Abstract
The importance of the equine thoracolumbar vertebral column in orthopaedic disorders is well recognized and diagnostic imaging becomes more feasible, but little is known about variations in the anatomical configuration within breeds. In this descriptive post‐mortem study, anatomical variations in three widely differing breeds: Warmblood horses, Shetland ponies and semi‐feral Konik horses are described. The caudal cervical (C), thoracic (T), lumbar (L) and sacral (S) regions of the vertebral column of 30 Warmblood horses, 29 Shetland ponies and 18 Konik horses were examined using computed tomography and visualized by volume rendering. Homologous/morphologic variations in the caudal cervical area were frequently seen in Warmblood horses (43%), which was significantly more than in the other breeds (p < 0.001). The as standard described equine formula of 18 T, 6 L and 5 S vertebrae was seen in 78% of Konik horses, but only in 53% Warmblood horses and 38% Shetland ponies, which was significantly different (p < 0.05). Overall, Shetland ponies showed a higher tendency of thoracoization, lumbarization and more variations in the number of vertebrae and pairs of ribs. Ankylosed intertransverse joints (ITJs) between transverse processes of the lumbar vertebrae were most common between the second last and last lumbar vertebra and prevalence was significantly higher in Shetland ponies (61%), than in Warmblood horses (38%) and Konik horses (7%) (p < 0.0001). Cranial to the second last lumbar vertebra there were fewer ITJs ankylosed (14%) in Warmblood horses (p < 0.0095), and this decrease in number of ankylosed ITJs was different compared to the change in ankylosed ITJs in Shetland ponies (p < 0.005). ITJs occurred asymmetrically in 15% (12/77) of the cases. A limitation of the study was that clinical data of the horses were only incompletely available, precluding any conclusions about the potential clinical implications of anatomical variations. Knowledge of variation in osseous anatomy of the equine thoracolumbar vertebral column is important for the interpretation of diagnostic imaging. To assess the functional importance and clinical relevance of this variation, follow‐up studies are necessary.
Keywords: horse, Konik, Shetland pony, spine, vertebrae, Warmblood
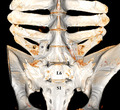
Volume rendering (VR) reconstruction image of the ventral aspect of the lumbar vertebrae of a Warmblood horse (gelding, 8y7m) with sacralization of the last lumbar (L6) vertebra by fusion with the first sacral vertebra (S1), and ankylosis of the right intertransverse joint between L4 and L5 (*)
1. INTRODUCTION
Musculoskeletal disorders are by far the most important reason to consult an equine veterinarian (Loomans et al., 2007). These disorders are almost all related to malfunctioning of components of either the appendicular or the axial musculoskeletal system. Whereas there are myriads of publications on the prevalence and treatments of diseases of the appendicular musculoskeletal system, reports on the kinematics (Hardeman et al., 2020), diagnostic imaging (Erichsen et al., 2004; Henson et al., 2007; Meehan et al., 2009; Veraa et al., 2016; Zimmerman et al., 2011, 2012), specific diseases of the vertebral column (Bergmann et al., 2018; Girodroux et al., 2009; Meehan et al., 2009), pathologic features (Jeffcott, 1980; Stubbs et al., 2010; VanderBroek et al., 2016) and different treatment modalities (Muñoz et al., 2019; Pfau et al., 2017) of the axial musculoskeletal system have started to trickle in only relatively recently, probably because of the availability of advanced diagnostic imaging modalities nowadays.
However, still much basic information which is needed for the deepening of our understanding of how the equine axial musculoskeletal system works, and how disorders of this system may be approached from a therapeutic or preventative perspective, is lacking. This includes data on the normal anatomy of the vertebral column and its anatomical variations. Recently, interesting work on osseous variations of the equine cervical vertebral column has been published (Veraa et al., 2016), but little is known about the other parts of the axial skeleton. In the early 1960 s, the numerical variation of vertebrae in Przewalski horses was reported, and compared to Shetland ponies, zebras, donkeys, Arabian horses, hybrids and a number of undefined domestic horses (Stecher, 1961a). Three decades later, anatomic variations of the lumbosacropelvic area were described in a post‐mortem study of Thoroughbred racehorses (Haussler et al., 1997). There is hardly any data on anatomical variations in the thoracolumbosacral region of the vertebral column of modern Warmblood horses, which is the predominant breed in many equestrian disciplines outside racing.
In this study, we examined the osseous anatomy of the axial skeleton from the caudal cervical area (C6, C7) up to the last sacral / first caudal (Cd1) vertebra in Warmblood horses using post‐mortem computed tomography (CT). Developmental variations are described in terms of homeotic variation (variation in the relative number of types of vertebrae with preservation of the total number), meristic variation (variation in total number), homologous variation (variation in size and shape of vertebrae), and developmental variations in ribs, orientation of dorsal spinous processes and the intertransverse joints (ITJs). The aim was to document these variations in modern Warmblood horses and to compare them with two breeds deemed closer to the wild predecessor of the domesticated horse: the Shetland pony and semi‐feral Konik horses which are considered direct descendants of the now extinct European wild horse, the Tarpan (Janikowski, 1942; Pasicka, 2013). We hypothesize that substantial differences between breeds will exist. Apart from adding to the as‐yet meagre body of knowledge on the equine axial skeleton, any such differences may have implications for conclusions to be drawn from findings in clinical studies.
2. MATERIALS AND METHODS
2.1. Collection and preparation of specimens
From 2017 to 2020, 77 equine cadavers were collected and divided into three groups:
Group 1, Warmblood horses (n = 30, WH; 28 Dutch Warmbloods, 1 Oldenburger and 1 Westfalen) that were euthanized and donated for scientific research by their owners.
Group 2, Shetland ponies (n = 29, SP) that had been used in terminal research projects not related to the axial musculoskeletal system.
Group 3, Semi‐feral Konik horses (n = 18, KH) from a national park in the Netherlands that had been culled following a population management program.
Age, weight and sex were noted for all animals. For the WH, the history and the reason for euthanasia were additionally recorded (Table 1). Thoracolumbar vertebral columns including the complete ribcage (SP) or with all the proximal parts of the ribs still in place (WH and KH) were examined with computed tomography. The cadavers of groups 1 (WH) and 3 (KH) had to be reduced in size in order to get them pass through the gantry of the CT scanner. Both thoracic limbs were removed from halfway the scapula, pelvic limbs were removed after sectioning the femur at the proximal metaphysis. The size of the pelvis was reduced by removing both ischial tuberosities, including tissue of the biceps, semimembranosus, semitendinosus and gluteus medius muscles. Ribs were removed with exception of the proximal 15–20 cm in order to leave the articulation with the vertebrae intact. In the group of KH the complete cervical, thoracic, lumbar and sacral parts of the vertebral column were available. In the group of Warmblood horses, the head including the cranial and middle portion of the cervical vertebral column had been removed, leaving the caudal cervical vertebrae (C6 and C7), with the complete thoracic, lumbar and sacrocaudal part of the vertebral column.
TABLE 1.
History, age (mean, SD), body mass (mean, SD) and gender for Shetland ponies (SP), Konik horses (KH) and Warmblood horses (WH).
| Breed | Number | History | Age | Body mass (kg) | Gender | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | m | s | g | ||||
| SP | 29 | No disorders of the axial musculoskeletal system | 8.6 (3.8) years | 181 (22) | 27 | — | 2 | |
| KH | 18 | Unknown | 6.7 (5.6) years | 261 (50) | 10 | 8 | — | |
| WH | 30 | 13 | No disorders of the axial musculoskeletal system | 11.4 (6.9) years | 542 (98) | 6 | 2 | 5 |
| 15 | Chronic lameness | 11.8 (5.3) years | 583 (45) | 7 | — | 8 | ||
| 2 | Chronic back pain | 13.0 (5.9) years | 656 (56) | 2 | — | — | ||
Abbreviations: m, mare; s, stallion; g, gelding.
2.2. CT examination
All vertebral columns were scanned within 4 hours of euthanasia. The vertebral columns of the Shetland ponies were scanned in dorsal recumbency, those of the other horses in ventral recumbency in a custom‐designed CT tray. A 64‐slice sliding gantry CT (Siemens, Definition AS) was used; 140 kVp, mA varying according to focal cadaver size between 280 and 380, rotation time 0.7 s, reconstruction in bone algorithm B50 and 1 mm slice thickness (WW/WL 3000/600), reconstruction in soft tissue algorithm B30 and 2 mm slice thickness (WW/WL 300/50), matrix 512 × 512 and scan field‐of‐view variable according to cadaver size.
2.3. Data evaluation
The CT images were available in DICOM format in the PACS system (Agfa) and blindly evaluated by a board‐certified equine surgeon (TS); a board‐certified radiologist (SV) was consulted for images that were ambiguous. The images were reconstructed in multiplanar reconstruction and 3D for assessment of the vertebral column, in bone algorithm and window width 6000/window level 300. A traditional vertebral reference system was used and vertebrae were conventionally counted from the cranial reference point caudad. Homeotic and meristic variations were recorded. Next, each vertebral body was individually examined and homologous variations were recorded for the 6th and 7th cervical and all thoracic, lumbar and sacral vertebrae, and for the first caudal vertebra. Ribs were examined for homologous variations, left and right numbers were counted and compared.
The specimens were examined for presence of transitional vertebrae (defined as having characteristics of vertebrae belonging to an adjacent spinal segment), and classified in several categories. Lumbar vertebrae with articulating transverse processes were classified as thoracic (i.e. thoracoization of the first lumbar vertebra), and thoracic vertebrae with ankylosed or no articulations were categorized as lumbar (i.e. lumbarization of the last thoracic vertebra) (Haussler et al., 1997). Transitional vertebrae featuring combinations of articulating and non‐articulating costal/transverse processes were classified as thoracic if the process was elongated and cylindrical (i.e. the form of a thoracic costal process), and as lumbar if they were more comparable with the shape of a lumbar transverse process (short and horizontally flat). The lumbosacral transition was defined as lumbarized when the first sacral vertebra had a transverse process or when an intervertebral disc between S1 and S2 was present. Sacralization of a lumbar vertebra was defined to be present when the vertebra had transverse processes that articulated with the pelvis, when there was fusion of lumbar vertebral bodies or transverse processes, or in case of absence of a caudal intervertebral disc (between L6 and S1). Sacrocaudal transitional vertebrae were defined as fusion of the sacrum with the first caudal vertebra (Haussler et al., 1997). The vertebra with the anticlinal spinous process was recorded. Also, the interspinal spaces between the dorsal spinous processes of the last two caudal lumbar vertebrae and the first sacral vertebra were categorized as either ‘open’, when the adjacent dorsal spinous processes were parallel or diverging dorsally, or as ‘closed’ when the processes were converging dorsally (Haussler et al., 1997; Stubbs et al., 2006). The absence or presence of intertransverse joints (ITJs) at the lumbar vertebral column and lumbosacral junction was recorded. If present, laterality (left or right) and condition (normal or ankylosis) were noted. Presence of a visible joints space was defined as normal, and ankylosis was defined as complete fusion of a joint. For the exact localization of the ITJs, taking into account the differences in total number of lumbar vertebrae (5, 6 or 7), we chose descriptions with a caudal to cranial reference point; ‘between the last lumbar and first sacral vertebra’, ‘between the second last and last lumbar vertebra’ and ‘cranial to the second last lumbar vertebra’. By using these descriptions, ITJs in specimens with 5, 6 or 7 lumbar vertebrae could be described and compared.
2.4. Data analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc.). Differences between breeds were estimated with (exact) logistic regression procedures (PROC LOGISTIC). p‐values of the likelihood ratio test <0.05 were considered significant. If percentages were either 0% or 100%, that is, all or none of the horses showing the outcome of interest, exact p‐values were used since these cannot be estimated with ordinary logistic regression.
3. RESULTS
3.1. Numbers of vertebrae
In 53% (41/77) of the specimens, the commonly accepted formula of 18 thoracic, 6 lumbar and 5 sacral vertebrae (Getty, 1975) was present; however, with large differences between breeds (Table 2). This formula was more prevalent in KH (78%) than in WH (53%) and SP (38%) (p < 0.05). Not all sacral vertebrae could be counted in 8 SP (8 of 29; 28%), 6 WH (6 of 30; 20%) and 1 KH (1 of 18; 6%), as some of those fell outside the field of view of the CT scans. Breeds did not differ in the prevalence of the standard number of 18 thoracic vertebrae (97% for WH, 94% in KH and 90% in SP). The same was true for lumbar vertebrae; with 6 present in 79%, 80% and 89% for SP, WH and HK respectively. Konik horses, however, were far more consistent in having five sacral vertebrae (94%) than WH and SP with 62% and 67% (p < 0.05). Of all specimens with a known total number of thoracic, lumbar and sacral vertebrae (n = 62), 82% had 29 vertebrae (71% in SP, 88% in WH and 88% in KH; p = 0.30). The atypical specimens (18%, 11/62) had 28 or 30 vertebrae. Of these, all SP had 30 vertebrae, in KH and WH both variations were seen.
TABLE 2.
Numbers of vertebrae recorded per breed. The grey row indicates the generally accepted formula for the numbers of thoracic, lumbar and sacral vertebrae in the horse
| Vertebral formula | Breed | Variation | |||||
|---|---|---|---|---|---|---|---|
| Thoracic | Lumbar | Sacral | Sum of thoracic, lumbar and sacral vertebrae | Shetland ponies (29) | Konik horses (18) | Warmblood horses (30) | |
| 17 | 6 | — | — | 1 | — | ||
| 17 | 7 | 5 | 29 | 1 | H | ||
| 18 | 5 | 5 | 28 | 1 | M | ||
| 18 | 5 | 6 | 29 | 3 | 1 | 5 | H |
| 18 | 6 | 4 | 28 | 1 | M | ||
| 18 | 6 | 5 | 29 | 11 (38%)* | 14 (78%)** | 16 (53%)* | |
| 18 | 6 | — | — | 5 | 1 | 6 | — |
| 18 | 6 | 6 | 30 | 5 | 1 | M | |
| 18 | 7 | 5 | 30 | 1 | M | ||
| 18 | 7 | — | — | 1 | — | ||
| 19 | 5 | 6 | 30 | 1 | M | ||
| 19 | 6 | 5 | 30 | 1 | M | ||
| 19 | 6 | — | — | 1 | — | ||
Different asterisks within rows denote a significant difference (p < 0.05) between the T18 and L6 and S5 formula versus all other formulae.
Abbreviations: H, homeotic; M, meristic; —, no data available.
3.2. Homologous variations
Homologous variations were found in the last two caudal cervical vertebrae. Uni‐ or bilateral transposition of the caudal part of the transverse process of the 6th cervical vertebra to the ventral aspect of the transverse process of the 7th cervical vertebra was significantly more frequently encountered in Warmblood horses (21/49 vertebrae; 43%) than in Shetland ponies (3/48; 6%) or Konik horses (0/36; 0%) (p < 0.001). Unilateral absence of a transverse process of C6 was accompanied by ipsilateral presence of the ventral part of a transverse process on C7. Similarly, bilateral absence of the transverse process of C6 was seen with bilateral presence of the transverse process on C7 (Table S1).
In two Shetland ponies, spina bifida occulta (neural arch defect with incomplete fusion of the spinous process without a skin defect) was present. In the first pony, the absence of laminae with the dorsal spinous process of L5 resulted in an open dorsal aspect of neural arch (Figure 1a–c). In this animal, the dorsal spinous process of L4 was extraordinarily large. Another Shetland pony had an incomplete fusion of the L5 lamina at the base of the dorsal spinous process (Figure 1d).
FIGURE 1.
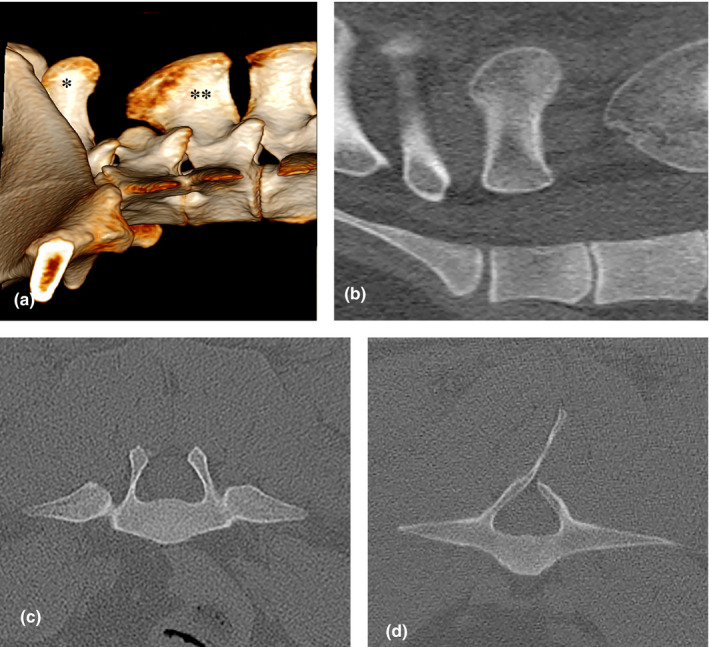
(a) Volume rendering (VR) reconstruction image of the lateral aspect of the caudal lumbar vertebrae of a Shetland pony (mare, 15y6m) with absence of the spinous process of L5, between an abnormally large dorsal spinous process of L4 (**) and a normal shaped spinal process of L6 (*); (b) sagittal plane CT image of the same pony; (c) transverse image of the same pony at the level of L5, showing an absence of the dorsal spinous process; (d) transverse CT image of an incomplete neural arch at L5 in another Shetland pony (mare, 8y1m)
3.3. Ribs
Most specimens (n = 70) had 18 pairs of normal symmetrically developed thoracic ribs. Two Shetland ponies had 17 thoracic vertebrae with a corresponding number of normally developed ribs. One of these animals had 6 lumbar vertebrae, in the other pony the 18th thoracic vertebra was lumbarized, leading to seven lumbar vertebrae (1 lumbarized and 6 normal). In line with the first right transverse process, and almost parallel to last normal rib, an 81‐mm‐long and 7‐mm‐thick osseous rib remnant was present (Figure 2a). On the left side, but in line with the second transverse process, another 65‐mm‐long and 2‐mm‐thick rib remnant was visible (Figure 2a). Three specimens, one Shetland pony, one Konik horse and one Warmblood horse had 19 rib pairs. The Shetland pony had 18 thoracic and 6 lumbar vertebrae with the first lumbar vertebra having an extra pair of ribs (Figure 2b). Both the Konik and Warmblood horse had 19 thoracic vertebrae (with 19 rib pairs), with 5 lumbar and 6 sacral vertebrae. In two other specimens, development of the ribs was asymmetrical. One Shetland pony had 18 left and 19 right ribs. In this specimen with 19 thoracic and 6 lumbar vertebrae, a transverse process was present on the left side of the 19th thoracic vertebra. One Warmblood horse had 18 normal ribs on the right side, but on the left side the 1st thoracic vertebra articulated to only a small rib remnant.
FIGURE 2.
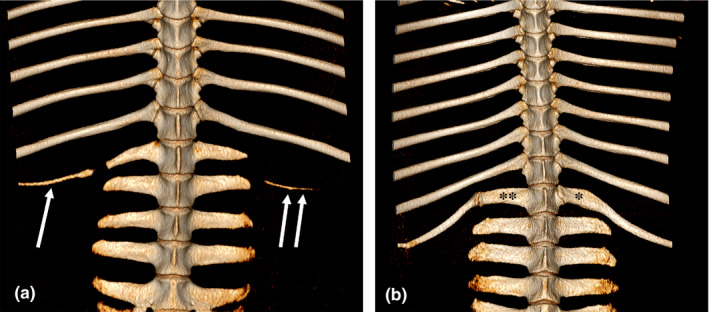
Volume rendering (VR) reconstruction images of the ventral aspect of the caudal thoracic and lumbar vertebrae of two Shetland ponies. (a) (Mare, 4y5m): lumbarization of the last thoracic vertebra. In line with the first right transverse process a remnant of a rib is present (single arrow), on the left side in line with the second transverse process another rib remnant (double arrows) is visible. b) (Mare, 6y): thoracoization of the first lumbar vertebra. Note the difference in location (*,**) of the costovertebral articulations between left and right side
3.4. Transitional vertebrae
Transitional vertebrae were most frequently encountered in Shetland ponies (Table S2). Lumbarization of the last thoracic vertebra was encountered in two (7%) Shetland ponies (Figure 2a). In one case this resulted in a reduction to 17 rib pairs. In the other, the anomaly resulted in 18 ribs and 7 transverse processes on the left side, and 19 ribs with 6 transverse processes on the right side. Thoracoization of the first lumbar vertebra was seen in one (3%) Shetland pony (Figure 2b), resulting in 19 ribs bilaterally. Sacralization of the last lumbar vertebra was seen in one (3%) Warmblood (Figure 3). Sacrocaudal transition of the first caudal vertebra (fusion to sacrum) was encountered in 33% of Shetland ponies, 29% of Warmblood horses and 6% of Konik horses.
FIGURE 3.
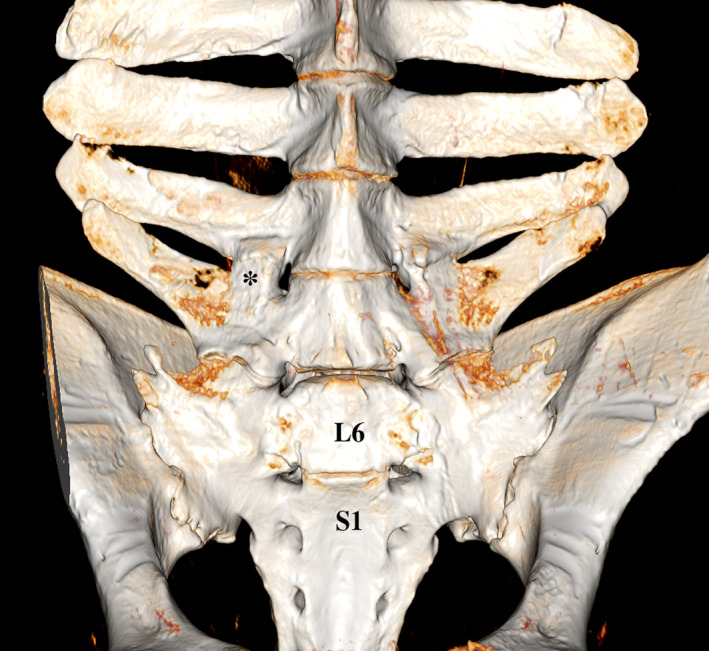
Volume rendering (VR) reconstruction image of the ventral aspect of the lumbar vertebrae of a Warmblood horse (gelding, 8y7m) with sacralization of the last lumbar (L6) vertebra by fusion with the first sacral vertebra (S1), and ankylosis of the right intertransverse joint between L4 and L5 (*)
3.5. Anticlinal vertebra
The predominant anticlinal vertebra in all three breeds was the 15th thoracic vertebra (60% in Shetland ponies, 61% in Warmbloods, 53% in Konik horses). In Shetland ponies, the anticlinal vertebra varied between T14 and T17, in Konik horses T15 and T16 and in Warmblood horses T14 and T16 (Table 3).
TABLE 3.
Location of anticlinal thoracic (T) vertebrae in Shetlands ponies, Konik and Warmblood horses
| Anticlinal vertebra | ||||
|---|---|---|---|---|
| T14 | T15 | T16 | T17 | |
| Shetland ponies (29) | 2 | 17 (59%) | 8 | 2 |
| Konik horses (18) | — | 11 (61%) | 7 | — |
| Warmblood horses (30) | 5 | 16 (53%) | 9 | — |
3.6. Dorsal spinous processes
The interspinal space between the dorsal spinous processes of the last two caudal lumbar vertebrae and the first sacral vertebra was variable and could be categorized as: closed‐open or open‐open. The closed‐open configuration was encountered in 46/77 (60%) vertebral columns; and more frequent in Warmblood horses (23/30, 77%) than in Shetland ponies (15/29, 52%) or Konik horses (8/18, 44%) (p < 0.05). Thirty‐five specimens had 6 lumbar vertebrae and 11 specimens had 5 lumbar vertebrae. The open‐open configuration was recorded in the other 31 vertebral columns (40%), and lower in Warmblood horses (7/30, 23%) than in Shetland ponies (14/29, 48%) and Konik horses (10/18, 56%) (p < 0.05). Twenty‐eight specimens with this open‐open configuration had six lumbar vertebrae, the other three specimens had seven lumbar vertebrae.
3.7. Intertransverse joints
There were in total 367 intertransverse joints (ITJs) in the caudal lumbosacral vertebral column (Table 4). In 22% (32/148) of Shetland ponies and in 23% (36/154) of Warmblood horses, the ITJs were located ‘cranial to the second last lumbar vertebra’, which is significantly different from the Konik horses (p < 0.0001), in which none (0/65) were found in that location. Between the ‘last lumbar and first sacral vertebra’ only three (2%: 3/154) of the ITJs were ankylosed, one unilaterally in a Shetland pony and one bilaterally in a Warmblood horse. The bilateral ankylosis in the Warmblood was accompanied by sacralization of L6 to S1 (Figure 3). There was no sacralization visible in the Shetland pony. Between the ‘second last and last lumbar vertebra’, a total of 60 ankylosed ITJs were found (30 left, 30 right). This was significantly different between breeds, with 62% (36/58) in Shetland ponies, 38% (22/58) in Warmblood horses and 7% (2/29) in Konik horses (p < 0.0001).
TABLE 4.
Location and condition (ankylosed (A) vs. normal (N)) of intertransverse joints in the lumbar region and at the lumbosacral junction in Shetland ponies (SP), Konik horses (KH) and Warmblood horses (WH) classified as specimens with 5, 6 and 7 lumbar vertebrae respectively
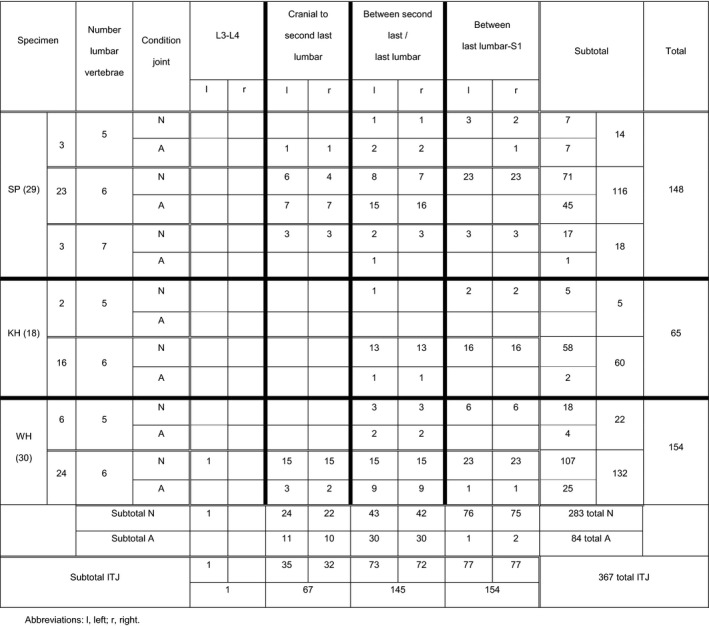
‘Cranial to the second last lumbar vertebra’ 50% (16/32) of the ITJs were ankylosed in Shetland ponies, which differed significantly from the 14% (5/35) found in Warmblood horses (p < 0.005). There was also a significant difference in prevalence of ankylosed ITJs within Warmblood horses between the positions ‘second last to last lumbar vertebra’ and ‘cranial to the second last lumbar vertebra’ with 38% and 14%, respectively (p < 0.05). Within Shetland ponies this difference was not significant.
Most of the ITJs (n = 362) were symmetrically distributed (Figure 4a,b). In total, 12/77 (16%) horses showed asymmetry, but this was not different (p = 0.32) between breeds. Two Shetland ponies, with six lumbar vertebrae had unilateral, normal ITJs between L4 and L5 (Table S3). One Konik horse with five lumbar vertebrae had a unilateral normal ITJ at L5–L6 (Figure 4b), and two Warmblood horses with six lumbar vertebrae had unilateral ITJs between L3 and L4 and L4 and L5 that were, respectively, normal and ankylosed (Table S3). Asymmetric manifestations of the ITJs were observed in four Shetland ponies: between L4 and L5 (n = 1), L5 and S1 (n = 1), L6 and L7 (n = 1) and L4 and L5, as well as L5 and L6 (n = 1) (Table S3); and in three Warmblood horses: between L4 and L5 (n = 1), L5 and L6 (n = 1) and L4 and L5, as well as L5 and L6 (n = 1) (Table S3).
FIGURE 4.
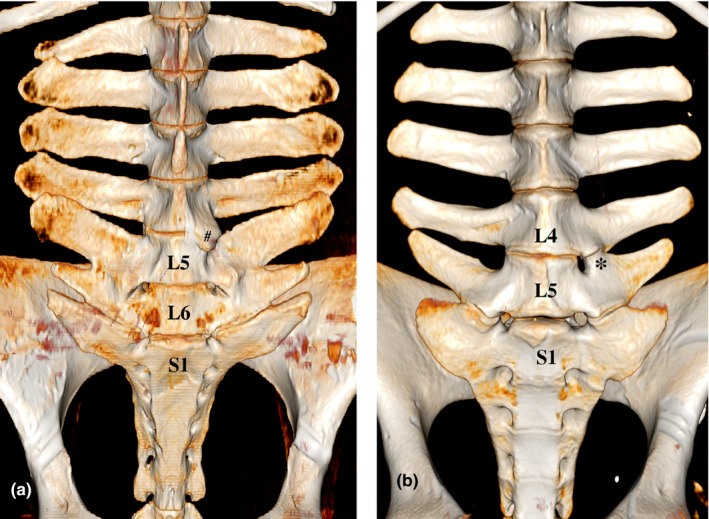
Volume rendering (VR) reconstruction images of the ventral aspect of the caudal thoracic and lumbar vertebrae of two specimens. (a) Warmblood horse (mare, 22y3m) with symmetrical distribution of normal and ankylosed intertransverse joints between L6 and S1, L5 and L6 respectively. Note: Left ventrolateral spondylosis between L4 and L5 (#), (b) Konik horse (stallion, 1y) with unilateral presence of a left intertransverse joint at L4‐L5 (*). Note: this specimen has five lumbar vertebrae
4. DISCUSSION
The outcome of this study is that a relatively large variation exists in the osseous anatomy of the equine caudal cervical and thoracolumbosacral vertebral column both between breeds and at the individual level in Warmblood horses, Konik horses and Shetland ponies. Of these breeds, it were the Koniks that featured most the formula that is commonly described as the standard for the horse (Getty, 1975), with both other breeds showing substantially more aberrations from the standard, be it in different forms. The reason why the Koniks appeared to have fewer vertebral changes cannot be deduced from this observational study; it may be that they are closer to the original undomesticated, European wild horse, the Tarpan. However, it should be kept in mind that the Tarpan became extinct in the late 19th century (Janikowski, 1942) and that the current Konik breed was ‘bred back’. Research on the original breeds that were used to create the Tarpan and perhaps the Asian wild horse, the Przewalski horse, might provide answers.
Homeotic variation was least in the Konik horses and significantly more in the other two breeds. The standard numbers of six lumbar and five sacral vertebrae that were most common in the Konik and Warmblood horses were also found by Haussler et al. (1997) and Stubbs et al. (2006) in Thoroughbred racehorses. Here, the Shetland ponies showed the most variation, which contrasted to earlier work by Stecher in the early 1960s, who reported a remarkable stability in both Shetland pony and Grevy zebra (Stecher, 1962b). In the current study, 2 Shetland specimens had 17 thoracic vertebrae, 1 combined with 6 and the other with 7 lumbar vertebrae, configurations that have not been reported before. These findings are in contrast with Stecher's statement that numerical variations in vertebrae of one spinal region are often compensated in an adjacent region, leaving the total number similar (Haussler et al., 1997; Stecher, 1962b). In our study, 18% of the specimens (11/62) had a total number of thoracic, lumbar and sacral vertebrae of 28 or 30, instead of the standard 29.
Overall, Shetland ponies showed the highest variability in the number of transitional vertebrae, ribs, position of the anticlinal spinous process, and number and condition of intertransverse joints. This observational study cannot provide an explanation for this, but it may be conjectured that there might be a relationship with the chondrodystrophic (abnormal development of cartilage, which causes the long bones of the body to grow at an abnormal rate and results in shortened legs) character of the breed (Nolte et al., 2019), and/or the relatively frequent occurrence of atavisms like a persistent ulna or fibula (Rafati et al., 2016). In companion animals many breed‐specific spinal malformations are known, some of them in chondrodystrophic breeds (De Decker et al., 2019; Rohdin et al., 2018; Westworth & Sturges, 2010). Another explanation for variations in lateral element morphology (transverse processes and ribs) could be the Hox code. These Hox genes set junctions in vertebral series along the cranio‐caudal axis (Burke, 2000; Nowicki & Burke, 2000), and can cause vertebral malformations when the genome deletes or changes. For Warmblood horses, Konik horses and Shetland ponies, further research in this area is necessary, but recently, in four German Warmblood breeds the HoxB complex has already been marked as a target for genetic selection (Nolte et al., 2019). And, therefore, it could be possible that changes in breeding policies over the past 60 years, in which selection for favourable traits may have inadvertently led to the introduction of undesired, clinically not immediately visible traits, such as vertebral morphological/homologous variations. Occurrence of vertebral transitions is also known to be influenced by breeding programs in canine breeds like German Shepherd, Lhasa Apso and Shih Tzu (Widmer & Thrall, 2018). Stecher did not report vertebral transitions like thoracoization and lumbarization, which were frequent in our study (Stecher, 1962b). Thoracolumbar transitions were found by Haussler in a large percentage (22%) in Thoroughbred racehorses (Haussler et al., 1997). Vanderbroek reported only thoracoization and no lumbarization in mixed breeds (VanderBroek et al., 2016). In the current study, sacralization of the last lumbar vertebra was only seen in one Warmblood specimen. This variation was not reported by Haussler in Thoroughbreds (Haussler et al., 1997). However, Stubbs did mention sacralization in this breed (Stubbs et al., 2006) and Vanderbroek et al. reported three cases in a mixed‐breed population of 56 horses (VanderBroek et al., 2016). Sacrocaudal transition was noted in 24% (15/62) of the specimens in our study, one Konik horse and seven cases in both Warmblood horses and Shetland ponies. Stecher and Goss and Haussler reported comparable figures in domestic horses (Stecher, 1962b; Stecher & Goss, 1961) and Thoroughbreds (Haussler et al., 1997). All the studies show that thoracolumbar transitions are normal in the horse, with differences in frequency most likely related to differences in sample populations.
Homologous variations in transverse processes at C6 and C7 were seen with high frequency in Warmblood horses (43%). This is comparable to the 34% reported by Veraa et al., (2016), who had a very similar sample population, consisting of 93% (65/70) Dutch Warmblood horses, versus 93% (28/30) in this study. The complete absence of this type of variation in Konik horses and its presence in Shetland ponies have not been reported earlier. A gene defect has been detected in mice that gave comparable homologous variations (Kappen, 2016).
The number and distribution of ITJs significantly varied between the breeds. In Konik horses, the distribution of ITJs was uniform with all of them localized caudally to the second last lumbar vertebra. In both other breeds, there were also ITJs cranial to the second last lumbar vertebra. A positive association between number of ITJs and the number of lumbar vertebrae was reported in other studies (Haussler et al., 1997; Stecher, 1962c; Stecher & Goss, 1961), which was partially corroborated in our study with more ITJs in specimens with 6 lumbar vertebrae than in specimens with 5; however, Shetland ponies with 7 lumbar vertebrae featured fewer ITJs. The asymmetrical distribution of ITJs we found was in line with earlier work of Stecher and Haussler (Haussler et al., 1997; Stecher, 1962b,c; Stecher & Goss, 1961). An interesting finding was that in Shetland ponies almost two thirds of the ITJs (62%) were ankylosed between the second last and last lumbar vertebrae, against only one‐third (38%) ITJs in Warmblood horses. Cranial to the second last lumbar vertebra still half of the ITJs were ankylosed in Shetland ponies; in Warmblood horses this was significantly less (14%). There are clear species differences here, as still another study using 17 vertebral columns of mixed breeds reported a relatively high percentage of 59% of L5‐L6 ankylosed ITJs and a lower percentage of 23% of L4‐L5 fusions (Townsend & Leach, 1984). In a study in Thoroughbreds, these figures were 25% and 3% respectively (Haussler et al., 1997). The figures show that ITJs are common in the horse; however, there are clear breed differences in their topographical distribution.
Determination of the clinical effect of the very variable and diverse manifestations of the different osseous structures of the caudal cervical and thoracolumbosacral equine vertebral column—and hence the interpretation of diagnostic imaging—is challenging and not possible based on the data in this study. Neither a complete history nor clinical data were available for the vast majority of the animals included, which is an important limitation of the study. Another limitation is that the imaging technique used, computed tomography, gives detailed information about the osseous anatomy but not about the soft tissues.
Little work has been done on the correlation of variations in spinal anatomy with differences in back mobility, impairment of athletic function or predisposition to development of back disease. What has been shown in Warmblood horses is a rather large horse specificity with respect to range of motion of spinal segments (Faber et al., 2000, 2001; Hardeman et al., 2020). These differences in kinematics of the equine vertebral column within and between breeds could possibly be related to anatomical variations, inducing differences in motion and/or stability of an individual vertebral column (Townsend & Leach, 1984). Intertransverse joints may play a prominent role here. It is generally accepted that normal ITJs have a stabilizing function by limiting the range of lumbar motion, and eliminating movement when completely ankylosed (Haussler et al., 1997; Stecher, 1962c; Stecher & Goss, 1961; Townsend & Leach, 1984). In this way, the asymmetry in ITJs might also be possibly related to asymmetry in back motion (Faber et al., 2000). Even when this hypothesis could be proven true, there remain several questions to be answered, like why Shetland ponies, the smallest breed in this study, show the highest numbers of ankylosed joints, why Koniks have so few ITJs and hardly any ankylosed ITJ and why Warmblood horses show more ITJs but less ankylosed ones? The latter issue may be linked to a strong selection for back mobility in performance horses; however, further research is necessary if these statements are to be substantiated.
In conclusion, this explorative, descriptive study shows an unexplained high frequency of variations in the osseous anatomy of the caudal cervical and the thoracolumbar vertebral column in several breeds of horses. These variations differ considerably per breed and are also not identical to data collected earlier in the Thoroughbred racehorse. Therefore, extrapolation from one breed to another is difficult and caution is needed. In a more general sense, awareness about the apparently large natural variation in anatomical appearance of the equine vertebral column may help equine clinicians, physiotherapists and chiropractors in their interpretation and evaluation of clinical and diagnostic findings and is also important in the ongoing quest for better understanding of equine back pathology.
AUTHOR CONTRIBUTIONS
T.J.P.S., S.V., P.R.W. and H.B. designed the study. T.J.P.S. acquired data. T.J.P.S. and S.V. evaluated the CT images. E.A.M.G. and T.J.P.S. performed statistical analysis. T.J.P.S., S.V., E.A.M.G., P.R.W. and H.B. drafted and critically revised the manuscript. All authors approved the final version of the manuscript.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
We want to thank Staatsbosbeheer, the Dutch State Forestry Agency, for providing access to the culled Konik Horses. We also are thankful to L.M.C. van den Boom and M.J. Lock from the Pathologic Diagnostic Centre, Faculty of Veterinary Medicine at Utrecht University for the dissection of the Warmblood and Konik horses that were used in this study.
REFERENCES
- Bergmann, W., Bergknut, N., Veraa, S., Gröne, A., Vernooij, H., Wijnberg, I.D. et al. (2018) Intervertebral disc degeneration in warmblood horses: morphology, grading, and distribution of lesions. Veterinary Pathology, 55(3), 442–452. [DOI] [PubMed] [Google Scholar]
- Burke, A.C. (2000) Genes and the global patterning. Current Topics in Developmental Biology, 47, 155–181. [PubMed] [Google Scholar]
- De Decker, S., Packer, R.M.A., Cappello, R., Harcourt‐Brown, T.R., Rohdin, C., Gomes, S.A. et al. (2019) Comparison of signalment and computed tomography findings in French Bulldogs, Pugs, and English Bulldogs with and without clinical signs associated with thoracic hemivertebra. Journal of Veterinary Internal Medicine, 33(5), 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen, C., Eksell, P., Holm, K.R., Lord, P. & Johnston, C. (2004) Relationship between scintigraphic and radiographic evaluations of spinous processes in the thoracolumbar spine in riding horses without clinical signs of back problems. Equine Veterinary Journal, 36(6), 458–465. [DOI] [PubMed] [Google Scholar]
- Faber, M., Johnston, C., Schamhardt, H., Weeren, R.V., Roepstorff, L. & Barneveld, A. (2001) Basic three‐dimensional kinematics of the vertebral column of horses trotting on a treadmill. American Journal of Veterinary Research, 62, 757–764. [DOI] [PubMed] [Google Scholar]
- Faber, M., Schamhardt, H., Van Weeren, R., Johnston, C., Roepstorff, L. & Barneveld, A. (2000) Basic three‐dimensional kinematics of the vertebral comlumn of horses walking on a treadmill. American Journal of Veterinary Research, 61(4), 399–406. [DOI] [PubMed] [Google Scholar]
- Getty, R. (Ed.) (1975) Ch. 15 Equine osteology. In: Sisson and Grossman’s the anatomy of the domestic animals, 5th edition. Philadelphia: W.B. Saunders Co., pp. 255–348. [Google Scholar]
- Girodroux, M., Dyson, S. & Murray, R. (2009) Osteoarthritis of the thoracolumbar synovial intervertebral articulations: clinical and radiographic features in 77 horses with poor performance and back pain. Equine Veterinary Journal, 41(2), 130–138. [DOI] [PubMed] [Google Scholar]
- Hardeman, A.M., Byström, A., Roepstorff, L., Swagemakers, J.H., van Weeren, P.R. & Braganca, F.M.S. (2020) Range of motion and between‐measurement variation of spinal kinematics in sound horses at trot on the straight line and on the lunge. PLoS One, 15(2), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler, K.K., Stover, S.M. & Willits, N.H. (1997) Developmental variation in lumbosacropelvic anatomy in Thoroughbred racehorses. American Journal of Veterinary Research, 58(10), 1083–1091. [PubMed] [Google Scholar]
- Henson, F.M.D., Lamas, L., Knezevic, S. & Jeffcott, L.B. (2007) Ultrasonographic evaluation of the supraspinous ligament in a series of ridden and unridden horses and horses with unrelated back pathology. BMC Veterinary Research, 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janikowski, T. (1942) The wild horse of Poland. Nature, 150(3815), 681–682. [Google Scholar]
- Jeffcott, L.B. (1980) Disorders of the thoracolumbar spine of the horse–a survey of 443 cases. Equine Veterinary Journal, 12(4), 197–210. [DOI] [PubMed] [Google Scholar]
- Kappen, C. (2016) Developmental patterning as a quantitative trait: genetic modulation of the hoxb6 mutant skeletal phenotype. PLoS One, 11(1), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomans, J.B.A., Stolk, P.W.T., van Weeren, P.R., Vaarkamp, H. & Barneveld, A. (2007) A survey of the workload and clinical skills in current equine practices in The Netherlands. Equine Veterinary Education, 19(3), 162–168. [Google Scholar]
- Meehan, L., Dyson, S. & Murray, R. (2009) Radiographic and scintigraphic evaluation of spondylosis in the equine thoracolumbar spine: a retrospective study. Equine Veterinary Journal, 41, 800–807. [DOI] [PubMed] [Google Scholar]
- Muñoz, A., Saitua, A., Becero, M., Riber, C., Satué, K., Medina, A.S.D. et al. (2019) The use of the water treadmill for the rehabilitation of musculoskeletal injuries in the sport horse. Journal of Veterinary Research (Poland), 63(3), 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte, W., Thaller, G. & Kuehn, C. (2019) Selection signatures in four German warmblood horse breeds: tracing breeding history in the modern sport horse. PLoS One, 14(4), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki, J.L. & Burke, A.C. (2000) Hox genes and morphological identity: axial versus lateral patterning in the vertebrate mesoderm. Development, 127(19), 4265–4275. [DOI] [PubMed] [Google Scholar]
- Pasicka, E. (2013) Polish konik horse ‐ characteristics and historical background of native descendants of tarpan. Acta Scientiarum Polonorum Medicina Veterinaria, 12(2–4), 25–38. [Google Scholar]
- Pfau, T., Simons, V., Rombach, N., Stubbs, N. & Weller, R. (2017) Effect of a 4‐week elastic resistance band training regimen on back kinematics in horses trotting in‐hand and on the lunge. Equine Veterinary Journal, 49, 829–835. [DOI] [PubMed] [Google Scholar]
- Rafati, N., Andersson, L.S., Mikko, S., Feng, C., Raudsepp, T., Pettersson, J. et al. (2016) Large deletions at the SHOX locus in the pseudoautosomal region are associated with skeletal atavism in shetland ponies. G3 Genes|Genomes|Genetics, 6(7), 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdin, C., Häggström, J., Ljungvall, I., Nyman Lee, H., De Decker, S., Bertram, S. et al. (2018) Presence of thoracic and lumbar vertebral malformations in pugs with and without chronic neurological deficits. Veterinary Journal, 241, 24–30. [DOI] [PubMed] [Google Scholar]
- Stecher, R.M. (1961a) Numerical variation in the vertebrae of the prjevalsky horse. Mammalia, 25(2), 192–194. [Google Scholar]
- Stecher, R.M. (1962b) Lateral facets and lateral joints in the lumbar spine of the horse‐ a descriptive and statistical study. American Journal of Veterinary Research, 23(96), 939–947. [PubMed] [Google Scholar]
- Stecher, R.M. (1962c) Anatomical variations of the spine. Journal of Mammalogy, 43(2), 205–219. [Google Scholar]
- Stecher, R.M. & Goss, L.J. (1961) Ankylosing lesions of the spine of the horse. Journal of the American Veterinary Medical Association, 138(5), 248–255. [Google Scholar]
- Stubbs, N.C., Hodges, P.W., Jeffcott, L.B., Cowin, G., Hodgson, D.R. & Mcgowan, C.M. (2006) Functional anatomy of the caudal thoracolumbar and lumbosacral spine in the horse. Equine Veterinary Journal, 38(Suppl), 393–399. 10.1111/j.2042-3306.2006.tb05575.x [DOI] [PubMed] [Google Scholar]
- Stubbs, N.C., Riggs, C.M., Hodges, P.W., Jeffcott, L.B., Hodgson, D.R., Clayton, H.M. et al. (2010) Osseous spinal pathology and epaxial muscle ultrasonography in Thoroughbred racehorses. Equine Veterinary Journal, 42(Suppl. 38), 654–661. [DOI] [PubMed] [Google Scholar]
- Townsend, H.G.G. & Leach, D.H. (1984) Relationship between intervertebral joint morphology and mobility in the equine thoracolumbar spine. Equine Veterinary Journal, 16(5), 461–465. [DOI] [PubMed] [Google Scholar]
- VanderBroek, A., Stubbs, N.C. & Clayton, H.M. (2016) Osseous pathology of the synovial intervertebral articulations in the equine thoracolumbar spine. Journal of Equine Veterinary Science, 44, 67–73. [Google Scholar]
- Veraa, S., Bergmann, W., van den Belt, A.J., Wijnberg, I. & Back, W. (2016) Ex vivo computed tomographic evaluation of morphology variations in equine cervical vertebrae. Veterinary Radiology and Ultrasound, 57(5), 482–488. [DOI] [PubMed] [Google Scholar]
- Westworth, D.R. & Sturges, B.K. (2010) Congenital spinal malformations in small animals. Veterinary Clinics of North America ‐ Small Animal Practice, 40(5), 951–981. [DOI] [PubMed] [Google Scholar]
- Widmer, W.R. & Thrall, D.E. (2018). Canine and feline vertebrae. In: Thrall, D.E. (Ed.) Textbook of veterinary diagnostic radiology, 7th edition, Elsevier Inc. 10.1016/b978-0-323-48247-9.00026-7 [DOI] [Google Scholar]
- Zimmerman, M., Dyson, S. & Murray, R. (2011) Comparison of radiographic and scintigraphic findings of the spinous processes in the equine thoracolumbar region. Veterinary Radiology and Ultrasound, 52(6), 661–671. [DOI] [PubMed] [Google Scholar]
- Zimmerman, M., Dyson, S. & Murray, R. (2012) Close, impinging and overriding spinous processes in the thoracolumbar spine: the relationship between radiological and scintigraphic findings and clinical signs. Equine Veterinary Journal, 44(2), 178–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3


