Abbreviations
- AA
amino acid
- AB
animal based
- AF
antifibrotic
- AO
antioxidant
- DNL
de novo lipogenesis
- FA
fatty acid
- IM
immunomodulatory
- IR
insulin resistance
- LP
lipoprotective
- MAFLD
metabolic associated fatty liver disease
- MUFA
monounsaturated fatty acid
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PB
plant based
- PUFA
polyunsaturated fatty acid
- RCT
randomized controlled trial
- SFA
saturated fatty acid
- Vit
vitamin
Metabolic associated fatty liver disease (MAFLD) is the most common cause of chronic liver disease in the United States. Formally known as nonalcoholic fatty liver disease (NAFLD), MAFLD is a spectrum of metabolic fatty liver disorders that histologically can range from hepatic steatosis, steatosis with inflammation called steatohepatitis (e.g., nonalcoholic steatohepatitis [NASH]), and persistent inflammation leading to fibrosis and ultimately cirrhosis. These histological states can exist simultaneously and exhibit chronic‐relapsing behavior.1
MAFLD is closely associated with metabolic syndrome and its ensuing complications. As a result, the prevalence of MAFLD mirrors the rising obesity epidemic.1 The traditional Western diet has been attributed as one major cause for this observation. It is energy dense and nutrient poor. It is characterized by processed foods, red meats, sugary beverages, and animal and dairy products.2
The liver lies at the epicenter of metabolism within the human body. Digested nutrients are predominately absorbed in the small bowel, where they enter the portal circulation and arrive at the liver for further processing. The macronutrient and micronutrient composition of a diet has been shown to either promote or protect against the development of MAFLD3, 4 (Fig. 1). MAFLD pathogenesis is multifactorial; however, it often begins with the dysregulation of homeostatic mechanisms that deviate the balance toward hepatic fatty acid (FA) synthesis and storage over oxidation within and export from the liver. This process is heavily influenced by nutritional and hormonal inputs.5 Excess and imbalanced macronutrient intake triggers insulin resistance (IR) and a proinflammatory state. In contrast, the role that micronutrients play is less clear. The putative role of micronutrients is supportive and believed to exert their effects by exacerbating deregulation of lipid homeostasis and antioxidant (AO) pathways.3, 4
FIG 1.
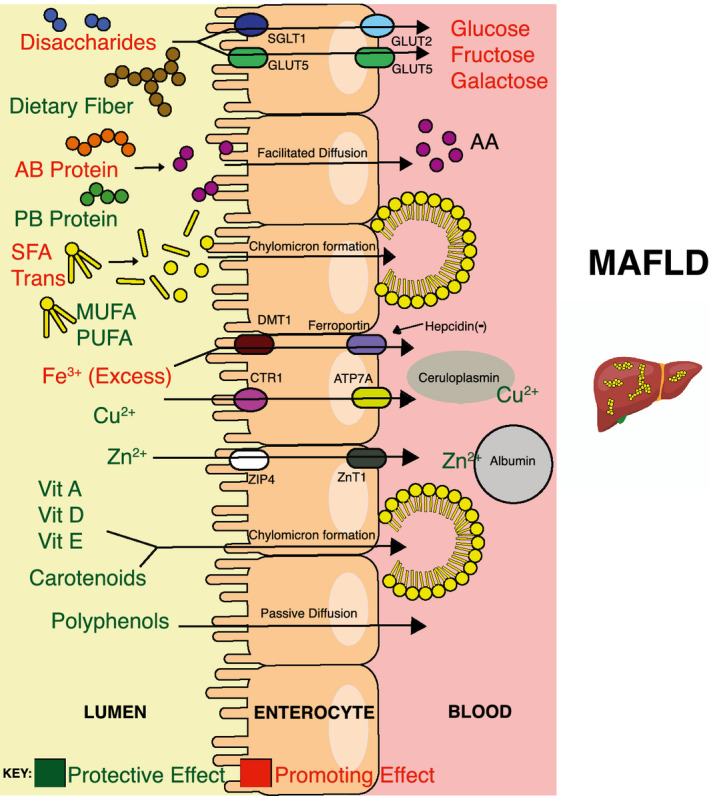
Relevant macronutrient and micronutrient absorption in the small bowel and their influences on MAFLD. Nutrients use active and/or passive transport to enter enterocytes and subsequently into the bloodstream/lymphatics, where many experience uptake and storage in the liver.
Macronutrients
The liver uses three major macronutrients for energy: carbohydrates, fats, and proteins. The various macronutrient roles in MAFLD are summarized in Table 1.
TABLE 1.
Macronutrient Metabolism in the Liver and Role in MAFLD
| Type | Hepatic Role | Dietary Findings and/or Effects of Supplementation |
|---|---|---|
| Carbohydrates | ||
| Simple sugars | Largest storage of glycogen. Homeostatic regulator of blood glucose. Converts excess carbohydrates into FAs, cholesterol, and bile salts. | High consumption leads to metabolic syndrome in human studies. Fructose is considered to be a strong DNL inducer. Fructose is noted to cause intestinal wall injury, leading to translocation of bacterial products and inflammation.4, 6 |
| Dietary fiber | Dietary intake reduces steatosis and adiposity in animal studies. Supplementation improved transaminases and insulin levels in a small human study.7 | |
| Fats | ||
| Saturated | Produces, uptakes, releases to peripheral tissues, stores, and oxidizes FAs depending on hormonal feedback. | A systemic review found a 10% risk reduction in coronary events for every 5% of energy replaced with PUFA instead of SFA. SFA intake is believed to contribute to impaired glutathione metabolism and oxidative stress.8 |
| MUFA | The relationship between MUFA intake and MAFLD development has been mixed.4 One notable RCT showed greater improvement in liver fat, hemoglobin A1c, and blood pressure in individuals with diabetes given an isocaloric, MUFA‐enriched diet in comparison with a high‐carbohydrate/high‐fiber/low‐glycemic‐index diet.9 | |
| PUFA (omega‐6) | Studies show high intake of omega‐6 >> omega‐3 in diet in patients with MAFLD. Common essential omega‐6 is linoleic acid. Linoleic acid metabolizes into arachidonic acid, which ultimately leads to production of proinflammatory, prothrombotic, and proaggregatory mediators.2, 4 | |
| PUFA (omega‐3) | Common essential omega‐3 is linolenic acid, which metabolizes into eicosapentaenoic acid and docosahexaenoic acid, which lead to production of anti‐inflammatory mediators.4 One systemic review showed supplementation with omega‐3 long‐chain PUFAs showed improvement in steatosis, metabolic risk factors, and liver enzymes in MAFLD. Supplementation was not found to benefit outcomes in improving NASH histology.10 | |
| Trans | Rat models fed a high‐trans‐fat diet over 4 months demonstrated steatosis, hyperinsulinemia, and severe necroinflammation.4, 6 | |
| Protein | ||
| Animal source | Synthesizes and breaks down AAs. Favors AAs for building hormones, cytokines, coagulants, transport proteins, etc. Can use as energy source when other macronutrients are limited. | Diets higher in animal protein sources increase risk for metabolic syndrome. Has higher SFA and trans fat content. Associated with increase in all‐cause mortality.11 |
| PB | Studies in humans show reduction in hepatic fat and inflammatory markers when protein sources from animals are replaced with plant sources. The Mediterranean diet has been used as an example.2, 4, 6 |
The liver removes nearly two‐thirds of the glucose and remaining monosaccharides absorbed from the gastrointestinal tract. Depending on the energy needs of the body, glucose is stored in the form of glycogen or oxidized for energy. Given limited storage capacity in the liver, excess carbohydrates are converted into triglycerides through the process of de novo lipogenesis (DNL). DNL is one of the main drivers of hepatic steatosis.5, 12
Simple sugars, particularly fructose, have been shown to predispose to the development of MAFLD in both animal and human studies. Fructose metabolism activates DNL as it can independently create triglycerides by bypassing one of the most regulated enzymatic steps in glycolysis with phosphofructokinase.4, 12 High‐fructose diets have been observed to influence signaling pathways that increase oxidative stress and inflammation in rats.13 In the pediatric population, one small randomized controlled trial (RCT) demonstrated that implementation of a diet low in free sugar resulted in significant improvement in hepatic steatosis.14 In adults, observational studies involving low‐carbohydrate diets have shown benefit in liver lipids in short‐term follow‐up. However, the emphasis has largely been on the hypocaloric nature of the diet because low‐fat/high‐carbohydrate versus low‐carbohydrate/high‐fat diets have produced comparable results.2, 15
Dietary fiber is another form of carbohydrate that has well‐documented beneficial effects on promoting weight loss and improving general markers of metabolic syndrome. In addition, there is growing research that dietary fiber may also influence gut microbiota and help promote an anti‐inflammatory environment within MAFLD.6
Fats
Lipids can arrive to the liver from dietary sources, lipolysis from adipocytes, and DNL. In fed states, lipids can be synthesized and stored peripherally in adipocytes. Over time, overnutrition will lead to adipocyte hypertrophy and the gradual release of proinflammatory cytokines that ultimately precipitate IR. IR has been strongly implicated in the formation of hepatic steatosis and progression of MAFLD.5
FAs can be categorized into saturated (SFAs), monounsaturated (MUFAs), and polyunsaturated FAs (PUFAs). Diets high in SFA are associated with increased hepatic fat deposition and oxidative stress within the liver. Alternatively, it has been shown that diets that replace SFA with MUFA help to increase FA oxidation and reduce lipogenesis in the liver in patients with MAFLD.2, 4 PUFAs can also affect the progression of MAFLD. The two most studied are omega‐3 (anti‐inflammatory) and omega‐6 (proinflammatory) FAs. An imbalance of omega‐6 FA over omega‐3 FA has been observed in patients with MAFLD.2, 6
Although the number of well‐conducted studies is limited, the Mediterranean diet has been the most studied. Protective nutritional benefits of the Mediterranean diet in MAFLD are shown in Fig. 2. It has been shown to display significant reduction in steatosis, liver enzymes, and markers of cardiovascular disease even under isocaloric conditions.15
FIG 2.
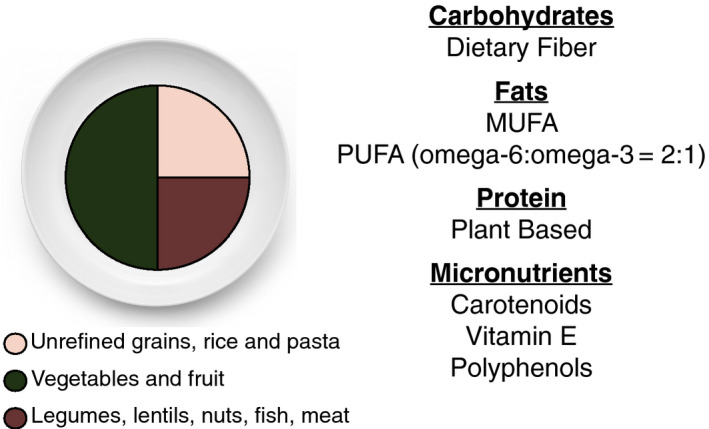
Food source with meal composition and major macronutrients and micronutrients present within a traditional Mediterranean diet.
Protein
Being the least energy dense, proteins are preferably used by the liver for synthesis over catabolism.12 Studies that have investigated the effects that proteins have on MAFLD are mixed. There have been concerns about confounding effects of other macronutrients that are either present or replaced by proteins in a diet. There are observational reports that note proteins from plant sources over animal sources help improve hepatic fat and IR and promote better glycemic control.4, 6
Micronutrients
Micronutrients are nutrients that the body requires in minuscule amounts to perform its physiological functions. Experimental studies have shown that micronutrients demonstrate many AO, anti‐inflammatory, and immunomodulatory (IM) properties.3 The various micronutrient roles in MAFLD are summarized in Table 2.
TABLE 2.
Micronutrient Metabolism in the Liver and Role in MAFLD
| Micronutrient | Hepatic Role in Homeostasis | Dietary Findings and/or Effects of Supplementation |
|---|---|---|
| Zinc | Albumin production, which binds and transports 75%‐80% of zinc in the plasma. | Serum levels low. One small RCT showed zinc supplementation for 3 months with a weight‐loss diet helped improve IR and oxidative stress in patients with MAFLD. No additional improvement in lipid profile compared with the control group.16 Has AO, LP, and AF properties.3 |
| Iron | Produces the regulatory hormone hepcidin that controls the export of iron from absorptive, recycling, and storage cells. | Iron stores elevated in MAFLD. Studies are mixed. One small RCT showed improvement in liver enzymes and histological damage to those who underwent phlebotomy.17 Another RCT found no improvement in hepatic steatosis, IR, liver injury with reduction in ferritin from phlebotomy.18 Has IM and LP properties. |
| Copper | Produces the transport protein ceruloplasmin. It is a cofactor for several AO enzymes. | Reduced hepatic levels found in patients with MAFL.19 Rats fed copper‐depleted diets developed severe hepatic steatosis, oxidative stress, and lipotoxicity. Has AO and LP properties.3 |
| Vitamin A | Production of bile acids for absorption via chylomicrons. Storage for majority of vitamin A in the body. Involved in hepatic lipid metabolism. | Low retinoic acid levels associated with increased steatosis and IR in patients with MAFLD.20 Animal studies found steatohepatitis from defects in mitochondrial beta‐oxidation. Has LP properties.3 |
| Vitamin D | Production of bile acids for absorption via chylomicrons. | Low serum levels are associated with MAFLD per a small systemic review of RCTs. Supplementation may improve lipid profile and markers of inflammation. Effects on glycemic index, IR, weight, and liver enzymes were not found to be significant.21 Pronounced steatohepatitis, upregulation of proinflammatory cytokines, fibrogenic factors, and lipotoxicity seen in rat models. Has AO, AF, IM, and LP properties.3 |
| Vitamin E | Production of bile acids for absorption via chylomicrons. | Low serum levels. Many clinical trials have demonstrated improvement in liver enzymes and liver histology for patients with MAFLD and NASH supplemented with vitamin E.22, 23 Has AO, LP, and AF properties.3 |
| Vitamin C | Liver not directly involved in homeostasis. Water‐soluble and absorbed passively in the gut. Known predominately for its AO and coenzymatic roles within cells. | Role in MAFLD is controversial. Low levels are mildly associated in the pediatric population but association in adults is mixed.4, 24 Studies aimed at better elucidating this relationship are lacking.3 |
| Polyphenols | Absorption via gut largely passive but will experience conjugation reactions in the liver that help facilitate metabolism and/or elimination. Production of bile acids for absorption for larger molecules. | Low levels seen in MAFLD. One RCT saw improvement in liver enzymes, steatosis grade, and inflammatory markers in patients supplemented with resveratrol for 3 months.25 Has AO, IM, and anti‐inflammatory properties.3 |
| Carotenoids | Production of bile acids for absorption via chylomicrons. | Low levels seen in patients with NASH.23 |
| Beta‐carotene supplementation in rats improved liver injury histology and steatosis.4 Has AO and anti‐inflammatory properties.3 |
A healthy liver provides storage and production of key binding, transport, and regulatory proteins that help maintain micronutrient homeostasis. Although micronutrient effects on MAFLD pathogenesis have not been clearly delineated, active liver disease is believed to alter micronutrient levels that lead to increased oxidative stress and inflammation. Micronutrient‐deficient and/or excess states have been observed in MAFLD, which has prompted therapeutic correction.
Therapeutic intervention involving micronutrients that positively affect liver injury histology in patients with MAFLD has produced varied results.3, 4 Zinc deficiency has been observed in MAFLD. One recent study demonstrated improvement in liver enzymes, IR, and markers of oxidative stress in supplemented patients in conjunction with a weight‐loss diet.16
Vitamin D has been known for its anti‐inflammatory, AO, and IM effects in rat models.3 One systemic review found that patients who supplemented with vitamin D in MAFLD resulted in only modest improvement in mediators of inflammation and lipid profile. However, changes in liver enzymes, body weight, IR, and markers of glycemic control were not present.21
Iron excess has been implicated in MAFLD. Interventions to reduce iron overload, such as phlebotomy, have generated debate. One clinical trial showed that reducing ferritin levels through phlebotomy had no effect on liver enzymes, steatosis, or IR,18 whereas other studies have demonstrated positive findings, such as liver enzyme reduction and improved histology, when compared with lifestyle changes alone.17
The effects of vitamin E are well documented. High‐dose vitamin E (>400 IU/day) has been noted to improve liver enzymes, body weight, and NASH steatosis when compared with placebo. However, there are concerns about the safety of routine vitamin E supplementation because of reported increased overall mortality and the development of hemorrhagic stroke and prostate cancer in several large meta‐analyses.26
Conclusion
MAFLD can be described as the hepatic manifestation of a metabolic syndrome within the liver. It often begins from the result of chronic exposure to nutrient‐poor diets. Synthesis and accumulation of hepatic fat eventually lead to inflammation and IR, which ultimately drive progression of disease. Micronutrient homeostasis is often disturbed in MAFLD, which likely adds to the cascade of inflammation and oxidative stress. Although corrective therapeutic interventions are being explored, the effects that micronutrients have on MAFLD pathophysiology still remain elusive. High‐dose vitamin E supplementation, however, is one exception. Independent of caloric intake, macronutrient and micronutrient composition of diets can influence MAFLD. Diets lower in simple sugars and fructose, SFA, and animal protein; higher in MUFA, PUFA, and fiber; and supplemented with vitamin E have been shown to improve markers of disease in MAFLD.
Potential conflict of interest: Nothing to report.
References
- 1.Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol 2020;17:387‐388. [DOI] [PubMed] [Google Scholar]
- 2.Berná G, Romero‐Gomez M. The role of nutrition in non‐alcoholic fatty liver disease: pathophysiology and management. Liver Int 2020;40(Suppl. 1):102‐108. [DOI] [PubMed] [Google Scholar]
- 3.Pickett‐Blakely O, Young K, Carr RM. Micronutrients in nonalcoholic fatty liver disease pathogenesis. Cell Mol Gastroenterol Hepatol 2018;6:451‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berná G, Álvarez‐Amor L, Martín F. Geometry of nutrition: nutrients and NAFLD progression. In: Romero‐Gomez M, eds. NAFLD and NASH: Biomarkers in Detection, Diagnosis and Monitoring. Cham: Springer International Publishing; 2020:49‐67. [Google Scholar]
- 5.Bechmann LP, Hannivoort RA, Gerken G, et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012;56:952‐964. [DOI] [PubMed] [Google Scholar]
- 6.Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients 2019;11:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daubioul CA, Horsmans Y, Lambert P, et al. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutr 2005;59:723‐726. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta‐analysis of randomized controlled trials. PLoS Medicine 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozzetto L, Prinster A, Annuzzi G, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 2012;35:1429‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musa‐Veloso K, Venditti C, Lee HY, et al. Systematic review and meta‐analysis of controlled intervention studies on the effectiveness of long‐chain omega‐3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev 2018;76:581‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etemadi A, Sinha R, Ward MH, et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH‐AARP Diet and Health Study: population based cohort study. BMJ 2017;357:j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stryer L, Berg J, Tymoczko J, et al. Biochemistry. New York, NY: W.H. Freeman/McMillan Learning; 2019. [Google Scholar]
- 13.Roglans N, Vilà L, Farré M, et al. Impairment of hepatic Stat‐3 activation and reduction of PPARalpha activity in fructose‐fed rats. Hepatology 2007;45:778‐788. [DOI] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Ugalde‐Nicalo P, Welsh JA, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA 2019;321:256‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeed N, Nadeau B, Shannon C, et al. Evaluation of dietary approaches for the treatment of non‐alcoholic fatty liver disease: a systematic review. Nutrients 2019;11:3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fathi M, Alavinejad P, Haidari Z, et al. The effects of zinc supplementation on metabolic profile and oxidative stress in overweight/obese patients with non‐alcoholic fatty liver disease: a randomized, double‐blind, placebo‐controlled trial. J Trace Elem Med Biol 2020;62:126635. [DOI] [PubMed] [Google Scholar]
- 17.Valenti L, Fracanzani AL, Dongiovanni P, et al. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol 2014;20:3002‐3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams LA, Crawford DH, Stuart K, et al. The impact of phlebotomy in nonalcoholic fatty liver disease: a prospective, randomized, controlled trial. Hepatology 2015;61:1555‐1564. [DOI] [PubMed] [Google Scholar]
- 19.Aigner E, Strasser M, Haufe H, et al. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol 2010;105:1978‐1985. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Chen H, Wang J, et al. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr 2015;102:130‐137. [DOI] [PubMed] [Google Scholar]
- 21.Hariri M, Zohdi S. Effect of vitamin D on non‐alcoholic fatty liver disease: a systematic review of randomized controlled clinical trials. Int J Prev Med 2019;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Hadi H, Vettor R, Rossato M. Vitamin E as a treatment for nonalcoholic fatty liver disease: reality or myth? Antioxidants (Basel) 2018;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erhardt A, Stahl W, Sies H, et al. Plasma levels of vitamin E and carotenoids are decreased in patients with nonalcoholic steatohepatitis (NASH). Eur J Med Res 2011;16:76‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madan K, Bhardwaj P, Thareja S, et al. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol 2006;40:930‐935. [DOI] [PubMed] [Google Scholar]
- 25.Faghihzadeh F, Adibi P, Rafiei R, et al. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res 2014;34:837‐843. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]


