e2. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrimi M, Aalouane R, Aarab C, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. Epub 2020 Jun 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronai C, Hamaoka-Okamoto A, Baker AL, et al. Coronary Artery Aneurysm Measurement and Z Score Variability in Kawasaki Disease. Journal of the American Society of Echocardiography. 2016;29(2):150–157. doi: 10.1016/j.echo.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-CoV-2 – induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130(11):5942–50. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moraleda C, Serna-Pascual M, Soriano-Arandes A, et al. Multi-inflammatory syndrome in children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Spain. Clin Infect Dis. 2021;72(9):e397–e401. doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–36. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 53.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–69. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10(1):69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhanalakshmi K, Venkataraman A, Balasubramanian S, et al. 2020 (Moraleda) Clin Infect Dis – Multi-Inflammatory Syndrome in Children related to SARSCoV-2 in Spain. Cureus. 2020;12(11):1–7. [Google Scholar]
- 56.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–46. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–47. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mariani R, Liu H. Severe transient pancytopenia with dyserythropoiesis and dysmegakaryopoiesis in COVID-19 – associated MIS-C. Blood. 2020;136(25):2964. doi: 10.1182/blood.2020009479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19 : Version 1. 2020;72(11):1791–805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panupattanapong S, Brooks EB. New spectrum of COVID-19 manifestations in children: Kawasaki-like syndrome and hyperinflammatory response. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc039. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Lipton M, Mahajan R, Kavanagh C, et al. AKI in COVID-19 – associated multi-system inflammatory syndrome in children (MIS-C) Kidney. 2021;2(4):611–18. doi: 10.34067/KID.0005372020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozlu SG, Bayhan GI. Acute kidney injury in multisystem inflammatory syndrome in children (MIS-C) SN Compr Clin Med. 2021;3(1):36–37. doi: 10.1007/s42399-020-00722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alabbas A, Kirpalani A, Morgan C, et al. Canadian Association of Paediatric Nephrologists COVID-19 rapid response: guidelines for management of acute kidney injury in children. Can J Kidney Healt Dis. 2021;8:2054358121990135. doi: 10.1177/2054358121990135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sethi SK, Rana A, Adnani H, et al. Kidney involvement in multisystem in-flammatory syndrome in children: a pediatric nephrologist’s perspective. Clin Kidney J. 2021 Apr;:1–12. doi: 10.1093/ckj/sfab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sica R, Pennoni S, Penta L, et al. New onset of hepatic steatosis post-severe multisystem inflammatory syndrome in children (MIS-C): A case report. Int J Environ Res Public Health. 2021;18(13):6961. doi: 10.3390/ijerph18136961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez A, Cantor A, Rudolph B, et al. Liver involvement in children with SARSCOV-2 infection: Two distinct clinical phenotypes caused by the same virus. Liver Int. 2021 doi: 10.1111/liv.14887. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cantor A, Miller J, Zachariah P, et al. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: A single-center report. Hepatology. 2020;72(5):1522–27. doi: 10.1002/hep.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kabeerdoss J, Pilania RK, Karkhele R, et al. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: Immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41(1):19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson JM, Newburger JW. Multisystem inflammatory syndrome in children in association with COVID-19. Circulation. 2020;142(5):437–40. doi: 10.1161/CIRCULATIONAHA.120.048726. [DOI] [PubMed] [Google Scholar]
- 71.Niaz T, Hope K, Fremed M, et al. Role of a pediatric cardiologist in the COVID 19 pandemic American Academy of Pediatrics American Society of Echocardiography. Pediatr Cardiol. 2021;42(1):19–35. doi: 10.1007/s00246-020-02476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niño-taravilla C, Espinosa-Vielma YP, Otaola-arca H. Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 treated with tocilizumab. Pediatr Rep. 2020;12(3):142–48. doi: 10.3390/pediatric12030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Navaeifar MR, Shahbaznejad L, Sadeghi Lotfabadi A, Rezai MS. COVID-19-associated multisystem inflammatory syndrome complicated with giant coronary artery aneurysm. Case Rep Pediatr. 2021;2021:8836403. doi: 10.1155/2021/8836403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramanathan K, Antognini D, Combes A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet. 2020;395:1771–78. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–8. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlapbach LJ, Andre MC, Grazioli S, et al. Best practice recommendations for the diagnosis and management of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMSTS; multisystem inflammatory syndrome in children, MIS-C) in Switzerland. Front Pediatr. 2021;9:667507. doi: 10.3389/fped.2021.667507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385(1):11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]


