Abbreviations
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase‐to‐platelet ratio index
- ARFI
acoustic radiation force impulse
- AST
aspartate aminotransferase
- AUD
alcohol use disorder
- AUROC
area under the receiver operating curve
- BBV
bloodborne virus
- CI
confidence interval
- ELF
Enhanced Liver Fibrosis
- FIB‐4
Fibrosis‐4
- GP
general practitioner
- LBT
liver blood test
- LFT
liver function test
- MR
magnetic resonance
- NAFLD
nonalcoholic fatty liver disease
- NFS
NAFLD Fibrosis Score
- NPV
negative predictive value
- PPV
positive predictive value
- Sens
sensitivity
- Spec
specificity
Liver disease remains a significant health burden worldwide and is increasingly driven by excess body weight and alcohol consumption.1
Morbidity and mortality related to liver disease are largely dependent on the presence of advanced fibrosis; however, in its early stages, detection may be difficult, and consequently liver disease is frequently diagnosed only when hepatic decompensation occurs.
Therefore, the early detection of advanced fibrosis is of critical importance in minimizing the rising morbidity and mortality from liver disease.
However, the traditional approach to liver disease detection has relied largely on the recognition of abnormal liver function tests (LFTs) with subsequent follow‐up serological testing to diagnose specific diseases.
This is problematic for two reasons. First, abnormal LFTs are frequently overlooked in primary care settings, and a fibrosis risk assessment is typically undertaken only in specialist hepatology clinics; second, LFTs are normal in about 20% of patients with cirrhosis. Therefore, without an appreciation in primary care of risk factors for advanced fibrosis and the poor positive predictive value (PPV) of abnormal LFTs in its identification, opportunities to recognize serious liver disease will continue to be missed.
The Challenge of Detecting Liver Disease in The Population
Although 10% to 20% of all liver blood test (LBT) panels contain at least one abnormality,2 just 3% to 5% translate into a specific or significant liver disease.3 This low “conversion rate” can lead to a false sense of reassurance that adverse outcomes are rare and explain why, in one UK study, just 50% of abnormal liver tests were ever followed up.2 These observations contribute to late diagnosis and highlight the urgent need to support primary care to find “the needles in the haystack.”
Given around one in five patients with cirrhosis has normal LFTs, fibrosis testing also should be considered in scenarios with a high pretest probability, such as the finding of fatty liver on imaging (especially in the context of type 2 diabetes mellitus), excess alcohol consumption, or unexplained splenomegaly and/or thrombocytopenia.
Any fibrosis testing approach on a population basis requires the use of a test that is cheap, readily available in a range of health care settings, accurate, and reproducible.
It remains unlikely that whole population screening is feasible, either from a cost‐effectiveness or a clinical service standpoint. Hence a more targeted, but assertive, approach to fibrosis detection is required, focusing on those at greatest risk as already outlined. A proposed initial approach is outlined in Fig. 1.
FIG 1.
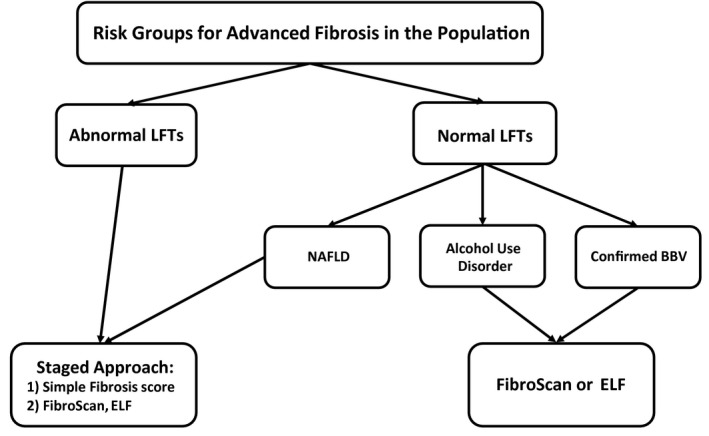
Target groups and methods of liver fibrosis screening in the population.
There remains a fine balance to be struck here between not relying on a specific liver diagnosis to be made before fibrosis assessment, yet still providing a framework to diagnose and treat specific conditions, such as viral or autoimmune hepatitis, regardless of whether advanced fibrosis is likely.
Methods of Fibrosis Assessment
When considering which fibrosis testing approach to take, the key is being able to identify these high‐risk populations and test at scale. This requires improved knowledge in primary care and robust pathways that do not require an existing liver diagnosis but help facilitate one. Information technology is also critical, in particular the ability to automate or “reflexively” undertake a fibrosis risk assessment. Building these tools into electronic clinical management systems will improve case capture and act as a positive feedback loop to further raise awareness and knowledge in primary care.
There are four major categories of fibrosis assessment, ranging from simple “scores” derived from commonly measured laboratory tests and/or clinical features to measurement of serum biomarkers of fibrosis through to measurement of liver stiffness via differing elastography methods and, finally, liver biopsy. These are summarized in Fig. 2.
FIG 2.
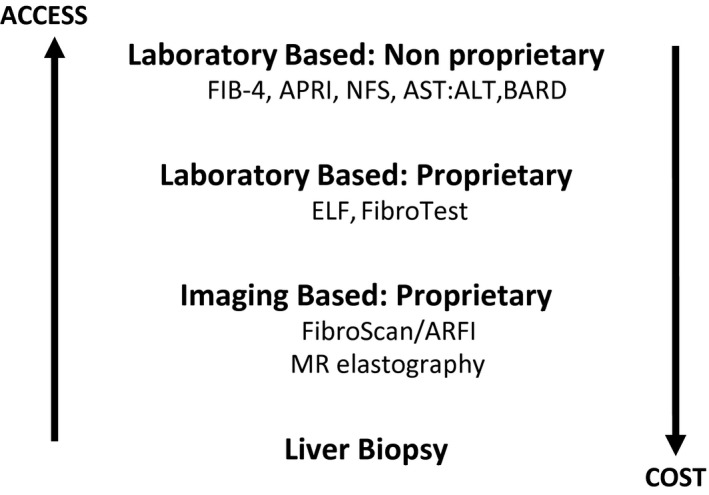
Methods of fibrosis assessment and their cost/access gradients.
Given the extremely low cost and near‐universal availability (certainly in comparison with other modalities), nonproprietary, simple fibrosis scores represent the best initial strategy.
The negative predictive value (NPV) of most of these fibrosis assessment tools in secondary care nonalcoholic fatty liver disease (NAFLD) cohorts is high at approximately 90%,4 and their performance characteristics are summarized in Table 1. In community settings, where disease prevalence is lower, the NPV is likely to be higher still. However, the major limitations of simple fibrosis scores are their poor PPV, and so they are more useful to exclude, rather than predict, advanced fibrosis.
TABLE 1.
Performance Characteristics of Different Fibrosis Tests for the Diagnosis of Advanced Fibrosis in NAFLD
| Test | AUROC (95% CI) | Cutoff | Sens (%) | Spec (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| AST:ALT ratio | 0.83 (0.74‐0.91) | 0.8 | 74 | 78 | 44 | 93 |
| 52 | 90 | 55 | 89 | |||
| APRI | 0.67 (0.54‐0.8) | 1 | 27 | 89 | 37 | 84 |
| BARD score | 0.77 (0.68‐0.87) | 2 | 89 | 44 | 27 | 95 |
| FIB‐4 score | 0.86 (0.78‐0.94) | 1.30 | 85 | 65 | 36 | 95 |
| 3.25 | 26 | 98 | 75 | 85 | ||
| NAFLD | 0.81 (0.71‐0.91) | −1.455 | 78 | 58 | 30 | 92 |
| Fibrosis score | 0.676 | 33 | 98 | 79 | 86 |
Reproduced with permission from Gut.4 Copyright 2010, British Society of Gastroenterology.
Consequently, attention has turned to two‐stage approaches, whereby the majority without fibrosis can have this reliably excluded by simple fibrosis scores, leaving more costly and/or labor‐intensive methods, such as Enhanced Liver Fibrosis (ELF) and/or FibroScan, to help confirm an initial suspicion raised by the simple score.5
Which Population Fibrosis Detection Model to Apply?
Although there are retrospective head‐to‐head comparisons of simple fibrosis scores among secondary care cohorts in specific diseases, there are none for unselected, primary care populations. Even then, any difference in performance characteristics is marginal, and all have a high NPV.4 Similarly, there is no current evidence to support any combination of tests as part of a two‐stage approach to fibrosis detection.
An important question is whether fibrosis detection strategies should be specific to different underlying etiologies of liver disease, or whether a once‐size‐fits‐all strategy can be used. In support of the former, UK guidelines recommend a different (one‐stage) approach via FibroScan for assessing fibrosis in people with an alcohol use disorder (AUD) but a different (two‐stage) approach using Fibrosis‐4 (FIB‐4) and FibroScan in NAFLD. A Danish study demonstrated that an ELF test also can reliably exclude fibrosis in AUD, but that transient elastography remained the best noninvasive predictor of fibrosis.6 In primary care patients diagnosed with NAFLD, a recent two‐stage pathway using FIB‐4 and then ELF demonstrated increased cirrhosis detection and a significant reduction in specialist referrals.7
In contrast with this disease‐specific approach, three different community‐based fibrosis detection models have recently been reported from the United Kingdom.8, 9, 10 These models are summarized in Table 2. Although heterogenous regarding entry criteria, degree of automation, and methods of fibrosis detection, they all demonstrate an ability to evaluate large populations and improve the detection of specific liver diseases and advanced fibrosis/cirrhosis compared with traditional models of care. In addition, two studies8, 9 demonstrate cost‐effectiveness. Another common theme identified in these studies is a disappointingly low engagement or compliance with the developed pathway of between 40% and 50%.9, 10 Reasons for this probably include the low profile of liver disease in primary care and a subsequent lack of understanding regarding the risk for fibrosis. Consequently, further education is required to improve population liver disease detection. This is relevant because fibrosis assessment pathways reliant on a diagnosis being made7 could act as a barrier to fibrosis detection. In contrast, those reliant on abnormal LFTs9, 10 run the risk of missing significant liver disease as exemplified by the Nottingham model, which demonstrated that 30% of the cirrhosis detected would not have been so had it been solely reliant on abnormal LFTs.8
TABLE 2.
Summary of Community‐Based Fibrosis Detection Studies
| Study | Target Group | Population Fibrosis Detection Method | Numbers Assessed/Compliance | Outcomes |
|---|---|---|---|---|
| Scarred Liver Project6 (Nottingham, England) | Population | Risk factors | 25,018 screened | 14% kPa 8‐15 |
| FibroScan | 3688 at risk | 6% kPa >15 | ||
| 1239 FibroScans | 39 new cirrhosis diagnoses | |||
| GP compliance unknown | Cost‐effective | |||
| Camden & Islington7 (London, England) | NAFLD | FIB‐4 | 1452 screened | 81% ↓ in referrals |
| ELF | 275 referred | 29% advanced fibrosis | ||
| GP compliance 55% | 14.5% cirrhosis | |||
| Intelligent Liver Function8 (Dundee, Scotland) | Population | Abnormal LBT | 229 intervention group | 45% ↑ in diagnosis |
| FIB‐4 | 64 with abnormal LBTs | Cost‐effective | ||
| FibroScan | GP compliance 50% | Suggests a diagnosis | ||
| Gwent AST Project9 (Gwent, Wales) | Population | Abnormal ALT | 17,770 abnormal ALT | 28% kPa 8‐15 |
| Reflex AST:ALT ratio | 2117 AST:ALT > 1 (12%) | 29% kPa >15 | ||
| FibroScan | 348 FibroScans | 192 advanced fibrosis | ||
| GP compliance 40% | 81% ↑ in cirrhosis diagnosis |
Summary
Assessment of fibrosis is critical in identifying those with significant liver disease in the population via clear pathways, those with abnormal liver tests, those with fatty liver, and/or those with excess alcohol consumption. Incorporation of these pathways, or at least the fibrosis aspects of them, into clinical management systems can facilitate recognition of specific diseases, as well as “reflex” calculation of simple fibrosis scores.
Engagement with primary care is also vital to bridge the primary care‐hospital provider gap and improve adherence to pathways, which is low in published series.7, 8, 9, 10
No one fibrosis test or combination thereof is perfect, or superior to another, and all have their merits and demerits. Although recent data suggest the aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio is not as accurate as FIB‐4, again this comes from a secondary care NAFLD cohort,11 and comparative data of the effectiveness of differing components of population fibrosis detection strategies are unlikely to be available in the short term. In contrast, data from Gwent10 show that use of FIB‐4 on an unselected population leads to a 4‐fold increase in FibroScan requirements using a threshold of >1.3 and a 2‐fold increase if >3.25 is used.
Despite these challenges, consistent implementation, at scale, using any of the cheap and widely available simple fibrosis scores as the first step followed by FibroScan or ELF will improve the diagnosis of advanced liver disease in those with abnormal LFTs or NAFLD compared with traditional models of care. However, models should not be reliant just on those with abnormal LFTs, and risk factor analysis followed by FibroScan or ELF testing will further improve population detection rates.
The choice of fibrosis test (or combination thereof) also should be based on local factors: prevalence of differing liver diseases, clinical expertise, geographical and capacity constraints, availability of technology, and patient wishes. Finally, iteration of pathways is necessary to constantly refine approaches designed to meet the challenge of identifying significant liver disease in the general population.
Potential conflict of interest: A.D.Y. consults for, advises, and is on the speakers’ bureau for Norgine Pharmaceuticals. He advises and is on the speakers’ bureau for Falk Pharma. He consults for and advises Intercept.
REFERENCES
- 1.Asrani S, Devarbhavi H, Eaton S, et al. Burden of liver diseases worldwide. J Hepatol 2019;70:151‐171. [DOI] [PubMed] [Google Scholar]
- 2.Donnan PT, McLernon D, Dillon JF, et al. Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record‐linkage population cohort study and decision analysis (ALFIE). Health Technol Assess 2009;13:iii‐iv, ix‐xi, 1‐134. [DOI] [PubMed] [Google Scholar]
- 3.Lilford RJ, Bentham L, Girling A, et al. Birmingham and Lambeth Liver Evaluation Testing Strategies (BALLETS): A Prospective Cohort Study. Health Technol Assess 2013;17:i‐xiv, 1‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPherson S, Stewart SF, Henderson E, et al. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- 5.Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut 2018;67:6‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiele M, Madsen BS, Hansen JF, et al. Accuracy of the enhanced liver fibrosis test vs Fibrotest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 2018;154:1369‐1379. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non‐alcoholic fatty liver disease. J Hepatol 2019;71:371‐378. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers J, Wilkes E, Harris R, et al. The development and Implementation of a commissioned pathway for the identification and stratification of liver disease in the community. Frontline Gastroenterol 2020;11:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillon JF, Miller MH, Robinson EM, et al. Intelligent liver function testing (iLFT): a trial of automated diagnosis and staging of liver disease in primary care. J Hepatol 2019;71:699‐706. [DOI] [PubMed] [Google Scholar]
- 10.Yeoman AD, Samuel D, Yousuf F, et al. Introduction of “reflex” AST testing in primary care increases detection of advanced liver disease: The Gwent AST project (GAP). J Hepatol 2020;73:S19. [Google Scholar]
- 11.McPherson S, Hardy T, Dufour J‐F, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2016;112:740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]


