Abstract
A growing body of research is focused on the extinction of parasite species in response to host endangerment and declines. Beyond the loss of parasite species richness, host extinction can impact apparent parasite host specificity, as measured by host richness or the phylogenetic distances among hosts. Such impacts on the distribution of parasites across the host phylogeny can have knock-on effects that may reshape the adaptation of both hosts and parasites, ultimately shifting the evolutionary landscape underlying the potential for emergence and the evolution of virulence across hosts. Here, we examine how the reshaping of host phylogenies through extinction may impact the host specificity of parasites, and offer examples from historical extinctions, present-day endangerment, and future projections of biodiversity loss. We suggest that an improved understanding of the impact of host extinction on contemporary host–parasite interactions may shed light on core aspects of disease ecology, including comparative studies of host specificity, virulence evolution in multi-host parasite systems, and future trajectories for host and parasite biodiversity.
This article is part of the theme issue ‘Infectious disease macroecology: parasite diversity and dynamics across the globe’.
Keywords: host–parasite interaction, phylogenetic ecology, coextinction, virulence evolution, infectious diseases
1. Introduction
The Earth's biodiversity is in the midst of a crisis, with current rates of extinction that are conservatively 100 times faster than the normal background rate [1]. Yet we are only beginning to understand the true scope of this crisis. Mammals are among the most well-documented groups, and over a quarter of all mammal species are threatened with extinction [2]. The loss of any one species will also impact affiliated species, including mutualists, commensals and parasites, and when associations are obligate, we risk cascading extinctions. The intimate interactions between parasites and their hosts have led to the suggestion that parasites may comprise the majority of endangered species [3], and increasing advocacy for the inclusion of parasites in global conservation planning [4]. Yet even within mammals, one of the best-sampled host groups, it is unclear how many parasite species may be lost with future host extinctions [5,6], what effect losses of hosts and their parasites will have on the ecological structure of communities or how patterns that we observe in contemporary communities may be related to losses of hosts and parasites in the recent past.
Parasites play critical roles in ecological communities through impacts on host populations and structuring food webs [7,8]. However, in comparison with their hosts, parasite extinctions are notoriously difficult to observe [9], though some can be inferred through analysis of ancient samples [10,11], or through co-phylogenetic analyses [12]. Beyond direct observation, the coextinction of parasites along with the loss of their hosts [13] has been studied via comparative analyses of threatened and non-threatened hosts [14,15], and simulations that identify likely coextinctions resulting from future host extinctions [3,16–19]. Both of these approaches commonly assume a complete extinction of parasites when, and only when, all of their documented hosts have gone extinct [20]. However, multi-host parasites may require multiple hosts to maintain a net reproductive rate greater than one, suggesting that the extinction of even a single host may imperil a parasite [21–23]. Moreover, the extinction of some of their hosts also impacts the ecology and evolution of multi-host parasites through altering the adaptive landscape across available hosts [24]. Host extinction therefore not only has the potential to result in parasite extinction, but may alter host specificity and shift the evolutionary landscapes shaping future parasite evolution. Predicting the impacts of host extinctions on host specificity becomes especially muddied when we expand our notion of host specificity beyond the number of host species infected.
Host specificity is a fundamental property of parasites and can be quantified by the richness, evenness or the ecological or evolutionary diversity of host species that a parasite infects [25]. Parasite species can display various degrees of specificity, from infecting a single host species (i.e. a specialist parasite) to infecting multiple host species (i.e. a generalist parasite). Among generalist parasites, the degree of specificity can also vary dramatically. Using phylogenetic distances among hosts to measure specificity, a parasite infecting the same number of hosts may infect only closely related hosts or infect hosts from across multiple, distantly related clades [25,26]. The degree of host specificity is a product of historical associations of parasites with their hosts, including processes of co-speciation and parasites shifting to infect novel hosts [27,28]. Identifying the set of host species that a parasite could infect given suitable opportunity (i.e. the potential host range of a parasite) allows us to infer ancestral host–parasite associations [29] and make crucial predictions of the potential for emergence in novel hosts [30,31] and likely impacts following cross-species transmission [32–34].
Predictions of unobserved host–parasite associations are often based on an assumption that present-day associations accurately reflect potential host ranges [31,35,36]. However, host range is a dynamic property of parasites that evolves through cospeciation, host shifts, and the gains and losses of hosts over varying timescales [37–41]. Changes in parasite host specificity as a result of host-switching and shifting geographic ranges have attracted considerable attention by researchers [42–47], whereas extinction history has tended to be overlooked. Similar to the impact of host-switches, if recent historical host extinctions have reshaped contemporary host–parasite associations, we may be misled as to the intrinsic specificity of parasites. For example, the extinction of an evolutionarily distinct host may shift our perception of a parasite from being a phylogenetic generalist to a phylogenetic specialist. We use the term ‘apparent specificity’ to reflect host specificity inferred from current documented host–parasite associations. Identifying the ways in which host specificity may have been influenced by past host extinction is important for quantifying risks of parasites establishing on novel hosts, and predicting how selection on multi-host parasites may shift in response to future host extinctions.
Here, we examine how host extinction may shape patterns and perceptions of host specificity and alter emergent patterns of parasite diversity and distribution at broader scales. We first summarize theoretical predictions on the consequences of host extinction, then showcase examples of these through the lenses of both historical mammal extinctions and projected future extinctions based on contemporary threat status. Although these patterns are complex, we highlight how host extinction can lead to both increases and decreases in apparent parasite host specificity, demonstrate how host specificity may be impacted by non-random host extinction and consider implications for projecting how host specificity might respond to future host extinctions. Finally, we discuss the impacts of host extinction on parasite ecology and evolution, with a focus on altering costs of generalism versus specialism, parasite fitness, transmission potential and virulence evolution. While current coextinction theory largely addresses parasite extinction resulting from host extinction, we suggest that expanding this framework to include contemporary measures of host specificity and theory underlying co-adaptation and virulence evolution in multi-host systems will be crucial to understanding how biodiversity loss impacts infectious diseases more broadly.
2. Proximate impacts of host extinction on parasite host specificity
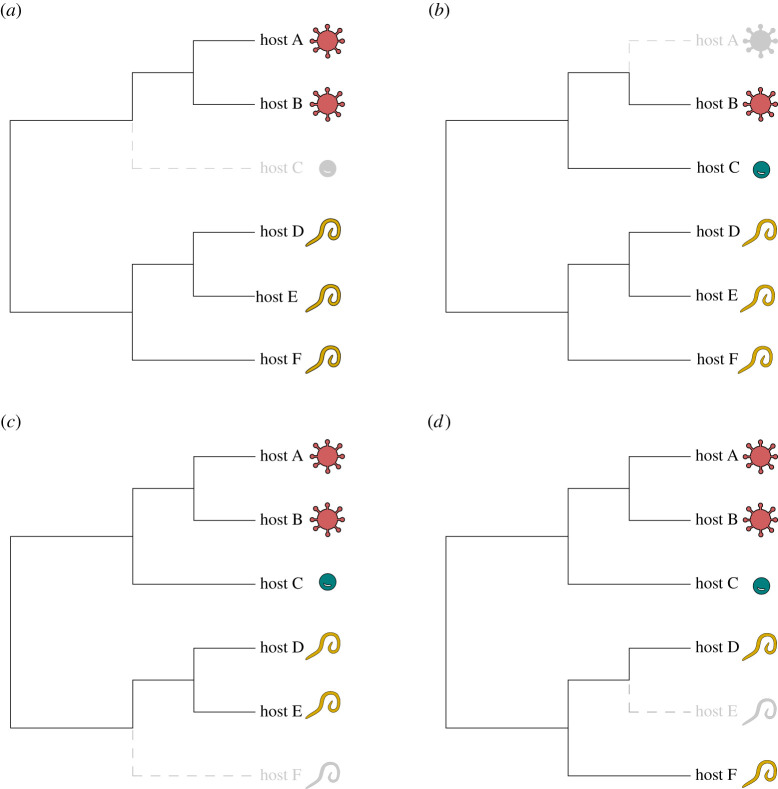
The concept of parasite coextinction was first formulated as the extinction of a host-specific parasite with the loss of its sole host [13,48] (figure 1a). While assumed to be quite common, coextinction events are rarely documented [49]. A classic example of coextinction is the loss of the host-specific California condor louse (Colpocephalum californici) which went extinct after California condors (Gymnogyps californianus) became extinct in the wild and surviving individuals were deloused during a captive breeding and reintroduction programme [50]. Beyond coextinction, host extinction may result in a formerly multi-host parasite being constrained to infect a single host species (figure 1b). This was the case for two species of passenger pigeon louse (Columbicola extinctus and Campanulotes defectus) that parasitized both the passenger pigeon (Ectopistes migratorius) and another closely related species [3,51]. Ironically, this was initially presented as a classic example of parasite coextinction as these two parasite species had only been described on the passenger pigeon and were presumed extinct with the pigeon [13], and only later were they found alive and parasitizing another host species. In hindsight, if the full host ranges had been known, these parasites would have been considered to be multi-host parasites and now constrained to single-host specialists after the extinction of the passenger pigeon. For parasites that infect more than two hosts, host extinction in the absence of host jumps will always reduce host richness, thus increasing perceived taxonomic specialization. However, the loss of a host species may increase or decrease the average phylogenetic distances among extant hosts (figure 1c,d), shifting our perception of the phylogenetic host breadth of the parasite. The directionality of the shift in phylogenetic host breadth is highly context dependent, which we explore further below.
Figure 1.
Examples of how host extinction can impact parasite specificity. Each shape represents a hypothetical parasite species, with their positions reflecting interactions with hosts alongside the host phylogeny. Each extinction scenario involves the loss of one host species (depicted by grey dashed lines). Depending on the original set of host–parasite interactions, the extinction of a host species may result in the loss of a single-host parasite, an example of coextinction (a), the reclassification of a former generalist to a single-host parasite (b), or more subtle changes in which the average phylogenetic distances among hosts may decrease (c) or increase (d) among the remaining hosts. (Online version in colour.)
3. Ghosts of hosts past
Building a greater understanding of coextinction and our perceptions of contemporary patterns of the host specificity of parasites may be achieved through studies of historical host extinctions. Looking to the past, we may find support for parasite extinctions following known host extinctions and identify cases in which historical extinctions likely influenced contemporary host specificity. As host species are pruned from the tree of life, those that survive can become increasingly isolated in the phylogeny, especially if they are nested within clades where extinction has been rampant [52]. The apparent phylogenetic specificity of the parasites found on them will therefore also change over time.
One approach to quantify how host extinction drives the phylogenetic distances among species is through the measure of evolutionary distinctiveness (ED) [53]. This measure, widely used in conservation prioritization [53–57], divides the total branch lengths of a phylogenetic tree among the tips. Each species is apportioned an amount of phylogenetic diversity, typically measured in millions of years of evolution, based on the sum of the branch lengths from the tip to the root of the tree, discounted by the number of shared descendents subtending from each branch. In this way, species that branched off deeper in the tree and have few or no extant relatives are considered to have high ED, whereas species in a young clade that recently underwent rapid speciation without much extinction would have low ED.
ED has been shown to be negatively related to parasite species richness per host [26,58], indicating that hosts more isolated in the mammal phylogeny have fewer parasites. This may result from different (and non-mutually exclusive) processes. The phylogenetic distance among hosts is negatively related to the propensity for parasite sharing [59–61], such that hosts isolated in the phylogeny may be less likely to be infected by multi-host parasites. One mechanism for this is the tendency for high ED hosts to have unique physiologies or life histories which may make them less likely to gain parasite species via host-switching events [62]. A less considered explanation is that more evolutionarily distinct hosts may have lost parasites because of the extinction of closely related species which acted as maintenance hosts. Following from the idea that single-host parasites will be lost with the extinction of their sole hosts, clades that have undergone large numbers of species extinctions are likely to have seen the coextinction of multi-host but clade-specific parasites. Thus, surviving hosts have both fewer close relatives (high ED) and fewer clade-specific parasites which would otherwise be maintained in more species-rich clades via frequent cross-species transmission.
While increasing ED may result in a reduction in parasite species richness per host, the remaining parasites may become apparent phylogenetic specialists or generalists depending on the initial host–parasite interactions before extinction (figure 1). For example, the loss of a host's close relatives might leave parasites stranded on these newly isolated hosts, if they are unable to evolve to infect additional host species (see [63]). In this case, host extinction may result in an increase of single-host parasites on distinct hosts (figure 1b) or they may appear to have lowered phylogenetic host specificity if parasite populations still persist on more distantly related hosts (figure 1d). Alternatively, if evolutionarily distinct hosts are more likely to be threatened with extinction [64], these hosts today may have already undergone severe population declines in the recent past, and thus host fewer specialist or generalist parasites, depending on host and parasite life histories [14,15].
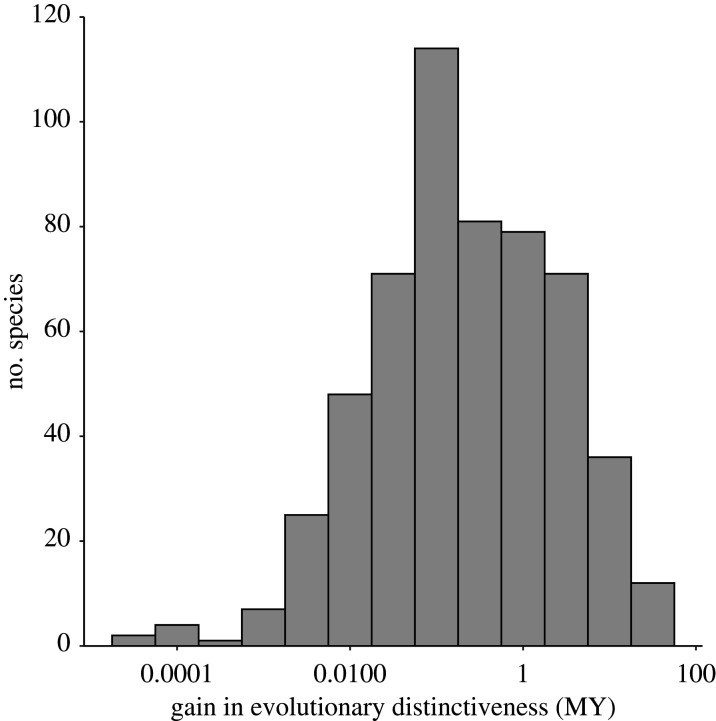
To explore empirical examples in which host extinction may have impacted contemporary patterns of host specificity, we pair a global database of contemporary mammal host–parasite interactions ([65], based on data amalgamated from [66–69]) with data on mammal host extinctions [70] and the Phylogenetic Atlas of Mammal Macroecology (PHYLACINE) [71]. PHYLACINE includes harmonized data on mammal traits, geographic distributions and phylogenetic relationships for all mammals since the last interglacial period (approx. 130 000 years ago until present), including extinct species. We use these data to identify illustrative examples, and demonstrate concepts that may be expanded upon to investigate the impact of host extinction on parasite specificity. With these data, we can calculate the ED of species before and after extinction, taking their difference as a measure of gains in ED and their increasing phylogenetic isolation. Over this time period, there are 352 documented mammal extinctions, which resulted in ED shifts for 551 extant mammals (figure 2). The majority of these ED gains are less than 1 million years (figure 2), but some species have seen large gains in ED on the order of tens of millions of years of added distinctiveness (table 1). As these hosts have lost close relatives, we suggest that the impacts of historical host extinction on parasite host specificity may be gleaned from investigating the ecology and evolution of parasites surviving on them. In the next section, we use a case study of an elephant tapeworm to demonstrate how this approach may generate new hypotheses of how host extinction may impact host specificity through altering parasite distributions, and ultimately shift selection pressures on surviving parasites.
Figure 2.
Distribution of gains in ED for extant mammal species resulting from mammal extinctions over the past 130 000 years. Gains in ED were calculated using the ‘equal-splits’ approach [53,72] and by subtracting contemporary ED measures per species from ED calculated including extinct taxa. Data from the PHYLACINE dataset [71] and Faurby & Svenning [70]
Table 1.
Extant mammal species with the largest gains in ED over the past 130 000 years (see figure 2 for the full distribution).
| species | common name | ED extant | ED pre-extinction | ED gain |
|---|---|---|---|---|
| Elephas maximus | Asian elephant | 47.69 | 10.00 | 37.69 |
| Solenodon cubanus | Cuban solenodon/almiqui | 66.45 | 32.60 | 33.85 |
| Dugong dugon | dugong | 60.50 | 30.86 | 29.64 |
| Loxodonta africana | African bush elephant | 47.69 | 19.86 | 27.83 |
| Macrotis lagotis | greater bilby | 45.85 | 19.73 | 26.12 |
| Tachyglossus aculeatus | short-beaked echidna | 74.61 | 49.04 | 25.57 |
| Hippopotamus amphibius | common hippopotamus | 33.28 | 9.14 | 24.14 |
| Zaglossus bruijnii | western long-beaked echidna | 39.05 | 16.62 | 22.43 |
| Tapirus indicus | Malayan tapir | 40.52 | 20.37 | 20.15 |
| Choloepus didactylus | Linnaeus's two-toed sloth | 25.59 | 7.31 | 18.27 |
4. Geographic discontinuity and the mystery of the elephant tapeworm
The species with the largest increase in ED is the Asian elephant (Elephas maximus), the only extant member of its genus. The Asian elephant is more closely related to extinct mammoths than African elephants (Loxodonta africana) [73], another species with large ED gains over the past 130 000 years (table 1). Currently listed by the IUCN as Endangered and with a declining population trend [74], Asian elephants are known to host at least 36 parasite species, 22 of which are only documented with this host species [65]. Among these parasites is the elephant tapeworm (Anoplocephala manubriata). Both Asian and African elephants are host to the eponymous cestode [75,76], even though these host species live on different continents, with no part of their geographic ranges overlapping. This raises a number of questions as to the ecology and evolutionary history of A. manubriata, and how disconnected species across the globe are infected by the same parasite. Although the taxonomy and biology of this parasite are rarely studied, the elephant tapeworm has been shown to use oribatid mites as obligate intermediate hosts [75], and phylogenetic analysis of tapeworms taken from Asian elephants were placed as sister taxa to Anoplocephala sp. infecting equids [76].
One possible explanation for the unusual distribution of A. manubriata might be circumglobal transmission. Some intermediate hosts of elephant tapeworms have distributions that span continents [75]. As oribatid mites commonly occur in soil communities, their general mechanisms of dispersal are relatively unknown, but some species have the ability to survive long-distance wind dispersal [77] and are speculated to undergo trans-oceanic dispersal via seabirds or ocean currents [78]. Although tapeworm populations in Asian and African elephants may be connected through rare cross-continental dispersal events, an alternative (and non-mutually exclusive) explanation is that the host range of the elephant tapeworm we see today is a relic of historical host extinctions.
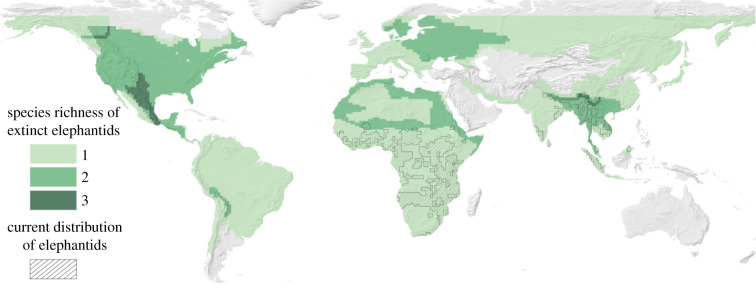
Over the past 50 000 years, we have seen the extinction of a suite of megafauna [79], including elephantids that roamed throughout Eurasia (figure 3) [71,73], which may have acted as alternative hosts and bridged the now disconnected ranges of African and Asian elephants [73]. Would these lost elephantids also have been host to the elephant tapeworm? If so, the elephant tapeworm may be an example of a parasite which has seen a reduction in host richness, but an increase in the mean evolutionary distance among its hosts (figure 1d). If true, elephantid extinctions may have changed the host landscape such that the elephant tapeworm is now isolated on two distinct and disjunct host populations. Unfortunately, precise data on historical ranges of hosts is unavailable beyond hindcasted distributional models encompassing large amounts of uncertainty, even for species with prolific fossil records [80]. In the case of the elephant tapeworm, the current distributions of elephant species do not overlap and would not be connected if extinct elephantids roamed the world today (figure 3). However, examining the hindcasted distribution of the woolly mammoth (Mammuthus primigenius) (see [81]), this species (and potentially the historical distribution of other extinct elephantids) is likely to have bridged the distributions of the African and Asian elephants.
Figure 3.
Distributions of species richness of extinct species from the Elephantidae family (green shades) and of current species of elephants (black stripes). The distribution of Asian elephants and African elephants would not be bridged by extinct elephantids in today's climate. Extinct species include Cuvieronius hyodon, Elephas antiquus, Elephas cypriotes, Elephas iolensis, Elephas maximus, Elephas mnaidriensis, Elephas namadicus, Elephas naumanii, Elephas tiliensis, Loxodonta africana, Mammut americanum, Mammuthus columbi, Mammuthus exilis, Mammuthus primigenius, Notiomastodon platensis, Stegodon florensis, Stegodon orientalis and Stegodon trigonocephalus. Data are from PHYLACINE 1.2 [71]. Distributions for species are based on models of where these species would live presently and without anthropogenic pressures, indicating that species richness of extinct elephants indicates where those species would live today, not where they were historically distributed. (Online version in colour.)
A more prosaic explanation is that the elephant tapeworm story is simply a case of mistaken identity; that elephant tapeworms in Asian and African elephants are morphologically similar, yet genetically distinct species. Expanding the study by Perera et al. [76] to explicitly include tapeworms from African elephants would perhaps resolve this. Currently, poor parasite taxonomy challenges our ability to reconstruct historical and contemporary patterns of parasite sharing, with viruses being particularly problematic as they were historically defined by the host in which they were isolated [82]. As the availability of parasite phylogenies become increasingly available (see [83]), we will be able to identify cases in which parasite evolution is driven by host extinction.
5. Non-random extinction and the reshaping of host and parasite assemblages
Extinction is a non-random process, with some clades and some areas more extinction prone than others [84–87]. Since the Cenozoic, mammals have faced extinction as a result of anthropogenic pressures, and climatic and environmental change [80,81]. These impacts have affected large-sized species more intensely [88], and their intensity is non-randomly distributed across space [89]. Today larger-bodied host species and host species with narrow geographic ranges or climatic niche tolerances suffer from disproportionately greater extinction risk [90–95]. Because the attributes that predispose some species to a higher risk of extinction than other species are typically conserved on the evolutionary tree of hosts, the process of extinction can result in a large loss of phylogenetic diversity [1,96] and reshape the phylogenetic tree structure of survivors [97]. These same host traits also covary with parasite richness across host species [98], for example, primates and carnivores with larger body sizes and larger geographic ranges also tend to host more parasite species [99,100]. Thus, the process of extinction may jointly reshape extant host phylogenetic structure and within-host parasite diversity, both mediated through host species traits. However, the direction of trait effects can be complicated: although both large geographic extent and larger body size are associated with higher parasite diversity, hosts with large ranges have reduced extinction risk, whereas hosts with large body size have higher extinction risk.
While host trait predictors of parasite richness have been explored for different parasite taxa [98,101], less work has explored how host traits contribute to variation in the richness of specialist versus generalist parasites. Observations that the relationship between host extinction risk and the ratio of specialist to generalist parasites differs [15] suggest that drivers of parasite loss may differ between these classes of parasite, and thus we might also predict drivers of parasite richness would differ similarly. Testing this prediction requires that we have a robust metric of parasite specificity that is insensitive to recent host extinctions. Exploring how contemporary parasite specificity varies with host traits can provide a potential signal of the effect of non-random host extinction. However, it may simply be infeasible to separate the effects of host traits on determining parasite encounter and transmission from the longer term evolutionary consequences of extinction-driven specialization.
Theory may be of some assistance in separating these effects, clarifying implicit assumptions and guiding future predictions. For example, simple mathematical models suggest that large-bodied hosts are more likely to be infected by generalist parasites than small-bodied hosts. This is based on an assumption that large-bodied hosts are a better resource for parasites, thus making the cost of generalism (poorer adaptation to any individual host) easier to pay [102]. This would suggest that biased extinctions of large-bodied hosts may more likely result in increases in apparent specificity, rather than in coextinction. However, this model also identifies cases where that pattern could reverse, and large-bodied hosts would be more likely to be infected by specialist parasites. Empirically, there is evidence for large-bodied hosts being more heavily infected by generalist parasites in some systems [102] and more heavily infected by specialist parasites in other systems [103–105].
6. Ghosts of future extinctions
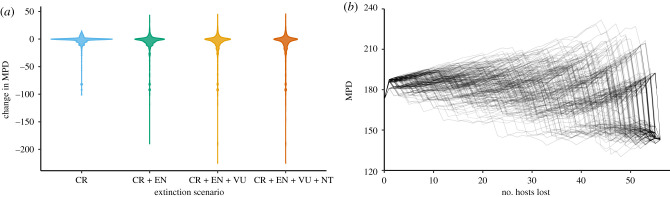
Considering that the loss of even a single host may impact the apparent host specificity of parasites in multiple ways (figure 1), it is difficult to outline clear predictions for formal comparative analyses investigating the impact of extinction on present-day host specificity. The shift in the phylogenetic signature of a parasite across the host phylogeny will depend on which host species is lost from the phylogeny, and different parasites will be impacted differently with the loss of the same host species, depending on their initial phylogenetic host range. However, we may study the impacts of extinction on host specificity through the lens of the current biodiversity crisis. Parallel to earlier studies examining the potential for parasites to go extinct with the loss of their hosts [16,50,106], we may similarly erode existing host–parasite networks and examine resulting impacts on host specificity; however, these approaches tend to ignore the potential for parasite host-switches. To demonstrate, we can examine future impacts of biodiversity loss on the host specificity of mammal parasites by removing sets of hosts based on their IUCN status, with all critically endangered hosts removed first, followed by those in categories with the decreasing risk of extinction (figure 4a). Exploring the mean pairwise phylogenetic distance among hosts (MPD) as a metric of host specificity, we see that the majority of parasites experience little change with future host extinctions, but there are a few with large changes in MPD. As additional hosts with a lower risk of extinction are lost, more extreme reductions in MPD are seen, while other parasites will see increases in MPD.
Figure 4.
(a) Changes in host specificity measured as the mean pairwise phylogenetic distance among hosts (MPD) as hosts are removed according to their IUCN status (CR, critically endangered; EN, endangered; VU, vulnerable; NT, near threatened). Extinction scenarios from left to right remove additional mammal hosts according to their current status. To improve visibility, changes in MPD of zero are removed before plotting. These represent parasites with MPD unchanged by future extinction events. Domesticated species and DD species are not assessed by the IUCN and were assigned a status of LC, thus retaining them in each extinction scenario. The host phylogeny and IUCN statuses are taken from PHYLACINE and paired with the host–parasite association data in Farrell et al. [65]. (b) Changes in host specificity of Trypanosoma cruzi measured as the MPD among hosts as hosts are increasingly lost via extinction. Each line represents a single simulation with a different randomized order of extinction for documented hosts, excluding humans and domesticated species. We use T. cruzi to illustrate this because it infects a large number and phylogenetic diversity of host species; 200 simulations are depicted. (Online version in colour.)
In the previous example, all hosts are removed simultaneously, based on their risk of extinction, but in reality, host extinctions will have an ordering, which will result in different trajectories for changes in phylogenetic host specificity as hosts are lost. Figure 4b illustrates the variable trajectories that shifts in MPD can take as the hosts for a single parasite go extinct. Each line represents a single randomized order of host extinction, indicating that the order of host extinction may result in increases or decreases in apparent specificity. While this is a simple example to illustrate this phenomenon, future studies may examine these patterns in increasingly realistic contexts of non-random and projected host extinctions, or consider simulated extinctions in the context of a host community network and incorporating additional interactions among hosts.
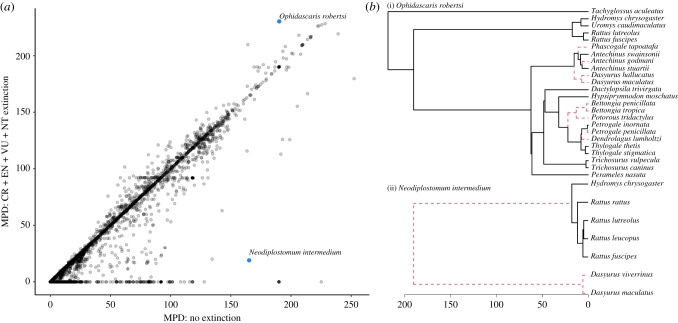
To further explore projected changes in host specificity for particular parasites, we examine differences in MPD as a measure of host specificity among extant hosts and after projected host extinction (figure 5a). Assuming a simulated extinction event leaving only hosts assessed as least concern (LC) or data deficient (DD) by the IUCN, we see that the majority of parasites fall on the 1 : 1 line, indicating that future extinctions will not have a consistent directional impact on phylogenetic host specificity. Nonetheless, phylogenetic specificity will change for a large number of parasites. Among those parasites impacted, some generalists will be reduced to single-host parasites (those with MPD of zero after host extinction), some will become ‘apparent specialists' (reduction in MPD) and others will become ‘apparent generalists’ (gains in MPD). Examples of increasing apparent specialism and generalism can be seen with extinctions among the hosts of the nematode Ophidascaris robertsi and the trematode Neodiplostomum intermedium (figure 5b). Both parasites infect Australian mammals including marsupials and native placental rats. However, future host extinctions are likely to trim away internal branches among hosts of O. robertsi leading to increased phylogenetic distances, while all of the marsupial hosts of N. intermedium will be lost and lead to greatly increased phylogenetic specificity. Although the number of projected host extinctions is high, the ecology of O. robertsi may be relatively unimpacted as extinctions do not prune large swathes of the host tree, multiple sister taxa are projected to survive, and mammals are only intermediate hosts for this parasite which uses pythons as a definitive host [107]. However, as N. intermedium uses mammals as definitive hosts, the large phylogenetic distances between Australian eutherian rats and marsupial hosts could mean that the projected extinction of the Dasyurus hosts will dramatically shift the selective landscape of the parasite.
Figure 5.
(a) Host specificity of parasites, measured as the mean pairwise phylogenetic distance (MPD) among contemporary hosts (x-axis), and assuming the extinction of all mammals except those categorized as LC or DD by the IUCN (y-axis). (b) Examples of future host extinctions on the phylogenetic relationships among hosts for (i) Ophidascaris robertsi and (ii) Neodiplostomum intermedium. Extinct lineages are denoted by red dashed lines and represent the loss of species assigned IUCN categories other than LC (host phylogeny and IUCN statuses are taken from PHYLACINE and paired with the host–parasite association data in Farrell et al. [65]). Scale bar represents millions of years. (Online version in colour.)
7. Impacts of host extinction on parasite ecology and evolution
Host extinction and the coextinction of dependent parasites will impact the structure and function of ecosystems [3,19,108] and may shift the distributions of zoonotic diseases [18]. In addition to complete host loss, there can be large impacts due to changes in host populations as they decline to extinction. These include reductions in host and parasite abundance, leading to reduced population densities or range sizes. At the extreme, for parasites that are ‘obligate’ multi-host parasites (where ‘obligate’ refers to a situation where the net reproductive rate of the parasite on any individual host is less than one, so that parasite maintenance requires multiple hosts; [23]), host population declines may lead to parasite extinction well before any host actually goes extinct. We are already seeing evidence of such changes in many host populations [2,109,110], and these host declines have been marked by the loss of parasites in threatened species [14,111] and changes in the proportion of generalist versus specialist parasites in some host groups [15]. In the latter case, these changes likely result from shifting intra- and interspecific contact rates among hosts, which may have proximate impacts such as shifting parasite distributions, population sizes and relative rates of host exposure. While it is clear that host extinction will influence parasite abundance, whether extinction increases or decreases transmission will depend on specifics of the system and how it impacts the relative abundance of competent hosts. In instances where parasites lose hosts that support onward transmission, we may see reduced transmission potential, whereas the extinction of off-target or dead-end hosts may allow for the maintenance of robust parasite populations within more competent reservoir hosts. Further, parasite life histories, such as transmission mode, may evolve in tandem with shifting host specificity [112], and are likely to mediate this effect. For many parasites, transmission is only weakly or not impacted by reductions in host density, and in extreme cases, such as vector-borne or strongly frequency-dependent transmission, reduced host density can improve transmission [113,114].
(a) . Transmission frequency
Whether host extinction increases or decreases parasite transmission will impact changes on evolutionary timescales [115] and may impose new selection pressures on parasite evolution [116]. For example, host extinction may limit gene flow among previously connected parasite populations, promoting specialization of parasites on their newly isolated hosts. For many infectious organisms, and especially those with short generation times such as viruses and bacteria, this isolation could lead to allopatric speciation, a process that would be reflected in congruent tree shapes in co-phylogenetic analyses [83,117]. This process of host extinction leading to parasite specialization and speciation may be quite common, but the lack of robust parasite fossil records and data on historical hosts make this difficult to identify. Future co-phylogenetic methods may benefit by modelling the impacts of host extinctions, as reconstructions may be differentially impacted by the loss of closely versus distantly related host species [118]. For relatively long-lived parasites, such as cestodes, including the elephant tapeworm discussed above, we may be able to identify examples where parasites are in the process of speciation. The longevity of adult tapeworms in their definitive hosts is quite variable, surviving from weeks to multiple decades up to the lifespan of the host [119]. The long generation times of some tapeworms might not allow sufficient time for divergence following historical extinctions and subsequent geographic isolation of their host species. This may be the case for the elephant tapeworm, but further research on maximum longevity, population genetics, and phylogenetic analyses of both the Asian and African populations would be needed.
(b) . Costs of generalism
As host extinction drives increasing phylogenetic isolation of host species, this is likely to alter the costs of generalism, potentially promoting further parasite specialization and speciation, and also shift the optima for virulence and transmission across extant hosts [33,62,120]. Multi-host parasites are often assumed to experience a cost of generalism, the increased transmission opportunities associated with additional host species trading off against fitness benefits gained by specializing on any particular host species [62,120–122]. Costs of generalism can take two forms; one is a more global cost in which having multiple hosts reduces the potential for coevolution with any one host, meaning generalists may not be as well adapted to their hosts, on average, when compared to specialist parasites. The other form that a cost of generalism may take is greater variation in fitness across hosts, with parasite adaptation to novel hosts resulting in reduced fitness in original hosts [123], with the magnitude of this trade-off increasing with the phylogenetic distance between hosts [62]. Due to either or both of these costs, generalist parasites are therefore likely to have lower fitness in any given host than is possible in a single-host relationship, which is offset by the demographic advantage of an expanded reservoir of available hosts [124]. In this context, the influence of host extinction on parasite mean fitness will depend precisely on which hosts are lost, the evolutionary distances between extant hosts and the types of costs of generalism that were being paid (e.g. if they were reasonably well adapted to any host in the system).
(c) . Virulence
Parasite fitness relies on successful transmission, which requires the exploitation of host resources and ultimately results in damage to hosts, termed ‘virulence’. For many parasites, greater host exploitation facilitates increased transmission, but if viruence is too high, then the transmission may be reduced due to shorter infection duration [125,126]. For multi-host parasites, there may be a unique optimal virulence that maximizes transmission on each individual host [124]. If parasites are constrained to a single level of virulence (i.e. they cannot plastically adjust their strategy to the current host), then parasites will evolve an intermediate virulence, influenced by the relative contribution of each host species to the total force of infection, that maximizes fitness across their host species, but achieves optimal virulence in none [24]. By changing the epidemiological contribution of each species, host extinction is likely to shift the selective landscape for parasites, leading to changes in virulence as parasites adapt to track the optimal virulence of the surviving hosts.
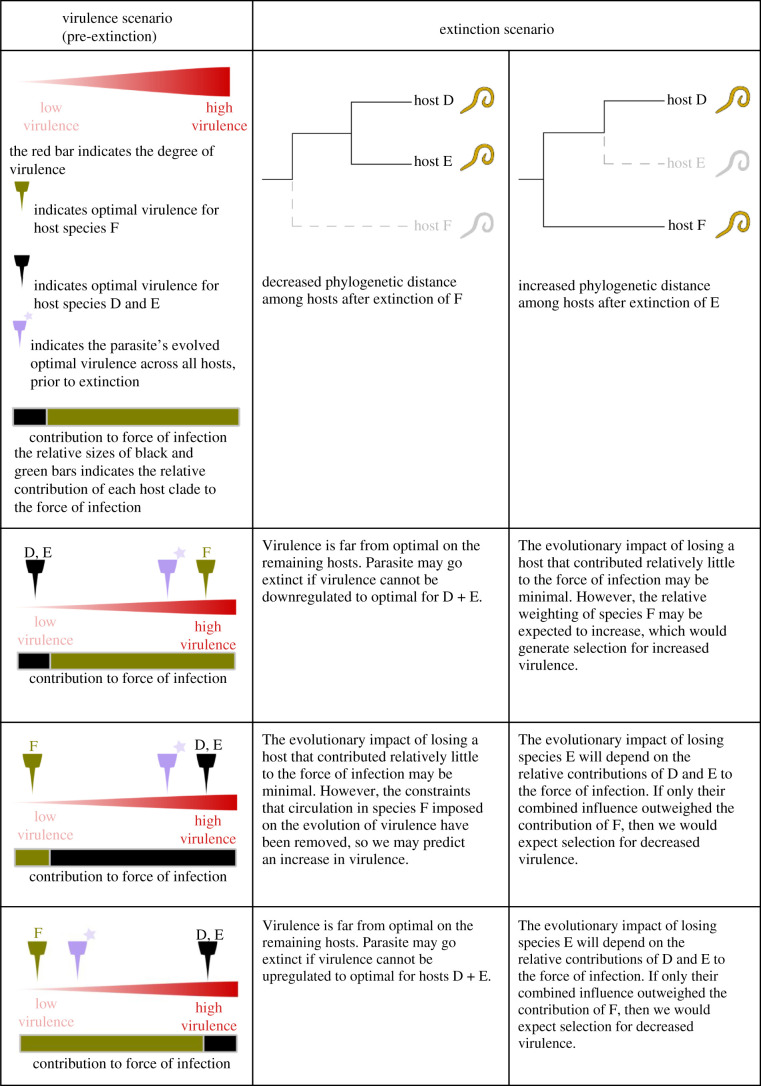
Depending on the relative contributions of different host species to transmission, as well as the optimal virulence within each, the extinction of a particular species may lead to the evolution of increased or decreased virulence on remaining hosts. In table 2, we explore possible evolutionary outcomes of host extinction assuming three host species, the potential for onward transmission in each host, and a single optimal virulence expressed in each host species that maximizes total transmission. Few empirical studies have examined how phylogenetic distance among hosts is linked to parasite virulence, but studies of zoonoses and multi-host domesticated animal parasites found that increased evolutionary distance among hosts is associated with greater potential for virulence, but at the cost of reduced transmission [33,34]. Predicting the evolution of virulence in multi-host systems is a complex challenge, but as biodiversity loss dramatically restructures host–parasite associations, and humans become increasingly isolated in the tree of life, understanding how parasite virulence may evolve in response to host extinction is increasingly important.
Table 2.
Examples of how parasite virulence might evolve in response to host extinction. The first column indicates the initial state of each system prior to extinction, including the optimal virulence for each host clade if this was the sole host, and the evolved optimal virulence expressed across all hosts. In these examples, optimal virulence is skewed towards the single-species optimum for the host clade that contributes the most to the force of infection. The second and third columns outline the shifts in the system resulting from two extinction scenarios in which the phylogenetic distances among hosts is either decreased or increased. With the extinction of a given host, in general, we would expect virulence to evolve towards the optimal virulence for the remaining species, though this is dependent on the initial state of the system. This framework closely follows the theory in Williams [24].
 |
8. Conclusion
The current biodiversity crisis is reshaping the tree of life, shifting realized parasite host specificities and the adaptive landscapes of contemporary parasites. Here, we demonstrate that the impacts of host extinction on phylogenetic measures of host specificity are context-specific, with host extinction potentially leading to both increases and decreases in generalism of parasites. We suggest that these changes in specificity are likely to have complex impacts on parasite evolution, including further evolution of specialist or generalist strategies, and the shifts in parasite virulence. We show that past extinctions may have reshaped host–parasite associations, and thus care should be taken when drawing inference from present-day patterns of host specificity. In the case of more recent host extinctions, parasites today may appear more or less specialized, masking an intrinsic ability to infect novel host species, and altering our perceptions of their potential host ranges.
Just as past extinctions have shaped present-day host–parasite interactions, ongoing biodiversity loss will continue to shape disease dynamics into the future. Beyond extinction, climate change-induced range shifts may promote host–parasite sharing and novel interactions never seen before in evolutionary history [127]. Infectious diseases act as synergistic drivers of host extinction, with impacts due to infectious diseases increasing as populations decline to extinction [128]. Host extinction is likely to decrease global parasite richness through the coextinction of specialist parasites [3], but generalist parasites are most often associated with host declines [129]. The relative loss of specialist parasites may remove protective effects of co-adapted parasites and expose hosts to more virulent parasites through the reduction of immune cross-protection and opening of new niches for generalist parasites [130]. When shifting to novel hosts, parasites may display increased virulence due to a lack of coevolutionary history between host and parasite [131], and host extinctions may also select for increased parasite virulence in some systems, exacerbating disease-mediated host declines. While the current theory is well developed for single-host single-parasite systems, expanding on theories of host specificity, co-adaptation, and virulence evolution in multi-host systems is crucial for better understanding how biodiversity loss impacts infectious diseases, and mitigating disease impacts as we navigate the current biodiversity crisis. We note that many of the concepts discussed here for host–parasite systems may also be applied to symbionts in general, offering new avenues for future research into the cascading impacts of host extinction.
Data accessibility
Data and R scripts to reproduce the figures are available at https://github.com/DiseaseMacroecology/ghost-host and doi:10.6084/m9.figshare.14573787.
Authors' contributions
M.J.F. wrote the manuscript and produced the figures with input from all authors.
Competing interests
We declare we have no competing interests.
Funding
We thank the Macroecology of Infectious Disease Research Coordination Network, jointly funded by NSF, NIH and USDA (grant no. NSF DEB 1316223), for facilitating discussion among the authors, and for supporting M.J.F. as a postdoctoral research associate. S.H. was supported by the German Science Foundation (grant no. DFG, HU 2748/1-1). I.M.C. acknowledges funding from the Spanish Ministry for Science and Innovation (grant no. PID2019-109711RJ-I00). M.J.F. is currently supported by the University of Toronto Ecology & Evolutionary Biology Postdoctoral Fellowship.
References
- 1.Davis M, Faurby S, Svenning J-C. 2018Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proc. Natl Acad. Sci. USA 115, 11 262-11 267. ( 10.1073/pnas.1804906115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IUCN. 2020The IUCN Red List of threatened species. Version 2020-3. See https://www.iucnredlist.org.
- 3.Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS. 2009The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. R. Soc. B 276, 3037-3045. ( 10.1098/rspb.2009.0413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson CJ, et al. 2020A global parasite conservation plan. Biol. Conserv. 250, 108596. ( 10.1016/j.biocon.2020.108596) [DOI] [Google Scholar]
- 5.Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W. 2008Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl Acad. Sci. USA 105(Supplement 1), 11 482-11 489. ( 10.1073/pnas.0803232105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson CJ, Dallas TA, Alexander LW, Phelan AL, Phillips AJ. 2020What would it take to describe the global diversity of parasites? Proc. R. Soc. B 287, 20201841. ( 10.1098/rspb.2020.1841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson P, Greenman J. 1998Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387-390. ( 10.1016/s0169-5347(98)01475-x) [DOI] [PubMed] [Google Scholar]
- 8.Dunne JA, et al. 2013Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biol. 11, e1001579. ( 10.1371/journal.pbio.1001579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galetti M, et al. 2018Ecological and evolutionary legacy of megafauna extinctions: anachronisms and megafauna interactions. Biol. Rev. 93, 845-862. ( 10.1111/brv.12374) [DOI] [PubMed] [Google Scholar]
- 10.Taglioretti V, Fugassa MH, Sardella NH. 2015Parasitic diversity found in coprolites of camelids during the Holocene. Parasitol. Res. 114, 2459-2464. ( 10.1007/s00436-015-4442-y) [DOI] [PubMed] [Google Scholar]
- 11.Lafferty KD, Hopkins SR. 2018Unique parasite ADNA in moa coprolites from New Zealand suggests mass parasite extinctions followed human-induced megafauna extinctions. Proc. Natl Acad. Sci. USA 115, 1411-1413. ( 10.1073/pnas.1722598115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doña J, Johnson KP. 2020Assessing symbiont extinction risk using cophylogenetic data. Biol. Conserv. 250, 108705. ( 10.1016/j.biocon.2020.108705) [DOI] [Google Scholar]
- 13.Stork NE, Lyal CHC. 1993Extinction or ‘co-extinction’ rates? Nature 366, 307. ( 10.1038/366307a0)8247122 [DOI] [Google Scholar]
- 14.Altizer S, Nunn CL, Lindenfors P. 2007Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 76, 304-314. ( 10.1111/j.1365-2656.2007.01214.x) [DOI] [PubMed] [Google Scholar]
- 15.Farrell MJ, Stephens PR, Berrang-Ford L, Gittleman JL, Davies TJ. 2015The path to host extinction can lead to loss of generalist parasites. J. Anim. Ecol. 84, 978-984. ( 10.1111/1365-2656.12342) [DOI] [PubMed] [Google Scholar]
- 16.Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. 2004Species coextinctions and the biodiversity crisis. Science 305, 1632-1634. ( 10.1126/science.1101101) [DOI] [PubMed] [Google Scholar]
- 17.Strona G, Galli P, Fattorini S. 2013Fish parasites resolve the paradox of missing coextinctions. Nat. Commun. 4, 1718. ( 10.1038/ncomms2723) [DOI] [PubMed] [Google Scholar]
- 18.Harris NC, Dunn RR. 2013Species loss on spatial patterns and composition of zoonotic parasites. Proc. R. Soc. B 280, 20131847. ( 10.1098/rspb.2013.1847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallas T, Cornelius E. 2015Co-extinction in a host-parasite network: identifying key hosts for network stability. Sci. Rep. 5, 13185. ( 10.1038/srep13185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colwell RK, Dunn RR, Harris NC. 2012Coextinction and persistence of dependent species in a changing world. Ann. Rev. Ecol. Evol. Syst. 43, 183-203. ( 10.1146/annurev-ecolsys-110411-160304) [DOI] [Google Scholar]
- 21.Holt RD, Dobson AP, Begon M, Bowers RG, Schauber EM. 2003Parasite establishment in host communities. Ecol. Lett. 6, 837-842. ( 10.1046/j.1461-0248.2003.00501.x) [DOI] [Google Scholar]
- 22.Dobson A. 2004Population dynamics of pathogens with multiple host species. Am. Nat. 164(S5), S64-S78. ( 10.1086/424681) [DOI] [PubMed] [Google Scholar]
- 23.Fenton A, Streicker DG, Petchey OL, Pedersen AB. 2015Are all hosts created equal? Partitioning host species contributions to parasite persistence in multihost communities. Am. Nat. 186, 610-622. ( 10.1086/683173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams PD. 2012New insights into virulence evolution in multigroup hosts. Am. Nat. 179, 228-239. ( 10.1086/663690) [DOI] [PubMed] [Google Scholar]
- 25.Poulin R, Krasnov BR, Mouillot D. 2011Host specificity in phylogenetic and geographic space. Trends Parasitol. 27, 355-361. ( 10.1016/j.pt.2011.05.003) [DOI] [PubMed] [Google Scholar]
- 26.Park AW, et al. 2018Characterizing the phylogenetic specialism–generalism spectrum of mammal parasites. Proc. R. Soc. B 285, 20172613. ( 10.1098/rspb.2017.2613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page RDM. 1993Parasites, phylogeny and cospeciation. Int. J. Parasitol. 23, 499-506. ( 10.1016/0020-7519(93)90039-2) [DOI] [Google Scholar]
- 28.Cooper N, Griffin R, Franz M, Omotayo M, Nunn CL. 2012Phylogenetic host specificity and understanding parasite sharing in primates. Ecol. Lett. 15, 1370-1377. ( 10.1111/j.1461-0248.2012.01858.x) [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Aquino A. 2016Phylogenetic framework for coevolutionary studies: a compass for exploring jungles of tangled trees. Curr. Zool. 62, 393-403. ( 10.1093/cz/zow018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woolhouse MEJ, Gowtage-Sequeria S. 2005Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842-1847. ( 10.3201/eid1112.050997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmasri M, Farrell MJ, Davies TJ, Stephens DA. 2020A hierarchical Bayesian model for predicting ecological interactions using scaled evolutionary relationships. Ann. Appl. Stat. 14, 221-240. ( 10.1214/19-AOAS1296) [DOI] [Google Scholar]
- 32.Brierley L, Pedersen AB, Woolhouse MEJ. 2019Tissue tropism and transmission ecology predict virulence of human RNA viruses. PLoS Biol. 17, e3000206. ( 10.1371/journal.pbio.3000206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell MJ, Davies TJ. 2019Disease mortality in domesticated animals is predicted by host evolutionary relationships. Proc. Natl Acad. Sci. USA 116, 7911-7915. ( 10.1073/pnas.1817323116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guth S, Visher E, Boots M, Brook CE. 2019Host phylogenetic distance drives trends in virus virulence and transmissibility across the animal–human interface. Phil. Trans. R. Soc. B 374, 20190296. ( 10.1098/rstb.2019.0296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker DJ, et al. 2020Predicting wildlife hosts of betacoronaviruses for SARS-CoV-2 sampling prioritization. bioRxiv111344. ( 10.1101/2020.05.22.111344) [DOI]
- 36.Wardeh M, Sharkey KJ, Baylis M. 2020Integration of shared-pathogen networks and machine learning reveals the key aspects of zoonoses and predicts mammalian reservoirs. Proc. R. Soc. B 287, 20192882. ( 10.1098/rspb.2019.2882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vienne DMd, Refrégier G, López-Villavicencio M, Tellier A, Hood ME, Giraud T. 2013Cospeciation vs host-shift speciation: methods for testing, evidence from natural associations and relation to coevolution. New Phytol. 198, 347-385. ( 10.1111/nph.12150) [DOI] [PubMed] [Google Scholar]
- 38.Wells K, Clark NJ. 2019Host specificity in variable environments. Trends Parasitol. 35, 452-465. ( 10.1016/j.pt.2019.04.001) [DOI] [PubMed] [Google Scholar]
- 39.Doña J, Serrano D, Mironov S, Montesinos-Navarro A, Jovani R. 2019Unexpected bird–feather mite associations revealed by DNA metabarcoding uncovers a dynamic ecoevolutionary scenario. Mol. Ecol. 28, 379-390. ( 10.1111/mec.14968) [DOI] [PubMed] [Google Scholar]
- 40.Braga MP, Landis MJ, Nylin S, Janz N, Ronquist F. 2020Bayesian inference of ancestral host–parasite interactions under a phylogenetic model of host repertoire evolution. Syst. Biol. 69, 1149-1162. ( 10.1093/sysbio/syaa019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braga MP, Janz N, Nylin S, Ronquist F, Landis MJ. 2021Evolution of butterfly-plant networks over time, as revealed by Bayesian inference of host repertoire. bioRxiv 429735. ( 10.1101/2021.02.04.429735) [DOI]
- 42.Charleston MA, Robertson DL. 2002Preferential host switching by primate lentiviruses can account for phylogenetic similarity with the primate phylogeny. Syst. Biol. 51, 528-535. ( 10.1080/10635150290069940) [DOI] [PubMed] [Google Scholar]
- 43.Hoberg EP, Brooks DR. 2008A macroevolutionary mosaic: episodic host-switching, geographical colonization and diversification in complex host–parasite systems. J. Biogeogr. 35, 1533-1550. ( 10.1111/j.1365-2699.2008.01951.x) [DOI] [Google Scholar]
- 44.Johnson KP, Weckstein JD, Meyer MJ, Clayton DH. 2011There and back again: switching between host orders by avian body lice (Ischnocera: Goniodidae). Biol. J. Linn. Soc. 102, 614-625. ( 10.1111/j.1095-8312.2010.01612.x) [DOI] [Google Scholar]
- 45.Doña J, Proctor H, Mironov S, Serrano D, Jovani R. 2018Host specificity, infrequent major host switching and the diversification of highly host-specific symbionts: the case of Vane-Dwelling feather mites. Glob. Ecol. Biogeogr. 27, 188-198. ( 10.1111/geb.12680) [DOI] [Google Scholar]
- 46.Engelstädter J, Fortuna NZ. 2019The dynamics of preferential host switching: host phylogeny as a key predictor of parasite distribution. Evolution 73, 1330-1340. ( 10.1111/evo.13716) [DOI] [PubMed] [Google Scholar]
- 47.Schatz AM, Park AW. 2021Host and parasite traits predict cross-species parasite acquisition by introduced mammals. Proc. R. Soc. B 288, 20210341. ( 10.1098/rspb.2021.0341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Windsor DA. 1990Heavenly hosts. Nature 348, 104. ( 10.1038/348104c0)2234071 [DOI] [Google Scholar]
- 49.Rózsa L, Vas Z. 2015Co-extinct and critically co-endangered species of parasitic lice, and conservation-induced extinction: should lice be reintroduced to their hosts? Oryx 49, 107-110. ( 10.1017/S0030605313000628) [DOI] [Google Scholar]
- 50.Dunn RR. 2009Coextinction: anecdotes, models, and speculation. In Holocene extinctions (ed. Turvey ST), pp. 167-180. Oxford, UK: Oxford University Press. [Google Scholar]
- 51.Price RD, Clayton DH, Adams RJ. 2000Pigeon lice down under: taxonomy of Australian campanulotes (Phthiraptera: Philopteridae), with a description of C. durdeni n. sp. J. Parasitol. 86, 948-950. ( 10.1645/0022-3395(2000)086[0948:PLDUTO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 52.Pavoine S, Bonsall MB, Davies TJ, Masi S. 2019Mammal extinctions and the increasing isolation of humans on the tree of life. Ecol. Evol. 9, 914-924. ( 10.1002/ece3.4630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redding DW, Mooers AØ. 2006Incorporating evolutionary measures into conservation prioritization. Conserv. Biol. 20, 1670-1678. ( 10.1111/j.1523-1739.2006.00555.x) [DOI] [PubMed] [Google Scholar]
- 54.Isaac NJB, Turvey ST, Collen B, Waterman C, Baillie JEM. 2007Mammals on the EDGE: conservation priorities based on threat and phylogeny. PLoS ONE 2, e296. ( 10.1371/journal.pone.0000296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redding DW, DeWolf CV, Mooers AØ. 2010Evolutionary distinctiveness, threat status, and ecological oddity in primates: EDGE ranking of primates. Conserv. Biol. 24, 1052-1058. ( 10.1111/j.1523-1739.2010.01532.x) [DOI] [PubMed] [Google Scholar]
- 56.Pearse WD, et al. 2015Beyond the EDGE with EDAM: prioritising British plant species according to evolutionary distinctiveness, and accuracy and magnitude of decline. PLoS ONE 10, e0126524. ( 10.1371/journal.pone.0126524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrault N, Farrell MJ, Davies TJ. 2017Tongues on the EDGE: language preservation priorities based on threat and lexical distinctiveness. R. Soc. Open Sci. 4, 171218. ( 10.1098/rsos.171218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang S, Drake JM, Gittleman JL, Altizer S. 2015Parasite diversity declines with host evolutionary distinctiveness: a global analysis of carnivores. Evolution 69, 621-630. ( 10.1111/evo.12611) [DOI] [PubMed] [Google Scholar]
- 59.Davies TJ, Pedersen AB. 2008Phylogeny and geography predict pathogen community similarity in wild primates and humans. Proc. R. Soc. B 275, 1695-1701. ( 10.1098/rspb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang S, Bininda-Emonds ORP, Stephens PR, Gittleman JL, Altizer S. 2014Phylogenetically related and ecologically similar carnivores harbour similar parasite assemblages. J. Anim. Ecol. 83, 671-680. ( 10.1111/1365-2656.12160) [DOI] [PubMed] [Google Scholar]
- 61.Braga MP, Razzolini E, Boeger WA. 2015Drivers of parasite sharing among neotropical freshwater fishes. J. Anim. Ecol. 84, 487-497. ( 10.1111/1365-2656.12298) [DOI] [PubMed] [Google Scholar]
- 62.Antonovics J, Boots M, Ebert D, Koskella B, Poss M, Sadd BM. 2013The origin of specificity by means of natural selection: evolved and nonhost resistance in host–pathogen interactions. Evolution 67, 1-9. ( 10.1111/j.1558-5646.2012.01793.x) [DOI] [PubMed] [Google Scholar]
- 63.Johnson KP, Weckstein JD, Bush SE, Clayton DH. 2011The evolution of host specificity in dove body lice. Parasitology 138, 1730-1736. ( 10.1017/S0031182010001770) [DOI] [PubMed] [Google Scholar]
- 64.Cadotte MW, Davies TJ. 2010Rarest of the rare: advances in combining evolutionary distinctiveness and scarcity to inform conservation at biogeographical scales. Divers. Distrib. 16, 376-385. ( 10.1111/j.1472-4642.2010.00650.x) [DOI] [Google Scholar]
- 65.Farrell MJ, Elmasri M, Stephens D, Davies TJ. 2020Predicting missing links in global host-parasite networks. bioRxiv965046. ( 10.1101/2020.02.25.965046) [DOI]
- 66.Gibson DI, Bray RA, HarrisEA (compilers). 2005Host-parasite database of the Natural History Museum, London. See https://www.nhm.ac.uk/research-curation/scientific-resources/taxonomy-systematics/host-parasites/. [Google Scholar]
- 67.Wardeh M, Risley C, McIntyre MK, Setzkorn C, Baylis M. 2015Database of host-pathogen and related species interactions, and their global distribution. Sci. Data 2, 150049. ( 10.1038/sdata.2015.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. 2017Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646-650. ( 10.1038/nature22975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephens PR, et al. 2017Global mammal parasite database version 2.0. Ecology 98, 1476. ( 10.1002/ecy.1799) [DOI] [PubMed] [Google Scholar]
- 70.Faurby S, Svenning J-C. 2016Resurrection of the island rule: human-driven extinctions have obscured a basic evolutionary pattern. Am. Nat. 187, 812-820. ( 10.1086/686268) [DOI] [PubMed] [Google Scholar]
- 71.Faurby S, Davis M, Pedersen RØ, Schowanek SD, Antonelli A, Svenning JC. 2018PHYLACINE 1.2: the phylogenetic atlas of mammal macroecology. Ecology 99, 2626. ( 10.1002/ecy.2443) [DOI] [PubMed] [Google Scholar]
- 72.Redding DW. 2003Incorporating genetic distinctness and reserve occupancy into a conservation prioritisation approach. Masters thesis, University of East Anglia, Norwich, UK.
- 73.Palkopoulou E, et al. 2018A comprehensive genomic history of extinct and living elephants. Proc. Natl Acad. Sci. USA 115, E2566-E2574. ( 10.1073/pnas.1720554115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choudhury A, et al. 2008Elephas maximus. The IUCN Red List of threatened species 2008. ( 10.2305/IUCN.UK.2008.RLTS.T7140A12828813.en) [DOI]
- 75.McAloon MF. 2004Oribatid mites as intermediate hosts of Anoplocephala manubriata, cestode of the Asian elephant in India. Exp. Appl. Acarol. 32, 181-185. ( 10.1023/B:APPA.0000021795.02103.d0) [DOI] [PubMed] [Google Scholar]
- 76.Perera KUE, Wickramasinghe S, Perera BVP, Bandara KBAT, Rajapakse RPVJ. 2017Redescription and molecular characterization of Anoplocephala manubriata, Railliet et al., 1914 (Cestoda: Anoplocephalidae) from a Sri Lankan wild elephant (Elephas maximus). Parasitol. Int. 66, 279-286. ( 10.1016/j.parint.2017.02.007) [DOI] [PubMed] [Google Scholar]
- 77.Lehmitz R, Russell D, Hohberg K, Christian A, Xylander WER. 2011Wind dispersal of oribatid mites as a mode of migration. Pedobiologia 54, 201-207. ( 10.1016/j.pedobi.2011.01.002) [DOI] [Google Scholar]
- 78.Starý J, Block W. 1998Distribution and biogeography of oribatid mites (Acari: Oribatida) in Antarctica, the sub-Antarctic islands and nearby land areas. J. Natl Hist. 32, 861-894. ( 10.1080/00222939800770451) [DOI] [Google Scholar]
- 79.Barnosky AD. 2008Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc. Natl Acad. Sci. USA 105(Suppl. 1), 11 543-11 548. ( 10.1073/pnas.0801918105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lorenzen ED, et al. 2011Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359-364. ( 10.1038/nature10574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nogués-Bravo D, Rodríguez J, Hortal J, Batra P, Araújo MB. 2008Climate change, humans, and the extinction of the woolly mammoth. PLoS Biol. 6, e79. ( 10.1371/journal.pbio.0060079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fauquet CM. 2008Taxonomy, classification and nomenclature of viruses. In Encyclopedia of virology (eds D Bamford, M Zuckermann), pp. 9-23. San Diego, CA: Academic Press. [Google Scholar]
- 83.Pfenning-Butterworth A, Davies J, Cressler C. 2021Identifying co-phylogenetic hotspots for zoonotic disease. Phil. Trans. R. Soc B 376, 20200363. ( 10.1098/rstb.2020.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Purvis A, Agapow P-M, Gittleman JL, Mace GM. 2000Nonrandom extinction and the loss of evolutionary history. Science 288, 328-330. ( 10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 85.Cardillo M, Mace GM, Gittleman JL, Purvis A. 2006Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157-4161. ( 10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fritz SA, Purvis A. 2010Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits: selectivity in extinction risk. Conserv. Biol. 24, 1042-1051. ( 10.1111/j.1523-1739.2010.01455.x) [DOI] [PubMed] [Google Scholar]
- 87.Davidson AD, Shoemaker KT, Weinstein B, Costa GC, Brooks TM, Ceballos G, Radeloff VC, Rondinini C, Graham CH. 2017Geography of current and future global mammal extinction risk. PLoS ONE 12, e0186934. ( 10.1371/journal.pone.0186934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cardillo M, et al. 2005Multiple causes of high extinction risk in large mammal species. Science 309, 1239-1241. ( 10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 89.Morales-Castilla I, Olalla-Tárraga MÁ, Purvis A, Hawkins BA, Rodríguez MÁ. 2012The imprint of Cenozoic migrations and evolutionary history on the biogeographic gradient of body size in New World mammals. Am. Nat. 180, 246-256. ( 10.1086/666608) [DOI] [PubMed] [Google Scholar]
- 90.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947-1952. ( 10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardillo M, Mace GM, Gittleman JL, Jones KE, Bielby J, Purvis A. 2008The predictability of extinction: biological and external correlates of decline in mammals. Proc. R. Soc. B 275, 1441-1448. ( 10.1098/rspb.2008.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davidson AD, Hamilton MJ, Boyer AG, Brown JH, Ceballos G. 2009Multiple ecological pathways to extinction in mammals. Proc. Natl Acad. Sci. USA 106, 10 702-10 705. ( 10.1073/pnas.0901956106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olden JD, Hogan ZS, Vander Zanden MJ. 2007Small fish, big fish, red fish, blue fish: size-biased extinction risk of the world's freshwater and marine fishes. Glob. Ecol. Biogeogr. 16, 694-701. ( 10.1111/j.1466-8238.2007.00337.x) [DOI] [Google Scholar]
- 94.Böhm M, Williams R, Bramhall HR, McMillan KM, Davidson AD, Garcia A, Bland LM, Bielby J, Collen B. 2016Correlates of extinction risk in squamate reptiles: the relative importance of biology, geography, threat and range size. Glob. Ecol. Biogeogr. 25, 391-405. ( 10.1111/geb.12419) [DOI] [Google Scholar]
- 95.Collins KS, Edie SM, Hunt G, Roy K, Jablonski D. 2018Extinction risk in extant marine species integrating palaeontological and biodistributional data. Proc. R. Soc. B 285, 20181698. ( 10.1098/rspb.2018.1698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heard SB, Mooers AØ. 2000Phylogenetically patterned speciation rates and extinction risks change the loss of evolutionary history during extinctions. Proc. R. Soc. Lond. B 267, 613-620. ( 10.1098/rspb.2000.1046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davies TJ, Yessoufou K. 2013Revisiting the impacts of non-random extinction on the tree-of-life. Biol. Lett. 9, 20130343. ( 10.1098/rsbl.2013.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamiya T, O'Dwyer K, Nakagawa S, Poulin R. 2014What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts: determinants of parasite species richness. Biol. Rev. 89, 123-134. ( 10.1111/brv.12046) [DOI] [PubMed] [Google Scholar]
- 99.Nunn CL, Altizer S, Jones KE, Sechrest W. 2003Comparative tests of parasite species richness in primates. Am. Nat. 162, 597-614. ( 10.1086/378721) [DOI] [PubMed] [Google Scholar]
- 100.Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, Gittleman JL. 2007Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Glob. Ecol. Biogeogr. 16, 496-509. ( 10.1111/j.1466-8238.2006.00301.x) [DOI] [Google Scholar]
- 101.Morand S. 2015(Macro-) evolutionary ecology of parasite diversity: from determinants of parasite species richness to host diversification. Int. J. Parasitol. 4, 80-87. ( 10.1016/j.ijppaw.2015.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walker JG, Hurford A, Cable J, Ellison AR, Price SJ, Cressler CE. 2017Host allometry influences the evolution of parasite host-generalism: theory and meta-analysis. Phil. Trans. R. Soc. B 372, 20160089. ( 10.1098/rstb.2016.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sasal P, Trouvé S, Müller-Graf C, Morand S. 1999Specificity and host predictability: a comparative analysis among monogenean parasites of fish. J. Anim. Ecol. 68, 437-444. ( 10.1046/j.1365-2656.1999.00313.x) [DOI] [Google Scholar]
- 104.Desdevises Y, Morand S, Legendre P. 2002Evolution and determinants of host specificity in the genus Lamellodiscus (Monogenea). Biol. J. Linn. Soc. 77, 431-443. ( 10.1046/j.1095-8312.2002.00114.x) [DOI] [Google Scholar]
- 105.Krasnov BR, Morand S, Mouillot D, Shenbrot GI, Khokhlova IS, Poulin R. 2006Resource predictability and host specificity in fleas: the effect of host body mass. Parasitology 133, 81-88. ( 10.1017/S0031182006000059) [DOI] [PubMed] [Google Scholar]
- 106.Dunn RR. 2005Modern insect extinctions, the neglected majority. Conserv. Biol. 19, 1030-1036. ( 10.1111/j.1523-1739.2005.00078.x) [DOI] [Google Scholar]
- 107.Gonzalez-Astudillo V, Knott L, Valenza L, Henning J, Allavena R. 2019Parasitism by Ophidascaris robertsi with associated pathology findings in a wild koala (Phascolarctos cinereus). Vet. Rec. Case Rep. 7, e000821. ( 10.1136/vetreccr-2019-000821) [DOI] [Google Scholar]
- 108.Lafferty KD. 2012Biodiversity loss decreases parasite diversity: theory and patterns. Phil. Trans. R. Soc. B 367, 2814-2827. ( 10.1098/rstb.2012.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.UNEP. 2019Biodiversity—global environment outlook (GEO-6): healthy planet, healthy people. Chapter 6. See https://wedocs.unep.org/xmlui/handle/20.500.11822/27659.
- 110.WWF. 2020Living planet report 2020—bending the curve of biodiversity loss (eds Almond REA, Grooten M, Petersen T). Gland, Switzerland: WWF. [Google Scholar]
- 111.Herrera J, Moody J, Nunn C. 2021Predictions of primate-parasite coextinction. Phil. Trans. R. Soc. B 376, 20200355. ( 10.1098/rstb.2020.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Antonovics J, et al. 2017The evolution of transmission mode. Phil. Trans. R. Soc. B 372, 20160083. ( 10.1098/rstb.2016.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bjørnstad ON, Finkenstädt BF, Grenfell BT. 2002Dynamics of measles epidemics: estimating scaling of transmission rates using a time series SIR model. Ecol. Monogr. 72, 169-184. ( 10.1890/0012-9615(2002)072[0169:DOMEES[2.0.CO;2) [DOI] [Google Scholar]
- 114.Hopkins SR, Fleming-Davies AE, Belden LK, Wojdak JM. 2020Systematic review of modelling assumptions and empirical evidence: does parasite transmission increase nonlinearly with host density? Methods Ecol. Evol. 11, 476-486. ( 10.1111/2041-210X.13361) [DOI] [Google Scholar]
- 115.Day T, Parsons T, Lambert A, Gandon S. 2020The price equation and evolutionary epidemiology. Phil. Trans. R. Soc. B 375, 20190357. ( 10.1098/rstb.2019.0357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, Ellis McKenzie F. 2012Ross, Macdonald, and a theory for the dynamics and control ofmosquito-transmitted pathogens. PLoS Pathog. 8, e1002588. ( 10.1371/journal.ppat.1002588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clayton DH, Bush SE, Goates BM, Johnson KP.. 2003Host defense reinforces host–parasite cospeciation. Proc. Natl Acad. Sci. USA 100, 15 694-15 699 (doi:10.1073/pnas.2533751100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santichaivekin S, Yang Q, Liu J, Mawhorter R, Jiang J, Wesley T, Wu Y-C, Libeskind-Hadas R. 2020EMPRess: a systematic cophylogeny reconciliation tool. Bioinformatics btaa978 ( 10.1093/bioinformatics/btaa978) [DOI] [PubMed] [Google Scholar]
- 119.Sandground JH. 1936On the potential longevity of various helminths with a record for a species of Trichostrongylus in man. J. Parasitol. 22, 464-470. ( 10.2307/3271690) [DOI] [Google Scholar]
- 120.Leggett HC, Buckling A, Long GH, Boots M. 2013Generalism and the evolution of parasite virulence. Trends Ecol. Evol. 28, 592-596. ( 10.1016/j.tree.2013.07.002) [DOI] [PubMed] [Google Scholar]
- 121.Woolhouse MEJ. 2001Population biology of multihost pathogens. Science 292, 1109-1112. ( 10.1126/science.1059026) [DOI] [PubMed] [Google Scholar]
- 122.Parrish CR, Holmes EC, Morens DM, Park E-C, Burke DS, Calisher CH, Laughlin CA, Saif LJ, Daszak P. 2008Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 72, 457-470. ( 10.1128/MMBR.00004-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ebert D. 1998Experimental evolution of parasites. Science 282, 1432-1436. ( 10.1126/science.282.5393.1432) [DOI] [PubMed] [Google Scholar]
- 124.Gandon S. 2004Evolution of multihost parasites. Evol. Int. J. Org. Evol. 58, 455-469. ( 10.1111/j.0014-3820.2004.tb01669.x) [DOI] [PubMed] [Google Scholar]
- 125.Anderson RM, May RM. 1982Coevolution of hosts and parasites. Parasitology 85, 411-426. (doi:10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- 126.Frank SA. 1996Models of parasite virulence. Q. Rev. Biol. 71, 37-78. (doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- 127.Morales-Castilla I, Pappalardo P, Farrell M, Aguirre AA, Huang S, Gehman AL, Dallas T, Gravel D, Davies TJ. 2021Forecasting parasite sharing under climate change. Phil. Trans. R. Soc. B 376, 20200360. ( 10.1098/rstb.2020.0360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heard MJ, Smith KF, Ripp KJ, Berger M, Chen J, Dittmeier J, Goter M, Mcgarvey ST, Ryan E. 2013The threat of disease increases as species move toward extinction: disease and extinction. Conserv. Biol. 27, 1378-1388. (doi:10.1111/cobi.12143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pedersen AB, Jones KE, Nunn CL, Altizer S. 2007Infectious diseases and extinction risk in wild mammals. Conserv. Biol. 21, 1269-1279. (doi:10.1111/j.1523-1739.2007.00776.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lloyd-Smith JO. 2013Vacated niches, competitive release and the community ecology of pathogen eradication. Phil. Trans. R. Soc. B 368, 20120150. (doi:10.1098/rstb.2012.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Woolhouse MEJ, Haydon DT, Antia R. 2005Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 20, 238-244. (doi:10.1016/j.tree.2005.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and R scripts to reproduce the figures are available at https://github.com/DiseaseMacroecology/ghost-host and doi:10.6084/m9.figshare.14573787.