Abstract
Background:
Ramucirumab as monotherapy or in combination with paclitaxel is a second-line treatment option recommended for patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma. However, real-world data from large study cohorts focused on ramucirumab plus paclitaxel in gastric cancer are limited.
Methods:
The study population comprised all patients with gastric or GEJ cancer who received ramucirumab plus paclitaxel in South Korea between 1 May 2018 and 31 December 2018. We included patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum and fluoropyrimidine-containing combination chemotherapy.
Results:
In total, 1063 patients were included in the present study. The objective response rate and disease control rate were 15.1% and 57.7%, respectively. The median progression-free survival was 4.03 months (95% confidence interval, 3.80–4.27) and the median overall survival was 10.03 months (95% confidence interval, 9.33–10.73). Grade 3 or higher treatment-related adverse events with incidence of ⩾5% were neutropenia (35.1%) and anemia (10.5%). Based on multivariable analysis, overall survival was negatively associated with Eastern Cooperative Oncology Group performance status ⩾2, weight loss ⩾10% in the previous 3 months, GEJ of primary tumor, poor or unknown histologic grade, number of metastatic sites ⩾3, presence of peritoneal metastasis, no prior gastrectomy, and time to second-line since first-line treatment <6 months.
Conclusion:
Our large-scale, nationwide, real-world data analysis of an unselected real-world population adds evidence for the efficacy and safety of second-line ramucirumab plus paclitaxel in patients with locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma.
Keywords: gastric cancer, paclitaxel, ramucirumab, real-world data
Introduction
Gastric cancer is the sixth most common cancer and the second most common cause of cancer-related death worldwide.1 The incidence of gastric cancer is highest in East Asian countries, accounting for more than 70% of the total global gastric cancer cases.1 Despite recent declines in the incidence of gastric cancer, it remains the most commonly diagnosed cancer and the fourth leading cause of cancer-related death in South Korea.2 Recently published guidelines for the treatment of unresectable locally advanced or metastatic gastric cancer recommend first-line treatment with fluoropyrimidine- and platinum-based combination regimens, with the addition of trastuzumab for patients who have human epidermal growth factor 2 (HER2)-positive tumors.3–5 Despite these treatment options, the prognosis for patients with unresectable locally advanced or metastatic gastric cancer remains dismal.
Vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2)-mediated signaling and angiogenesis contribute to the development and progression of gastric cancer.6,7 Ramucirumab is a VEGFR-2-specific human IgG1 monoclonal antibody approved worldwide as monotherapy, or in combination with paclitaxel, for the treatment of patients with previously treated gastric or gastroesophageal junction (GEJ) adenocarcinoma, based on the results of two phase III trials, REGARD and RAINBOW.8,9 While ramucirumab monotherapy was marginally effective in the REGARD trial, using ramucirumab in combination with paclitaxel is preferred as second-line therapy in patients with good performance status.3–5,10 The pivotal phase III RAINBOW trial demonstrated statistically significant benefits of ramucirumab plus paclitaxel compared with placebo plus paclitaxel in overall survival (OS; median 9.6 versus 7.4 months), progression-free survival (PFS; median 4.4 versus 2.9 months), and objective response rate (ORR; 28% versus 16%) among patients with advanced gastric or GEJ adenocarcinoma previously treated with platinum and fluoropyrimidine.9 The Korean Gastric Cancer Treatment Guidelines recommend ramucirumab plus paclitaxel as a second-line treatment for advanced gastric or GEJ adenocarcinoma,5 and it is currently widely used in South Korea.
Although randomized controlled trials (RCTs), such as the RAINBOW trial, are undoubtedly important for generating data on drug efficacy and safety, they have limited generalizability for the broader actual patient population because only less than 1% of candidate cancer patients participate in RCTs. Real-world data (RWD) from routine clinical practice have become an important tool in adding information to the results of RCTs. However, RWD from large study cohorts focusing on the ramucirumab plus paclitaxel combination in gastric cancer are limited.
Therefore, we conducted a nationwide real-world study to evaluate the efficacy, safety, treatment patterns, and potential factors associated with survival in patients with gastric or GEJ adenocarcinoma who received second-line ramucirumab plus paclitaxel in a real-world setting (KCSG ST19-16).
Patients and methods
Study design and population
In this nationwide, non-interventional study, the efficacy and safety of second-line ramucirumab plus paclitaxel was retrospectively evaluated in a real-world setting in patients with gastric or GEJ adenocarcinoma from South Korea. Second-line ramucirumab plus paclitaxel in patients with locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma has been covered by insurance since 1 May 2018 in South Korea. The study population included all patients with gastric or GEJ cancer who received ramucirumab plus paclitaxel in South Korea between 1 May 2018 and 31 December 2018. We used eligibility criteria similar to the RAINBOW trial by selecting patients with gastric or GEJ adenocarcinoma who received second-line ramucirumab plus paclitaxel treatment. Eligible patients were ⩾18 years of age, had locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma, and experienced disease progression during first-line fluoropyrimidine and platinum-containing combination chemotherapy or within 6 months of the last dose of fluoropyrimidine and platinum-containing adjuvant chemotherapy. Patients who started treatment with ramucirumab alone or who received ramucirumab or paclitaxel before 1 May 2018 were excluded.
The Korean Cancer Society Group (KCSG) conducted this study with funding from the Health Insurance Review & Assessment Service. The study was performed in accordance with the principles of the Good Clinical Practice and was approved by the institutional review board (IRB) of each hospital (Supplemental material Table 1 online). The IRBs of all participating hospitals waived the requirement for informed consent from the patients due to the retrospective nature of this study. This study is registered with ClinicalTrials.gov identifier: NCT04192734.
Study outcomes
Predefined outcomes of the study included OS, PFS, ORR, and treatment-related adverse events (TRAEs). OS was defined as the time from the start date of ramucirumab plus paclitaxel treatment to the date of death or the last follow-up visit for patients still alive. If it was not known whether a patient had died, observations were censored at the last-known-alive date as captured in the medical records. PFS was defined as the time from the start date of ramucirumab plus paclitaxel treatment to disease progression or death from any cause. Tumor response was assessed by investigators using the Response Evaluation Criteria in Solid Tumors criteria (version 1.1).11 ORR indicated the proportion of patients who had achieved complete response (CR) or partial response (PR) as the best overall response. The disease control rate (DCR) indicated the proportion of patients who had achieved CR, PR, or stable disease (SD) (including non-CR/non-progressive disease for non-measurable disease) as the best response. TRAEs were listed as possibly, probably, or definitely related to the ramucirumab or paclitaxel, and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).12 TRAEs were retrospectively collected based on the patients’ medical records and laboratory results.
Data collection
A retrospective review was performed using the medical records of patients with gastric or GEJ adenocarcinoma who received second-line ramucirumab plus paclitaxel. Data included baseline patient and tumor characteristics, treatment data, tumor response, TRAEs, laboratory data, and death. All data were anonymously collected and were managed using an electronic data capture system consisting of filters and a query-generating system to guarantee reliability and control for missing and inconsistent data, as well as errors. Data recorded within 31 December 2019 were collected in our analysis.
Statistical analysis
Data are reported as number and percentage for categorical variables and mean and standard deviation for continuous variables. Chi-squared tests were used to compare percentages and two sample t-tests were used to compare mean values. PFS and OS were estimated using the Kaplan–Meier method. An exploratory analysis of prognostic factors for predicting survival associated with ramucirumab plus paclitaxel was performed using both univariable and multivariable analyses. The potential prognostic factors for predicting survival associated with ramucirumab plus paclitaxel were chosen based on theoretical considerations, as recommended in the literature:8,9 age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, weight loss in the previous 3 months, site of the primary tumor, disease measurability, World Health Organization histologic grade, number of metastatic sites, presence of peritoneal metastasis, prior gastrectomy, and time to second-line ramucirumab plus paclitaxel since first-line treatment. The hazard ratios and corresponding 95% confidence intervals (CIs) were estimated using Cox’s proportional hazards regression model.
Analyses of outcomes included all patients, excluding missing values. All statistical analyses were two-sided, and p < 0.05 was considered statistically significant. Kaplan–Meier analysis was conducted using the R software (version 3.4.2; www.R-project.org/) survival package and the survival curve was drawn using the survminer package. All other analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
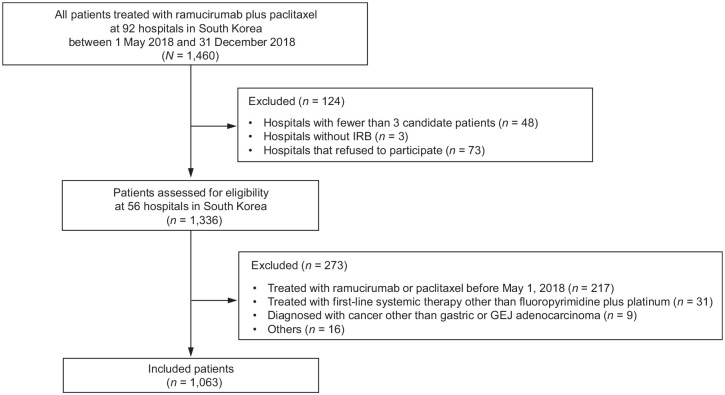
We retrospectively considered all 1460 consecutive, unselected patients treated with ramucirumab plus paclitaxel at 92 hospitals in South Korea between 1 May 2018 and 31 December 2018. Among them, 1336 patients at 56 hospitals were assessed for eligibility and 1063 eligible patients with advanced gastric or GEJ adenocarcinoma who received second-line ramucirumab plus paclitaxel were included in this analysis (Figure 1).
Figure 1.
Study population and the process of patient identification.
IRB, institutional review board; GEJ, gastroesophageal junction.
Baseline patient and tumor characteristics are presented in Table 1. The patients had a median age of 60 years (range 18–88 years) at the start of ramucirumab plus paclitaxel treatment. ECOG performance status at the initiation of the ramucirumab plus paclitaxel was 0 or 1 in 924 (87.5%) patients, but 97 patients (9.2%) had ECOG performance status 2, 3, or 4. Most patients (89.4%) had gastric adenocarcinoma as the primary tumor. Poorly differentiated adenocarcinomas or poorly cohesive carcinomas accounted for 66.8% of cases, and 15.5% presented with HER2-positive disease. All patients received first-line fluoropyrimidine and platinum-based chemotherapy and almost all patients (99.8%) received fluoropyrimidine and platinum doublet chemotherapy.
Table 1.
Baseline characteristics of patients.
| All patients N= 1063 Number of patients (%) |
|||
|---|---|---|---|
| Missing value | Analyzed patients | Result | |
| Age, years | 8 (0.8) | 1055 (99.2) | |
| Median (range) | 60 (18–88) | ||
| Sex | 8 (0.8) | 1055 (99.2) | |
| Male | 724 (68.6) | ||
| Female | 332 (31.4) | ||
| ECOG performance status | 8 (0.8) | 1055 (99.2) | |
| 0 | 145 (13.7) | ||
| 1 | 779 (73.8) | ||
| 2 | 85 (8.1) | ||
| 3 | 11 (1.0) | ||
| 4 | 1 (0.1) | ||
| Unknown | 34 (3.2) | ||
| Weight loss in the previous 3 months | 0 (0) | 1063 (100) | |
| <10% | 760 (71.5) | ||
| ⩾10% | 106 (10.0) | ||
| Unknown | 197 (18.5) | ||
| Site of primary tumor | 13 (1.2) | 1050 (98.8) | |
| Gastric | 939 (89.4) | ||
| Gastroesophageal junction | 44 (4.2) | ||
| Unknown | 67 (6.4) | ||
| Disease measurability | 14 (1.3) | 1049 (98.7) | |
| Measurable | 797 (76.0) | ||
| Non-measurable | 252 (24.0) | ||
| WHO histologic grade | 0 (0) | 1063 (100) | |
| Tubular adenocarcinoma | 788 (74.1) | ||
| Well differentiated | 37 (3.4) | ||
| Moderately differentiated | 290 (27.3) | ||
| Poorly differentiated | 437 (41.1) | ||
| Unknown | 14 (1.3) | ||
| Other | 10 (0.9) | ||
| Mucinous carcinoma | 14 (1.3) | ||
| Poorly cohesive carcinoma | 275 (25.7) | ||
| HER2 status | 8 (0.8) | 1055 (99.2) | |
| Negative | 831 (78.8) | ||
| Positive | 163 (15.5) | ||
| Unknown | 61 (5.8) | ||
| Number of metastatic sites | 25 (2.3) | 1038 (97.6) | |
| 0–2 | 854 (82.3) | ||
| ⩾3 | 184 (17.7) | ||
| Peritoneal metastasis | 0 (0) | 1063 (100) | |
| Yes | 556 (52.3) | ||
| No | 507 (47.7) | ||
| Presence of ascites | 8 (0.8) | 1055 (99.2) | |
| Yes | 207 (19.6) | ||
| No | 848 (80.4) | ||
| Prior gastrectomy | 8 (0.8) | 1055 (99.2) | |
| Yes | 597 (56.6) | ||
| No | 458 (43.4) | ||
| Time to second-line ramucirumab plus paclitaxel since first-line treatment | 19 (1.8) | 1044 (98.2) | |
| <6 months | 470 (45.0) | ||
| ⩾6 months | 574 (55.0) | ||
| Previous first-line treatment | 14 (1.3) | 1049 (98.7) | |
| Trastuzumab plus FP or XP | 129 (12.3) | ||
| XELOX | 452 (43.1) | ||
| FOLFOX | 291 (27.7) | ||
| XP or SP | 104 (9.9) | ||
| DCF | 2 (0.2) | ||
| Others | 71 (6.8) | ||
DCF, docetaxel, cisplatin, and 5-fluorouracil; ECOG, Eastern Cooperative Oncology Group; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; FP, 5-fluorouracil and cisplatin; HER2, human epidermal growth factor receptor 2; SP, S-1 and cisplatin; WHO, World Health Organization; XELOX, capecitabine and oxaliplatin; XP, capecitabine and cisplatin.
Efficacy
Among 1053 patients, the first dose of ramucirumab and paclitaxel was reduced in 107 (10.2%) and 315 (29.9%) patients, respectively (Supplemental Table 2). Poor performance status was the most common cause for a reduction of the first dose of ramucirumab plus paclitaxel. In patients with ECOG performance statuses above 2, the rate of reduction of the first dose of ramucirumab and paclitaxel was significantly higher (ramucirumab, 9.1% versus 21.6%; paclitaxel, 26.5% versus 60.8%) and the first mean dose of ramucirumab and paclitaxel was lower than those in patients with ECOG performance statuses of 0 or 1 (ramucirumab, 443.85 mg versus 394.55 mg; paclitaxel, 119.37 mg versus 102.41 mg) (Supplemental Table 3).
Among 1044 patients, 443 (42.4%) underwent subsequent dose reductions after the first dose of ramucirumab plus paclitaxel was administered, and ramucirumab and paclitaxel accounted for approximately 22% and 78% of dose reductions, respectively (Table 2). Adverse events (AEs; 60.1%) were the most common cause for a reduction after the first dose of ramucirumab plus paclitaxel.
Table 2.
Dose reductions after first dose of ramucirumab plus paclitaxel.
| All patients N= 1063 | |||
|---|---|---|---|
| Missing value | Analyzed patients | Result | |
| Dose reduction | 19 (1.8) | 1044 (98.2) | |
| Yes | |||
| Number of patients (%) | 443 (42.4) | ||
| Number of events | 727 | ||
| No | |||
| Number of patients (%) | 601 (57.6) | ||
| Drug, number of events (%) | 0 (0) | 727 (100) | |
| Ramucirumab | 162 (22.3) | ||
| Paclitaxel | 565 (77.7) | ||
| Reason for dose reduction, number of events (%) | 0 (0) | 727 (100) | |
| Adverse events | 437 (60.1) | ||
| Subject decision | 73 (10.0) | ||
| Other | 124 (17.1) | ||
| Unknown | 93 (12.8) | ||
Tumor response was available for 1048 (98.6%) patients in the study (Table 3). The best overall responses to ramucirumab plus paclitaxel were CR in nine patients (0.9%), PR in 149 patients (14.2%), SD in 447 patients (42.7%) and progressive disease in 268 patients (25.6%). The proportion of patients who achieved an objective response and disease control were 15.1% (95% CI 13.0–17.4) and 57.7% (95% CI 48.4–60.8), respectively.
Table 3.
Best overall response.
| All patients N = 1063 Number of patients (%) |
|||
|---|---|---|---|
| Missing value | Analyzed patients | Result | |
| Best overall response | 15 (1.4) | 1048 (98.6) | |
| Complete response | 9 (0.9) | ||
| Partial response | 149 (14.2) | ||
| Stable disease | 447 (42.7) | ||
| Progressive disease | 268 (25.6) | ||
| Not evaluable | 175 (16.7) | ||
| Objective response ratea | 158 (15.1; 13.0–17.4) | ||
| Disease control rateb | 605 (57.7; 48.4–60.8) | ||
Objective response rate is defined as the proportion of patients with complete response (CR) or partial response (PR) as best overall response.
Disease control rate is CR+PR+stable disease (including non-CR/non-progressive disease).
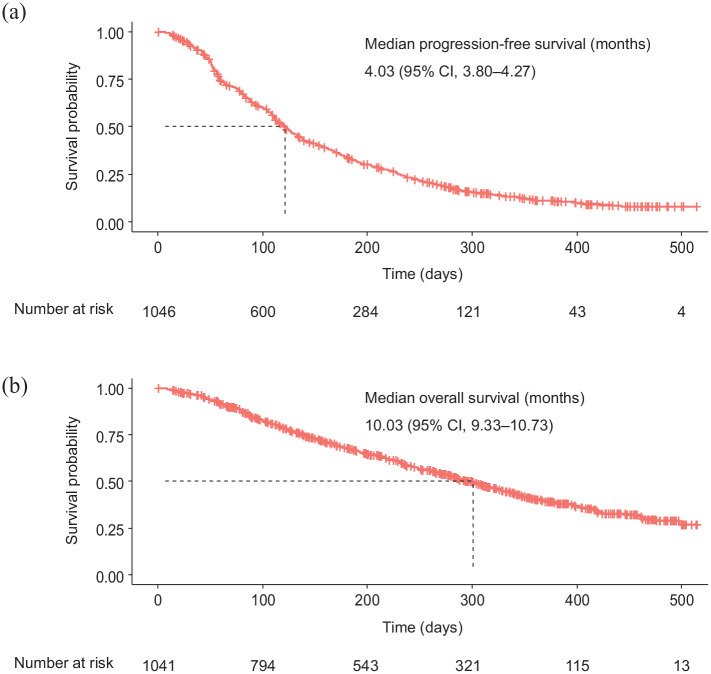
At the data cutoff date, 865 (82.7%) of 1046 patients had PFS events and 529 (50.8%) of 1041 patients had died. Based on a median follow-up of 7.0 months (range 0–17.2 months), the median PFS was 4.03 months [95% CI 3.80–4.27; Figure 2(a)] and the median OS was 10.03 months [95% CI 9.33–10.73; Figure 2(b)].
Figure 2.
Kaplan–Meier estimates of (a) progression-free survival and (b) overall survival.
CI, confidence interval.
At the data cutoff date, 998 (95.0%) of 1050 patients had discontinued ramucirumab plus paclitaxel, mainly due to disease progression (67.9%; Supplemental Table 4). After discontinuation of ramucirumab plus paclitaxel, post-discontinuation systemic antineoplastic therapy was administered to 497 (47.1%) of 1055 patients (Supplemental Table 5). The most commonly used post-discontinuation therapies (PDTs) were irinotecan-based regimens and anti-programmed cell death-1 antibodies (nivolumab or pembrolizumab).
Safety
Incidence of TRAEs is presented in Table 4. The most common TRAE at any grade was neutropenia (44.7%), while the incidence of febrile neutropenia was low (4.5%). Other common TRAEs at any grade included anemia (41.8%), neuropathy (29.1%), fatigue (25.9%), and anorexia (25.0%). Grade 3 or higher TRAEs with incidence ⩾5% were neutropenia (35.1%) and anemia (10.5%). AEs of special interest potentially associated with the VEGF pathway were infrequent and included hypertension (2.1%), proteinuria (3.0%), and gastrointestinal hemorrhage (2.7%) or perforation (0.9%). Discontinuation due to TRAEs was observed in only 53 (5.3%) of 998 patients who discontinued ramucirumab plus paclitaxel permanently (Supplemental Table 4).
Table 4.
Treatment-related adverse events of ramucirumab plus paclitaxel.
| All patients N = 1063 Number of patients (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment-related adverse eventsa | Missing value | Analyzed patients | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Hematologic adverse events | |||||||
| Neutropenia | 12 (1.1) | 1051 (98.9) | 25 (2.4) | 76 (7.2) | 205 (19.4) | 166 (15.7) | |
| Febrile neutropenia | 9 (0.8) | 1054 (99.2) | 37 (3.5) | 4 (0.4) | 6 (0.6) | ||
| Anemia | 10 (0.9) | 1053 (99.1) | 84 (8.0) | 246 (23.3) | 106 (10.0) | 5 (0.5) | 0 (0) |
| Thrombocytopenia | 9 (0.8) | 1054 (99.2) | 89 (8.4) | 29 (2.7) | 32 (3.0) | 13 (1.2) | |
| Non-hematologic adverse events | |||||||
| Anorexia | 10 (0.9) | 1053 (99.1) | 129 (12.2) | 117 (11.1) | 17 (1.6) | 1 (0.1) | 0 (0) |
| Nausea | 10 (0.9) | 1053 (99.1) | 100 (9.4) | 41 (3.9) | 14 (1.3) | ||
| Vomiting | 11 (1.0) | 1052 (99.0) | 54 (5.1) | 32 (3.0) | 15 (1.4) | 1 (0.1) | 0 (0) |
| Fatigue | 11 (1.0) | 1052 (99.0) | 144 (13.6) | 108 (10.2) | 22 (2.1) | ||
| Stomatitis | 11 (1.0) | 1052 (99.0) | 44 (4.2) | 35 (3.3) | 7 (0.7) | 1 (0.1) | 0 (0) |
| Neuropathy | 10 (0.9) | 1053 (99.1) | 150 (14.2) | 125 (11.8) | 32 (3.0) | 1 (0.1) | 0 (0) |
| Diarrhea | 11 (1.0) | 1052 (99.0) | 91 (8.6) | 28 (2.7) | 4 (0.4) | 0 (0) | 0 (0) |
| Infection | 11 (1.0) | 1052 (99.0) | 6 (0.6) | 6 (0.6) | 11 (1.0) | 1 (0.1) | 8 (0.8) |
| Alopecia | 11 (1.0) | 1052 (99.0) | 16 (1.5) | 40 (3.8) | |||
| Constipation | 11 (1.0) | 1052 (99.0) | 20 (1.9) | 2 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Myalgia | 11 (1.0) | 1052 (99.0) | 7 (0.7) | 11 (1.0) | 3 (0.3) | ||
| Skin rash | 11 (1.0) | 1052 (99.0) | 8 (0.8) | 7 (0.7) | 0 (0) | ||
| Abdominal pain | 11 (1.0) | 1052 (99.0) | 6 (0.6) | 4 (0.4) | 2 (0.2) | ||
| Fever | 11 (1.0) | 1052 (99.0) | 8 (0.8) | 4 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Epistaxis | 11 (1.0) | 1052 (99.0) | 8 (0.8) | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| Adverse events of special interest | |||||||
| Hypertension | 11 (1.0) | 1052 (99.0) | 5 (0.5) | 11 (1.0) | 6 (0.6) | 0 (0) | 0 (0) |
| Proteinuria | 11 (1.0) | 1052 (99.0) | 15 (1.4) | 13 (1.2) | 4 (0.4) | ||
| GI hemorrhage | 11 (1.0) | 1052 (99.0) | 2 (0.2) | 3 (0.3) | 22 (2.1) | 1 (0.1) | 0 (0) |
| GI perforation | 12 (1.1) | 1051 (98.9) | 3 (0.3) | 4 (0.4) | 1 (0.1) | 1 (0.1) | |
| Wound complication | 11 (1.0) | 1052 (99.0) | 4 (0.4) | 1 (0.1) | 1 (0.1) | 0 (0) | 0 (0) |
| Thromboembolic event | 11 (1.0) | 1052 (99.0) | 2 (0.2) | 8 (0.8) | 2 (0.2) | 0 (0) | 0 (0) |
Treatment-related adverse events listed as possibly, probably, or definitely related to the ramucirumab or paclitaxel.
GI, gastrointestinal.
Prognostic factors
A two-step exploratory analysis including univariable and multivariable analyses was conducted to identify potential factors for predicting PFS and OS associated with ramucirumab plus paclitaxel (Table 5). Multivariable analyses were conducted using the stepwise Cox model with the inclusion of all prespecified factors and identified eight significantly independent predictors for reduced PFS and OS: ECOG performance status ⩾2 (median PFS, 2.1 versus 4.2 months, p = 0.003; median OS, 5.1 versus 10.5 months, p < 0.001), weight loss ⩾10% in the previous 3 months (median PFS, 2.6 versus 4.1 months, p = 0.004; median OS, 5.5 versus 10.3 months, p < 0.001), GEJ of the primary tumor (median PFS, 3.7 versus 4.1 months, p = 0.018; median OS, 8.1 versus 10.3 months, p = 0.01), poor or unknown histologic grade (median PFS, 3.7 versus 4.7 months, p = 0.072; median OS, 8.6 versus 11.9 months, p = 0.005), number of metastatic sites ⩾3 (median PFS, 3.2 versus 4.3 months, p = 0.005; median OS, 6.4 versus 10.8 months, p < 0.001), presence of peritoneal metastasis (median PFS, 3.7 versus 4.6 months, p = 0.031; median OS, 8.2 versus 11.4 months, p = 0.003), no prior gastrectomy (median PFS, 3.6 versus 5.2 months, p < 0.001; median OS, 8.3 versus 12.8 months, p < 0.001), and time to second-line since first-line treatment <6 months (median PFS, 3.2 versus 5.1 months, p < 0.001; median OS, 7.5 versus 12.2 months, p < 0.001).
Table 5.
Univariable and multivariable analyses for progression-free survival and overall survival.
| No. | Progression-free survival | Overall survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median, months (95% CI) | Univariable | Multivariable | Median, months (95% CI) | Univariable | Multivariable | ||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| Age, years | |||||||||||
| <70 | 865 | 3.9 (3.7–4.2) | Ref. | Ref. | 10.0 (9.2–10.7) | Ref. | Ref. | ||||
| ⩾70 | 190 | 4.6 (4.0–5.2) | 0.96 (0.81–1.11) | 0.631 | 1.03 (0.85–1.24) | 0.784 | 9.6 (7.9–13.3) | 0.99 (0.79–1.25) | 0.961 | 1.12 (0.88–1.44) | 0.361 |
| Sex | |||||||||||
| Male | 724 | 4.3 (4.0–4.6) | 0.90 (0.78–1.03) | 0.132 | 0.89 (0.76–1.05) | 0.157 | 10.3 (9.5–11.2) | 0.87 (0.72–1.04) | 0.125 | 0.89 (0.73–1.09) | 0.261 |
| Female | 331 | 3.6 (3.4–3.9) | Ref. | Ref. | 9.3 (8.1–10.9) | Ref. | Ref. | ||||
| ECOG performance status | |||||||||||
| 0–1 | 924 | 4.2 (4.0–4.6) | Ref. | Ref. | 10.5 (9.7–11.2) | Ref. | Ref. | ||||
| ⩾2 | 97 | 2.1 (1.8–3.3) | 1.64 (1.30–2.05) | <0.001 | 1.45 (1.14–1.84) | 0.003 | 5.1 (3.3–6.3) | 2.07 (1.57–2.71) | <0.001 | 1.92 (1.44–2.55) | <0.001 |
| Unknown | 34 | 3.6 (2.2–4.3) | 1.55 (1.05–2.27) | 0.027 | 1.24 (0.83–1.85) | 0.290 | 7.1 (5.5–9.8) | 1.61 (0.99–2.62) | 0.054 | 1.28 (0.77–2.13) | 0.341 |
| Weight loss in the previous 3 months | |||||||||||
| <10% | 760 | 4.1 (3.9–4.5) | Ref. | Ref. | 10.3 (9.5–11.4) | Ref. | Ref. | ||||
| ⩾10% | 106 | 2.6 (2.1–3.5) | 1.55 (1.24–1.93) | <0.001 | 1.42 (1.12–1.80) | 0.004 | 5.5 (4.0–8.1) | 1.94 (1.49–2.51) | <0.001 | 1.76 (1.3–2.32) | <0.001 |
| Unknown | 197 | 4.5 (3.7–5.1) | 0.99 (0.83–1.18) | 0.898 | 1.11 (0.91–1.35) | 0.303 | 10.6 (9.6–12.2) | 0.99 (0.78–1.26) | 0.929 | 1.19 (0.92–1.55) | 0.185 |
| Site of the primary tumor | |||||||||||
| Gastric | 939 | 4.1 (3.9–4.3) | Ref. | Ref. | 10.3 (9.5–11.2) | Ref. | Ref. | ||||
| GEJ | 44 | 3.7 (2.6–4.6) | 1.34 (0.98–1.84) | 0.068 | 1.50 (1.07–2.09) | 0.018 | 8.1 (5.3–10.3) | 1.54 (1.05–2.24) | 0.025 | 1.69 (1.13–2.52) | 0.01 |
| Disease measurability | |||||||||||
| Measurable | 797 | 4.0 (3.0–4.3) | Ref. | Ref. | 9.6 (8.9–10.5) | Ref. | Ref. | ||||
| Non-measurable | 252 | 4.0 (3.6–4.7) | 0.96 (0.82–1.13) | 0.647 | 1.03 (0.87–1.23) | 0.730 | 11.2 (9.5–13.4) | 0.92 (0.75–1.12) | 0.396 | 0.96 (0.76–1.21) | 0.749 |
| WHO histologic gradea | |||||||||||
| Well/moderate | 384 | 4.7 (4.2–5.8) | Ref. | Ref. | 11.9 (10.6–14.0) | Ref. | Ref. | ||||
| Poor/unknown | 679 | 3.7 (3.5–4.0) | 1.31 (1.14–1.51) | <0.001 | 1.15 (0.99–1.35) | 0.072 | 8.6 (7.8–9.5) | 1.57 (1.30–1.89) | <0.001 | 1.34 (1.09–1.64) | 0.005 |
| Number of metastatic sites | |||||||||||
| 0–2 | 854 | 4.3 (4.0–4.6) | Ref. | Ref. | 10.8 (10.0–11.6) | Ref. | Ref. | ||||
| ⩾3 | 184 | 3.2 (2.8–3.6) | 1.45 (1.23–1.73) | <0.001 | 1.30 (1.08–1.57) | 0.005 | 6.4 (5.5–7.9) | 1.77 (1.44–2.18) | <0.001 | 1.53 (1.22–1.92) | <0.001 |
| Peritoneal metastasis | |||||||||||
| Yes | 556 | 3.7 (3.4–4.0) | 1.27 (1.11–1.45) | 0.001 | 1.18 (1.02–1.37) | 0.031 | 8.2 (7.3–9.5) | 1.49 (1.25–1.77) | <0.001 | 1.35 (1.10–1.64) | 0.003 |
| No | 507 | 4.6 (4.2–5.3) | Ref. | 11.4 (10.3–12.7) | Ref. | Ref. | |||||
| Prior gastrectomy | |||||||||||
| Yes | 458 | 5.2 (4.5–5.8) | Ref. | 12.8 (11.2–14.1) | Ref. | Ref. | |||||
| No | 597 | 3.6 (3.3–3.8) | 1.62 (1.41–1.86) | <0.001 | 1.56 (1.34–1.81) | <0.001 | 8.3 (7.7–9.2) | 1.70 (1.42–2.03) | <0.001 | 1.54 (1.27–1.88) | <0.001 |
| Time to second-line ramucirumab plus paclitaxel since first-line treatment | |||||||||||
| <6 months | 470 | 3.2 (2.9–3.6) | 1.68 (1.47–1.93) | <0.001 | 1.52 (1.31–1.76) | <0.001 | 7.5 (6.3–8.3) | 1.86 (1.57–2.21) | <0.001 | 1.65 (1.37–1.99) | <0.001 |
| ⩾6 months | 574 | 5.1 (4.5–5.6) | Ref. | Ref. | 12.2 (11.0–13.6) | Ref. | Ref. | ||||
Well/moderate histologic grade is defined as well differentiation, tubular adenocarcinoma with moderately differentiation, mucinous carcinoma, and mixed adenocarcinoma. Poor/unknown histologic grade is defined as tubular adenocarcinoma with poor differentiation, poorly cohesive carcinoma, and unknown.
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; HR, hazard ratio; Ref., reference; WHO, World Health Organization.
Discussion
To the best of our knowledge, this is the first large-population-based report of outcomes focused on the combination of ramucirumab and paclitaxel in patients with gastric or GEJ adenocarcinoma. Our large-scale, nationwide, real-world KCSG ST19-16 study of an unselected real-world population adds evidence for the efficacy and safety of second-line ramucirumab plus paclitaxel in patients with locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma.
The results revealed that the median PFS was 4.03 months (95% CI 3.80–4.27), the median OS was 10.03 months (95% CI 9.33–10.73), and ORR and DCR were 15.1% and 57.7%, respectively. Several differences in efficacy and safety were identified between the pivotal RAINBOW RCT or previous real-world studies and our KCSG ST19-16 study (Supplemental Table 6).9,13–16 The median OS in the present study was similar to that in the RAINBOW overall population at 9.6 months,9 but lower than that in the East Asian patients in RAINBOW at 12.1 months.13 This result reflects the real-world nature of the present study, revealing an efficacy gap between RCT and RWD, which is not a new concept.14–16 Population-based RWD represent a wide variation among patients, including elderly or fragile patients and patients with comorbidity who usually do not participate in RCTs. Patients in the present study had much worse performance before ramucirumab plus paclitaxel compared with those in RAINBOW. In the present study, patients with an ECOG performance status of 0 constituted 13.7% of all patients compared with 35% in RAINBOW, and the present study population included 97 patients (9.2%) with an ECOG performance status ⩾2, whereas all patients in RAINBOW scored 0 or 1. Conversely, the OS outcome was higher in the present study than among the non-East Asian patients in RAINBOW at 8.5 months.13 Differences in survival outcomes between Eastern and Western patients with gastric cancer are well known,17–24 and tumor biology, ethnicity, healthcare insurance or reimbursement systems, and the use of subsequent PDT have been suggested as possible explanations for this difference.25,26 Specifically, post-disease progression factors, such as PDT, most likely affected survival outcomes;21,23,27 the proportion of patients receiving third-line or further-line therapy is higher in East Asia than in the US and Europe.13,27,28 This hypothesis is supported by our exploratory survival analyses evaluating the impact of PDT on survival: patients who received any PDTs after ramucirumab plus paclitaxel survived longer than patients who did not (median OS, 11.47 versus 7.27 months, p < 0.001; Supplemental Figure 1). Considering previous real-world studies, patients in the present study who were treated with ramucirumab plus paclitaxel had a higher OS than patients treated with ramucirumab alone or ramucirumab plus paclitaxel in previous studies.14–16 These survival differences are likely to be related not only to the differences between Eastern and Western patients but also to the better efficacy of combination therapy with paclitaxel compared with monotherapy. Thus, combination therapy of ramucirumab and paclitaxel is strongly recommended as a second-line treatment, unless paclitaxel is contraindicated.
The best overall response was lower in our study than in RAINBOW (ORR, 15.1% versus 28.0%; DCR 57.7% versus 80.0%). It has been already established that RWD-based studies have a lower tumor response rate than RCTs,14 for which one of the reasons is related to the inability to maintain the dose intensity of cancer drug in the real-world population.29,30 In the present study, a dose reduction was implemented at the first dose ramucirumab and paclitaxel in approximately 10% and 30% of all patients, respectively. This result is associated with RWD-based studies and does not occur in RCTs with a fixed recommended dose administered at the first dose. After the first dose of ramucirumab plus paclitaxel, subsequent dose reductions were implemented in approximately 40% of all patients, and ramucirumab and paclitaxel accounted for approximately 22% and 78% of dose reductions, respectively. Dose reductions were more frequently associated with the cytotoxic agent paclitaxel than with the molecularly targeted agent ramucirumab. The most common reason for a dose reduction of the first dose was a poor performance status rather than old age, and after the first dose, AEs were the most common reason for dose reductions.
The present study and previous studies revealed a similar safety profile of ramucirumab plus paclitaxel.9,14–16 In general, no significant difference was observed in the incidence of hematologic AEs between our study and RAINBOW. The most common hematologic AEs were neutropenia and anemia, but the incidence of febrile neutropenia or grade ⩾3 anemia was low. However, non-hematologic AEs or VEGFR pathway-related AEs were much less frequent in the present study than in RAINBOW. Unlike hematologic AEs derived from laboratory data, non-hematologic AEs are subjective symptoms such as fatigue, gastrointestinal symptoms, and neuropathy, which are difficult to evaluate in detail during routine clinical practice. Furthermore, hypertension and proteinuria were the most common ramucirumab-related AEs of special interest that reportedly occurred in 15–25% and 5–15% of patients in previous clinical trials, respectively, but in less than 5% of patients in the present study. Hypertension and proteinuria are difficult to detect unless regular blood pressure measurements and urinalysis are performed because they do not cause symptoms and signs during the early stage. Non-hematologic AEs and AEs that do not manifest as specific symptoms or signs are underestimated in real-world clinical settings. Thus, application of patient-reported outcomes for evaluating non-hematologic AEs and careful monitoring for AEs of special interest is needed.
To evaluate factors potentially prognostic for OS in RWD, we assessed baseline clinicopathologic parameters similar to the parameters used in RAINBOW. Based on our multivariable analyses, ECOG performance status ⩾2, weight loss ⩾10% in the previous 3 months, GEJ of primary tumor, poor or unknown histologic grade, number of metastatic sites ⩾3, presence of peritoneal metastasis, no prior gastrectomy, and time to second-line since first-line treatment <6 months were negatively associated with OS. Because these factors were prognostic for locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma itself, differences were not observed between RCTs and RWD-based studies.8,9,13,27,31
The main strength of the present study was the real-world approach; it included nearly all patients with gastric or GEJ adenocarcinoma treated with second-line ramucirumab plus paclitaxel in South Korea. Therefore, the study population was representative of the routine clinical practice in South Korea. Although some small-scale real-world studies have investigated the outcomes of ramucirumab plus paclitaxel,13–15 our sample size was larger and the RWD focused on patients treated with ramucirumab plus paclitaxel, similar to the RAINBOW RCT. However, the present study also had limitations. First, it was inherently limited due to the retrospective observational methodology based on chart review, including missing data, possible under-reporting of AEs, and the lack of control group patients receiving paclitaxel alone, all of which might have affected the efficacy and safety data analysis. Second, assessment of disease progression was based exclusively on assessment by the treating physician and may have been influenced by local diagnostic practices. There may have also been inconsistencies in the time points at which disease progression was assessed. Third, 76.5% of patients in this study were confirmed to have died based on medical records, but for 23.5% of patients, whether they survived or died was unknown due to loss of follow-up. However, significant differences were not observed in the major prognostic factors between patients with and without accurate death information (Supplemental Table 7).
In conclusion, the KCSG ST19-16 study provides the largest RWD-based analysis indicating a favorable efficacy and safety profile of second-line ramucirumab plus paclitaxel for locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma. Furthermore, the present RWD analysis supports the scientific evidence derived from the previous RAINBOW RCT and smaller retrospective analyses. Further analysis assessing the real burden including patient-reported outcomes, quality of life, healthcare resources or oncology practice claim data, and using databases other than hospital medical records will enhance the value of RWD in the management of gastric cancer.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211042812 for Ramucirumab plus paclitaxel as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma: a nationwide real-world outcomes in Korea study (KCSG-ST19-16) by Hye Sook Han, Bum Jun Kim, Hee-Jung Jee, Min-Hee Ryu, Se Hoon Park, Sun Young Rha, Jong Gwang Kim, Woo Kyun Bae, Keun-Wook Lee, Do-Youn Oh, In-Ho Kim, Sun Jin Sym, So Yeon Oh, Hyeong Su Kim, Ji-Hye Byun, Dong Sook Kim, Young Ju Suh, Hyonggin An and Dae Young Zang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pptx-2-tam-10.1177_17588359211042812 for Ramucirumab plus paclitaxel as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma: a nationwide real-world outcomes in Korea study (KCSG-ST19-16) by Hye Sook Han, Bum Jun Kim, Hee-Jung Jee, Min-Hee Ryu, Se Hoon Park, Sun Young Rha, Jong Gwang Kim, Woo Kyun Bae, Keun-Wook Lee, Do-Youn Oh, In-Ho Kim, Sun Jin Sym, So Yeon Oh, Hyeong Su Kim, Ji-Hye Byun, Dong Sook Kim, Young Ju Suh, Hyonggin An and Dae Young Zang in Therapeutic Advances in Medical Oncology
Acknowledgments
This work was supported by the Korean Cancer Study Group and the Health Insurance Review & Assessment Service. The views expressed are those of the authors and not necessarily those of the Health Insurance Review & Assessment Service. We thank all investigators and their support staff who generously participated in this work.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability: Data supporting the results presented in this study are available from the corresponding author upon reasonable request and with permission of the Health Insurance Review & Assessment Service.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Health Insurance Review & Assessment Service.
ORCID iD: Dae Young Zang  https://orcid.org/0000-0002-2602-7848
https://orcid.org/0000-0002-2602-7848
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hye Sook Han, Department of Internal Medicine, Chungbuk National University College of Medicine, Chungbuk National University Hospital, Cheongju, Chungcheongbuk-do, Republic of Korea.
Bum Jun Kim, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang-si, Gyeonggi-do, Republic of Korea.
Hee-Jung Jee, Department of Biostatistics, College of Medicine, Korea University, Seoul, Republic of Korea.
Min-Hee Ryu, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Se Hoon Park, Division of Hematology–Oncology, Department of Medicine, Sungkyunkwan University Samsung Medical Center, Seoul, Republic of Korea.
Sun Young Rha, Songdang Institute for Cancer Research, Yonsei University College of Medicine; Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine; Brain Korea 21 PLUS Project for Medical Sciences, Yonsei University College of Medicine, Seoul, Republic of Korea.
Jong Gwang Kim, Department of Oncology/Hematology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Republic of Korea.
Woo Kyun Bae, Department of Internal Medicine, Chonnam National University Medical School and Hwasun Hospital, Hwasun, Jeollanam-do, Republic of Korea.
Keun-Wook Lee, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Republic of Korea.
Do-Youn Oh, Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Republic of Korea.
In-Ho Kim, Department of Internal Medicine, The Catholic University of Korea, Seoul St. Mary’s Hospital, Seoul, Republic of Korea.
Sun Jin Sym, Division of Hematology and Oncology, Department of Internal Medicine, Gachon University Gil Hospital, Incheon, Republic of Korea.
So Yeon Oh, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea.
Hyeong Su Kim, Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Republic of Korea.
Ji-Hye Byun, Innovation Research Department, Health Insurance Review and Assessment Service, Wonju, Republic of Korea.
Dong Sook Kim, Review & Assessment Research Department, Health Insurance Review and Assessment Service, Wonju, Republic of Korea.
Young Ju Suh, Department of Biomedical Sciences, College of Medicine, Inha University, Incheon, Republic of Korea.
Hyonggin An, Department of Biostatistics, College of Medicine, Korea University, Seoul, Republic of Korea.
Dae Young Zang, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, 22 Gwanpyeong-ro 170 beon-gil Dongan-gu, Anyang-si, Gyeonggi-do 14068, Republic of Korea.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics. 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, Won YJ, Park YR, et al. ; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat 2020; 52: 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019; 30: 19–33. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer 2019; 19: 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieto E, Ferraraccio F, Orditura M, et al. Expression of Vascular Endothelial Growth Factor (VEGF) and Epidermal Growth Factor Receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol 2008; 15: 69–79. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Dobashi Y, Hatakeyama Y, et al. Clinicopathological significance of Platelet-Derived Growth Factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-β phosphorylation, and microvessel density in gastric cancer. BMC Cancer 2010; 10: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs CS, Tomasek J, Yong CJ, et al. ; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383: 31–39. [DOI] [PubMed] [Google Scholar]
- 9.Wilke H, Muro K, Van Cutsem E, et al. ; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014; 15: 1224–1235. [DOI] [PubMed] [Google Scholar]
- 10.Cotes Sanchís A, Gallego J, Hernandez R, et al. Second-line treatment in advanced gastric cancer: data from the Spanish AGAMENON registry. PLoS One 2020; 15: e0235848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (2017, accessed December 2019).
- 13.Muro K, Oh SC, Shimada Y, et al. Subgroup analysis of East Asians in RAINBOW: a phase 3 trial of ramucirumab plus paclitaxel for advanced gastric cancer. J Gastroenterol Hepatol 2016; 31: 581–589. [DOI] [PubMed] [Google Scholar]
- 14.Jung M, Ryu MH, Oh DY, et al. Efficacy and tolerability of ramucirumab monotherapy or in combination with paclitaxel in gastric cancer patients from the expanded access program cohort by the Korean Cancer Study Group (KCSG). Gastric Cancer 2018; 21: 819–830. [DOI] [PubMed] [Google Scholar]
- 15.Paulson AS, Hess LM, Liepa AM, et al. Ramucirumab for the treatment of patients with gastric or gastroesophageal junction cancer in community oncology practices. Gastric Cancer 2018; 21: 831–844. [DOI] [PubMed] [Google Scholar]
- 16.Di Bartolomeo M, Niger M, Tirino G, et al. Ramucirumab as second-line therapy in metastatic gastric cancer: real-world data from the RAMoss study. Target Oncol 2018; 13: 227–234. [DOI] [PubMed] [Google Scholar]
- 17.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011; 47: 2306–2314. [DOI] [PubMed] [Google Scholar]
- 18.Ford HE, Marshall A, Bridgewater JA, et al. ; COUGAR-02 Investigators. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014; 15: 78–86. [DOI] [PubMed] [Google Scholar]
- 19.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013; 31: 4438–4444. [DOI] [PubMed] [Google Scholar]
- 20.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011; 29: 3968–3976. [DOI] [PubMed] [Google Scholar]
- 21.Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012; 30: 2119–2127. [DOI] [PubMed] [Google Scholar]
- 22.Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 23.Sawaki A, Ohashi Y, Omuro Y, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer 2012; 15: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KW, Maeng CH, Kim TY, et al. A phase III study to compare the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer who failed in first-line therapy (KCSG ST10-01). Oncologist 2019; 24: 18-e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohtsu A, Yoshida S, Saijo N.Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol 2006; 24: 2188–2196. [DOI] [PubMed] [Google Scholar]
- 26.Kim R, Tan A, Choi M, et al. Geographic differences in approach to advanced gastric cancer: is there a standard approach? Crit Rev Oncol Hematol 2013; 88: 416–426. [DOI] [PubMed] [Google Scholar]
- 27.Shitara K, Muro K, Shimada Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 2016; 19: 927–938. [DOI] [PubMed] [Google Scholar]
- 28.Carter GC, Kaltenboeck A, Ivanova J, et al. Real-world treatment patterns among patients with advanced gastric cancer in South Korea. Cancer Res Treat 2017; 49: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabernero J, Ohtsu A, Muro K, et al. Exposure-response analyses of ramucirumab from two randomized, phase III trials of second-line treatment for advanced gastric or gastroesophageal junction cancer. Mol Cancer Ther 2017; 16: 2215–2222. [DOI] [PubMed] [Google Scholar]
- 30.Kim TY, Yen CJ, Al-Batran SE, et al. Exposure-response relationship of ramucirumab in East Asian patients from RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 2018; 21: 276–284. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs CS, Muro K, Tomasek J, et al. Prognostic factor analysis of overall survival in gastric cancer from two phase III studies of second-line ramucirumab (REGARD and RAINBOW) using pooled patient data. J Gastric Cancer 2017; 17: 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211042812 for Ramucirumab plus paclitaxel as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma: a nationwide real-world outcomes in Korea study (KCSG-ST19-16) by Hye Sook Han, Bum Jun Kim, Hee-Jung Jee, Min-Hee Ryu, Se Hoon Park, Sun Young Rha, Jong Gwang Kim, Woo Kyun Bae, Keun-Wook Lee, Do-Youn Oh, In-Ho Kim, Sun Jin Sym, So Yeon Oh, Hyeong Su Kim, Ji-Hye Byun, Dong Sook Kim, Young Ju Suh, Hyonggin An and Dae Young Zang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pptx-2-tam-10.1177_17588359211042812 for Ramucirumab plus paclitaxel as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma: a nationwide real-world outcomes in Korea study (KCSG-ST19-16) by Hye Sook Han, Bum Jun Kim, Hee-Jung Jee, Min-Hee Ryu, Se Hoon Park, Sun Young Rha, Jong Gwang Kim, Woo Kyun Bae, Keun-Wook Lee, Do-Youn Oh, In-Ho Kim, Sun Jin Sym, So Yeon Oh, Hyeong Su Kim, Ji-Hye Byun, Dong Sook Kim, Young Ju Suh, Hyonggin An and Dae Young Zang in Therapeutic Advances in Medical Oncology