Abstract
Background:
Combined hepatocellular-cholangiocarcinoma liver tumors (cHCC-CCA) with pathologic differentiation of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma within the same tumor are not traditionally considered for liver transplantation due to perceived poor outcomes. Published results are from small cohorts and single centers. Through a multi-center collaboration, we performed the largest analysis to date of the utility of liver transplantation for cHCC-CCA.
Study Design:
Liver transplant and resection outcomes for HCC (n=2998) and cHCC-CCA (n=208) were compared in a 12-center retrospective review (2009–2017). Pathology defined tumor type. Tumor burden was based on radiologic Milan criteria at time of diagnosis and applied to cHCC-CCA for uniform analysis. Kaplan-Meier survival curves and log-rank test were used to determine overall survival and disease-free survival. Cox regression was used for multivariate survival analysis.
Results:
Liver transplant for cHCC-CCA (n=67) and HCC (n=1814) within Milan had no significant difference in overall survival (5-yr cHCC-CCA 70.1%, HCC 73.4%, p=0.806) despite higher cHCC-CCA recurrence rates (23.1% vs 11.5% 5-years, p<0.001). Irrespective of tumor burden, cHCC-CCA tumors undergoing liver transplant had significantly superior overall survival (p=0.047) and disease-free survival (p<0.001) compared to resection. For cHCC-CCA within Milan, liver transplant was associated with improved disease-free survival over resection (70.3% vs 33.6% 5-years, p<0.001).
Conclusions:
Regardless of tumor burden, outcomes following liver transplant are superior to resection for patients with cHCC-CCA. Within Milan criteria, liver transplant for cHCC-CCA and HCC results in similar overall survival justifying consideration of transplantation due to the higher chance of cure with liver transplantation in this traditionally excluded population.
Précis
Within Milan criteria, liver transplant for combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CCA) results in similar overall survival to transplant for hepatocellular carcinoma justifying consideration of transplantation for cHCC-CCA due to the higher chance of cure with liver transplantation in this traditionally excluded population.
Introduction
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) liver tumors have features of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA). Other terminologies for cHCC-CCA tumors are biphenotypic, mixed hepatocellular-cholangiocarcinoma, cholangiolocellular carcinoma, and cholangiohepatoma. cHCC-CCA comprises of approximately only 2% of all primary liver tumors. The pathologic diagnosis of cHCC-CCA is made when the tumors have cells that have dual phenotypic expression on one cell type. Although this tumor type was first recognized in the literature over 100 years ago (1), the diagnosis and terminology have recently been standardized in an international consensus document (2).
For patients with cHCC-CCA tumors identified pre-operatively, the majority are directed towards resection. However, the 5-year survival for resection of cHCC-CCA tumors is poor, ranging from 20–40% (3). Liver transplant may have improved outcomes compared to resection as is seen in patients with HCC eligible for transplant (4). With varied degrees of long-term outcomes in resection and transplant, there has been an attempt to better classify tumors and identify outcomes (2).
When meeting specific criteria, HCC has been widely acceptable for liver transplantation due to good outcomes following liver transplantation (5, 6). There are also positive outcomes following liver transplantation for hilar cholangiocarcinoma on a protocol with strict criteria (7, 8). Historically, liver transplantation for intrahepatic cholangiocarcinoma has been associated with poor long term outcomes (9–14). Similar to intrahepatic cholangiocarcinoma, cHCC-CCA tumors are not traditionally considered for liver transplantation due to perceived poor outcomes in prior published results from small cohorts and single centers (9, 15–17). Some studies grouped cHCC-CCA together with intrahepatic cholangiocarcinoma, which may skew the results negatively (9, 18). Retrospective large database reviews have indicated worse outcomes of liver transplant for cHCC-CCA compared to liver transplant for HCC but lack important granular data such as tumor burden and pre-operative therapy (17). In some single-center small cohorts, liver transplantation for cHCC-CCA has satisfactory outcomes equivalent to HCC (10, 18–21). Currently, patients with cHCC-CCA are not eligible for deceased donor organ allocation prioritization based on the Model for End Stage Liver Disease (MELD) system in the United States.
Several factors have made standardizing treatment for cHCC-CCA difficult. First, due to similarity in radiographic features of the two tumor types, it is difficult to differentiate cHCC-CCA from HCC on imaging (15, 22, 23). Unlike HCC, where radiographic features are diagnostic without biopsy, imaging criteria for the diagnosis of cHCC-CCA tumors have not been standardized, which may lead to pre-operative mis-classification when biopsy is not used (16, 24). Secondly, the pathologic diagnosis has been varied from including cases with two separate tumors (one HCC and one intrahepatic cholangiocarcinoma), a single “collision” tumor with two different cell types of both HCC and intrahepatic cholangiocarcinoma mixed in one tumor, or tumors that have expression of both HCC and cholangiocarcinoma (2, 25, 26). Thirdly, cHCC-CCA is rare compared to HCC making it difficult for single institutions to acquire a significant number of cases for study (3, 27).
Due to mixed reports in the literature of small mostly single-center cohorts, we sought to overcome the limitations of prior studies through a multi-center collaboration. We performed the largest retrospective cohort analysis to date of the utility of liver transplantation for cHCC-CCA. The aim of this study is to assess outcomes of liver transplantation for cHCC-CCA. We hypothesize that if size and number of cHCC-CCA tumors are comparable to HCC cases that are currently considered transplant-eligible, liver transplantation provides better outcomes than resection. Due to the limited supply of livers for transplantation, outcomes following liver transplantation for cHCC-CCA must be comparable to other indications for liver transplantation. Recognizing this, our second hypothesis is that for small cHCC-CCA, liver transplantation will be acceptable with outcomes that are not statistically worse than liver transplantation for HCC.
Methods
Data Collection
Liver transplantation and resection outcomes were compared in a 12-center retrospective review for cases of HCC and cHCC-CCA (2009–2017). Data were collected at each center through retrospective chart review. The Washington University in St. Louis institutional review board (IRB) approved this study as did each individual IRB for each center. Data use agreements were signed between centers. Limited datasets were transferred securely to Washington University in St. Louis.
Case Definition
Tumors were defined as either HCC or cHCC-CCA based on final surgical pathology report from the participating center. The actual pathology slides were not re-reviewed; however, the pathology reports for all centers were centrally reviewed to ensure consistency in classification. Tumor burden was determined based on initial imaging report. The Milan criteria and University of California San Francisco (UCSF) criteria were applied to cHCC-CCA as well as HCC for uniform analysis. Within Milan criteria was defined as: a solitary lesion with diameter less than or equal to 5 cm or up to three lesions, each with a diameter less than or equal to 3 cm and no evidence of gross vascular invasion (5). UCSF criteria was defined as: a solitary lesion smaller than or equal to 6.5 cm or up to three lesions with the largest lesion smaller than or equal to 4.5 cm and a total tumor burden 8 cm or less (28).
Analysis Plan
The aim of the study was to study the outcomes of liver transplantation for cHCC-CCA, which we approached using three different analyses: 1) outcomes of resection of cHCC-CCA and liver transplantation for cHCC-CCA, 2) outcomes of resection of cHCC-CCA and HCC, and 3) outcomes of liver transplantation for cHCC-CCA and HCC. Within each analysis category, we also completed sub-analyses for cases based on tumor burden: beyond UCSF criteria, beyond Milan criteria but within UCSF criteria, and within Milan criteria.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism v 5.0 (GraphPad Software, Inc. La Jolla, CA) and SPSS version 26 (IBM SPSS Statistics, IBM Corporation, Armonk, NY). Categorical variables were compared using Fisher’s exact test or Chi Square test as applicable. Student’s T test was used to compare continuous variables. Survival graphs were plotted using Kaplan Meier curves and survival rates were compared using Log Rank test. Cox regression method was used to run multivariate survival analysis. For every comparison, difference with a p value <0.05 was considered statistically significant.
Results
The cohort consisted of 3206 surgically managed patients with primary liver tumor (HCC (n=2998) and cHCC-CCA (n=208)). Gender and age were available for the entire cohort. Race data was missing in 423 (13.2%) cases and distribution of missing data was almost similar in all groups. Among the patients receiving resection, HCC cohort had significantly higher proportion of males and cHCC-CCA cohort had higher proportion of Caucasian patients (Table 1).
Table 1.
Demographic Characteristics
| Variable | cHCC-CCA | HCC | p Value |
|---|---|---|---|
| Full cohort | |||
| Total, n | 208 | 2998 | |
| Sex, m, n (%) | 146 (70.2) | 2296 (76.6) | 0.036 |
| Race, Caucasian, n (%) | 132 (72.1) | 1551 (59.7) | <0.001 |
| Age, y, mean±SD | 60.2 ± 11.4 | 60.5 ± 9.2 | 0.598 |
| BMI, kg/m2, mean±SD | 29.3 ± 6.6 | 28.6 ± 5.8 | 0.150 |
| TACE, n (%) | 66 (33.0) | 1486 (60.9) | <0.001 |
| Y90, n (%) | 8 (4.3) | 65 (2.6) | 0.193 |
| Ablation, n (%) | 13 (7.0) | 465 (19.0) | <0.001 |
| Within Milan cases, n (%) | 125 (60.1) | 2183 (73.6) | <0.001 |
| Beyond Milan, within-UCSF cases, n (%) | 31 (14.9) | 278 (9.4) | 0.009 |
| Beyond UCSF cases, n (%) | 52 (25.0) | 505 (17.0) | 0.004 |
| Resection | |||
| Total, n | 109 | 824 | |
| Sex, m, n (%) | 71 (65.1) | 622 (75.5) | <0.001 |
| Race, Caucasian, n (%) | 74 (68.5) | 256 (46.8) | <0.001 |
| Age, y, mean±SD | 61.6 ± 12.8 | 62.0 ± 12.5 | 0.792 |
| BMI, kg/m2, mean±SD | 29.0 ± 6.9 | 28.1 ± 6.1 | 0.219 |
| Cirrhosis, n (%) | 32 (29.4) | 424 (51.5) | <0.001 |
| TACE, n (%) | 4 (3.7) | 24 (7.8) | 0.146 |
| Y90, n (%) | 2 (1.9) | 5 (1.7) | 0.889 |
| Ablation, n (%) | 2 (1.9) | 8 (2.7) | 0.639 |
| Within Milan cases, n (%) | 58 (53.2) | 369 (44.8) | 0.097 |
| Beyond Milan, within-UCSF cases, n (%) | 17 (15.6) | 116 (14.1) | 0.670 |
| Beyond UCSF cases, n (%) | 34 (31.2) | 339 (41.1) | 0.046 |
| Transplant | |||
| Total, n | 99 | 2174 | |
| Sex, m, n (%) | 75 (75.8) | 1674 (77.0) | 0.774 |
| Race, Caucasian, n (%) | 58 (77.3) | 1295 (63.1) | 0.012 |
| Age, y, mean±SD | 58.5 ± 9.5 | 60.0 ± 7.6 | 0.070 |
| BMI, kg/m2, mean±SD | 29.8 ± 6.3 | 29.9 ± 5.7 | 0.180 |
| Liver disease, n (%) | |||
| HCV | 56 (56.6) | 1192 (54.8) | 0.813 |
| NASH | 18 (18.2) | 203 (9.3) | 0.006 |
| Alcoholic | 11 (11.1) | 216 (9.9) | 0.834 |
| None | 2 (2.0) | 7 (0.3) | 0.070 |
| TACE, n (%) | 62 (67.4) | 1462 (68.6) | 0.801 |
| Y90, n (%) | 6 (7.5) | 60 (2.8) | 0.014 |
| Ablation, n (%) | 11 (13.9) | 457 (21.3) | 0.113 |
| Within Milan cases, n (%) | 67 (67.7) | 1814 (84.7) | <0.001 |
| Beyond Milan, within-UCSF | 14 (14.1) | 162 (7.6) | 0.017 |
| cases, n (%) | |||
| Beyond UCSF cases, n (%) | 18 (18.2) | 166 (7.7) | <0.001 |
| cHCC-CCA | Resection | Transplant | |
| Total, n | 109 | 99 | |
| Sex, m, n (%) | 71 (65.1) | 75 (75.8) | 0.095 |
| Race, Caucasian, n (%) | 74 (68.5) | 58 (77.3) | 0.191 |
| Age, y, mean±SD | 61.6 ± 12.8 | 58.5 ± 9.5 | 0.049 |
| BMI, kg/m2, mean±SD | 29.0 ± 6.9 | 29.8 ± 6.3 | 0.421 |
| TACE, n (%) | 4 (3.7) | 62 (67.4) | <0.001 |
| Y90, n (%) | 2 (1.9) | 6 (7.5) | 0.058 |
| Ablation, n (%) | 2 (1.9) | 11 (13.9) | 0.001 |
| Within Milan cases, n (%) | 58 (53.2) | 67 (67.7) | 0.033 |
| Beyond Milan, within-UCSF cases, n (%) | 17 (15.6) | 14 (14.1) | 0.769 |
| Beyond UCSF cases, n (%) | 34 (31.2) | 18 (18.2) | 0.030 |
cHCC-CCA, combined hepatocellular carcinoma-cholangiocarcinoma; NASH, non-alcoholic steatohepatitis; TACE, transarterial chemoembolization; UCSF, University of California-San Francisco
Locoregional Therapy
Though pre-operative Trans-arterial Chemoembolization (TACE) and Ablation were significantly more common in HCC cases than in cHCC-CCA cases in the entire cohort of 3206 cases, this difference between the groups was not seen when the cohort was divided by the type of surgery. In patients undergoing transplant, Yittrium-90 (Y-90) was the only locoregional therapy (LRT) used more frequently in cHCC-CCA patients (7.5%) than in HCC patients (2.8%) with 7.5% of cHCC-CCA patients and 2.8% of HCC patients having Y-90 prior to transplant (p=0.014) (Table 1).
Pre-Transplant Diagnosis and Tumor Burden of cHCC-CCA Cases
There were 208 patients with cHCC-CCA based on final surgical pathology. In the pre-operative setting only 11 (5.3%) had a pre-operative diagnosis of cHCC-CCA. Nine received resection and two underwent liver transplantation (Table 2). Of the 11 pre-operatively diagnosed cHCC-CCA tumors, five were beyond Milan criteria at the time of diagnosis (of which only two were beyond UCSF) and none had major vascular invasion. One of those five received transplant (beyond Milan, but within UCSF at diagnosis with no measurable disease after Y90) whereas four received a resection. Remaining six patients with pre-operative diagnosis of cHCC-CCA were within Milan. One of those six received a transplant whereas other five were resected. Total 125 patients with cHCC-CCA on final surgical pathology received a surgery with a pre-operative imaging diagnosis of HCC. Forty-four patients were beyond Milan (26 beyond UCSF) at the time of diagnosis. Twenty-seven (14 beyond UCSF) received transplant (Table 3).
Table 2.
Preoperative Diagnoses of Combined Hepatocellular Carcinoma-Cholangiocarcinoma Tumors
| Preop tumor diagnosis | Data | Liver transplantation, n | Resection, n |
|---|---|---|---|
| Biphenotypic tumor, n (%) | 11 (5.3) | 2 | 9 |
| Cholangiocarcinoma, n (%) | 24 (11.5) | 1 | 23 |
| Hepatocellular carcinoma, n (%) | 125 (60.1) | 84 | 41 |
| Indeterminate lesion, n (%) | 33 (15.9) | 6 | 27 |
| Other tumors, n (%) | 10 (4.9) | 2 | 8 |
| Incidental tumor, n (%) | 2 (1.0) | 2 | 0 |
| Unknown diagnosis, n (%) | 3 (1.4) | 2 | 1 |
| Total, n | 208 | 99 | 109 |
Table 3:
Tumor Burden of Combined Hepatocellular Carcinoma-Cholangiocarcinoma Tumors Based on Preoperative Imaging
| Tumor burden at diagnosis | N | Received transplant, N (pre-diagnosed as) | Received resection, N (Pre-diagnosed as) |
|---|---|---|---|
| Beyond UCSF | 48 | 16 (14 HCC; 1 Indeterminate, 1 Other) | 32 (9 iCCA; 12 HCC; 2 cHCC-CCA; 5 Indeterminate; 4 Other) |
| Beyond Milan, within-UCSF | 30 | 14 (13 HCC; 1 cHCC-CCA) | 16 (4 iCCA; 5 HCC; 2 cHCC-CCA; 5 Indeterminate) |
| Within Milan | 122 | 64 (1 iCCA; 56 HCC; 1 cHCC-CCA; 5 Indeterminate; 1 Other) | 58 (9 iCCA; 24 HCC; 5 cHCC-CCA; 16 Indeterminate; 4 Other) |
| Tumor size/ number details unknown | 6 | 3 (1 HCC; 2 Unknown) | 3 (1 iCCA; 1 Indeterminate; 1 Unknown) |
| Incidental tumor | 2 | 2 | 0 |
HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; cHCC-CCA, combined hepatocellular-intrahepatic cholangiocarcinoma
Entire Cohort Outcomes
In the entire cohort, total 2273 patients underwent liver transplantation (99 cHCC-CCA; 2174 HCC) and 933 patients underwent resection (109 cHCC-CCA; 824 HCC). When comparing all cases regardless of surgical treatment strategy, there was significantly better overall survival and disease-free survival in the HCC group compared to the cHCC-CCA group. Additionally, the recurrence rate was significantly lower in the HCC group (all p<0.001).
In the multivariable analysis of the full cohort, only cHCC-CCA as the type of tumor (HR = 1.63 [95% CI = 1.28 – 2.09], p<0.001) and transplant versus resection (HR = 0.71 [95% CI = 0.57 – 0.90], p=0.005) were the independent variables affecting the overall survival. Overall survival was worse in cHCC-CCA cases and better in transplant cases. Similarly, recurrence rate was higher in cHCC-CCA cases (HR = 1.36 [95% CI = 1.03 – 1.79], p=0.03) than in HCC cases, and lower in transplantation (HR = 0.16 [95% CI = 0.12 – 0.21], p<0.001) versus resection.
cHCC-CCA Cohort: Resection of cHCC-CCA and liver transplantation for cHCC-CCA
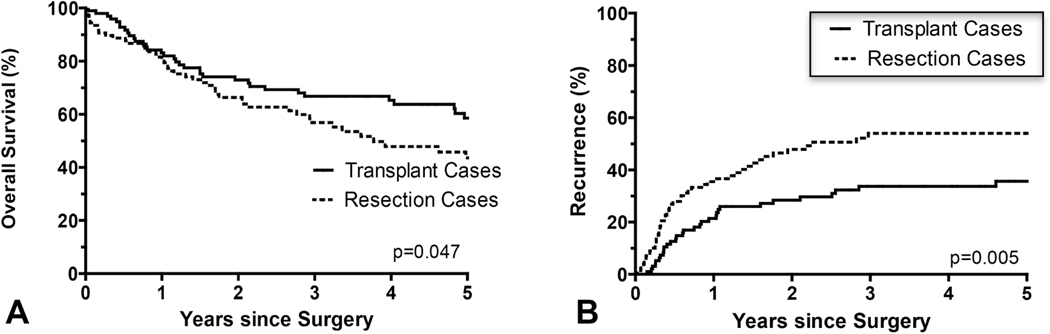
Within the cHCC-CCA cohort, 99 transplant recipients and 109 patients that underwent resection were compared. Irrespective of tumor burden, cHCC-CCA tumors undergoing liver transplantation had significantly superior overall survival (p=0.047) (Figure 1a) and disease free-survival (p<0.001) (Figure 1b) compared to resection.
Figure 1.
(A) The overall survival rate was statistically significantly higher (p=0.047) and the (B) disease recurrence rate significantly lower (p=0.005) in patients with combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CCA) with liver transplantation compared to resection.
On multivariable analysis, though tumor burden within Milan Criteria was the only variable that independently affected the overall survival (HR = 0.53 [95% CI = 0.34 – 0.84], p=0.007). Transplantation (HR = 0.50 [95% CI = 0.25 – 1.02], p=0.047) as well as tumor burden within Milan Criteria (HR = 0.51 [95% CI = 0.31 – 0.83], p=0.007) were associated with decreased disease recurrence. To investigate further the impact of disease burden and transplant versus resection, we performed sub-analyses by disease burden: beyond UCSF criteria, within UCSF criteria but beyond Milan Criteria, and within Milan criteria.
Resection versus liver transplantation for cHCC-CCA with tumor burden beyond UCSF criteria
Among the 52 patients with cHCC-CCA beyond UCSF criteria, there was no significant difference in overall survival (p=0.780), disease-free survival (p=0.105) or recurrence rate (p=0.152) between transplant (n=18) and resection (n=34) cases.
Resection versus liver transplantation for cHCC-CCA with tumor burden beyond Milan, but within UCSF criteria
Similar to cHCC-CCA tumors beyond UCSF criteria, there was no statistically significant difference in outcomes for patients with cHCC-CCA tumor burden beyond Milan criteria but within UCSF criteria (all p>0.2); however, numbers in this cohort were small (transplant n=14, resection n=17).
Resection versus liver transplantation for cHCC-CCA with tumor burden within Milan criteria
At one year, liver transplantation and resection for cHCC-CCA had similar overall survival (89.1% transplant vs. 89.3% resection). While it did not reach statistical significance, there was disparate overall survival at 5 years (liver transplantation 70.1%; resection 49.7%; p=0.078). However, for cHCC-CCA within Milan criteria, liver transplantation (n=67) was associated with lower recurrence rate (5-year 23.1% vs 45.7%, p=0.009) and improved disease-free survival over resection (n=58) (5-year 70.3% vs 33.6%, p<0.001).
Resection Cohort: Resection of cHCC-CCA and Resection of HCC
In the cohort undergoing resection (109 cHCC-CCA; 824 HCC), irrespective of tumor burden there was better overall survival for HCC than cHCC-CCA (p=0.020) but no difference in disease-free survival or recurrence rates.
Resection for tumor burden beyond UCSF criteria
For cases beyond UCSF criteria, there was no statistically significant difference in the overall survival (p=0.136), recurrence rate (p=0.231) and disease-free survival (p=0.079) between HCC (n=339) and cHCC-CCA (n=34) groups. However, there was disparity between the two groups with overall survival of 40.1% at 5 years in the HCC group compared to 26.9% at 5 years in the cHCC-CCA cohort.
Resection of tumor burden beyond Milan, but within UCSF criteria
Similarly, there was no statistically significant difference in outcome for patients with cHCC-CCA (n=17) or HCC (n=116) beyond Milan criteria but within UCSF criteria undergoing resection. Overall survival, disease-free survival and recurrence rate were similar in this cohort (all p>0.088).
Resection of tumor burden within Milan criteria
In the cohort of cases that underwent resection and were within Milan criteria (58 cHCC-CCA; 369 HCC), overall survival was significantly better for HCC cases (93.2%, 77.4% and 66.6% vs 89.3%, 67.7% and 49.7% at 1-, 3- and 5-years, p=0.015). However, there was no statistically significant difference between the two tumor types regarding disease free-survival and recurrence rate (all p>0.493).
Transplant Cohort: Liver transplantation for cHCC-CCA and liver transplantation for HCC
Liver transplantation for HCC (n=2174) was associated with better overall outcomes than liver transplantation for cHCC-CCA (n=99). Patients transplanted for HCC had better overall survival, disease-free survival, and lower recurrence rates after liver transplantation (all p < 0.007) than patients transplanted for cHCC-CCA.
In multivariable analysis of the transplant cohort, only tumor type (HCC vs cHCC-CCA) and tumor burden were independently associated with overall survival. For the entire transplant cohort, overall survival was worse for patients with cHCC-CCA (HR = 1.65 [95% CI = 1.16 – 2.36], p=0.005) and better in patients with within Milan criteria (HR = 0.40 [95% CI = 0.24 – 0.69], p=0.001). Also, on multivariable analysis of the transplant cohort, cHCC-CCA was the only independent variable with significantly higher disease recurrence (HR = 3.14 [95% CI = 2.03 – 4.85], p<0.001).
Due to these findings of the association of tumor type and tumor burden with outcomes after transplantation, we analyzed the transplant cohort based on tumor burden.
Liver transplantation for tumor burden beyond UCSF criteria
One hundred eighty four patients (18 cHCC-CCA; 166 HCC) with tumor burden beyond UCSF criteria underwent liver transplantation with statistically significantly better overall survival, disease free-survival and lower recurrence rate (all p<0.001) for patients with HCC beyond UCSF criteria compared to patients with cHCC-CCA beyond UCSF criteria. Five-year overall survival for HCC was 67.3% versus 15.8% for cHCC-CCA.
Liver transplantation for tumor burden beyond Milan, but within UCSF criteria
Fourteen patients with cHCC-CCA and 162 patients with HCC with tumor burden beyond Milan criteria but within UCSF criteria underwent liver transplantation. There was no statistically significant difference in overall survival. 5-year overall survival for cHCC-CCA was 64.3% and HCC was 67.3%, (p=0.094). There was a statistically significant difference with longer disease-free survival for HCC, as well as a lower recurrence rate (p=0.013 and <0.001 respectively).
Liver transplantation for tumor burden within Milan criteria
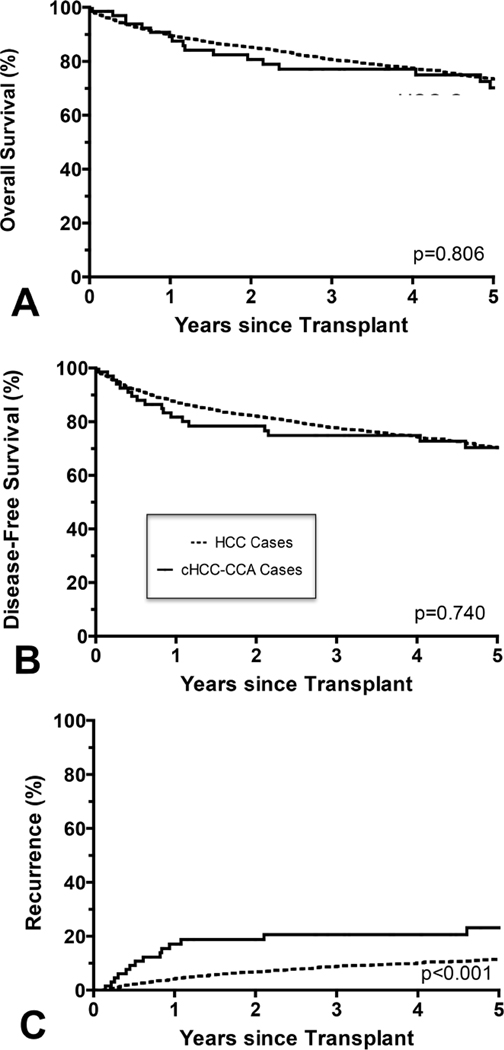
The majority of patients that underwent liver transplantation had tumor burden within Milan criteria (67 cHCC-CCA, 1814 HCC) (Table 4). In this cohort, there was no statistically significant difference in overall survival (5-yr cHCC-CCA 70.1%; HCC 73.4%) (Figure 2a) or disease-free survival (Figure 2b) (both p>0.7). There was a statistically significant difference in recurrence rates with HCC having a lower recurrence rate (11.5% at 5 years) compared to cHCC-CCA (23.1% at 5 years) (p<0.001) (Figure 2c).
Table 4:
Outcomes for Liver Transplantation for Cases within Milan Criteria
| Years since operation | Overall survival, % | Disease-specific survival, % | Disease-free survival, % | Recurrence rate, % | ||||
|---|---|---|---|---|---|---|---|---|
| cHCC-CCA | HCC | cHCC-CCA | HCC | cHCC-CCA | HCC | cHCC-CCA | HCC | |
| 1 | 89.1 | 89.8 | 90.5 | 98.3 | 81.7 | 87.3 | 17.1 | 4.2 |
| 3 | 77.1 | 80.7 | 81.6 | 94.5 | 74.8 | 77.7 | 20.6 | 8.8 |
| 5 | 70.1 | 73.4 | 76.4 | 91.9 | 70.1 | 70.3 | 23.1 | 11.5 |
| Median | N/A | 13.0 | N/A | N/A | N/A | 12.7 | N/A | N/A |
| p Value | 0.806 | <0.001 | 0.740 | <0.001 | ||||
cHCC-CCA cases, n=67; HCC cases, n=1814
HCC, hepatocellular carcinoma; cHCC-CCA, combined hepatocellular-intrahepatic cholangiocarcinoma
Figure 2.
(A) In patients with tumor burden within Milan criteria, the overall survival rate for combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CCA) was comparable to the overall survival rate for HCC (p=0.806). (B) Similarly, there was no significant difference in disease-free survival (p=0.740). (C) The recurrence rate was significantly higher for cHCC-CCA within Milan criteria compared to HCC within Milan Criteria lower (p<.001).
Discussion
Liver transplantation for cHCC-CCA was associated with better overall survival and disease-free survival as well as lower recurrence rates than resection for cHCC-CCA, irrespective of the tumor burden (all p<0.05). Specifically, for cHCC-CCA within Milan criteria, liver transplantation was associated with improved disease-free survival when compared to resection for cHCC-CCA. This is consistent with findings for transplant versus resection for HCC (4). Ideally, all medically and socially eligible cHCC-CCA patients should undergo liver transplantation given the superior outcomes and better long-term survival compared to resection. However, given the shortage of available livers for transplantation, it is imperative that outcomes for liver transplant for cHCC-CCA are equivalent to patients undergoing transplant for other currently accepted indications for liver transplantation. We compared the outcomes of liver transplantation for cHCC-CCA to transplantation for HCC. When comparing liver transplantation for cHCC-CCA and HCC within Milan, despite higher cHCC-CCA recurrence rates, there was no significant difference in overall survival. For greater tumor burden, survival outcomes were worse for patients with cHCC-CCA than HCC.
A study of the SEER and UNOS databases concluded worse outcomes for transplant for patients with cHCC-CCA compared to HCC, and similar outcomes for resection and transplant for cHCC-CCA; however, that study did not differentiate outcomes based on size and lacked granular data due to the retrospective use of large databases. There was no data on pre-operative locoregional therapy (17). In our analysis, we found that there was a difference in liver transplant outcomes for cHCC-CCA based on pre-operative tumor burden, and patients with cHCC-CCA beyond Milan criteria had worse outcomes following transplantation than those with similarly sized HCC. Unlike this study, overall survival for transplantation of tumors within Milan criteria in our study was comparable between HCC and cHCC-CCA. Differences in our results and the results from this previously published study could be because of their inability to differentiate outcomes based on tumor burden.
Another small cohort compared survival for liver transplantation for cHCC-CCA with HCC within Milan criteria in only 12 patients and found worse outcomes than patients with HCC but better outcomes than those with intrahepatic cholangiocarcinoma. Interestingly, this group could not identify pre-operative tumor markers or imaging criteria to differentiate these tumors from HCC (15). Similar to this small study, we found a difference in transplant outcomes for the entire cohort regardless of tumor burden, but when analyzing the data in subsets based on tumor burden, we found that liver transplantation for cHCC-CCA was comparable to transplant for HCC within Milan criteria. Additionally, very small number of cases was a limitation of that study.
Some studies have similarly reported positive outcomes for patients with cHCC-CCA undergoing liver transplantation. Facciuto et al. from Mount Sinai, in a retrospective review, analyzed 32 patients with intrahepatic cholangiocarcinoma and mixed cHCC-CCA on pathology (only half were cHCC-CCA), and found 78% 5-year survival following liver transplant in patients with early-stage tumors within Milan criteria (18). Sapisochin et al. published a multicenter study from Spain of 27 patients with cHCC-CCA (15 patients) or intrahepatic cholangiocarcinoma (total 27 patients) matched 1:2 with controls of HCC patients. While discordant outcomes were seen in the intrahepatic cholangiocarcinoma group compared to those with HCC, the cHCC-CCA patients had outcomes similar to the HCC controls with 5-year survival of 78% for the cHCC-CCA group (9). Furthermore, patients with single small tumors (2 cm or less) had better outcomes than patients with multinodular tumors or tumors > 2 cm (10).
Lunsford et al. matched 12 patients with cHCC-CCA 3:1 with patients with HCC based on pre-transplant characteristics and found that low-grade, well to moderately differentiated cHCC-CCA have good outcomes with low risk of recurrence (20). One limitation of this study is that pathologic findings are not universally available pre-transplant for cHCC-CCA given the difficulty in differentiation from HCC based on imaging findings. Similarly, Antwi et al. with 19 cHCC-CCA patients found that overall survival post liver transplant was good for patients with either cHCC-CCA or HCC (21). Each of these small single-center reports corroborates our findings in this larger multicenter cohort of positive outcomes for transplantation for cHCC-CCA, especially for cHCC-CCA with favorable features and tumor burden within Milan criteria.
Limitations
While this study has many merits of being a multicenter study with a large cohort, it was a retrospective review and has some associated limitations. As discussed in methods, the actual pathology slides were not centrally re-reviewed. Limitations to a central re-review included the transfer of actual slides, the burden to review over 3000 cases, and concern that limitations in slide availability might reduce the potential number of available cHCC-CCA cases. Additionally, we felt the study results should realistically be applicable with the pathology available at each transplant program. While the definition of cHCC-CCA cases is quite nuanced and the classification recently published (2), it would be unrealistic to make clinical management recommendations based on every primary liver tumor being centrally reviewed long-term. Additionally, some of these tumors undergo locoregional therapy, resection and transplantation without prior biopsy for pathologic diagnosis.
For similar reasons, we chose to use the tumor burden available on pre-operative imaging. We discussed using actual tumor burden from final pathology, but post-operative pathology is not a criterion that can be used in determining candidacy for liver transplantation. Transplant candidacy is determined based on pre-operative imaging, and this should be consistent for an indication for transplantation even for cHCC-CCA.
Future Study
Future research is needed to develop the ability to better diagnose cHCC-CCA via imaging criteria pre-operatively. Ideally, this would be similar to the currently used LIRADS or OPTN criteria for HCC. Based on our data, liver transplantation for patients with cHCC-CCA within Milan criteria, even those with a known pre-transplant diagnosis could be considered for liver transplantation. Further discussion is required regarding possible MELD exception points for patients with cHCC-CCA. Criteria for liver transplantation for patients with cHCC-CCA should be defined and prospective data should be collected on the outcomes of transplantation for cHCC-CCA.
Conclusions
Regardless of tumor burden, outcomes following liver transplantation are superior to resection for patients with cHCC-CCA. Within Milan criteria, liver transplantation for cHCC-CCA results in overall survival similar to liver transplantation for HCC justifying consideration of transplantation due to the higher chance of cure in this traditionally excluded population.
Acknowledgement
The authors would like to acknowledge Daniel Cloonan, MD for his artwork for the visual abstract.
Abbreviations:
- HCC
hepatocellular carcinoma
- cHCC-CCA
combined hepatocellular-cholangiocarcinoma
- iCCA
intrahepatic cholangiocarcinoma
- MELD
Model for End Stage Liver Disease
- UCSF
University of California San Francisco
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Chapman is a member of the Novartis Scientific Advisory Board, serves on the Board of Directors for Mid-America Transplant, and receives payment for intellectual property from Pathfinder Therapeutics. Dr Doyle is a member of the Novartis Scientific Advisory Board.
Selected for the 2020 Southern Surgical Association Program.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen RA, Lisa JR. Combined Liver Cell and Bile Duct Carcinoma. .Am. J. Pathol; 1949;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 2.Brunt E, Aishima S, Clavien P-A, et al. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. .Hepatology; 2018;68:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: Demographic, clinical, and prognostic factors. .Cancer; 2002;94:2040–2046. [DOI] [PubMed] [Google Scholar]
- 4.Chapman WC, Klintmalm G, Hemming A, et al. Surgical Treatment of Hepatocellular Carcinoma in North America: Can Hepatic Resection Still Be Justified? .J. Am. Coll. Surg. American College of Surgeons; 2015;220:628–637. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. .N. Engl. J. Med. M; 1996;334:693–700. [DOI] [PubMed] [Google Scholar]
- 6.Doyle MBM, Vachharajani N, Maynard E, et al. Liver Transplantation for Hepatocellular Carcinoma: Long-Term Results Suggest Excellent Outcomes. .J. Am. Coll. Surg. American College of Surgeons; 2012;215:19–28. [DOI] [PubMed] [Google Scholar]
- 7.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. .Gastroenterology.; 2012;143:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. .Liver Transplant; 2000;6:309–316. [DOI] [PubMed] [Google Scholar]
- 9.Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. Wiley Subscription Services, Inc., A Wiley Company; 2011;17:934–942. [DOI] [PubMed] [Google Scholar]
- 10.Sapisochin G, De Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: A spanish matched cohort multicenter study. .Ann. Surg; 2014;259:944–952. [DOI] [PubMed] [Google Scholar]
- 11.Lee DD, Croome KP, Musto KR, et al. Liver transplantation for intrahepatic cholangiocarcinoma. .Liver Transplant; 2018;24:634–644. [DOI] [PubMed] [Google Scholar]
- 12.Shimoda M, Farmer DG, Colquhoun SD, et al. Liver transplantation for cholangiocellular carcinoma: Analysis of a single-center experience and review of the literature. .Liver Transplant; 2001;7:1023–1033. [DOI] [PubMed] [Google Scholar]
- 13.Robles R, Figueras J, Turrión VS, et al. Spanish Experience in Liver Transplantation for Hilar and Peripheral Cholangiocarcinoma. .Ann. Surg; 2004;239:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong JC, Jones CM, Duffy JP, et al. Comparative Analysis of Resection and Liver Transplantation for Intrahepatic and Hilar Cholangiocarcinoma. . 2014;146:683–689. [DOI] [PubMed] [Google Scholar]
- 15.Panjala C, Senecal DL, Bridges MD, et al. The Diagnostic Conundrum and Liver Transplantation Outcome for Combined Hepatocellular-Cholangiocarcinoma. .Am. J. Transplant. Blackwell Publishing Inc; 2010;10:1263–1267. [DOI] [PubMed] [Google Scholar]
- 16.Elshamy M, Presser N, Hammad AY, et al. Liver transplantation in patients with incidental hepatocellular carcinoma/cholangiocarcinoma and intrahepatic cholangiocarcinoma: a single-center experience. .Hepatobiliary Pancreat. Dis. Int; 2017;16:264–270. [DOI] [PubMed] [Google Scholar]
- 17.Groeschl RT, Turaga KK, Gamblin TC. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. .J. Surg. Oncol; 2013;107:608–612. [DOI] [PubMed] [Google Scholar]
- 18.Facciuto ME, Singh MK, Lubezky N, et al. Tumors with intrahepatic bile duct differentiation in cirrhosis: Implications on outcomes after liver transplantation. .Transplantation; 2015;99:151–157. [DOI] [PubMed] [Google Scholar]
- 19.Maganty K, Levi D, Moon J, et al. Combined Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: Outcome After Liver Transplantation. .Dig. Dis. Sci. Springer; US; 2010;55:3597–3601. [DOI] [PubMed] [Google Scholar]
- 20.Lunsford KE, Court C, Seok Lee Y, et al. Propensity-Matched Analysis of Patients with Mixed Hepatocellular-Cholangiocarcinoma and Hepatocellular Carcinoma Undergoing Liver Transplantation. .Liver Transplant. AASLD; 2018;24:1384–1397. [DOI] [PubMed] [Google Scholar]
- 21.Antwi SO, Habboush YY, Chase LA, et al. Response to Loco-Regional Therapy Predicts Outcomes After Liver Transplantation for Combined Hepatocellular-Cholangiocarcinoma. .Ann. Hepatol. Elsevier BV; 2018;17:969–979. [DOI] [PubMed] [Google Scholar]
- 22.Sammon J, Fischer S, Menezes R, et al. MRI features of combined hepatocellular-cholangiocarcinoma versus mass forming intrahepatic cholangiocarcinoma. .Cancer Imaging; 2018;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potretzke TA, Tan BR, Doyle MB, et al. Imaging features of biphenotypic primary liver carcinoma (hepatocholangiocarcinoma) and the potential to mimic hepatocellular carcinoma: LIRADS analysis of CT and MRI features in 61 cases. .Am. J. Roentgenol.; 2016;207:25–31. [DOI] [PubMed] [Google Scholar]
- 24.Gigante E, Ronot M, Bertin C, et al. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. .Liver Int; 2019;39:2386–2396. [DOI] [PubMed] [Google Scholar]
- 25.Balitzer D, Joseph NM, Ferrell L, et al. Immunohistochemical and molecular features of cholangiolocellular carcinoma are similar to well-differentiated intrahepatic cholangiocarcinoma. .Mod. Pathol.; 2019;32:1486–1494. [DOI] [PubMed] [Google Scholar]
- 26.Bergquist JR, Groeschl RT, Ivanics T, et al. Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. .HPB.; 2016;18:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakka K, Jiang R, Alese OB, et al. Clinical outcomes of rare hepatocellular carcinoma variants compared to pure hepatocellular carcinoma. .J. Hepatocell. Carcinoma; 2019;Volume 6:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology; 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]