Abstract
Because the stomach in situ has few distinctive surface features and changes shape dramatically with food intake, we have used micro‐CT imaging combined with two distinct contrast agents to (1) characterize the pattern of arteries, potential landmarks, on the stomach wall and (2) evaluate how meal‐related shape changes affect the size of the different regions. Images generated with a contrast agent injected directly into the heart during perfusion enabled a thorough look at the organizational features of the stomach angioarchitecture. The stomach receives its blood supply primarily from two pairs of vessels, the gastric and gastroepiploic arteries. Each of the three regions of the stomach is delineated by a distinctive combination of arterial fields: the corpus, consistent with its dynamic secretory activity and extensive mucosa, is supplied by extensive arterial trees formed by the left and right gastric arteries, travelling, respectively, on the ventral and dorsal stomach surfaces. These major arteries course circularly from the lesser towards the greater curvature, distally along both left (or ventral) and right (or dorsal) walls of the corpus, and branch rostrally to supply the region. The muscular antrum is characterized by smaller arterial branches arising primarily from the right gastroepiploic artery that follows the distal greater curvature and secondarily from small, distally directed arteries supplied by the large vessels of the left and right gastric arteries. The forestomach, essentially devoid of mucosal tissue and separated from the corpus by the limiting ridge, is vascularized predominantly by a network of small arteries issued from the left gastroepiploic artery coursing around the proximal greater curvature, as well as from higher order and smaller branches issued by the gastric and celiac arteries. These distinctive arterial fields appear to distinguish the major gastric regions, irrespective of the degree of fill of the stomach. Volume assessments of stomach compartments were made from images of iodine‐stained stomachs. By varying the delay time between eating and perfusion, we were able to probe the emptying behavior of the stomach and demonstrate that the regions of the stomach empty at different rates, thus changing the relative dimensions of the organ regions. Notably, and despite these shape changes, the gastric arteries appear to form a regular, particularly recognizable, and lateralized pattern corresponding to the corpus that should be of use in guiding surgical and experimental interventions.
Keywords: antrum, celiac artery, corpus, duodenum, forestomach, gastric arteries, micro‐CT, stomach emptying
Because the stomach in situ has few distinctive surface features and changes shape dramatically with food intake, we have used microCT imaging combined with two distinct contrast agents to (1) characterize the pattern of arteries on the stomach wall and (2) quantify how meal‐related shape changes affect the size of the different regions. Despite shape changes, the gastric arteries appear to form a recognizable pattern that should be of use in guiding surgical and experimental interventions.
1. INTRODUCTION
Little information is available on the regional patterns of arterial fields located superficially or serosally on the stomach wall. More information is available on the basic pattern of vasculature between the abdominal aorta and the stomach (e.g., Froud, 1959; Greene, 1968; Guth & Leung, 1987; Schnitzlein, 1957; Shukla & Ellis, 2002; Vdoviakova et al., 2016) and on the microcirculation within the deeper gastric tissues (e.g., Gannon et al., 1982; Guth & Smith, 1975; Holzer et al., 1991; Moskalewski et al., 2002; Peti‐Peterdi et al., 1998; Piasecki & Wyatt, 1986; Schnitzlein, 1957), than on the architecture of the vessels running superficially on the stomach wall.
Information about the vessels coursing on surface of the stomach is especially needed because (a) surgeons and physiologists often have to rely on surface features to guide their surgical approach, (b) from the few superficial landmarks that are available, it is difficult to distinguish gastric regions of the stomach in situ in the living animal, (c) unmonitored compression of vascular supplies might follow experimental or surgical interventions, (d) understanding reproducible patterns might make it practical to implement near arterial infusions of hormonal or pharmacological agents, and (e) nerve bundles such as the abdominal vagus and the sympathetic projections to the gastric vessels throughout the stomach course for a distance near or on the blood vessels as neurovascular bundles, and these nerves could be compromised by vessel ligation or clamping.
More particularly, the rat is considered the most practical and widely used experimental model for surgical research (Lambert, 1965; Martins, 2003; Martins & Neuhaus, 2007; Vdoviakova et al., 2016), and yet the course of serosal blood vessels in the rat stomach (as well as that of other species) has remained almost undescribed. The arterial supply on the stomach wall has conventionally been represented as distributing in schematic, random, and arbitrary generic patterns around the periphery of the organ (see for example, (Greene, 1968; Robert, 1971; Stevens, 1988)). For surgeries and physiological experiments, the lack of realistic descriptions and the absence of adequate markers or regional definitions is problematic. Adjustments in size and shape that the stomach undergoes as food is consumed and then macerated for digestion and emptied into the duodenum presumably further complicate assessments of locations for surgical (e.g., bariatric surgeries) access in vivo, attachment of implantable devices, or situating of electrophysiological probes or transducers. Furthermore, few surface landmarks on the organ, other than the borders of the lesser and greater curvature and a hint, from some perspectives, of the deeply situated limiting ridge, are available to guide physiology or surgery of the stomach in situ, thus making accurate interventions or placements of electrodes, chemotrodes, strain gauges, or other transducers problematic.
In this publication, we describe the use of micro‐CT imaging to visualize, using different contrast agents, both the vasculature of the stomach, and the stomach as a whole. In contrast to previous schematic representations, we are able to see from the detailed micro‐CT images that the major vessels seem to be located in relatively consistent patterns in the rat, offering potential fiducial landmarks that could be used to guide experimental implants or surgeries.
2. MATERIALS AND METHODS
2.1. Animals
Two‐ to four‐month‐old male Sprague–Dawley rats (n = 73; RRID:RGD_737903; Envigo, Indianapolis, IN) with an average initial weight of 278 g (sd =19 g) were housed individually in shoebox cages with bedding material in an Association for Assessment and Accreditation of Laboratory Animal Care‐approved colony room, temperature (22–24°C) and humidity (40%–60%) controlled. The room was maintained on a 12:12‐hour light–dark schedule. Pelleted chow (2018 Teklad global 18% protein rodent diet; Envigo, Indianapolis, IN) and filtered tap water were provided ad libitum, except during feeding training. All husbandry practices conformed to the NIH Guide for the Care and Use of Laboratory Animals (8th edition) and were reviewed and approved by the Purdue University Animal Care and Use Committee. All efforts were made to minimize any suffering as well as the number of animals used.
2.2. Training and feeding
Beginning about one week before the day of each intended experiment, four rats in the desired weight range were trained to consume DietGel Recovery® (ClearH20). The rats were fasted overnight before being presented with DietGel® at 7 am and given 6 h to consume it, after which the remaining gel was removed and 5 g of regular chow was supplied for the rest of the day. After several repetitions the rats naturally consumed DietGel® following an overnight fast. On the morning of the planned experiment, following an overnight fast, a known weight of DietGel® was placed in each animal's cage; 30 min later the bowls containing DietGel® were removed and reweighed to determine the amount of DietGel® consumed by each rat; this time was designated t0. On experimental days, up to three rats consuming at least 6 g of DietGel® were then chosen for the experiment; any rat not eating sufficient DietGel® was returned to the general colony population.
2.3. Perfusion and dissection
Stomachs were stained for micro‐CT analysis in two ways. The first process was designed to stain the entire stomach (43 animals). An aqueous solution of 0.2% KI and 0.1% I 2 was made by dissolving KI and I 2 in ultrapure water. After a delay of 0–8 h from the conclusion of eating, an individual rat was weighed and euthanized with a lethal dose of a combination of ketamine and xylazine (275 mg/kg of ketamine and 27.5 mg/kg of xylazine). Once the animal was unresponsive to paw pinch, the abdomen and chest cavity were opened with minimal incisions, and heparin (0.5 ml; 1,000 units/ml) was injected into the heart, followed by transcardial perfusion as follows: 250 ml of 0.01‐M sodium phosphate‐buffered saline (PBS; pH 7.4; 40°C) was injected with a blunt 18‐ga needle threaded via the left ventricle into the aorta (25 ml/min using a peristaltic pump), with blood allowed to drain via a cut in the right atrium and inferior vena cava. During saline perfusion, a 5‐ or 8‐Fr feeding tube was threaded through the esophagus and into the stomach. Similarly, a 5‐Fr feeding tube was threaded retrogradely through a small hole cut in the duodenum about 3–4 cm distal to the pylorus and into the stomach via the pylorus. The purpose of the feeding tubes was to ensure that both the pylorus and lower esophageal sphincter remained open to enable subsequent flushing of stomach contents. After exsanguination, the perfusion medium was switched to 500 ml of a fixative solution of 4% solution of paraformaldehyde in PBS. Then, the stomach was removed intact along with approximately 2‐cm sections of the esophagus and duodenum and immersed in the KI/I2 solution on an agitator table. The stomach was held under the surface of the solution and gently manipulated to flush out any Dietgel® and replace the diet with solution, and also to ensure that all air was removed so the organ would not float but be fully immersed for the 24‐hour soak period.
The second process was designed to produce a stomach with a stained vasculature (30 animals). Before perfusion, the Microfil® compound (MV‐122; Flow Tech Inc.) and diluent were mixed (about 40 ml of total volume, preferred ratio: 3‐ml MV‐122 to 5‐ml diluent) in a disposable container and gently shaken or stirred to mix thoroughly but without producing bubbles. The ratio of compound to diluent was chosen empirically to ensure good penetration of the vasculature without the viscosity being so low that the mixture leaked out of the vasculature before curing.
After a delay that was varied systematically from 0 to 8 h from the conclusion of eating, an individual rat was euthanized and perfused as above. Approximately every minute during perfusion warm saline was administered in and around the abdominal cavity of the rat to minimize cooling. This procedure maximized vasodilation and subsequent penetration of the Microfil® material into the smaller vessels of the forestomach.
After an exsanguination perfusion of around 200‐ml saline, the curing agent was added to the Microfil® solution (5% by weight of volume of MV‐122 compound [excluding diluent]) and mixed gently but thoroughly for 1–2 min to achieve homogeneity.
After a total of 250 ml of saline had been perfused, the input to the pump was switched to the Microfil® solution, and the pump rate was reduced to 2 ml/min. Warm saline continued to be administered in and around the abdominal cavity of the rat to minimize cooling. Once all the Microfil® solution had been used, the pump was stopped, and both the perfusion line and the inferior vena cava (distal to the cut) were clamped to prevent back‐leakage.
After about a 70‐minute delay to ensure adequate cure of the Microfil® material, the abdomen was opened, and the stomach dissected out, gently removing the feeding tubes, and leaving about a centimeter each of esophagus and duodenum attached to the stomach, and also leaving in place the gastroepiploic arteries along the greater curvature of the corpus/antrum and the forestomach. The stomach was then immediately placed in a solution of 4% paraformaldehyde in 0.1‐M PBS and left for a period of at least 24 h.
2.4. Scanning
Before micro‐CT scanning, the stomach (either gastric tissues stained with iodine or vessels filled with Microfil® [or both, see below]) was removed from fix solution and rinsed in saline. To ensure that the stomach was of a reproducible shape and not collapsed during scanning, both duodenum and esophagus were tied off with thread after gently injecting saline into the stomach cavity via the esophagus until it was completely full and contained no air bubbles.
Micro‐CT scanning was performed with a Quantum GX2 scanner (Perkin Elmer), typically at 90 V/88 A. To enable the stomach to be supported in the preferred orientation without distortion, the stomach was suspended in a viscous solution of Type B gelatin (4%–5% in water) in a polypropylene container. The solution was liquid enough that the stomach was fully surrounded by gelatin without pockets of air, but viscous enough that the stomach was fully supported and did not move during scanning. Once scanning was complete, the stomach was thoroughly rinsed in PBS, the tie‐off threads were removed, and the stomach was replaced in fixative.
With some Microfil® infused stomachs, following an initial scan to reveal the vasculature alone, the stomach was also lightly stained in iodine solution for about 24 h, rinsed in PBS for 24 h to desaturate the iodine stain and then rescanned to provide an image of both the vasculature and the rest of the stomach (five animals).
Scan data were exported as DICOM files and visual images of both the vasculature alone and its combination with the stomach were produced using the open source software platform 3D Slicer (RRID:SCR_005619). In addition, Vesselucida 360 software (MBF Biosciences; RRID:SCR_017320) was used to create a 3D reconstruction of the vasculature. Images for presentation were generated from both 3D Slicer and from Vesselucida using screen capture and saved as tiff files. For figure generation, the tiff files were imported into Photoshop and resized with resampling if needed.
Stomach compartment volumes of iodine stained stomachs were measured using the Segment Editor and Segment Statistics modules in 3D Slicer. The limiting ridge was used to delineate the boundary between the forestomach and the corpus. No such clear demarcation exists between the corpus and antrum, so these two compartments were evaluated as one. Volumes were measured relative to the outer serosal surface since the inner lumenal surface was irregular and sometimes indistinct.
Data associated with this study (Powley et al., 2020a; Powley et al., 2020b) were collected as part of the Stimulating Peripheral Activity to Relieve Conditions (SPARC) project and are available through the SPARC Data Portal (RRID:SCR_017041) under a CC‐BY 4.0 license. More detailed experimental protocols are available through Protocols.io (dx.doi.org/10.17504/protocols.io.bafnibme and dx.doi.org/10.17504/protocols.io.95ih84e).
3. RESULTS
It is widely recognized that the basic pattern of blood vessels coursing from the descending aorta to supply the stomach is relatively similar for a variety of species. Between the aorta and the stomach, the pattern in the rat was consistent with the commonly reported arterial distribution. Specifically, the two pairs of major arteries coursing to, and supplying, the stomach are readily recognized: one pair of arteries represent the terminal branches of the celiac artery which divides at the distal esophagus to form the left and right gastric arteries that fork over the lesser curvature close to the lower esophageal sphincter and then branch so as to supply blood to much of the ventral (or left) and dorsal (or right) gastric wall. The right gastric artery often bifurcates from any of several different sites along the more distal branches of the celiac artery.
The second major pair of arteries, the gastroepiploics, issue from higher order branches of the celiac artery and course to perfuse the stomach from the greater curvature. This pair consists of the left gastroepiploic artery running on the rostral or proximal greater curvature and the right gastroepiploic artery coursing around the caudal or distal greater curvature of the antrum. Less conspicuous and apparently less critical are other smaller arteries given off in passing by branches formed from the secondary and tertiary branches of the celiac artery, specifically small arterioles from the splenic artery (to the forestomach) and the gastroduodenal artery (to the antrum).
The overall pattern of the gastric serosal wall angioarachitecture is illustrated in Figure 1. The case illustrated in Figure 1 also appears as an animation (Data S1). Notably, the vessels covering the corpus or middle (rostral to caudal) region of the stomach are exceptionally large, presumably capable of delivering considerable blood to the underlying mucosa which is responsible for most of the secretion in the stomach. These lateralized arteries supplying the corpus originate as the left (on the ventral wall) and right (on the dorsal wall) gastric arteries. The gastric arteries travel from the lesser towards the greater curvature along the distal limits of the corpus. As they travel across the stomach, the gastric arteries issue multiple large secondary and higher order branches that distribute out rostrally to supply virtually the entire corpus. The left and right gastric arteries are relatively symmetrical in distribution and comparable in their branching patterns.
FIGURE 1.
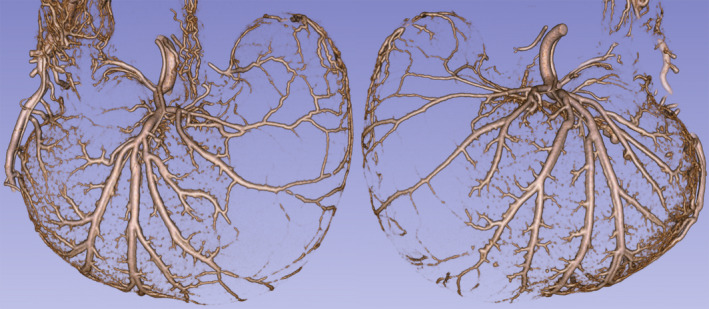
The angioarchitecture profile of the stomach of a recently fed rat delineated with a Microfil® label by micro‐CT imaging (55 kV; 144 µA; voxel size =90 µm). A post‐feeding delay of 0.5 hours occurred after a meal of 9.49‐g DietGel Recovery® was consumed. The profile on left is an exterior micro‐CT view of the ventral stomach wall, and the profile on the right is a comparable exterior view of the dorsal stomach wall of the same animal. The profiles are 3D Slicer reconstructions of micro‐CT scan DICOM files, cropped to include just dorsal or ventral features. The figure was created by screen capture of the 3D Slicer image followed by import into Photoshop where images were resized with resampling as needed. See also Data S1 for an animated 3D version
The gastroepiploic arteries, the other conspicuous pair bifurcating so as to supply blood to the stomach, differ from the gastric arteries in that they are far less symmetrical and are considerably smaller in diameter. The right gastroepiploic artery courses along the distal greater curvature, supplying the antrum and the duodenum with a network of small arteries and arterioles. Along the greater curvature, the right gastroepiploic artery gives off a series of smaller arteries that course towards the lesser curvature or esophagus, supplying the field of the antrum with vascular processes. The bulk of the antral supply of blood seems to arise from the right gastroepiploic artery coursing along the greater curvature, though the antrum also receives some blood supply from the modest numbers of moderately sized higher order arteries that separate from the two gastric arteries.
The left gastroepiploic artery, considerably rostral to the right gastroepiploic artery, courses along the greater curvature of the forestomach, providing smaller, higher order arteries to the forestomach. Whereas the two gastric arteries are relatively closely matched in size and distributions—basically symmetrical on a sagittal axis—the right and left gastroepiploic arteries distribute at different rostrocaudal levels, making them somewhat asymmetrical on a frontal axis, and are conspicuously different in size. The right gastroepiploic issues medium‐sized arteries to both the antrum and (extensively to the) duodenal bulb, whereas the left gastroepiploic artery issues much smaller arteries to the forestomach. The gastric arteries also contribute some vascular distribution to the forestomach, particularly in the “cap” of the forestomach where a conspicuous network of arterioles occurs (see below).
The MBF Biosciences’ software tool Vesselucida® was developed to trace the distributions and calibers of blood vessels, and we used it for that purpose. The complementary patterning of the arteries was particularly apparent when we employed Vesselucida® to start closer to the celiac artery source and to reconstruct the two conventional pairs of gastric surface arteries. Using Vesselucida®, we mapped the arterial fields on both the dorsal (left side of top panel) and the ventral (right side of top panel) including the gastric arteries fields (reconstructed in green), the right gastroepiploic artery (reconstructed in red), and the left gastroepiploic artery (reconstructed in purple) separately. Notably too, both of the gastroepiploic arteries are located on the midline and can be seen clearly supplying both the dorsal and ventral surfaces of the stomach. For the animal assessed in Figures 1 and 2 (upper panel) displays the Vesselucida® patterns of arteries and their relatively distinct perfusion fields, and when the specimen was counterstained with an overnight iodine soak, the shadow of the stomach walls becomes apparent (bottom panel, Figure 2).
FIGURE 2.
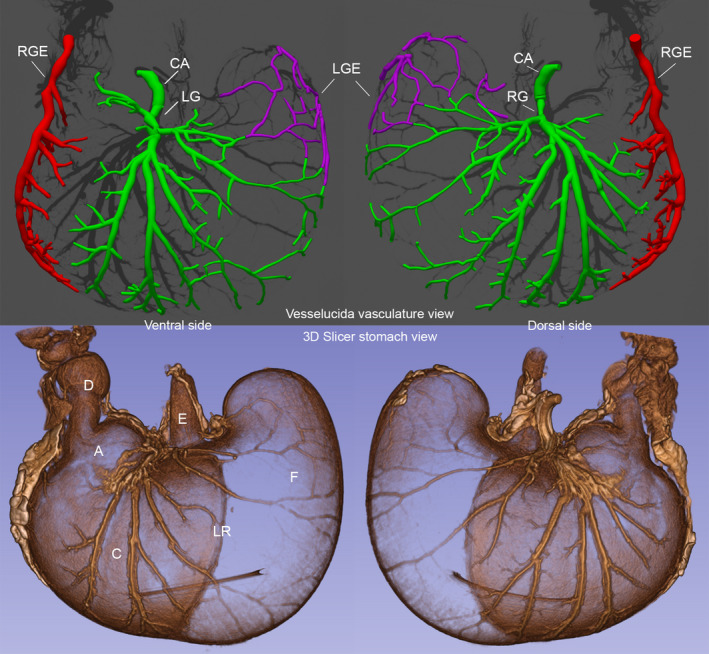
The angioarchitecture profile of a fed rat (same animal as in Figure 1) stomach delineated with a Microfil® label and micro‐CT imaging. A post‐feeding delay of 0.5 hours occurred after a meal of 9.49‐g Dietgel Recovery® was consumed. Upper panel: profile on left is an exterior micro‐CT (55 kV; 144 µA; voxel size =90 µm) view of the ventral stomach arteries mapped and color designated with MBF Biosciences’ Vesselucida®, and the profile on the right is a comparable exterior view of the dorsal stomach arteries mapped and color designated with Vesselucida® of the same animal (3D image cropped to include just dorsal or ventral features). Lower panel: micro‐CT images (90 kV; 88 µA; voxel size =90 µm) of Iodine counterstained profiles of the stomach in the upper panel. Perspectives the same as in the upper panel. The profiles are 3D Slicer reconstructions of micro‐CT scan DICOM files, cropped to include just dorsal or ventral features. The figure was created by screen capture of the 3D Slicer and Vesselucida® images followed by import into Photoshop where images were resized with resampling as needed See also Data S2 for an animated 3D version. Labels: A: antrum; C: corpus; F: forestomach; LR: limiting ridge; E: esophagus; D: duodenum; CA; celiac artery; LG: left gastric artery; RG: right gastric artery; LGE: left gastroepiploic artery; RGE right gastroepiploic artery
The stomach stores and partially digests food. Furthermore, the different regions of the stomach, in performing their different functions, have distinctly different thicknesses of wall tissues, muscularity, secretory specializations, and distensibility. An expectation that follows these facts is that, as the stomach empties into the intestines, the different regions of the stomach may empty at different rates. If so, then the stomach might well undergo nonlinear adjustments in regional sizes and such changes would presumably complicate surgical and physiological placements on the stomach wall. This effect of differential changes in size can be appreciated by comparing the Vesselucida® reconstructions of a stomach perfused when largely emptied of the last meal (6 h following a 5.94‐g meal; volume = 2.7 cm3 [close to the minimum observed, c.f. Figure 5]) in Figure 3 with the similar reconstruction in Figure 2 of an animal with considerable food still left in its stomach at perfusion (0.5 h after a 9.49‐g meal; volume = 9.4 cm3 [approximately ¾ of maximum volumes observed; c.f. Figure 5]). For example, the heavily muscular antrum and its right gastroepiploic arterial distribution undergo little size reduction, and the secretory corpus and its paired gastric arteries are relatively similar in the comparison of nearly empty stomach in Figure 3 and the relatively full stomach in Figure 2. In contrast, however, the forestomach of the nearly empty stomach in Figure 3 has effectively collapsed producing a dense network of arteries and arterioles in the “cap” region, whereas the forestomach and its arteries are relatively distended in the animal in Figures 1 and 2.
FIGURE 5.
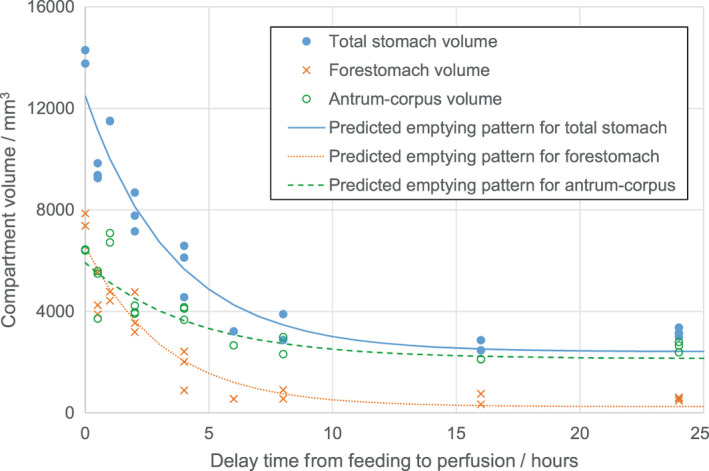
Total stomach volume and compartment volumes (forestomach and antrum/corpus) as a function of the delay time between meal completion and perfusion for meal sizes of 9–11 g only, together with illustrative lines corresponding to the fit to an exponential decay model of the form: where v = compartment volume, W = animal weight, M = meal size, T = delay time from meal to perfusion, and a, b, c and d are fit‐derived, derived from all the data for each compartment, calculated here for a 10‐g meal size with W set to the average animal weight for the set of animals. The data show that the forestomach (which is reflected in the whole stomach emptying pattern) undergoes a dramatic emptying over time (tending towards zero) whereas the antrum–corpus undergoes a much more modest emptying pattern consistent with the observation that food is stored primarily in the forestomach and digested and emptied—but not accumulated—in the antrum–corpus
FIGURE 3.
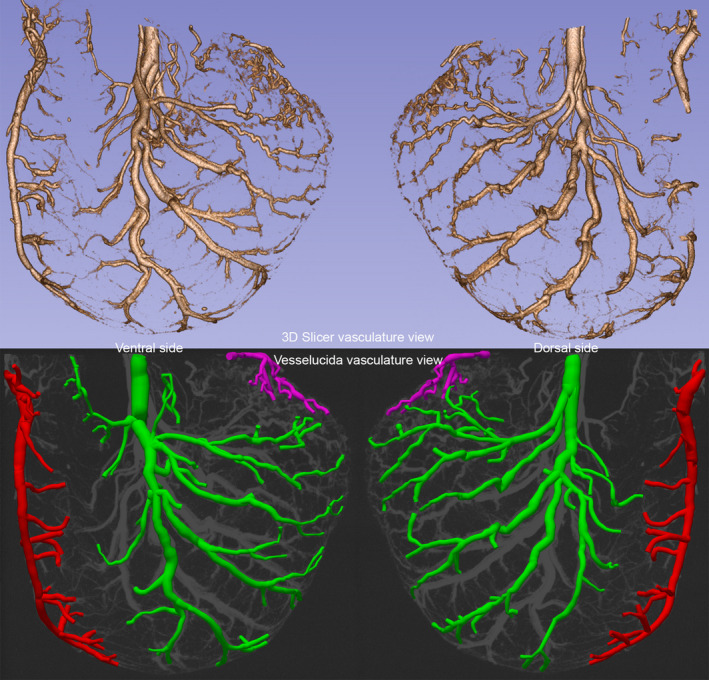
For comparison (with Figures 1 and 2), the micro‐CT angioarchitecture profile of a rat fed (smaller meal of 5.94 g of Dietgel Recovery®) and given 6 hours for stomach emptying before perfusion and preparation with the Microfil® label; and micro‐CT imaging (90 kV; 88 µA; voxel size = 90 µm). Upper panel: profile on left is an exterior micro‐CT view of the ventral stomach wall arteries, and the profile on the right is a comparable exterior view of the dorsal stomach wall arteries (3D image cropped to include just dorsal or ventral features as appropriate). The profiles are 3D Slicer reconstructions of micro‐CT scan DICOM files. Lower panel: profile on left is an exterior micro‐CT view of the ventral stomach arteries mapped with MBF Biosciences’ Vesselucida®, and the profile on the right is a comparable exterior view of the dorsal stomach arteries mapped with Vesselucida® of the same animal. The figure was created by screen capture of the 3D Slicer and Vesselucida® images followed by import into Photoshop where images were resized with resampling as needed
We also examined the relationship between the volume of the different gastric regions (presumably related to the amount of food remaining in the stomach) and the size of different meals and the delay times before perfusion, by gauging the size of the overall stomach, as well as the forestomach, and the antrum–corpus (see Figure 4 for illustrative micro‐CT images of iodine stained stomachs of different sizes). Note that we did not attempt to accurately determine the precise boundary between the corpus and the antrum because (a) this boundary was more indistinct and (b) the forestomach unequivocally emptied faster than the other regions (see Figure 5). Overall, the rate of volume reduction was similar for the forestomach and the entire stomach. The data in Figure 5 correspond to a narrow range of meal size (9–11 g) and also include illustrative lines corresponding to a best fit exponential decay model for each set of data (total stomach, forestomach and antrum–corpus, with meal size set to 10 g and animal weight set to the study population average of 238.2 g). Models were optimized using the entire dataset of 59 measurements with the Solver Add‐in within Microsoft Excel (GFG Nonlinear method with multistart option). The model used in Figure 5 is of the form where v=compartment volume; W = animal weight; M = meal size; T = delay time from meal to perfusion; and a, b, c, and d are constants. For parameters and statistical assessments of this model and a more complex alternative, see Data S3. The quantitative model parameters support the idea that emptying of the forestomach is more rapid than emptying of the antrum–corpus (c parameter is more negative for the forestomach than for the antrum–corpus). The minimum or baseline volume of the antrum–corpus is significantly larger than that of the forestomach. This reflects two factors: (1) the sizeable amount of tissue associated with the stomach wall in the corpus in particular; and (2) the consequence of the irregular internal surface of the stomach in the corpus due to the rugae.
FIGURE 4.
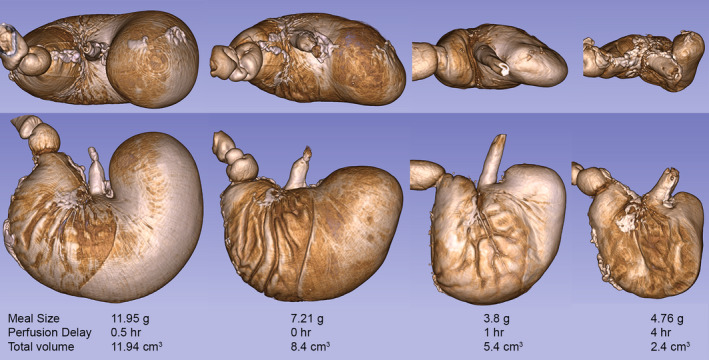
Illustrative micro‐CT images of a variety of iodine stained stomachs (no labeling of the vasculature) at different sizes from full to empty (90 kV; 88 µA; voxel size = 144 µm). The images were created in 3D Slicer from DICOM files. Each stomach is labeled to show the meal size, delay time to perfusion and measured total stomach size. The figure was created by screen capture of the 3D Slicer images followed by import into Photoshop where images were resized with resampling as needed
A final observation—one we had not anticipated—also deserves mention: we had not anticipated the degree to which small arterioles in the mucosa would apparently respond to a newly ingested meal. Immediately before perfusion, animals were given a half hour to eat plus a subsequent period for emptying. A few of the animals (11) were perfused at t = 0, or immediately after the half hour of access to DietGel Recovery®. Of those animals, some (n = 3) had weak or incomplete vascular fills. Of the remaining eight animals with complete vascular fills 6/8 exhibited a dramatic filling of the fine arterioles and vessels of the mucosa, illustrated in Figure 6, in which the inset panel demonstrates a high level of fill, in contrast to that seen, for example, in Figure 3, where the animal was perfused several hours after eating. The pattern is consistent with the idea that consumption of nutrients after an overnight fast triggers an initial gastric phase of digestion which involves a dramatic secretory response from the mucosal lining of the corpus. In the animals that were perfused anywhere from a half hour to an hour and a half after the meal, the dilation of the mucosal arterioles was already considerably reduced.
FIGURE 6.
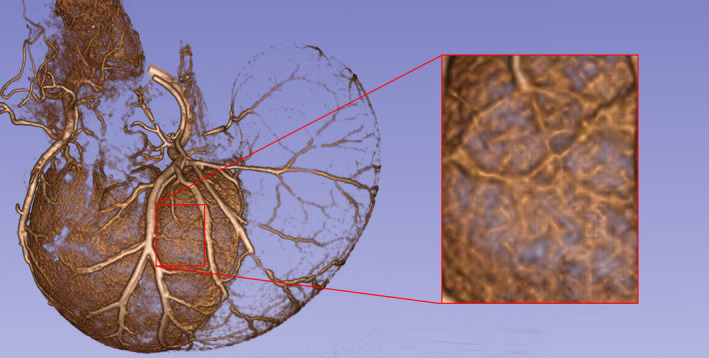
The micro‐CT angioarchitecture profile of an animal perfused immediately after the 30‐min feeding period (5.3‐g Gel consumed; 90 kV; 88 µA; voxel size = 90 µm). Animals perfused immediately after feeding characteristically exhibited labeling or filling of fine arterioles in the gastric mucosa throughout its distribution distinguished by the large vessels of the gastric arteries (see inset). The profile is a 3D Slicer reconstruction of micro‐CT scan DICOM files. The figure was created by screen capture of the 3D Slicer images followed by import into Photoshop where images were resized with resampling as needed
4. DISCUSSION
During rodent surgeries—experimental bariatric analyses, electrophysiology, physiology, nerve isolations, biopsies, etc.—from the relatively undifferentiated appearance of the surface of the stomach, it is often difficult to determine the regions such as the antrum, corpus, or forestomach (cf. Robert, 1971). Other than the overall profile of the organ (e.g., the greater and lesser curvature) and, at least in some illuminations, the limiting ridge in the gastric wall, the stomach is hard to divide unequivocally into regions (e.g., Lambert, 1965).
Ironically, conventional morphological sources have largely ignored the arterial architecture observed on the serosal sides of the stomach wall. Some sources ignore the vasculature of the stomach altogether (e.g., Stevens, 1988); others simply illustrate an inaccurate annulus of small vessels entering the stomach at the greater and lesser curvatures (e.g., Robert, 1971), and still others arbitrarily suggest larger vessels on the wall of the stomach that do not appear to be close approximations of the actually architecture (e.g., Greene, 1968). While the Greene text provides what is still perhaps the most comprehensive description of the angioarchitecture of the rat (152 total pgs., 177–329) “circulatory system,” it only devotes one figure (# 246) and nine lines of text to the arteries and veins connecting the descending aorta and the vena portae, respectively, to the stomach. The Greene volume does not attempt to characterize or name the arteries (or veins) on the surface of the stomach wall.
Furthermore, the lack of distinguishing markers that definitively identify the particular regions of the stomach is further complicated by the fact that the organ, in its role as a storage vessel, varies dramatically in size and regional dimensions as a function of deprivation or feeding (see Figures 4 and 5). Indeed, we speculate that this variability is partially responsible for apparent variability in the angioarchitecture of the stomach. To address the lack of adequate descriptions of the arterial supply of the stomach and potentially to address complications introduced by the changes in gastric shape associated with differences in gastric fill, we have examined the superficial arterial vessels of the stomach. The results suggest that the major vessels of the two canonical pairs of arteries supplying the stomach with blood might be used as fiducial markers to enhance reliable surgical exposures and placements for stomach interventions.
4.1. Angioarchitecture of rat stomach surface
Under the methodological conditions outlined above, in ongoing experiments in the laboratory, a regular and instructive angioarchitecture of the rat gastric vasculature was observed. Each of the three major divisions of the stomach, namely, the antrum, corpus, and forestomach, was well delineated by a distinctive and characteristic arterial field. The three fields were not so rigidly organized that the higher order arterial branches and arterioles were invariant (and they apparently have never been named). The gastric regions were, however, quite characteristic in terms of the general features of the major vessels.
The antrum, the muscular region of the distal stomach that grinds and macerates food mixed with gastric acid and enzymes and then pumps the resulting chyme into the duodenum, receives its primary supply from the right gastroepiploic artery. This artery, which courses on the greater curvature of the stomach, wraps small arterial branches around the body of the antrum. The higher order arterial branches were relatively small, apparently carrying a limited blood supply. A limited number of similarly small (or even smaller) arteries perfusing the antrum also course off the more rostrally located left and right gastric arteries associated with the corpus. Consistent with several estimates of the circulation required by muscle tissue (Kvietys, 2010; Peti‐Peterdi et al., 1998), the antrum appears to receive small vessels presumably capable of supplying the circulation needed for its primarily muscular function.
By contrast, the corpus receives numerous larger arterial branches issued by the relatively symmetrically organized left and right gastric arteries. These large arteries wrap over the organ, coursing from the lesser to the greater curvature. As the large vessels course along the distal border of the corpus, numerous secondary arteries are issued as larger branches coursing rostrally and towards the greater curvature.
The secretory functions of the corpus mucosa require far more circulation than smooth muscle activity. Peti‐Peterdi et al., (1998), for example, found in an analysis of 25 rats that 84% of the blood flow to the stomach supported the secretory activity of the mucosa–submucosa (in the corpus region), whereas only relatively 16% of the blood flow supplied the smooth muscle of the stomach. Such dissimilar proportions are consistent with the additional fact that the corpus receives the blood flow of two paired and large gastric arteries, whereas the antrum is supplied primarily by only a single smaller vessel (the right gastroepiploic artery) and the forestomach similarly is perfused primarily by a single vessel (the left gastroepiploic artery).
Whereas the antrum is heavily muscular and the corpus is heavily secretory, the forestomach or fundus is specialized for storage of food until the nutrient material is moved into the corpus for the initial stages of digestion. The forestomach is less muscular than the antrum and (in the rat) is lined with squamous epithelium, not with mucosa and a dynamic epithelial layer such as is found in the corpus. In keeping with what must be less demand for circulatory exchanges, each side of the forestomach is perfused by small arterial branches from the left gastroepiploic artery. In addition, the forestomach receives a few higher order arteriolar branches, with each side receiving arteriolar vessels from the ipsilateral (ventral from left; dorsal from right) gastric artery.
4.2. Factors obscuring arterial patterns
In spite of this regular pattern, as illustrated in our Microfil defined observations (see Figures 1, 3, and 6), multiple factors in previous reports seem to have made the arterial pattern often harder to discern and should be noted. One factor that may well have contributed to some lack of attention to the vascular architecture, as well as use of overly schematic representations of the stomach vasculature, is blood pressure. Frequently, both experimental animal stomachs and human stomachs have been drawn or schematized using postmortem or cadaver material to estimate features. Since blood pressure is at zero, or near zero, in such cases, the larger vessels are likely to be partially collapsed and the finest vessels may even be completely drained of blood cells and plasma. In all likelihood, additional, accurate representations of the stomach vasculature will need to be done with fill agents or other means of generating normal pressures and contrasts in the vessels. In light of this consideration, it is relevant that we found that there was a window of pump circulation rates and pressures for Microfil infusions into the vasculature. Slower perfusion speeds and/or smaller volumes of contrast agent left the major vessels only modestly full and the finer arteries often unfilled; higher perfusion speeds and/or volumes often produced the formation of swollen blebs or “aneurysm‐like” dilations, particularly in the finer vessels of the gastric mucosa, that appeared artifactual.
Furthermore, in the case of in vivo experiments, physiological factors operate to modulate vascular tone and often reduce vessel diameter to shunt blood away from some sites or to other regions both within the stomach and even between the different tissues and viscera. Physiological factors such as tissue oxygenation, hormones, sympathetic nervous system transmitters, paracrine signals released in the stomach wall, etc. can all change the vascular constriction or dilation pattern in a particular drainage field in the tissues (cf., for example, Kvietys, 2010). Such factors, which presumably normally work to modulate regional functions and link the food in the GI tract to the various need states and environmental stimuli the organism is negotiating at a given time. Certainly, factors influencing vasoconstriction and dilation may modulate the prominence of the stomach angioarchitecture during a surgical exposure as well as during normal physiological adjustments to local and immediate energy balance demands.
Finally, another set of factors originating from the limited work on the blood vessels of the rat stomach seem to have impeded analyses of the rat stomach angioarchitecture. The limited examinations of the rat and, in some discussions, the tendency to assume that the arterial patterns of rat and dog and human and other species are effectively interchangeable or can all be summarized with the same uncritical cross‐species description may have confused the attempts to evaluate circulation specifically in the rat.
4.3. Stomach fill and gastric fiducials
In natural environments, the rat is an opportunistic feeder, and its stomach can distend extensively to take in a large meal, when an occasion arises and as needed to store calories (Collier, 1985). With ad libitum access to food, the rat, a nocturnal species, eats multiple large meals in the dark and then tends to rest and to have only smaller and far fewer bouts of feeding during the diurnal phase (Kissileff, 2000; Zorrilla et al., 2005). The feeding regimen in the present experiment capitalized on this feeding pattern to deprive mildly animals overnight and then give them an opportunity to feed on a large ad libitum meal in the early morning. As predicted from the ecological and environmental picture of the rat's natural habitats, the animals in this experiment ingested large meals, stored much of the food in their stomachs, and then began emptying the organ over the course of the daylight hours. Correspondingly, when we sacrificed animals at different times of day after their large morning meal, their stomachs varied dramatically in size and shape. Immediately after a large meal, the stomach was considerably distended. Interestingly, shortly after the large meal, the mucosa of the corpus evidenced dilated arterioles, presumably reflecting the production and release of secretory fluids and hormones. Hours after the large meal, the stomach had emptied most nutrient material, the gastric size and shape were considerably reduced and folded, and the dilated arterioles of the corpus had disappeared.
Our micro‐CT scans of the stomach walls over the course of this store‐and‐empty cycle illustrate the dramatic differences in size and shape that occur, even in a free‐feeding environment. It is notable, as seen in Figure 4, that the commonly identified regions of the stomach (forestomach; corpus; antrum) varied in size and emptied at different rates. Finally, the distribution fields of the serosal blood supply—particularly the gastric arteries—appeared to define the different regions relatively accurately, even as those regions changed in size at different rates (see Figures 4 and 5).
In sum, for optimal accuracy locating sites on the stomach wall, the serosal vasculature as well as the organ landmarks and degree of stomach fill should be considered.
CONFLICT OF INTEREST
No conflicts of interest, financial or otherwise, are held by any of authors.
AUTHOR CONTRIBUTIONS
T.L.P. and D.M.J conceived and designed research; L.C. performed experiments; D.M.J. analyzed data and interpreted results of experiments; D.M.J. and L.C. prepared figures; T.L.P. drafted manuscript; T.L.P. and D.M.J. edited and revised manuscript; T.L.P., D.M.J. and L.C. approved the final version of the manuscript.
Supporting information
Video S1
Video S2
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Bartek Rajwa (Purdue Bindley Bioscience Center) for extensive expert help with biostatistics and with modeling gastric emptying; Dr. Andy Schaber, Director of the Imaging Facility, Purdue's Bindley Bioscience Center for discussions of the micro‐CT analyses; and Ms. Jennifer McAdams for all of her help with laboratory operations.
Jaffey, D.M., Chesney, L.&Powley, T.L. (2021) Stomach serosal arteries distinguish gastric regions of the rat. Journal of Anatomy, 239, 903–912. 10.1111/joa.13480
Funding information
As per NIH reporting guidelines, the research reported in this publication was supported by NIH DK27627 and by the Office of the Director, NIH under award OD023847.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Pennsieve Discover (https://doi.org/10.26275/jl5t‐xfgu; https://doi.org/10.26275/zxe9‐o3ss ).
REFERENCES
- Collier, G.H. (1985) Satiety: an ecological perspective. Brain Research Bulletin, 14, 693–700. [DOI] [PubMed] [Google Scholar]
- Froud, M.D. (1959) Studies on the arterial system of three inbred strains of mice. Journal of Morphology, 104, 441–478. [DOI] [PubMed] [Google Scholar]
- Gannon, B., Browning, J. & O'Brien, P. (1982) The microvascular architecture of the glandular mucosa of rat stomach. Journal of Anatomy, 135, 667–683. [PMC free article] [PubMed] [Google Scholar]
- Greene, E.C. (1968) Anatomy of the rat. New York: Hafner Publishing Co. [Google Scholar]
- Guth, P.H. & Leung, F.W. (1987) Physiology of the gastric circulation. In: Johnson, L.R. (Ed.) Physiology of the gastrointestinal tract (pp. 1031–1053). New York: Raven Press. [Google Scholar]
- Guth, P.H. & Smith, E. (1975) Neural control of gastric mucosal blood flow in the rat. Gastroenterology, 69, 935–940. [PubMed] [Google Scholar]
- Holzer, P., Livingston, E.H. & Guth, P.H. (1991) Sensory neurons signal for an increase in rat gastric mucosal blood flow in the face of pending acid injury. Gastroenterology, 101, 416–423. [DOI] [PubMed] [Google Scholar]
- Kissileff, H.R. (2000) Ingestive behavior microstructure, basic mechanisms and clinical applications. Neuroscience and Biobehavioral Reviews, 24, 171–172. [DOI] [PubMed] [Google Scholar]
- Kvietys, P.R. (2010) The gastrointestinal circulation. University of MIssissippi School of Medicine: Morgan & Claypool Life Sciences Publishers. [PubMed] [Google Scholar]
- Lambert, R. (1965) Surgery of the digestive system in the rat. Illinois: Charles C. Thomas, Springfield. [Google Scholar]
- Martins, P.N.A. (2003) The importance of experimental microstructure for transplantation. Acta Cirúrgica Brasileira, 18, 59–61. [Google Scholar]
- Martins, P.N. & Neuhaus, P. (2007) Surgical anatomy of the liver, hepatic vasculature and bile ducts in the rat. Liver International: Official Journal of the International Association for the Study of the Liver, 27, 384–392. [DOI] [PubMed] [Google Scholar]
- Moskalewski, S., Biernacka‐Wawrzonek, D., Klimkiewicz, J. & Zdun, R. (2002) Collecting and paramuscular venules in glandular mucosa of rat stomach. Annals of Anatomy ‐ Anatomischer Anzeiger, 184, 173–179. [DOI] [PubMed] [Google Scholar]
- Peti‐Peterdi, J., Kovacs, G., Hamar, P. & Rosivall, L. (1998) Hemodynamics of gastric microcirculation in rats. American Journal of Physiology, 275, H1404–H1410. [DOI] [PubMed] [Google Scholar]
- Piasecki, C. & Wyatt, C. (1986) Patterns of blood supply to the gastric mucosa. A comparative study revealing an end‐artery model. Journal of Anatomy, 149, 21–39. [PMC free article] [PubMed] [Google Scholar]
- Powley, T., Jaffey, D., Chesney, L., McAdams, J. & Rajwa, B. (2020a) MicroCT imaging of iodine‐stained rat stomachs from full to empty. Pennsieve Discover 10.26275/jl5t-xfgu [DOI]
- Powley, T., Jaffey, D., Chesney, L., McAdams, J. & Rajwa, B. (2020b) MicroCT imaging of rat stomach vaculature with Microfil MV‐122. Pennsieve Discover 10.26275/zxe9-o3ss [DOI]
- Robert, A. (1971) Proposed terminology for the anatomy of the rat stomach. Gastroenterology, 60, 344–345. [PubMed] [Google Scholar]
- Schnitzlein, H.N. (1957) Regulation of blood flow through the stomach of the rat. Anatomical Record, 127, 735–753. [DOI] [PubMed] [Google Scholar]
- Shukla, C.J. & Ellis, H. (2002) Normal and variant anatomy and collateral mesenteric circulation. In: Geroulakgs, G. & Cherry, K.J. (Eds.) Diseases of the visceral circulation (pp. 1–23). London: Arnold. [Google Scholar]
- Stevens, C.E. (1988) Comparative physiology of the vertebrate digestive system. Cambridge: Cambridge Univeristy Press. [Google Scholar]
- Vdoviaková, K., Petrovová, E., Maloveská, M., Krešáková, L., Teleky, J., Elias, M.Z.J. & & et al. (2016) Surgical anatomy of the gastrointestinal tract and its vasculature in the laboratory rat. Gastroenterology Research and Practice, 2016, 2632368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla, E.P., Inoue, K., Fekete, E.M., Tabarin, A., Valdez, G.R. & Koob, G.F. (2005) Measuring meals: structure of prandial food and water intake of rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 288, R1450–R1467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Supplementary Material
Data Availability Statement
The data that support the findings of this study are openly available in Pennsieve Discover (https://doi.org/10.26275/jl5t‐xfgu; https://doi.org/10.26275/zxe9‐o3ss ).


