Abstract
Background
A possible oncogenic role of human papillomavirus (HPV) in head and neck cancers (mainly oropharynx tumors) has been suggested. This significant association has been considered true for oropharynx tumors; however, the association between HPV infection and laryngeal carcinomas is yet to be established. The aim of this study was to evaluate the relationship between p16 expression and long-term overall, disease-free, and disease-specific survival (OS, DF, and DSS, respectively) in patients surgically treated for laryngeal carcinoma.
Materials and Methods
Seventy-four previously untreated laryngeal carcinoma patients who underwent surgical treatment were considered for this retrospective study. The tissue specimens were processed for immunohistochemical p16 protein (surrogate HPV marker) detection.
Results
Survival analysis of the p16 expression of the primary tumor showed that the 5-year OS rates were 90% and 29.7% for the p16-positive and negative groups, respectively (P = .003). The 5-year DFS and DSS also differed between both groups (P < .001), whereas the 5-year DSS seemed to be related to tumor/lymph node classification and p16 expression. However, only p16 expression was identified as an independent prognostic factor associated with OS and DSS.
Conclusions
Surgically treated p16-positive laryngeal cancer patients may represent a subset of patients with a better prognosis than their p16-negative counterparts.
Keywords: laryngeal cancer, p16 overexpression, human papillomavirus, disease-specific survival, cancer biomarkers
Introduction
Over the years, evidence on the possible oncogenic role of the human papillomavirus (HPV) in head and neck cancers has accumulated with previous studies.1 Namely, an important and consistent association between HPV and oropharyngeal tumors has emerged across different countries and ethnic groups.2-4
HPV involvement in head and neck cancers has been associated with a better prognosis and is linked to a better response to chemo-radiotherapy treatment.5 HPV-positive oropharyngeal tumors are therefore a subset of head and neck tumors, also referred to as HPV-related tumors, although the HPV prognostic value for tumors of other head and neck sites remains unclear. In that regard, immunohistochemical (IHC) staining for p16 protein is used as a surrogate marker for identifying HPV infection. p16 is a tumor suppressor protein encoded by the CDKN2A gene (9p21.3), which prevents progression into the cell cycle S phase by inhibiting cyclin D-dependent protein kinases (CDK4 and 6), thereby maintaining Rb in its hypo-phosphorylated state and preventing its dissociation from the E2F transcription factor. HPV tumorigenesis is primarily thought to be driven by the HPV E6 and E7 proteins, which decrease p53 and functional Rb tumor suppressor protein levels, leading to aberrant overexpression of the cell cycle protein p16INK4a.6 Although the relationship between HPV and laryngeal squamous cell carcinoma (SCC) remains controversial,7-11 the concept of multistep progression by the accumulation of numerous genetic mutations underlying the neoplastic transformation process and its transition from precancerous to invasive carcinoma has been reported.12,13 Thus, the aim of this study was to evaluate the prognostic role of p16 expression in laryngeal SCC patients treated with surgery on long-term overall survival, disease-free survival, and disease-specific survival (OS, DF, and DSS, respectively).
Materials and Methods
Patients
The Institutional Review Board approved the study before it was launched. Previously untreated laryngeal carcinoma patients who underwent surgical treatment between January 2009 and December 2014 at the Otolaryngology Department of Health Sciences, University of Catanzaro, were considered for this retrospective study. Meanwhile, the patients with metastatic disease or synchronous tumors at the time of diagnosis were excluded. For each patient, clinical-anamnestic data, including age, sex, primary tumor site, histopathology, and TNM staging and classification, based on the seventh Edition established by the American Joint Committee for Cancer, were collected from the hospital database. The data for the smoking and alcohol consumption habits of each patient were also included. To collect the data on smoking habits, non-smokers were defined as those who never smoked or had stopped smoking, whereas smokers were defined as patients who had smoked regularly before or were still active smokers. Additionally, the patients were asked about the number of cigarettes they smoked, which was used to calculate their respective pack years as well. Regarding the data on alcohol consumption, the patients who were reported to drink alcohol regularly were defined as alcohol drinkers, whereas the non-alcohol drinkers were defined as those who never drank alcohol or those who only drank on rare occasions with less than two drinks per day. Finally, the data recorded were related to any disease recurrence and locoregional or distant metastases detected during follow-up every 3 months.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue specimens were obtained from surgical pathology and processed at our Department of Pathology. Multiple sections (at least five sections), as previously described, were obtained from these tissue specimens.14 The slides were dewaxed in xylene, hydrated using graded ethanol mixtures, incubated for 30 minutes in .3% hydrogen peroxide (H2O2)/methanol to quench endogenous peroxidase activity, and rinsed for 20 minutes with phosphate-buffered saline (PBS; Bio-Optica, Milan, Italy). Afterward, these sections were heated (5 minutes × 3) in capped polypropylene slide-holders with citrate buffer (10 mM citric acid, .05% Tween 20, pH 6.0; Bio-Optica, Milan, Italy) using a microwave oven (750 W) to unmask the antigenic sites. The blocking step was performed before the primary antibody application with 5% bovine serum albumin (BSA; Sigma, Milan, Italy), a non-specific antibody binding blocking agent, in PBS for 1 hour in a humid chamber. The sections were incubated overnight at 4°C with rabbit polyclonal anti-p16 antibody (CINtec® INK4a; Roche Diagnostics, Indianapolis, Indiana, USA), which was ready for use in PBS (Sigma, Milan, Italy). On the following day, the secondary antibody, which was a biotinylated anti-rabbit antibody, was applied for 30 minutes at room temperature, followed by the avidin–biotin–peroxidase complex (Vector Laboratories, Burlingame, CA, USA) for another 30 minutes at the same temperature. The immunoreaction was visualized by incubating the sections for 4 minutes in .1% 3,3′-diaminobenzidine (DAB) and .02% H2O2 solution (DAB substrate kit, Vector Laboratories, CA, USA). The sections were lightly counterstained with Mayer’s hematoxylin (Histolab Products AB, Göteborg, Sweden), mounted in glycerol vinyl alcohol mounting solution (GVA, Zymed Laboratories, San Francisco, CA, USA), and observed under a Zeiss Axioplan light microscope (Carl Zeiss, Oberkochen, Germany).
Evaluation of Immunohistochemistry
Immunostained slides were separately evaluated using light microscopy by two pathologists who were blinded to patient identity, clinical status, and group identification. Specifically, the p16 staining status of the specimens was identified as either negative or positive. IHC staining was defined as the presence of brown chromogen within the nucleus, wherein p16 staining intensity was classified as negative (0), weak (1), moderate (2), or strong (3), as described previously.14 Additionally, the percentage of positive cells was used to score the specimens as follows: (1) none (score: 0); (2) 1%–10% stained cells (score: 1); (3) 11%–30% stained cells (score: 2); (4) 31%–50% stained cells (score: 3); (5) 51%–80% stained cells (score: 4); and (6) >80% stained cells (score: 5). Moreover, the staining intensity was multiplied by the percentage of positive cells to obtain the intensity reactivity score (IRS), which became the basis for the classification of our patients into three groups: (1) IRS = 0 (no expression), (2) IRS <10 (low expression), and (3) IRS >10 (high expression).
Statistical Analysis
Statistical analyses were performed with the MedCalc software (Belgium) using the chi-squared and Fisher’s exact tests, in which the data were described as means, medians, and standard deviations. Pearson’s chi-squared or Fisher’s exact test was used to identify the differences between the demographic and clinicopathologic data of the cohorts, and the survival analysis variables included age, T and N status, adjuvant therapy, tumor subsite, alcohol and smoking habits, and p16 status. The Kaplan–Meier method was also used for survival analysis, and the log-rank test was used to compare the survival curves between groups. Additionally, multivariate analysis was performed using multiple regression analysis to determine the independent prognostic factors in this study. Among these factors, OS was defined as the time interval from surgery to adjuvant treatment until death (from any cause), whereas DF and DSS were defined as the duration from treatment till locoregional recurrence and the duration from treatment till death due to the disease, respectively. All deaths due to other causes were considered censored, and all the analyses had a statistical significance set at P < .05.
Results
The study was carried out with 74 patients, consisting of 68 men and 6 women with a median age of 70 years (range: 41–88 years). Of the 74 patients, 66 (89.1%) were smokers, 8 were non-smokers, and 2 of them reported having stopped smoking. Regarding alcohol consumption, 58 (78.3%) patients were non-drinkers and 16 were drinkers. Regarding tumor location, 28 (37.8%) tumors had a transglottic location, 20 (27%) tumors had a supraglottic location, and 26 (35.2%) had glottic tumors. Histological grading of the respective tumors was G1 in 8 (10.8%), G2 in 44 (59.4%), and G3 (29.8%) in 22 patients. Regarding T and N status, 34 patients were classified as T1–T2 (16 glottic, 10 transglottic, and 8 supraglottic tumors), while 40 were classified as T3–T4 (22 transglottic, 10 glottic, and 8 supraglottic tumors). Moreover, the lymph node clinical status was classified as N0 in 26, N1 in 20, and N2 in 28 patients. A total of 56 patients underwent neck dissection and 19 underwent surgery plus radiotherapy.
p16 Expression
Nuclear p16 expression in tumor cells was detected in 20 of the 74 (27.02%) laryngeal carcinomas, wherein all cases showed high p16 expression (IRS > 10). As reported in the literature,14 we excluded the fraction of laryngeal SCC that had low p16 expression (IRS < 10). The correlation between clinical data and p16 expression is shown in Table 1, showing that p16 expression correlated significantly with N clinical classification.
Table 1.
Correlation Between p16 Expression and Clinic-Pathological Patient Data.
| Clinical data | N (%) | P16 expression | |
|---|---|---|---|
| Negative | Positive | ||
| Age (year) | |||
| ≤60 | 26 (35.1) | 20 | 6 |
| >60 | 48 (64.9) | 34 | 14 |
| P = 1.00 | |||
| Smoking habit | |||
| Smokers | 66 (89.1) | 50 | 16 |
| No-smokers | 8 (10.9) | 4 | 4 |
| P = .29 | |||
| Alcohol habit | |||
| Drinker | 58 (78.3) | 44 | 14 |
| No-drinker | 16 (21.7) | 10 | 6 |
| P = .64 | |||
| Primary site | |||
| Glottic | 26 (35.1) | 18 | 8 |
| Trans-supraglottic | 48 (64.9) | 36 | 12 |
| P = .71 | |||
| Histologic grade | |||
| G1 | 8 (10.9) | 8 | 0 |
| G2-G3 | 66 (89.1%) | 46 | 20 |
| P = .55a | |||
| T classification | |||
| T1-T2 | 34 (45.9) | 24 | 10 |
| T3-T4 | 40 (54.1%) | 30 | 510 |
| P = 1.00 | |||
| N clinical status | |||
| N0 | 26 (35.1) | 14 | 12 |
| N+ | 48 (68) | 30 | 8 |
| P = .02 | |||
| Treatment | |||
| Surgery alone | 36 | 22 | 14 |
| Surgery plus RT/CT | 38 | 34 | 6 |
| P = .14 | |||
aChi-squared test.
Survival Analysis
The median follow-up period was 52 months (range: 6–128 months), with 32 (43.2%) patients remaining alive. The 5-year OS was 45.9% (34/74), while both the 5-year DSS and DFS rates were 59.5% (44/74). The 5-year OS appears to be better in younger patients who are non-smokers with glottic tumors at an early T stage, clinically classified as N negative and p16-positive. In the univariate analysis, the age of less than 60 years and p16-positive status reached significance. On the other hand, the 5-year DSS seems to be related to the T and N classification and p16 expression (univariate analysis, P = .003) (Table 2). On multivariate analysis, only p16 expression was identified as an independent prognostic factor associated with OS and DSS (log-rank P = .009 and P = .001, respectively).
Table 2.
Univariate and Multivariate Analysis of Variables Correlated to 5-Year Overall Survival, Disease-Free Survival, and Disease-Specific Survival.
| Variables | N | 5-y overall survival | 5-y disease-specific survival 5-y disease-free survival | ||||
|---|---|---|---|---|---|---|---|
| % | P-value Univariate | P-value Multivariate | % | P-value Univariate | P-value Multivariate | ||
| Age (year) | .01 | .08 | .08 | .15 | |||
| ≤60 | 26 | 79.9 | 76.9 | ||||
| >60 | 48 | 29.1 | 50.0 | ||||
| Smoking habit | .06 | .07 | .17 | .98 | |||
| Smokers | 66 | 35.5 | 54.4 | ||||
| No-smokers | 8 | 50.0 | 100 | ||||
| Alcohol habit | .20 | .40 | .31 | .12 | |||
| Drinker | 58 | 44.8 | 55.1 | ||||
| No-drinker | 16 | 50.0 | 75.0 | ||||
| Localization | .14 | .32 | .25 | .84 | |||
| Glottic | 26 | 61.5 | 69.2 | ||||
| T-supraglottic | 48 | 44.8 | 54.7 | ||||
| Histologic grade | .82 | .72 | .81 | .31 | |||
| G1 | 8 | 45.4 | 50.0 | ||||
| G2-G3 | 66 | 50.0 | 60.1 | ||||
| T classification | .20 | .09 | .20 | .96 | |||
| T1-T2 | 34 | 58.8 | 70.5 | ||||
| T3-T4 | 40 | 35.0 | 50.0 | ||||
| N clinical status | .19 | .52 | .62 | .74 | |||
| N0 | 26 | 61.5 | 61.5 | ||||
| N+ | 48 | 37.5 | 58.3 | ||||
| Treatment | .41 | .21 | .20 | .96 | |||
| Surgery alone | 36 | 55.5 | 72.2 | ||||
| Surgery plus RT/CT | 38 | 36.8 | 47.3 | ||||
| P16 expression | .01 | .009 | .003 | .05 | |||
| Positive | 20 | 85.7 | 100 | ||||
| negative | 54 | 27.7 | 44.4 | ||||
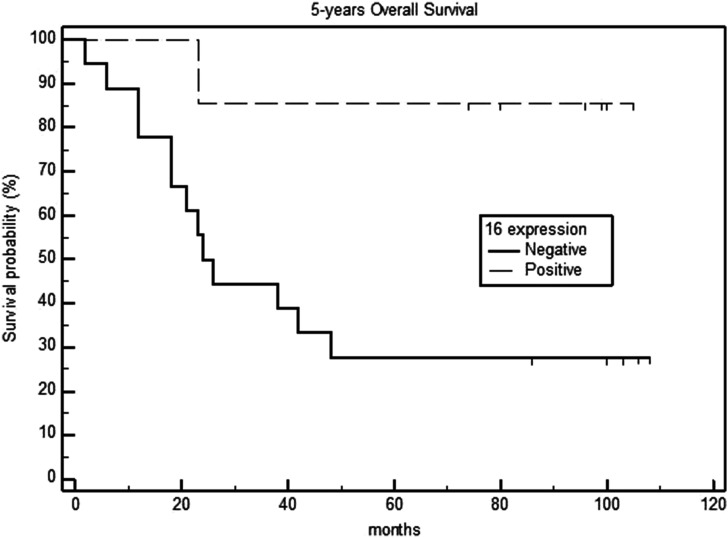
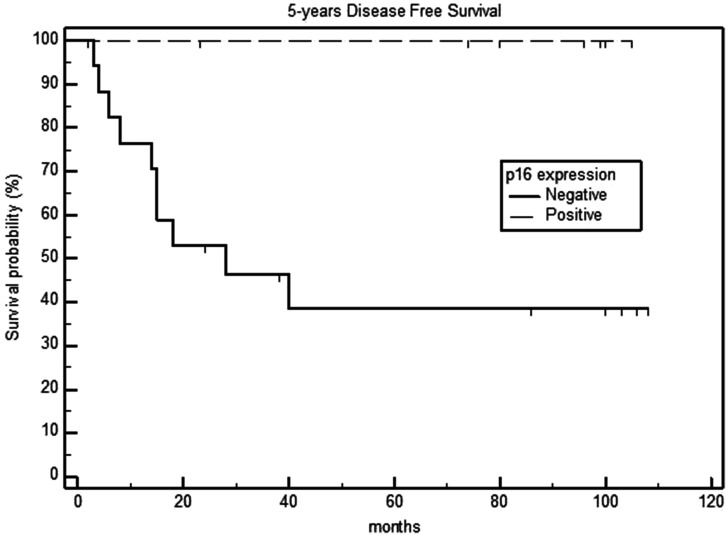
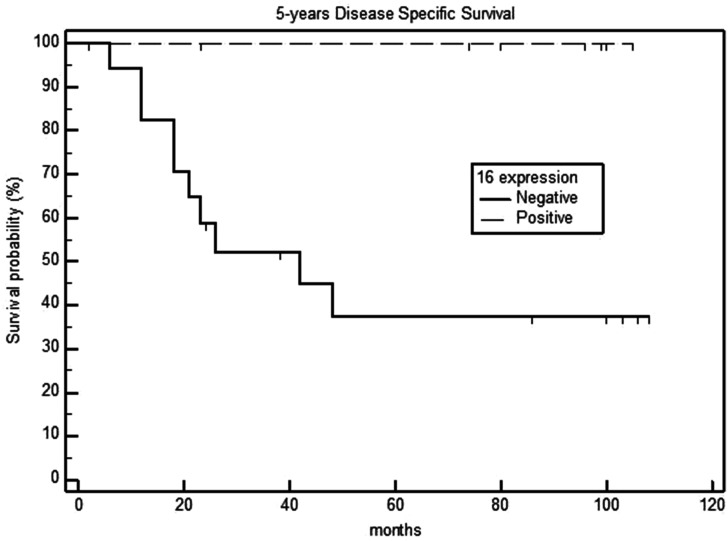
Survival analysis in relation to the p16 expression of primary tumors showed that the 5-year OS rates were 90% and 29.7% for the p16-positive and negative groups, respectively (P = .003) (Figure 1). Additionally, the 5-year DSS and DFS rates were 100% and 44.4% in the p16-positive and negative groups, respectively (P <.001) (Figures 2 and 3), with a median DFS of 19 months (range: 6–40 months).
Figure 1.
Five-year overall survival in p16-negative and positive laryngeal carcinomas.
Figure 2.
Five-year disease-free survival in p16-negative and positive laryngeal carcinomas.
Figure 3.
Five-year disease-specific survival in p16-negative and positive laryngeal carcinomas.
Specific Causes of Death
Thirty (40.5%) patients died of neoplastic causes. Six (20%) patients had tumor progression or recurrence, 8 (26.7%) had lymph node metastasis, and 16 (53.3%) had distant metastases or secondary tumors (lung, 8; esophageal, 4; liver, 1; brain tumor, 1; leukemia, 1; and prostate cancer with bone metastases, 1).
Discussion
In our study, we only considered laryngeal cancer patients without synchronous or metastatic tumors at diagnosis and were treated surgically, whereas 27.02% of the examined tumors showed p16 overexpression, showing data close to that found by other authors.1,15-17 Based on these results, the p16-positive patients had a more favorable prognosis than the p16-negative patients, and the p16-positive laryngeal carcinoma patients had less aggressive cancer behaviors and a lower risk of metastasis to the laterocervical and distant lymph nodes. On the other hand, the overall 5-year survival was found to be worse in older smokers and p16-negative patients, but the most relevant data seemed to emerge from the DSS analysis. P16-positive patients exhibit different biological behaviors with a lower propensity for progression and distant metastasis. In our study, DFS and DSS were 100% in p16-positive patients vs 44.4% in p16-negative patients, indicating that surgically treated p16-positive laryngeal patients represent a subset of patients with a better prognosis than p16-negative patients. Studies on the role of HPV in laryngeal tumors have reported widely divergent and discordant results for the prevalence and prognostic role of HPV infection. Furthermore, only a few studies have reported adequate follow-up and disease-specific survival durations.18 Thus, little is known about the prognostic significance of p16 overexpression in laryngeal tumors, and the studies conducted so far have considered patients undergoing different treatment modalities, with a few studies reporting on specific survival and causes of death.8,11,19 Hermandez et al.15 did not find a significant OS difference associated with HPV status, although they also did not report on the treatment modality and DSS. Similarly, in a study by Young et al.,19 there were no significant OS and DFS differences two years after treatment, even if none of the p16-positive patients developed distant metastases. Dahm et al.,8 in a study of 85 patients treated with different modalities, did not find significant OS differences; however, survival in p16-positive patients was better even if the difference did not reach statistical significance by two years. Moreover, our data are consistent with the results of Sanchez et al.18 and Chen et al.20 The first of these studies considered glottic cancer, mainly T1 treated surgically, and concluded that p16-positive tumors have a better disease-free survival with no relapses after a 2-year follow-up. A study by Chen et al. included 106 patients with laryngeal carcinoma treated surgically and reported a DSS and a DFS of 100% in p16-positive patients, although the study included patients with synchronous tumors at diagnosis. Despite our findings, the present study had certain limitations related to the limited number of patients taken into consideration, its retrospective nature, and the IHC evaluation of p16 expression as the only surrogate of HPV infection. However, the strengths of the study include the homogeneity of the selected patients, all of whom underwent surgical treatment within a limited period and long-term follow-up.
Conclusions
Surgically treated p16-positive laryngeal cancer patients may represent a subset of patients with a better prognosis than p16-negative patients. Laryngeal cancers can benefit from different treatment modalities depending on the subsite and staging; therefore, further studies with more patients who are selected homogeneously based on the type of treatment and adequate follow-up will be necessary to better understand the prognostic significance of p16 overexpression.
Footnotes
Authors Contributions: Eugenia Allegra is the corresponding author and was involved in the study design, data acquisition analysis and interpretation, and drafting of the manuscript. Maria Rita Bianco was involved in data acquisition, interpretation, and assisted with the preparation and revision of the manuscript. Maria Grasso was involved in data acquisition and statistical analysis; Chiara Mignogna and Rosario Caltabiano were involved in data acquisition and tumor pathology review. Lidia Puzzo was involved in data acquisition, interpretation, and assisted with the preparation and final version of the manuscript. All authors have read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement: This study was deemed exempt from the institutional review board. Approval and informed consent were waived. This study was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Availability of Data and Materials: Data supporting the results of this study can be obtained from the corresponding author under reasonable request.
ORCID iD
Eugenia Allegra https://orcid.org/0000-0002-5202-4333
References
- 1.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomark Prev. 2005;14(2):467-475. [DOI] [PubMed] [Google Scholar]
- 2.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Canc. 2017;140:1968-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellsagué X, Mena M, Alemany L. Epidemiology of HPV-positive tumors in Europe and in the world. Recent Results Cancer Res. 2017;206:27-35. [DOI] [PubMed] [Google Scholar]
- 4.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118-128. [DOI] [PubMed] [Google Scholar]
- 5.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366(6456):704-707. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi N, Ahmadi N, Chan MV, Huo YR, Sritharan N, Chin R. Laryngeal squamous cell carcinoma survival in the context of human papillomavirus: a systematic review and meta-analysis. Cureus. 2018;10(2):e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahm V, Haitel A, Kaider A, Stanisz I, Beer A, Lill C. Cancer stage and pack-years, but not p16 or HPV, are relevant for survival in hypopharyngeal and laryngeal squamous cell carcinomas. Eur Arch Oto-Rhino-Laryngol. 2018;275(7):1837-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duray A, Descamps G, Arafa M, et al. High incidence of high-risk HPV in benign and malignant lesions of the larynx. Int J Oncol. 2011;39(1):51-59. [DOI] [PubMed] [Google Scholar]
- 10.Gallo A, Degener AM, Pagliuca G, et al. Detection of human papillomavirus and adenovirus in benign and malignant lesions of the larynx. Otolaryngology-Head Neck Surg. 2009;141(2):276-281. [DOI] [PubMed] [Google Scholar]
- 11.Lam EWH, Chan MMH, Wai CKC, et al. The role of human papillomavirus in laryngeal cancer in Southern China. J Med Virol. 2018;90(6):1150-1159. [DOI] [PubMed] [Google Scholar]
- 12.Allegra E, Baudi F, La Boria A, Fagiani F, Garozzo A, Costanzo FS. Multiple head and neck tumours and their genetic relationship. Acta Otorhinolaryngol Ital. 2009;29:237-241. [PMC free article] [PubMed] [Google Scholar]
- 13.Allegra E, Trapasso S. Cancer stem cells in head and neck cancer. OncoTargets Ther. 2012;5:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allegra E, Caltabiano R, Amorosi A, Vasquez E, Garozzo A, Puzzo L. Expression of BMI1 and p16 in laryngeal squamous cell carcinoma. Head Neck. 2013;35(6):847-851. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez BY, Goodman MT, Lynch CF, et al. Human papillomavirus prevalence in invasive laryngeal cancer in the United States. PLoS One. 2014;9(12):e115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meshman J, Wang P-C, Chin R, et al. Prognostic significance of p16 in squamous cell carcinoma of the larynx and hypopharynx. Am J Otolaryngol. 2017;38(1):31-37. [DOI] [PubMed] [Google Scholar]
- 17.Bussu F, Sali M, Gallus R, et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker?. Br J Canc. 2013;108(5):1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sánchez Barrueco A, González Galán F, Villacampa Aubá JM, et al. P16 influence on laryngeal squamous cell carcinoma relapse and survival. Otolaryngology-Head Neck Surg. 2019;160(6):1042-1047. [DOI] [PubMed] [Google Scholar]
- 19.Young RJ, Urban D, Angel C, et al. Frequency and prognostic significance of p16INK4A protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Canc. 2015;112(6):1098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W-C, Chuang H-C, Lin Y-T, Huang C-C, Chien C-Y. Clinical impact of human papillomavirus in laryngeal squamous cell carcinoma: a retrospective study. PeerJ. 2017;5:e3395. [DOI] [PMC free article] [PubMed] [Google Scholar]