Abstract
Background: Sodium bicarbonate supplementation is a mainstay in the treatment of metabolic acidosis in patients with chronic kidney disease (CKD). Recent studies showed reduction of progression of CKD and reduced all-cause mortality. However, additional sodium loading could worsen arterial hypertension, a well-known contributor to progression of CKD. This patient-relevant and economically negative side effect is under-studied in prospective studies up until now.
Objective: The aim of this study was to analyze the effect of sodium bicarbonate treatment on arterial blood pressure at baseline and after 8 weeks.
Methods: The SoBic study is an ongoing randomized controlled trial, in which patients with CKD receive either a high dose of oral sodium bicarbonate or a rescue treatment, if necessary. We used standardized office blood pressure and 24-hour ambulatory blood pressure monitoring (24h-ABPM). Regression models were adjusted for estimated glomerular filtration rate and change of antihypertensives.
Results: 47 subjects were enrolled and the mean age was 57 (±14.6) years and 18 (38%) were female. In 43 randomized subjects with sufficiently performed 24h-ABPM neither systolic 24h-ABPM (2.522; 95%CI: −2.364, 7.408; mmHg) nor diastolic 24h-ABPM (0.868; 95%CI: −2.411, 4.147; mmHg) was affected by study group allocation. When looking at the effect of individual sodium bicarbonate dose on 24h-ABPM, the fully adjusted model suggested an increase of 0.047 (95%CI: −0.026, 0.119) mmHg by each mg/kg per day increase of sodium bicarbonate dose.
Conclusion: Sodium bicarbonate supplementation over 8 weeks did not significantly increase blood pressure measured by 24h-ABPM in CKD patients.
Trial Registration: EUDRACT Number: 2012-001824-36; 12/07/2012 (https://www.clinicaltrialsregister.eu).
Keywords: metabolic acidosis, sodium bicarbonate, blood pressure, 24-hour ambulatory blood pressure measurement, chronic kidney disease
Introduction
Metabolic acidosis regularly accompanies chronic kidney disease (CKD) and on long-term enhances several detrimental pathways leading to nephron loss and renal fibrosis. Evidence now exists showing that supplementing alkali can halt progression of CKD and more importantly can reduce all-cause mortality (1–3). A recent meta-analysis underscored these benefits, but also reported worsening of arterial hypertension or the requirement of increased antihypertensive therapy (4), another well-established contributor to progression of CKD. Sodium bicarbonate, the most frequently used alkaline supplement, results in substantial sodium loading in addition to alimentary sodium intake. Recently Bushinsky (5) discussed the adverse effect of sodium loading accompanied by alkali supplementation on arterial blood pressure and raised caution. This patient-relevant and economically negative side effect is under-studied in prospective studies up until now.
The SoBic study is an ongoing randomized controlled trial, in which patients with CKD receive either a high dose of oral sodium bicarbonate or a rescue dose, if necessary. The study protocol includes standardized office blood pressure (BP) measurements and 24-hour ambulatory blood pressure monitoring (24h-ABPM), the gold standard of BP monitoring. The aim of the study was to analyze the effect of sodium bicarbonate loading on arterial BP assessed by 24h-ABPM at baseline and after 8 weeks of sodium bicarbonate treatment. We hypothesized that sodium bicarbonate treatment has a dose-dependent effect on change in arterial BP from baseline to week 8.
Materials and Methods
Study Design and Participants
The SoBic study is a single-center, randomized controlled trial performed at the Nephrological outpatient service of the Medical University of Vienna. The study is described in detail elsewhere (6). In brief, patients with CKD stage G3 and G4 and chronic metabolic acidosis (serum ≤21 mmol/L) were randomized to either receive a high dose of oral sodium bicarbonate with a serum target level of 24 ± 1 mmol/L (intervention group) or receive a rescue therapy of sodium bicarbonate, if necessary, with a serum target level of 20 ± 1 mmol/L (rescue group). Study personnel and subjects were aware of the randomization assignment. Inclusion criteria were age over 18 years, renal function (measured by estimated glomerular filtration rate (eGFR) calculated by the four-variable Modification of Diet in Renal Disease (MDRD) study equation) between 60 and 15 mL/min per 1.73 m2, a venous serum of ≤21 mmol/L on two consecutive measurements (at least 1 day apart), and stable clinical condition of the patient. Exclusion criteria were malignant disease or <5 years of successful treatment of malignant disease (except dermal malignancies and carcinoma in situ of the cervix declared to be cured), patients with morbid obesity (body mass index >40 kg/m2), chronic inflammation (C reactive protein >10 mg/dL), immunosuppressive therapy of any kind, poorly controlled blood pressure (>150/90 mmHg despite the use of four agents), overt congestive heart failure, or known peanut or soy allergy (as given by the manufacturer of the study medication).
All methods were carried out in accordance with relevant guidelines and regulations.
The ethics committee of the Medical University of Vienna approved the study (EK Number: 1331/2012), the trial was registered at the EU registry for clinical studies (EUDRACT Number: 2012-001824-36; 12/07/2012; https://www.clinicaltrialsregister.eu), and all subjects gave written informed consent.
For the present study we included all subjects randomized until March 2019. Patients had office BP measurements at each visit and a 24h-ABPM at baseline and after 8 weeks. We hypothesized that sodium bicarbonate treatment has a dose-dependent effect on systolic (SBP) and/or diastolic BP (DBP). First, we compared change of mean 24h-ABPM (overall, day-time, and night-time) from baseline to week 8 between the intervention and the rescue group. Because the design of the SoBic study allows rescue sodium bicarbonate treatment in controls if necessary, we second investigated the change in 24h-ABPM from baseline to week 8 with respect to individual sodium bicarbonate dose (mg) per kilogram (kg) body weight per day. Third, we estimated the change in 24h-ABPM from baseline to week 8 with respect to low dose, moderate dose, and high sodium bicarbonate dose vs. no treatment independent of randomization.
The primary outcome was change in arterial BP (SBP and DBP) at week 8 compared to baseline. We defined that a mean change of equal or >7 mmHg in SBP (overall systolic 24h-ABPM) between baseline and week 8 is patient-relevant, as this represents the average SBP-lowering effect of approximately one antihypertensive agent (7). With a defined power of 80% and a type-1 error of 5% we would have to enroll 18 patients or more per group to be able to observe a change of 7 mmHg, if the null hypothesis was true.
Laboratory Measurements and Clinical Assessment
Serum creatinine was determined by means of the Jaffe method (IDMS-traceable, reference range <1.2 mg/dL, mg/dL × 88.4 = μmol/L). Bicarbonate measurements were performed in venous blood samples and determined by an ABL 700 Copenhagen Blood Gas Analyzer (Radiometer Copenhagen, Copenhagen, Denmark). Serum and urine electrolytes were assessed as detailed elsewhere (6). Urine pH has been measured by dipstick (5, 5.5, 6, 6.5, 7) analyzed by a validated device.
Body weight was measured on a validated scale each visit and presence of edema (yes/no) or worsening of present edema (yes/no) was assessed by an experienced clinician. Change in concomitant medication, especially antihypertensives and diuretics, was monitored at each visit. Number of antihypertensives is given on average (total number of antihypertensives/N) overall and per type of antihypertensive. Initiation of an additional antihypertensive/diuretic or increase of dosage is presented as “increase in antihypertensives/diuretics” (yes/no). Decrease of dose or cessation of an antihypertensive is presented as “decrease in antihypertensives/diuretics” (yes/no).
Sodium Bicarbonate
Study medication was supplied as sodium bicarbonate capsules à 840 mg in an extended-release product (Nephrotrans 840 mg, Medice Arzneimittel Pütter GmbH & Co. Iserlohn, Germany). One capsule contained 840 mg sodium bicarbonate (NaHCO3; 10.08 meq) and includes 230.16 mg of sodium. At each visit dosage could be modified according to the treatment algorithm and allocated study group, as detailed in Gaggl et al. (6) Compliance was measured by pill count each visit. The presented dose is the reported medication taken during the last 7 days prior to the visit at week 8, ideally as prescribed at visit 4 by the investigator. Sodium bicarbonate dose was presented as mg per kg body weight (at baseline) per day and as mg per day (effective dose). For further analysis we categorized subjects into treatment categories: no treatment, low dose (1–37.9 mg/kg/day), moderate dose (38–79.9 mg/kg/day), and high dose (≥80 mg/kg/day) of sodium bicarbonate. Boundaries for treatment categories were chosen to reflect the size of the effects expected across the range of exposure (8).
Blood Pressure Measurements
Office Blood Pressure Measurements
At each visit office BP was taken by a trained study nurse using a Boso medicus uno (Bosch + Sohn GmbH u. Co. KG, Jungingen, Germany) device. Measurements were taken 3 times at least 1 min apart after a rest of at least 10 min in a sitting position. The mean SBP and DBP value, respectively, of the 3 measurements was reported (mmHg). Measurements are summarized as mean SBP (mSBP) and DBP (mDBP) overall and for respective study groups. Differences in SBP and DBP were calculated by subtracting the baseline from week 8 measurement (mean Δ office BP = mean office BPweek8 – mean office BPbaseline).
24-Hour Ambulatory Blood Pressure Measurements
Twenty Four hours-ABPM was measured by oscillometry using a Mobil-O-Graph NG (I.E.A. GmbH, Stolberg, Germany) device at 15-min interval during the daytime (between 8:00–21:59) and 30-min interval during nighttime (between 22:00–7:59). Measurements were performed according to the guidelines of the European Society of Hypertension (9). We defined 24h-ABPM valid if more than 23 measurements during daytime and 11 during nighttime (random draw) were successful. A recent validation study showed that those are the minimum number of measurements needed to achieve a precision of ±2.5 mmHg in blood pressure monitoring (10). Total number of measurements was summarized and the overall (24h-ABPM), daytime (daytime-ABPM) and nighttime mean (nighttime-ABPM) and standard deviation (±SD) were calculated. Measurements are summarized as mean systolic and diastolic 24h-ABPM overall and for respective study groups. Differences in means were calculated by subtracting the baseline from week 8 measurement (mean Δ 24h-ABPM = mean 24h-ABPM week8 – mean 24h-ABPM baseline).
Statistical Analysis
Descriptive statistics are given in count (percent), mean (± standard deviation, SD), or median (interquartile range, IQR). Descriptive data is depicted by means of dot plots. Student's t-test for paired and unpaired groups were performed, as well as analysis of variances (ANOVA) to compare means across groups. To compare difference in mean Δ office BP and mean Δ 24h-ABPM we calculated unpaired t-tests. For not normally distributed data non-parametric tests were used to compare medians.
To assess change in blood pressure, first, we performed linear regression with respect to study group randomization (reference: rescue group; crude) adjusted for change of eGFR (mL/min per 1.73 m2; adjusted I), and increase of antihypertensive medication (reference: no change), and decrease of antihypertensive medication (reference: no change; adjusted II).
Second, we regressed individual sodium bicarbonate dose (mg/kg per day) on change of BP, adjusted as described above.
Third, dose dependent effects on change in BP were estimated by using treatment categories low, moderate, and high dose (reference: no treatment) in crude and adjusted models as given above.
All results are presented as point estimates and corresponding 95% confidence intervals (95%CI), and all results with a p-value lower than 0.05 were considered statistically significant. All statistical analysis was performed with R (R Core Team, 2016. “R: A language and environment for statistical computing”; R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
Results
Participants and Sodium Bicarbonate Dose
Until March 2019 47 subjects were enrolled in the SoBic study and had completed week 8 of the study protocol (Figure 1A). The mean age was 57 (±14.6) years and 18 (38%) were females. Ninety-six percent had a diagnosis of arterial hypertension at baseline [median time: 5 (IQR: 3–15) years] and 34% had diabetes [median time: 5.5 (IQR: 2.25–11) years]. Population demographics at baseline are given in Table 1A. No significant differences were observed at baseline across groups. Twenty-four patients were randomized to the intervention group and received a median daily dose of 63 mg/kg per day (IQR: 50–88) or an effective median dose of 5,040 mg/day (IQR: 4,200–6,930) at week 8. Of the 23 patients of the rescue group 10 (44%) received sodium bicarbonate (median sodium bicarbonate dose 21 mg/kg per day, IQR: 19–31; effective median dose 1,680 mg per day, IQR: 1,680–2,520) at week 8. Overall, 13 patients did not take sodium bicarbonate, 15 took a low dose (median: 24.71 mg/kg per day, IQR: 20.12–31.17/2,520 mg/day, IQR: 1,680–2,296), 10 a moderate dose (median: 59.94 mg/kg per day, IQR: 57.72–63/5,040 mg/day, IQR: 4,200–5,040), and 9 a high dose (median: 89.09 mg/kg per day, IQR: 87.91–99.47/6,720 mg/day, IQR: 5,880–7,560) at week 8 (Table 1B).
Figure 1.
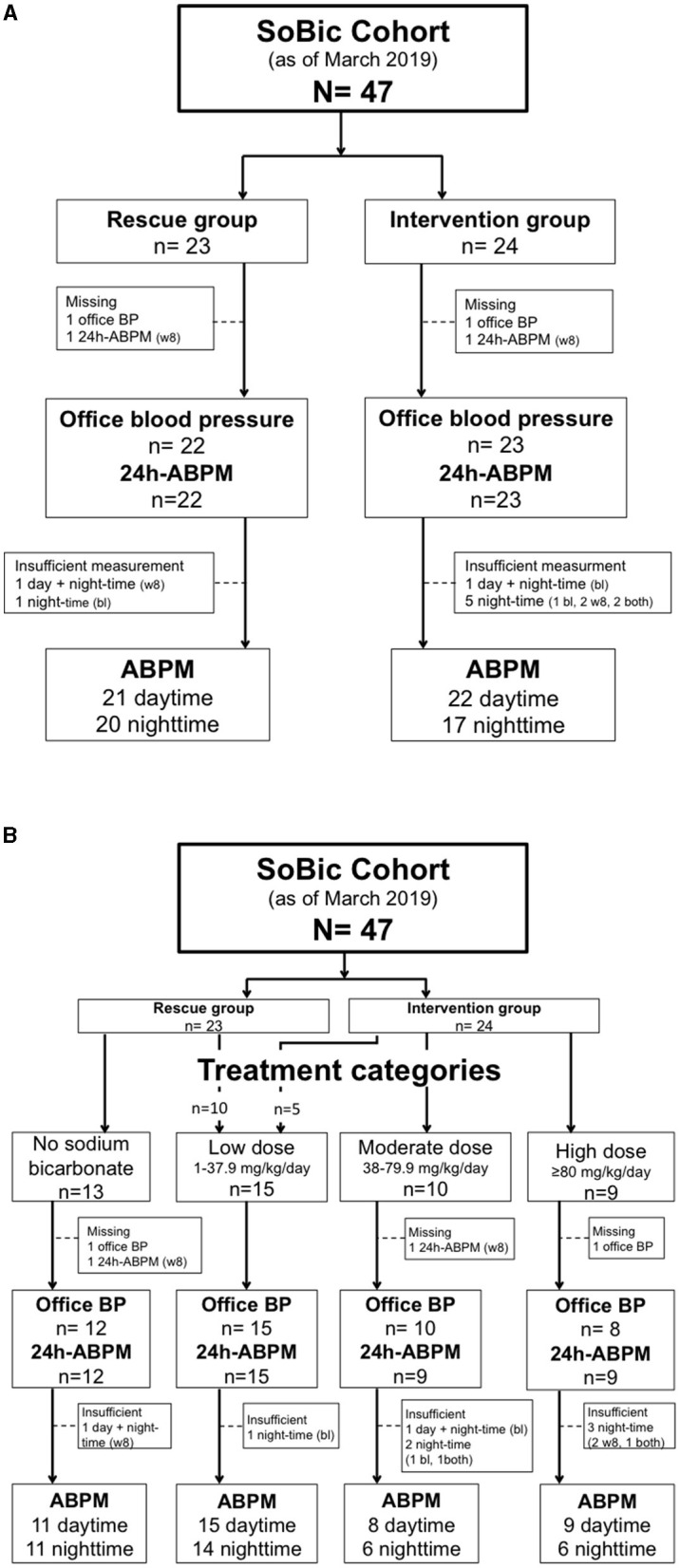
Patient flow (A) according to study group and (B) according to treatment categories. 24h-ABPM, 24-h blood pressure monitoring; bl, baseline measurement; w8, week 8 measurement.
Table 1A.
Basic demographic data at baseline [count (percent) and mean (standard deviation), respectively] with respect to study groups.
| Rescue group | Intervention group | |
|---|---|---|
| N | 23 (49%) | 24 (51%) |
| Sex (female) | 9 (39%) | 9 (38%) |
| Age (years) | 56 (±16.5) | 58 (±12.8) |
| BMI (kg/m2) | 27.4 (±4.26) | 28.8 (±6.22) |
| eGFR (mL/min per 1.73 m2) | 27 (±9.7) | 25.33 (±7.5) |
| Serum (mmol/L) | 19.2 (±1.5) | 18.8 (±1.8) |
| Urine sodium (mmol/24 h) | 186 (±88) | 190 (±79) |
| Urine pH | 5.5 (±1.1) | 5.3 (±0.54) |
| Number of antihypertensives | 64 (2.8 per subject) | 48 (2.0 per subject) |
| Type of antihypertensive | ||
| Diuretics | 15 (65%) | 8 (33%) |
| Calcium antagonist | 11 (48%) | 10 (42%) |
| ACEi/ARB | 19 (83%) | 18 (75%) |
| Beta blocker | 10 (45%) | 8 (33%) |
| Others | 9 (39%) | 4 (17%) |
| Underlying renal disease | ||
| Glomerulopathy | 7 (30%) | 5 (21%) |
| Vascular nephropathy | 7 (30%) | 4 (17%) |
| Diabetic nephropathy | 6 (26%) | 6 (25%) |
| Others | 3 (13%) | 9 (38%) |
| Comorbidities | ||
| Arterial hypertension | 22 (96%) | 23 (96%) |
| Diabetes | 7 (30%) | 9 (38 %) |
| Cardiovascular disease | 3 (13%) | 4 (17%) |
| Congestive heart failure | 3 (13%) | 1 (4%) |
| Peripheral occlusive disease | 4 (17%) | 3 (13%) |
| COPD/Asthma | 4 (17%) | 0 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease.
Table 1B.
Basic demographic data at baseline [count (percent), mean (standard deviation)] with respect to treatment category.
| Treatment category sodium bicarbonate (mg/kg per day) | |||||
|---|---|---|---|---|---|
| None | 1–37.9 | 38–79 | ≥80 | p-value | |
| N | 13 | 15 | 10 | 9 | |
| Study group | |||||
| Intervention | 0 | 5 | 10 | 9 | |
| Rescue | 13 | 10 | 0 | 0 | |
| Sex (female) | 5 (39%) | 6 (40%) | 1 (10%) | 6 (67%) | 0.092 |
| Age (years) | 56 (±17.3) | 58 (±15) | 61 (±8.8) | 53 (±16) | 0.741 |
| BMI (kg/m2) | 26 (±4.5) | 31 (±5.4) | 27 (±7) | 27 (±2.8) | 0.083 |
| eGFR (mL/min per 1.73 m2) | 30.3 (±10.3) | 24 (±7.2) | 25 (±9.1) | 25 (±6.7) | 0.338 |
| Serum (mmol/L) | 20.2 (±0.9) | 18.6 (±1.4) | 19 (±1.1) | 17.9 (±2.3) | 0.007 |
| Urine sodium (mmol/24 h) | 207 (±95) | 167 (±79) | 201 (±82) | 181 (±75) | 0.766 |
| Urine pH | 5.7 (1.34) | 5.2 (±0.5) | 5.2 (±0.3) | 5.6 (±0.7) | 0.281 |
| Number of antihypertensives | 35 (2.7 per subject) | 41 (2.7 per subject) | 21 (2.1 per subject) | 15 (1.7 per subject) | 0.001 |
| Type of antihypertensive | |||||
| Diuretics | 8 (62%) | 9 (60%) | 4 (40%) | 2 (22%) | |
| Calcium antagonist | 6 (46%) | 8 (53%) | 4 (40%) | 3 (33%) | |
| ACEi/ARB | 11 (85%) | 12 (80%) | 7 (70%) | 7 (78%) | |
| Beta blocker | 5 (39%) | 8 (53%) | 3 (30%) | 2 (22%) | |
| Others | 5 (39%) | 4 (27%) | 3 (30%) | 1 (11%) | 0.994 |
| Underlying renal disease | |||||
| Glomerulopathy | 4 (31%) | 3 (20%) | 1 (10%) | 4 (44%) | |
| Vascular nephropathy | 5 (39%) | 2 (13%) | 3 (30%) | 1 (11%) | |
| Diabetic nephropathy | 3 (23%) | 5 (33%) | 4 (40%) | 0 | |
| Others | 1 (8%) | 5 (33%) | 2 (20%) | 4 (44%) | 0.200 |
| Comorbidities | |||||
| Arterial hypertension | 12 (92%) | 15 (100%) | 10 (100%) | 8 (89%) | 0.436 |
| Diabetes | 4 (31%) | 4 (27%) | 8 (80%) | 0 | 0.002 |
| Cardiovascular disease | 2 (15%) | 2 (13%) | 3 (30%) | 0 | 0.389 |
| Congestive heart failure | 2 (15%) | 1 (7%) | 1 (10%) | 0 | 0.756 |
| Peripheral occlusive disease | 2 (15%) | 2 (13%) | 2 (20%) | 1 (11%) | 1.000 |
| COPD/Asthma | 2 (15%) | 2 (13%) | 0 | 0 | 0.529 |
Statistical testing was done by means of the Kruskal-Wallis rank sum test for continuous data and by the Fisher Exact test for categorical data.
BMI, body mass index; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease.
Effect of Sodium Bicarbonate on Blood Pressure
Mean office SBP and DBP at baseline were 129 (±16) and 80 (±13) mmHg and was higher in the intervention group at baseline (Δ SBP: 10 mmHg, Δ DBP: 8 mmHg; Table 2). Similar results were observed using 24h-ABPM, but the differences were less distinct (Δ systolic 24h-ABPM: 3 mmHg, Δ diastolic 24h-ABPM: 4 mmHg).
Table 2A.
Mean office and 24h-ambulatory blood pressure measurements (±SD, mmHg) with respect to study group and point in time, and mean blood pressure change (95%CI, mmHg).
| Rescue group | Intervention group | Differences between groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Week 8 | Change (95% CI) | n | Baseline | Week 8 | Change (95% CI) | p-value | |
| 24h-ABPM | 21 | 22 | |||||||
| Systolic | 122 ± 11.7 | 124 ± 11.6 | 2.1 (−0.79 to 4.90) | 125 ± 9.5 | 129 ± 11.7 | 4.58 (0.30 to 8.86) | 0.314 | ||
| Diastolic | 76 ± 6.8 | 77 ± 6.8 | 1.11 (−1.18 to 3.4) | 80 ± 6.2 | 82 ± 5.8 | 2.18 (−0.12 to 4.48) | 0.606 | ||
| Daytime-ABPM | 21 | 22 | |||||||
| Systolic | 123 ± 12.4 | 125 ± 10.2 | 2.1 (−1.12 to 5.24) | 126 ± 10.0 | 130 ± 11.4 | 3.88 (−0.62 to 8.37) | 0.500 | ||
| Diastolic | 77 ± 7.0 | 78 ± 6.8 | 1.5 (−1.0 to 3.95) | 81 ± 6.5 | 83 ± 6.3 | 2.46 (−1.9 to 5.10) | 0.577 | ||
| Nighttime-ABPM | 19 | 17 | |||||||
| Systolic | 119 ± 12.2 | 120 ± 14.5 | 1.49 (−1.65 to 4.63) | 119 ± 10.7 | 125 ± 14.7 | 5.72 (0.75 to 10.70) | 0.140 | ||
| Diastolic | 73 ± 8.7 | 74 ± 9.1 | 0.70 (−1.75 to 3.15) | 75 ± 7.8 | 77 ± 7.3 | 2.17 (−0.78 to 5.12) | 0.425 | ||
| Office BP | 22 | 23 | |||||||
| Systolic | 124 ± 17.0 | 132 ± 16.1 | 8.64 (1.00 to 16.28) | 134 ± 12.6 | 135 ± 16.1 | 1.35 (−4.31 to 7.01) | 0.119 | ||
| Diastolic | 76 ± 14.8 | 80 ± 12.0 | 6.36 (2.68 to 10.05) | 84 ± 10.1 | 81 ± 15.0 | −1.83 (−5.88 to 2.23) | 0.003 | ||
Missings: 24h- and daytime-ABPM 1 rescue, 1 intervention group; Nighttime-ABPM: 1 rescue, 5 intervention group; Office BP; 1 rescue, 1 intervention group.
Change between baseline and week 8 was tested by a paired t-test, difference in change across groups was tested by an unpaired t-test.
24h-ABPM, 24 h ambulatory blood pressure monitoring; Daytime ABPM, from 8:00 until 22:00 ambulatory blood pressure monitoring; Nighttime ABPM, from 22:01 until 7:59 ambulatory blood pressure monitoring; Office BP, office blood pressure measurement; 95% CI, 95 % confidence interval.
At baseline on average 2.4 antihypertensives were prescribed per subject, which was higher in the rescue group compared to the intervention group (2.8 vs. 2.0; Table 1).
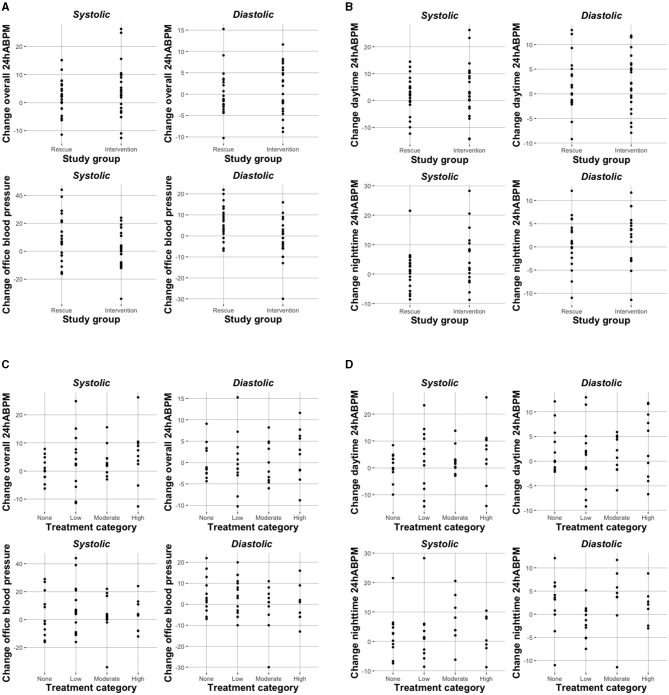
Overall, we observed an increase in mean SBP and DBP from baseline to week 8 independent of study group (except for Δ office DBP intervention group), but this was statistically significant only for the intervention group for 24h-ABPM (Δ: 4.58, 95%CI: 0.30–8.86, mmHg) and systolic nighttime-ABPM (Δ: 5.72, 95%CI: 0.75, 10.70; mmHg), respectively (Table 2A). When looking at different treatment categories a similar trend toward increase in SBP and DBP was observed (Table 2B), with an exception for office DBP for moderate (Δ: −1.70, 95%CI: −10.02, 6.62; mmHg) and high treatment categories (Δ: −0.13, 95%CI: −7.86, 7.61; mmHg), and for diastolic nighttime-ABPM in the low sodium bicarbonate group (Δ: −1.39, 95%CI: −4.33, 1.54; mmHg). Individual change of blood pressure with respect to study group and treatment category are depicted in Figures 2A,B.
Table 2B.
Mean office and 24h-ambulatory blood pressure measurements (± SD, mmHg) with respect to treatment categories and point in time and mean blood pressure change (95%CI, mmHg).
| Treatment category | Systolic blood pressure | Diastolic blood pressure | |||||
|---|---|---|---|---|---|---|---|
| n | Baseline | Week 8 | Change (95% CI) | Baseline | Week 8 | Change (95% CI) | |
| 24h-ABPM | |||||||
| None | 11 | 121 ± 12.1 | 122 ± 11 | 0.72 (−2.27 to 3.71) | 75 ± 3.8 | 77 ± 5.9 | 1.71 (−1.38 to 4.81) |
| Low | 15 | 124 ± 9.9 | 127 ± 12.5 | 3.03 (−2.27 to 8.34) | 78 ± 7.8 | 79 ± 7.3 | 0.4 (−2.53 to 3.33) |
| Moderate | 8 | 128 ± 8.7 | 134 ± 12.4 | 6.31 (−3.26 to 15.88) | 80 ± 6.1 | 83 ± 7.4 | 3.7 (−0.53 to 8.01) |
| High | 9 | 120 ± 11.1 | 124 ± 9.1 | 4.44 (0.17 to 8.71) | 80 ± 7.8 | 81 ± 4.7 | 1.83 (−2.35 to 6.01) |
| p-value | 0.214 | p-value | 0.488 | ||||
| Daytime-ABPM | |||||||
| None | 11 | 123 ± 13.4 | 124 ± 9.7 | 0.72 (−2.81 to 4.24) | 76 ± 3.6 | 79 ± 6.3 | 2.35 (−0.92 to 5.62) |
| Low | 15 | 125 ± 10.4 | 128 ± 11.2 | 2.69 (−2.96 to 8.34) | 79 ± 8.2 | 80 ± 7.9 | 0.96 (−2.48 to 4.4) |
| Moderate | 8 | 130 ± 10.7 | 135 ± 13.2 | 4.78 (−5.21 to 14.78) | 81 ± 7.7 | 84 ± 7.5 | 2.75 (−1.85 to 7.36) |
| High | 9 | 121 ± 9.9 | 125 ± 8.1 | 4.67 (−0.23 to 9.57) | 80 ± 7.5 | 83 ± 5.2 | 2.53 (−2.42 to 7.48) |
| p-value | 0.261 | p-value | 0.768 | ||||
| Nighttime-ABPM | |||||||
| None | 11 | 117 ± 11.4 | 119 ± 14.1 | 2.45 (−2.8 to 7.71) | 70 ± 8 | 73 ± 7.7 | 2.53 (−1.55 to 6.61) |
| Low | 14 | 121 ± 10.9 | 123 ± 15.8 | 1.96 (−3.18 to 7.10) | 76 ± 8.3 | 75 ± 8.5 | −1.39 (−4.33 to 1.54) |
| Moderate | 6 | 124 ± 8.9 | 133 ± 12.6 | 8.18 (−2.77 to 19.13) | 75 ± 7.3 | 80 ± 9.7 | 4.39 (−0.56 to 9.35) |
| High | 6 | 112 ± 11.2 | 116 ± 11.2 | 3.95 (−1.87 to 9.76) | 73 ± 8.7 | 76 ± 7.8 | 2.68 (−1.18 to 6.55) |
| p-value | 0.396 | p-value | 0.547 | ||||
| Office BP | |||||||
| None | 12 | 129 ± 14.9 | 131 ± 16.5 | 3.42 (−6.62 to 13.45) | 78 ± 17.3 | 79 ± 14.9 | 4.58 (−1.18 to 10.35) |
| Low | 15 | 125 ± 18.3 | 133 ± 13.5 | 8.07 (−1.68 to 17.82) | 76 ± 11.2 | 81 ± 7.8 | 4.07 (−0.84 to 8.98) |
| Moderate | 10 | 136 ± 9.4 | 139 ± 15.7 | 3.2 (−8.04 to 14.44) | 82 ± 6.7 | 81 ± 14.5 | −1.7 (−10.02 to 6.62) |
| High | 8 | 131 ± 16.6 | 132 ± 21 | 3.38 (−6.97 to 13.72) | 87 ± 13.7 | 84 ± 19.2 | −0.13 (−7.86 to 7.61) |
| p-value | 0.831 | p-value | 0.128 | ||||
Missings: 24h-ABPM and daytime-ABPM 2 none, 2 moderate; Nighttime-ABPM: 2 none, 4 moderate, 2 high; Office BP 1 none, 1 high.
Change between baseline and week 8 for respective treatment category was tested by a paired t-test, difference in change across treatment categories was tested by an analysis of variances (ANOVA).
Treatment category: none, no sodium bicarbonate: low (1–37.9 mg/kg/day), moderate (38–79.9 mg/kg/day), high (≥80 mg/kg/day).
24h-ABPM, 24 h ambulatory blood pressure monitoring; Daytime ABPM, from 8:00 until 22:00 ambulatory blood pressure monitoring; Nighttime ABPM, from 22:01 until 7:59 ambulatory blood pressure monitoring; Office BP, office blood pressure measurement; 95% CI, 95% confidence interval.
Figure 2.
Change in blood pressure (mmHg) with respect to study group (A,B) and treatment category (C,D).
In all 43 randomized subjects with sufficient 24h-ABPM neither systolic 24h-ABPM (2.522; 95%CI: −2.364, 7.408; mmHg) nor diastolic 24h-ABPM (0.868; 95%CI: −2.411, 4.147; mmHg) was affected by study group allocation (Table 3A). Adjustment for change of estimated glomerular filtration rate and change of antihypertensive medication did only slightly change estimates. In contrast, office BP measurements showed a decrease in BP for subjects being randomized to the intervention group (SBP: −8.241, 95%CI: −17.808, 1.327; DBP: −8.94, 95%CI: −14.458, −3.421; mmHg), however, only statistically significant for DBP.
Table 3A.
Association of systolic and diastolic blood pressure change (mmHg) with respect to study group.
| Regression models | |||||||
|---|---|---|---|---|---|---|---|
| Crude | Adjusted I | Adjusted II | |||||
| n | β | 95% CI | β | 95% CI | β | 95% CI | |
| Δ 24h-ABPM | 43 | ||||||
| Systolic | 2.522 | −2.364 to 7.408 | 2.585 | −2.361 to 7.532 | 2.632 | −2.515 to 7.800 | |
| Diastolic | 0.868 | −2.411 to 4.147 | 0.856 | −2.469 to 4.182 | 0.797 | −2.658 to 4.252 | |
| Δ Daytime-ABPM | 43 | ||||||
| Systolic | 1.816 | −3.411 to 7.042 | 1.905 | −3.379 to 7.189 | 2.119 | −3.362 to 7.599 | |
| Diastolic | 0.976 | −2.437 to 4.388 | 0.918 | −2.532 to 4.368 | 0.925 | −2.654 to 4.504 | |
| Δ Nighttime-ABPM | 37 | ||||||
| Systolic | 4.231 | −1.069 to 9.531 | 4.146 | −1.238 to 9.529 | 4.018 | −1.496 to 9.531 | |
| Diastolic | 1.470 | −2.069 to 5.009 | 1.214 | −2.230 to 4.659 | 1.303 | −2191 to 4.797 | |
| Δ Office BP | 45 | ||||||
| Systolic | −7.289 | −16.203 to 1.626 | −7.296 | −16.344 to 1.751 | −8.241 | −17.808 to 1.327 | |
| Diastolic | −8.19 | −13.375 to −3.004 | −8.35 | −13.577 to −3.124 | −8.94 | −14.458 to −3.421 | |
Crude, intervention group (target 24 ± 1 mmol/L) vs. rescue group [reference; group (target 20 ± 1 mmol/L)].
Adjusted I, Crude + change of eGFR from baseline to week 8 (mL/min per 1.73 m2).
Adjusted II, Adjusted I + increase and decrease of antihypertensive medication vs. no change of antihypertensive medication.
24h-ABPM, 24 h ambulatory blood pressure monitoring; Daytime ABPM, from 8:00 until 22:00 ambulatory blood pressure monitoring; Nighttime ABPM, from 22:01 until 7:59 ambulatory blood pressure monitoring; Office BP, office blood pressure measurement; β, point estimate (mmHg); 95% CI, 95% confidence interval.
When looking at the effect of individual sodium bicarbonate dose on 24h-ABPM, the fully adjusted model suggested an increase of 0.047 (95%CI: −0.026, 0.119) mmHg by each mg/kg per day increase of sodium bicarbonate dose. None of the estimates reached statistical significance, however, the opposite direction of effects for office BP vs. 24h-ABPM remained present (Table 3B).
Table 3B.
Association of systolic and diastolic blood pressure change (mmHg) with respect to sodium bicarbonate dose (mg/kg body weight).
| Regression models | |||||||
|---|---|---|---|---|---|---|---|
| Crude | Adjusted I | Adjusted II | |||||
| n | β | 95% CI | β | 95% CI | β | 95% CI | |
| Δ 24h-ABPM | 43 | ||||||
| Systolic | 0.047 | −0.022 to 0.116 | 0.047 | −0.023 to 0.117 | 0.047 | −0.026 to 0.119 | |
| Diastolic | 0.021 | −0.026 to 0.068 | 0.021 | −0.026 to 0.068 | 0.023 | −0.026 to 0.071 | |
| Δ Daytime-ABPM | 43 | ||||||
| Systolic | 0.046 | −0.028 to 0.119 | 0.045 | −0.03 to 0.12 | 0.046 | −0.033 to 0.122 | |
| Diastolic | 0.013 | −0.036 to 0.062 | 0.013 | −0.036 to 0.063 | 0.015 | −0.036 to 0.066 | |
| Δ Nighttime-ABPM | 37 | ||||||
| Systolic | 0.044 | −0.037 to 0.124 | 0.044 | −0.038 to 0.125 | 0.049 | −0.036 to 0.133 | |
| Diastolic | 0.026 | −0.027 to 0.079 | 0.026 | −0.025 to 0.077 | 0.022 | −0.030 to 0.075 | |
| Δ Office BP | 45 | ||||||
| Systolic | −0.024 | −0.161 to 0.112 | −0.024 | −0.163 to 0.114 | −0.036 | −0.183 to 0.11 | |
| Diastolic | −0.067 | −0.15 to 0.016 | −0.067 | −0.151 to 0.017 | −0.068 | −0.157 to 0.022 | |
Crude, sodium bicarbonate dose (mg /kg body weight).
Adjusted I, Crude + change of eGFR from baseline to week 8 (mL/min per 1.73 m2).
Adjusted II, Adjusted I + increase and decrease of antihypertensive medication vs. no change of antihypertensive medication.
24h-ABPM, 24 h ambulatory blood pressure monitoring; Daytime ABPM, from 8:00 until 22:00 ambulatory blood pressure monitoring; Nighttime ABPM, from 22:01 until 7:59 ambulatory blood pressure monitoring; Office BP, office blood pressure measurement; β, point estimate (mmHg); 95% CI, 95 % confidence interval.
When treatment categories low, moderate, and high were compared against no sodium bicarbonate treatment we couldn't show a clear dose-dependent effect on blood pressure and none of the models were statistically significant. Again, when looking at office BP a higher does of sodium bicarbonate was associated with a decrease in BP (n.s.; Table 3C and Figures 2C,D). In Supplementary Tables 1A,B we present age- and diuretic use- adjusted estimates for the relation of sodium bicarbonate loading and blood pressure.
Table 3C.
Association of systolic and diastolic blood pressure change (mmHg) with respect to treatment category (low, moderate, high; reference, none).
| Treatment category | Crude | Adjusted I | Adjusted II | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systolic | Diastolic | Systolic | Diastolic | Systolic | Diastolic | ||||||||
| n | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| 24h-ABPM | |||||||||||||
| Low | 15 | 2.316 | −4.095 to 8.726 | −0.63 | −4.953 to 3.693 | 2.21 | −4.332 to 8.752 | −0.601 | −5.016 to 3.814 | 2.212 | −4.515 to 8.939 | −0.55 | −5.08 to 3.98 |
| Moderate | 8 | 5.589 | −1.914 to 13.092 | 1.852 | −3.209 to 6.912 | 5.534 | −2.074 to 13.142 | 1.867 | −3.268 to 7.0 | 5.706 | −2.331 to 13.744 | 1.908 | −3.504 to 7.32 |
| High | 9 | 3.722 | −3.536 to 10.98 | 0.948 | −3.947 to 5.843 | 3.654 | −3.712 to 11.02 | 0.966 | −4.005 to 5.938 | 3.607 | −4.016 to 11.231 | 1.108 | −4.025 to 6.242 |
| Daytime-ABPM | |||||||||||||
| Low | 15 | 1.972 | −4.919 to 8.862 | −1.387 | −5.915 to 3.141 | 1.785 | −5.238 to 8.807 | −1.246 | −5.858 to 3.366 | 1.764 | −5.437 to 8.965 | −1.177 | −5.902 to 3.548 |
| Moderate | 8 | 4.064 | −4.001 to 12.13 | 0.404 | −4.896 to 5.705 | 3.966 | −4.201 to 12.132 | 0.479 | −4.885 to 5.842 | 4.351 | −4.254 to 12.955 | 0.617 | −5.029 to 6.262 |
| High | 9 | 3.951 | −3.851 to 11.753 | 0.177 | −4.95 to 5.304 | 3.832 | −4.076 to 11.739 | 0.267 | −4.925 to 5.46 | 3.652 | −4.509 to 11.813 | 0.433 | −4.922 to 5.787 |
| Nighttime-ABPM | |||||||||||||
| Low | 14 | −0.495 | −7.143 to 6.150 | −3.920 | −8.003 to 0.162 | −0.336 | −7.110 to 6.438 | −3.540 | −7.538 to 0.458 | −0.262 | −7.153 to 6.628 | −3.579 | −7.648 to 0.491 |
| Moderate | 6 | 5.728 | −2.645 to 14.102 | 1.860 | −3.28 to 7.003 | 5.748 | −2.733 to 14.229 | 1.908 | −3.073 to 6.914 | 6.083 | −2.672 to 14.839 | 1.739 | −3.431 to 6.910 |
| High | 6 | 1.492 | −6.881 to 9.866 | 0.150 | −4.992 to 5.293 | 1.588 | −6.050 to 10.080 | 0.378 | −4.638 to 5.390 | 2.255 | −6.482 to 10.981 | −0.112 | −5.171 to 5.148 |
| Office BP | |||||||||||||
| Low | 15 | 4.65 | −7.425 to 16.725 | −0.517 | −7.847 to 6.813 | 4.645 | −7.653 to 16.942 | −0.311 | −7.752 to 7.131 | 4.507 | −8.053 to 17.067 | −0.29 | −7.906 to 7.327 |
| Moderate | 10 | −0.217 | −13.567 to 13.133 | −6.283 | −14.387 to 1.82 | −0.219 | −13.744 to 13.307 | −6.205 | −14.389 to 1.979 | −1.139 | 15.431 to 13.153 | −6.567 | −15.233 to 2.1 |
| High | 8 | −0.042 | −14.273 to 14.189 | −4.708 | −13.347 to 3.93 | −0.044 | 14.467 to 14.379 | −4.606 | −13.333 to 4.122 | −0.96 | −15.955 to 14.035 | −4.616 | −13.71 to 4.477 |
Crude, treatment category of sodium bicarbonate: low (1–37.9 mg/kg/day), moderate (38–79.9 mg/kg/day), and high (≥80 mg/kg/day) vs. no treatment (reference); Adjusted I, Crude + change of eGFR from baseline to week 8 (mL/min per 1.73 m2); Adjusted II, Adjusted I + increase and decrease of antihypertensive medication vs. no change of antihypertensive medication.
24h-ABPM, 24 h ambulatory blood pressure monitoring; Daytime ABPM, from 8:00 until 22:00 ambulatory blood pressure monitoring; Nighttime ABPM, from 22:01 until 7:59 ambulatory blood pressure monitoring; Office BP, office blood pressure measurement; β, point estimate (mmHg); 95% CI, 95 % confidence interval.
Adverse Events, Antihypertensive Medication, and Compliance
Overall mean body weight changed by −0.2 (±3.64) kg, 0.13 (±3.52) kg in the intervention group and −0.5 (±3.8) kg in the rescue group (p = 0.717) from baseline to week 8. In neither the intervention group nor the rescue group development or worsening of peripheral edema was observed.
In six (13%) patients antihypertensive medication changed during the 8 weeks of follow-up: In one subject a thiazide diuretic was started instead of calcium antagonist treatment, and in another a calcium antagonist was started (both 50% of max. daily dose; both intervention group). Angiotensin- converting-enzyme inhibitor (ACEi) dose was increased by 6.25% of max. daily dose, while furosemide was decreased and doxazosin was stopped in another subject in the rescue group. In the remaining three patients (one intervention group) with treatment change doses were decreased (furosemide, ACEi and thiazide, ACEi and calcium antagonist). Overall, use of classes of antihypertensives decreased to 1.9 per person in the intervention group and 2.5 per person in the rescue group from baseline until week 8.
Study medication was well-tolerated and compliance was good, except for one female who didn't want to take as many capsules as would have been necessary to reach the target level (nine per day). Serum bicarbonate on average increased in the intervention group by 3.9 (95%CI: 3.04, 4.71) mmol/L and by 1.5 (95%CI: 0.47, 2.56) mmol/L in the rescue group. At week 8 median urinary pH was 7 (IQR: 6.5–7.25) in the intervention and 5 (IQR: 5–5.6) in the rescue group. Urine sodium excretion was only determined at week 4, but increased on average by 32.77 (95%CI: −4.44, 69.99) mmol/24 h in the intervention group and by 12.47 (95%CI: −7.24, 32.18) mmol/24 h in the rescue group.
Discussion
To the best of our knowledge this is the first study investigating the effect of sodium bicarbonate treatment on arterial blood pressure using the gold standard measurement technique of 24h-ABPM. Overall, we did not observe a statistically significant effect on blood pressure by sodium bicarbonate treatment. Change in antihypertensive medication and weight gain was negligible. However, 24h-ABPM increased consistently by approximately two to five mmHg between baseline and week 8 (Table 2A). This increase was present in systolic and diastolic measurements and was somewhat more distinct in the intervention group compared to the rescue group, as indicated by the positive regression coefficients (Table 3A). Regardless, we could not establish a dose-dependent effect of sodium bicarbonate on blood pressure, as the magnitude of BP increase didn't consistently rise by increasing dose category (Table 2B). For systolic 24h-ABPM effect sizes (β) approximately suggest doubling of BP increase from low to a moderate sodium bicarbonate dose in relation to subjects receiving no sodium bicarbonate, but decreased in the high bicarbonate dose group (Table 3C). Notably, none of our results reached statistical significance and the observed trends might be due to random error.
Striking are the differences between office and ambulatory measurements. Mean baseline office SBP was relatively low in the rescue group and did increase to comparable levels until week 8 (Figure 2A). This led to negative effect sizes for regression models looking at office BP change, suggesting a decrease of BP with increasing sodium bicarbonate dose. Our results highlight the limitations of office BP measurements even though when performed by a well-trained nurse using a standardized protocol.
The amount of sodium administered while correcting metabolic acidosis with sodium bicarbonate is substantial. In the current study the intervention group received a median daily dose of 63 mg/kg per day (0.76 mEq/kg per day), which translates to 6 capsules à 840 mg per day for an individual weighing 80 kg. In light of the recent evidence showing improvement of renal function and more importantly survival in sodium bicarbonate supplemented CKD patients with chronic acidosis (1), the uncertainty of safety with regard to worsening of arterial hypertension and changes in fluid status is prevailing in clinical practice (5).
Looking at our regression model a daily dose of 63 mg/kg sodium bicarbonate would lead to an on average increase of 3 mmHg systolic and 1.3 mmHg diastolic 24h-ABPM [systolic 24h-ABPM: +4.7 (95%CI: −2.2 to +11.6)] mmHg by 100 mg/kg per day orally administered sodium bicarbonate (Table 3B).
The question whether sodium or chloride is the driving force for arterial hypertension in salt-sensitive individuals is not novel. Indisputable is the recommendation for a low sodium chloride diet of <5 g per day [corresponding to <2 g (<90 mmol) of sodium per day] in patients with arterial hypertension and CKD (11). The average effect on SBP and DBP for the suggested limitation of salt intake is 4.7 mmHg (95%CI: 2.2–7.2) and 2.5 mmHg (95%CI: 1.8–3.3), respectively (12). The relationship between elevated ambulatory BP and all-cause and cardiovascular mortality is well-established (13). Although our estimates were not statistically significant, the shown blood pressure-reducing effect of a salt-restricted diet would be almost outweighed by the blood pressure-increasing effect of a regularly necessary sodium bicarbonate dose to counteract acidosis in CKD. However, a set of small randomized controlled trials including 5–10 subjects each specifically studied the effect of NaCl on blood pressure, sodium retention, and weight gain compared to NaHCO3 and sodium citrate, respectively (14–18). Under these experimental conditions, which included a very-low dietary sodium load of 10 mEq per day a sodium load accompanied by bicarbonate or citrate led to weight increase and sodium retention but not to increase of blood pressure. These observations are supported by animal experiments suggesting that the BP increasing effect is sufficiently induced by chloride alone (19–21), but more pronounced for NaCl (22, 23). The renin-angiotensin system, which is usually suppressed after a NaCl load and rise in blood pressure, is not affected by non-chloride sodium salts, both in animals (24–26) and men (27). However, with more realistic dietary salt loading of either 200 mEq per day of NaCl or 100 mEq per day of NaCl plus 100 mEq per day of NaHCO3 blood pressure increase was comparable between groups in a randomized crossover trial in 6 patients with CKD stage G5 (28). Furthermore, more recent studies tested the effect of sodium bicarbonate on blood pressure as a secondary outcome, but used placebo as a control substance. de Brito-Ashurst et al. observed no difference in blood pressure, but increased antihypertensives use in the study group (61 vs. 48%) after 24 months (2). Also no difference in BP was seen in a study testing sodium citrate compared to controls after over 30 months (29). A recent study in CKD stage G3 and G4 patients with a bicarbonate lower than 22 at baseline and a target bicarbonate of 24 to 26 for the intervention group, recorded “worsening of hypertension” in 35.1 % of the intervention group vs. 21% in the control group [p = 0.13; (30)]. “Worsening of edema” and diuretic use was significantly more frequent with a mean dose of sodium bicarbonate of 2.3 g/day (0.5 mEq/kg body weight per day) in the intervention group after the 6 months follow-up. Jeong et al. followed CKD stage G4 and G5 subjects over 10 months (31). The mean dose of sodium bicarbonate was 0.58 ± 0.42 mEq/kg in the intervention group. Blood pressure did neither change in CKD stage G4 nor stage G5 (treatment group: 3.4 ± 16.3/0.7 ± 11.5 mmHg; controls: −2.1 ± 22.3/−1.8 ± 12.3 mmHg) with similar use of antihypertensives and loop diuretics across groups. However, in both studies a trend toward worsening of arterial hypertension was seen and observed effects are comparable with our results in magnitude. Of note, daily average sodium bicarbonate load in our study was much higher in the intervention group, which could indicate that the increase in BP is due to other reasons independent of sodium bicarbonate load. The so far largest trial investigating sodium bicarbonate treatment in CKD, the UBI study, presented little information on BP monitoring and change during the course of the 36 months follow-up, but did not observe worsening of hypertension (1). On the contrary, the recently published BASE Pilot trial (32) observed a significant increase in SBP after 12 weeks of high-dose sodium bicarbonate treatment compared to the low-dose and placebo group. Although not statistically significant this effect remained until week 32, when mean SBP was lower in the high-dose than the low-dose group but still higher than the placebo group. Altogether this might give a hint toward a dose-dependent effect. Like in our study, Abramowitz et al. observed a non-dose dependent effect on SBP and DPB in their dose-finding study. Sodium bicarbonate was increased every 2 weeks by ~0.3 mEq per kg ideal body weight (33).
In light of the possible blood pressure increasing effect of sodium bicarbonate, the set of studies by Goraya et al. (34–37) who used a fruit and vegetable-rich diet to counteract acidosis in CKD, is even more remarkable. In patients with hypertensive nephropathy and different stages of CKD SBP was lower in subjects treated with the dietary intervention compared to sodium bicarbonate or standard care. Further indicators of cardiovascular risk as well-improved in the dietary group (34). However, whether sodium bicarbonate supplementation ameliorates cardiovascular outcomes ought to be shown (38, 39).
Taken together, our study is in line with former reports showing a statistically non-significant trend toward an increase of blood pressure in sodium bicarbonate treated patients. However, like others (32, 33) we did not observe a dose-dependent effect as is shown in Figure 2. Ultimately, we don't know the reason behind the slight increase in BP in our cohort. One can speculate that individual dietary sodium chloride consumption might have mediated increase in blood pressure in some individuals.
Our study was limited by a relatively small sample size, in addition to four insufficient daytime- and 10 nighttime-ABPMs. Furthermore, we did not perform dietary assessment in particular salt intake, but our study subjects received standard dietary assistance by a trained dietitian as needed. Correction of acidosis by sodium bicarbonates results in high pill burden, suggesting incomplete compliance. However, the short duration of follow-up gives confidence that studied subjects followed prescriptions. Objective measures such as urine pH, change and urinary sodium excretion suggested sufficient compliance.
In conclusion, sodium loading accompanied by sodium bicarbonate supplementation to counterbalance chronic metabolic acidosis in CKD did not significantly increase BP. Nevertheless, BP of sodium bicarbonate treated CKD patients should be monitored and 24h-ABPM might be recommended for this vulnerable patient cohort.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical University of Vienna. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MG, SR, and GS-P designed the study. CA, SR, AR, CS, MF, MG, and GS-P conducted the study and collected the data. MG, CA, DC, and GS-P drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge the help of Candace Joefield-Roka, Marlies Dames-Ludwig, Miriam Kunst, Ingrid Jachimow, Sylvia Taxer, Karin Voith, Danica Doric, Maria Villaluz, Gabriele Holzmann, and Violeta Tanacovic by collecting data and helping to conduct the SoBic-study.
Footnotes
Funding. This project was funded by the Medizinisch-Wissenschaftliche Fonds des Bürgermeisters der Bundeshauptstadt Wien (Projekt-Number: 17082). Study medication was provided by the manufacturer (MEDICE Pharma Pütter GmbH & Co. KG, Iserlohn, Germany) free of charge. The manufacturer had no role in the conduction of that study and preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.711034/full#supplementary-material
References
- 1.Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. (2019) 32:989–1001. 10.1007/s40620-019-00656-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. (2009) 20:2075–84. 10.1681/ASN.2008111205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wesson DE, Mathur V, Tangri N, Stasiv Y, Parsell D, Li E, et al. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet. (2019) 394:396–406. 10.1016/S0140-6736(19)31388-1 [DOI] [PubMed] [Google Scholar]
- 4.Navaneethan SD, Shao J, Buysse J, Bushinsky DA. Effects of treatment of metabolic acidosis in CKD: a systematic review and meta-analysis. Clin J Am Soc Nephrol. (2019) 14:1011–20. 10.2215/CJN.13091118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushinsky DA. Tolerance to sodium in patients with CKD-induced metabolic acidosis: does the accompanying anion matter? Am J Kidney Dis. (2019) 73:858–65. 10.1053/j.ajkd.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 6.Gaggl M, Cejka D, Plischke M, Heinze G, Fraunschiel M, Schmidt A, et al. Effect of oral sodium bicarbonate supplementation on progression of chronic kidney disease in patients with chronic metabolic acidosis: study protocol for a randomized controlled trial (SoBic-Study). Trials. (2013) 14:196. 10.1186/1745-6215-14-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naci H, Salcher-Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, et al. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. (2019) 53:859–69. 10.1136/bjsports-2018-099921 [DOI] [PubMed] [Google Scholar]
- 8.Rothman K, Greenland S, Lash TL. Modern Epidemiology: Data Analysis: Fundamentals of Epidemiologic Data Analysis. 3rd ed. Vol. 3, Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilinks; (2008). p. 217–8. [Google Scholar]
- 9.O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. (2003) 21:821–48. 10.1097/00004872-200305000-00001 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Tu W. Minimally sufficient numbers of measurements for validation of 24-hour blood pressure monitoring in chronic kidney disease. Kidney Int. (2018) 94:1199–204. 10.1016/j.kint.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung AK, Chang TI, Cushman WC, Furth SL, Ix JH, Pecoits-Filho R, et al. Blood pressure in chronic kidney disease: conclusions from a kidney disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. (2019) 95:1027–36. 10.1016/j.kint.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 12.Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. (2006) 24:215–33. 10.1097/01.hjh.0000199800.72563.26 [DOI] [PubMed] [Google Scholar]
- 13.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. (2018) 378:1509–20. 10.1056/NEJMoa1712231 [DOI] [PubMed] [Google Scholar]
- 14.Husted FC, Nolph KD, Maher JF. NaHCO3 and NaCl tolerance in chronic renal failure. J Clin Invest. (1975) 56:414–9. 10.1172/JCI108107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz TW, Al-Bander HA, Morris RC, Jr. “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med. (1987) 317:1043–8. 10.1056/NEJM198710223171702 [DOI] [PubMed] [Google Scholar]
- 16.Shore AC, Markandu ND, MacGregor GA. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J Hypertens. (1988) 6:613–7. 10.1097/00004872-198808000-00003 [DOI] [PubMed] [Google Scholar]
- 17.Sharma AM, Schattenfroh S, Thiede HM, Oelkers W, Distler A. Effects of sodium salts on pressor reactivity in salt-sensitive men. Hypertension. (1992) 19:541–8. 10.1161/01.HYP.19.6.541 [DOI] [PubMed] [Google Scholar]
- 18.Tomita Y, Ueno M, Tsuchihashi T, Muratani H, Kobayashi K, Takishita S, et al. Chloride ion plays an important role in sodium induced volume expansion in normal humans. Am J Hypertens. (1990) 3:485–7. 10.1093/ajh/3.6.485 [DOI] [PubMed] [Google Scholar]
- 19.Luft FC, Steinberg H, Ganten U, Meyer D, Gless KH, Lang RE, et al. Effect of sodium chloride and sodium bicarbonate on blood pressure in stroke-prone spontaneously hypertensive rats. Clin Sci. (1988) 74:577–85. 10.1042/cs0740577 [DOI] [PubMed] [Google Scholar]
- 20.Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC, Jr. Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension. (2005) 45:867–73. 10.1161/01.HYP.0000164628.46415.66 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Schmidlin O, Yi SL, Bollen AW, Morris RC, Jr. Genetically determined chloride-sensitive hypertension and stroke. Proc Natl. Acad Sci USA. (1997) 94:14748–52. 10.1073/pnas.94.26.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitescarver SA, Holtzclaw BJ, Downs JH, Ott CE, Sowers JR, Kotchen TA. Effect of dietary chloride on salt-sensitive and renin-dependent hypertension. Hypertension. (1986) 8:56–61. 10.1161/01.HYP.8.1.56 [DOI] [PubMed] [Google Scholar]
- 23.Wyss JM, Liumsiricharoen M, Sripairojthikoon W, Brown D, Gist R, Oparil S. Exacerbation of hypertension by high chloride, moderate sodium diet in the salt-sensitive spontaneously hypertensive rat. Hypertension. (1987) 9:III171–5. 10.1161/01.HYP.9.6_Pt_2.III171 [DOI] [PubMed] [Google Scholar]
- 24.Abboud HE, Luke RG, Galla JH, Kotchen TA. Stimulation of renin by acute selective chloride depletion in the rat. Circ Res. (1979) 44:815–21. 10.1161/01.RES.44.6.815 [DOI] [PubMed] [Google Scholar]
- 25.Kotchen TA, Galla JH, Luke RG. Failure of NaHCO3 and KHCO3 to inhibit renin in the rat. Am J Physiol. (1976) 231:1050–6. 10.1152/ajplegacy.1976.231.4.1050 [DOI] [PubMed] [Google Scholar]
- 26.Kotchen TA, Luke RG, Ott CE, Galla JH, Whitescarver S. Effect of chloride on renin and blood pressure responses to sodium chloride. Ann Intern Med. (1983) 98:817–22. 10.7326/0003-4819-98-5-817 [DOI] [PubMed] [Google Scholar]
- 27.Julian BA, Galla JH, Guthrie GP, Jr, Kotchen TA. Renin and aldosterone responses to short-term NaCl or NaHCO3 loading in man. J Lab Clin Med. (1982) 100:261–8. [PubMed] [Google Scholar]
- 28.Husted FC, Nolph KD. NaHCO3 and NaCl tolerance in chronic renal failure II. Clin Nephrol. (1977) 7:21–5. [PubMed] [Google Scholar]
- 29.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. (2010) 77:617–23. 10.1038/ki.2009.519 [DOI] [PubMed] [Google Scholar]
- 30.Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant. (2020) 35:121–9. 10.1093/ndt/gfy214 [DOI] [PubMed] [Google Scholar]
- 31.Jeong J, Kwon SK, Kim HY. Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press. (2014) 12:80–7. 10.5049/EBP.2014.12.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raphael KL, Isakova T, Ix JH, Raj DS, Wolf M, Fried LF, et al. A randomized trial comparing the safety, adherence, and pharmacodynamics profiles of two doses of sodium bicarbonate in CKD: the BASE pilot trial. J Am Soc Nephrol. (2020) 31:161–74. 10.1681/ASN.2019030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. (2013) 8:714–20. 10.2215/CJN.08340812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goraya N, Munoz-Maldonado Y, Simoni J, Wesson DE. Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol. (2019) 49:438–48. 10.1159/000500042 [DOI] [PubMed] [Google Scholar]
- 35.Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. (2014) 86:1031–8. 10.1038/ki.2014.83 [DOI] [PubMed] [Google Scholar]
- 36.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. (2012) 81:86–93. 10.1038/ki.2011.313 [DOI] [PubMed] [Google Scholar]
- 37.Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. (2013) 8:371–81. 10.2215/CJN.02430312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A, et al. Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study. Clin J Am Soc Nephrol. (2018) 13:1463–70. 10.2215/CJN.00380118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aigner C, Cejka D, Sliber C, Fraunschiel M, Sunder-Plassmann G, Gaggl M. Oral sodium bicarbonate supplementation does not affect serum calcification propensity in patients with chronic kidney disease and chronic metabolic acidosis. Kidney Blood Press Res. (2019) 44:188–99. 10.1159/000498975 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.