Abstract
Covid-19 pandemic has been a very serious cause of health concern worldwide. Thrombosis has been a critical manifestation in severe Covid-19 infection. The increased arterial and venous thrombosis in patients with Covid-19 is proving to be life threatening. Sticky platelet syndrome and sickle cell disease are genetic disorders with procoagulant nature of the disease, while in Glanzmann syndrome there is an enhanced bleeding tendency, with pathological defect leading to altered platelet aggregation and delayed clot formation. Considering the thrombotic episodes of Covid-19, we decided to review the literature on data bases such as PubMed and Medline for knowing the coagulant status in genetically associated diseases such as sticky platelet syndrome, sickle cell disease and Glanzmann syndrome. We planned to review various published studies with the aim to find whether the coagulant profiles in these conditions alter the thrombotic manifestations and prognosis if these patients contract Covid-19. Various research studies revealed that patients with sticky platelet syndrome develop arterial and venous thrombosis, while those with sickle cell disease are known to develop complications such as deep vein thrombosis and pulmonary embolism. Moreover, patients with Glanzmann syndrome who usually have a bleeding tendency also rarely present with severe venous and arterial thrombosis and pulmonary embolism. Patients with sticky platelet syndrome and sickle cell disease and,, occasionally those with Glanzmann syndrome have a higher risk for thrombosis if infected with Covid-19. More studies are needed to better understand the clinical manifestations and designing standard management protocol for patients with sticky platelet syndrome, sickle cell disease and Glanzmann syndrome who contract Covid-19 infections.
Keywords:Sticky platelet syndrome, sickle cell disease, Glanzmann syndrome, thrombosis, pulmonary embolism, Covid-19.
INTRODUCTION
Covid-19, an abbreviation for corona virus disease 2019, is caused by severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). Covid-19 pandemic, which originated in Wuhan, China, in 2019, has led to a severe socioeconomic and health devastation worldwide. The severity of Covid-19 manifestation across communities has been countered with a dedicated management approach, including social isolation, quarantine management of active cases and contacts, symptomatic medical management along with Covid-19 vaccination. For fulfilling this obligation towards effective public health services, every country requires adequate manpower of doctors and nurses, medicines, oxygen cylinders and ventilators. This results in an increasing global health burden, which is also heavily affecting national economies, especially those of developing and underdeveloped countries.
The signs and symptoms associated with Covid-19 infection include fever, dry cough, sore throat, body ache, headache, myalgia, fatigue, shortness of breath, diarrhea, and sometimes loss of smell, taste or appetite. Severe Covid-19 infection is associated with dyspnea along with hypoxemia, acute respiratory distress syndrome, pneumonia, thrombosis, thromboembolism, coagulopathy, arrhythmias and shock (1). Although the pandemic has been lasting for more than 18 months, mortality rates are still rising on a daily basis worldwide. Many countries are facing a second wave of the pandemic as a result of the rapid circulation of mutant strains of SARS-CoV-2 across continents. World Health Organization dashboard reported 146,054,107 confirmed cases and 3,092,410 deaths on the 25th of April 2021 (2).
Increased arterial and venous thrombosis is one of the most critical manifestations in critically ill Covid-19 patients. Considering the thrombotic episodes of Covid-19, we decided to review the literature in order to find out which was the coagulant status in genetically associated diseases such as sticky platelet syndrome, sickle cell disease and Glanzmann syndrome. We planned to review various published studies with the aim to find whether the coagulant profiles in these conditions altered the thrombotic manifestations and prognosis if these patients contracted Covid-19. Sticky platelet syndrome and sickle cell disease are genetic disorders with a procoagulant nature of the disease, while in Glanzmann syndrome there is an enhanced bleeding tendency, with the pathological defect leading to altered platelet aggregation and delayed clot formation.
Sticky platelet syndrome is an autosomal dominant disease and a rare clinical condition with unknown etiology; it is characterized by hyperaggregation of the platelets, with an increased risk of arterial and venous thrombosis and thromboembolism. Sticky platelet syndrome has been the causative factor in nearly 21% of all arterial thrombosis and 13% of venous thrombosis, with both conditions being of unknown origin (3).
Sickle cell disease is due to mutation of beta globin chain of hemoglobin in which glutamate is replaced by valine at the sixth amino acid position of the beta chain. According to epidemiological studies, around 20–25 million individuals worldwide have sickle cell disease, and about 50–80% of infants born with this disease in Africa die before the age of five years (4). Patients with sickle cell disease present with complains of recurrent chest infection, acute chest syndrome, painful vaso-occlusive crisis, stroke, deep vein thrombosis and pulmonary embolism (4).
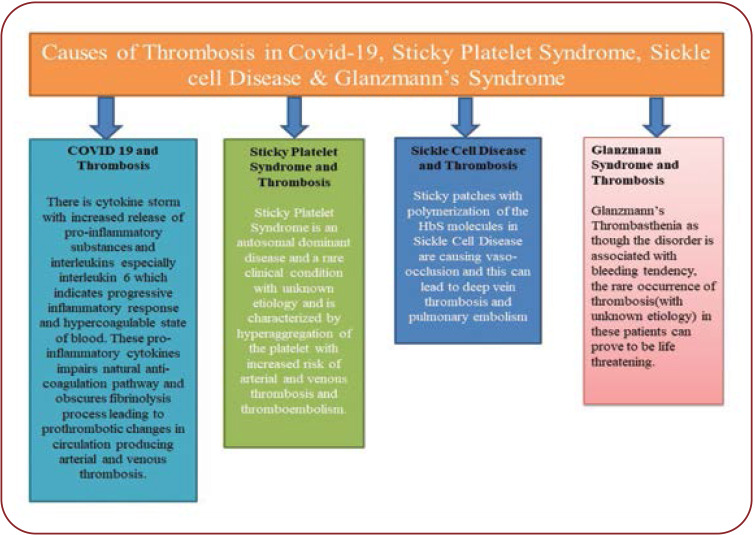
Glanzmann syndrome is a very rare autosomal recessive genetic disorder in which the platelets contain defective or low levels of glycoprotein IIb/IIIa: GPIIb-IIIa. These glycoproteins are receptors for fibrinogen. Due to this, fibrinogen bridging between platelets is not possible. This will lead to a severe bleeding tendency and defective haemostatic plug formation. Glanzmann thrombasthenia is prevalent in about 1:1,000,000 of the general population worldwide. But recent hospital-based research studies and reports have pointed out to an increasing number of cases with unexplained thrombosis, pulmonary embolism as well as severe proximal deep vein thrombosis in a Glanzmann thrombasthenia variant (5). The causes of thrombosis in Covid-19, sticky platelet syndrome, sickle cell disease and Glanzmann syndrome are showed in Figure 1.
These facts gave us an impetus to search the literature for understanding the clinical perspective of higher risk of thrombosis and thromboembolism in patients with sticky platelet syndrome, sickle cell disease and Glanzmann syndrome and whether these conditions may promulgate severe thrombosis if these patients get infected with Covid-19.
MATERIALS AND METHODS
We planned to have a thorough search of the literature of all recently published articles from various data bases such as Medline, PubMed and World Health Organization website. Sticky platelet syndrome and Glanzmann syndrome have not been very commonly discussed by researchers. Therefore, we decided to analyze various research and review papers published in English, describing pathological manifestations and management of sticky platelet syndrome, sickle cell disease and Glanzmann syndrome, which were conducted in the last three decades, using the above-mentioned key words. All articles published in line with national and international guidelines and recommendation of International Committee of Medical Journal Editors were included in our review.
DISCUSSION
Covid-19 and thrombosis
There has been a high risk of venous thromboembolism in patients with Covid-19, whose infection with SARS-CoV-2 was found to cause a hypercoagulable state. Research carried out on patients with Covid-19 hospitalized in intensive care units revealed that 31% to 79% of the developed thrombosis (6). There is a cytokine storm with increased release of pro-inflammatory substances and interleukins, mainly interleukin 6, which indicates a progressive inflammatory response and a hypercoagulable state of blood (7, 8). These pro-inflammatory cytokines impair the natural anti-coagulation pathway and obscures the fibrinolysis process, leading to prothrombotic changes in circulation and causing arterial and venous thrombosis. The impairment of prothrombotic changes is due to interaction of mononuclear cell with platelets and coagulation cascade leading to binding of thrombin with specific protease activated receptors. Moreover, increased levels of D-dimer, interleukin 6 and fibrin degradation product (FDP) have been associated with severe thrombosis in critically ill Covid-19 patients (8, 9).
Sticky platelet syndrome and thrombosis
Sticky platelet syndrome the autosomal dominant diseases have reported to be associated with unexplained thrombosis. Both arterial and venous thrombosis has been noted very commonly in patients with sticky platelet syndrome. Depending upon the hyperaggregability of this disease, the recognized forms of sticky platelet syndrome include types I, II and III. The hyperaggregability characteristics on conducting platelet aggregation test by addition of epinephrine and adenosine-diphosphate defines the three forms of sticky platelet syndrome. Platelet aggregation is observed by adding epinephrine and adenosine-diphosphate in type I, or epinephrine in type II, or adenosine-diphosphate in type III (3, 10). Despite the thrombotic nature of that disease, not much research has been carried out to explore its exact pathophysiology and to identify what could have caused the thrombosis. Patients with sticky platelet syndrome shall be exposed to a two-fold increased risk of developing thrombotic complications if they contract a Covid-19 infection. Sticky platelet syndrome has been identified as one of the main causes of life threatening thrombosis (11, 12). The sticky platelet syndrome being difficult to diagnose, we recommend that a complete coagulation profile should be conducted in any patient with Covid-19 infection reporting in OPD with either past history of any coagulation disorders or associated symptoms such as chest pain, dyspnea, swelling of lower limb, stroke, etc suggestive of thrombotic episodes.
Sickle cell disease and thrombosis
The characteristic manifestation of sickle cell disease includes increased adhesiveness of the sickled red blood cell to the vascular endothelium. Whenever sickle cell disease patients are exposed to hypoxia there is a polymerization of the HbS molecules, leading to an alteration in the shape of red blood cell, which makes them more prone to haemolysis. Moreover, the altered replacement of glutamate, which is carrying a negative charg,e with a neutral hydrophobic molecule of valine renders a sticky patch on the surface of the red blood cell. It has been noted that there was an expression of CD 47 and alpha 4 Beta 1 proteins on the surface cell membrane of red blood cell in patients with sickle cell disease (13, 14). The CD 47 protein binds to thrombospondin in circulation and initiates a cascade of reaction, eventually activating alpha 4 Beta 1 proteins, which binds to the vessel wall protein thrombospondin, being responsible for red blood cell adhesion. Thus, sticky patches with polymerization of the HbS molecules are causing vaso-occlusion, which can result in vein thrombosis and pulmonary embolism (14, 15). Thus, increased thrombotic episodes in sickle cell disease may further lead to a two-fold increased risk of sickle cell disease if those patients also contract a Covid-19 infection.
Glanzmann syndrome and coagulation profile
Glanzmann syndrome is an autosomal recessive disorder or may sometimes occur as an autoimmune disease, with patients presenting with bleeding episodes from minor bruising to severe bleeding. Platelet count and morphological features of platelets on microscopy are normal, but platelet aggregation study reveals an impaired aggregation with ADP, epinephrine or both, while aggregation after addition of ristocetin is normal. Assuming these facts, patients may have a favorable protection against developing thrombotic complications if they contract a Covid-19 infection (16, 17).
In contrast, a study conducted by Yves Gruel et al showed that severe thrombosis was possible in patients with Glanzmann thrombasthenia even if they had a severely deficient platelet agreeability, with the likely cause being attributed to prolonged airplane flight, which was thought to have triggered the occurrence of thrombosis. In patients with Glanzmann thrombasthenia, the contact phase of platelet adherence to the subendothelium was found to be normal and granule secretion occurred satisfactorily. Moreover, the sites where fibrin monomers bind on thrombasthenic platelets have been also found to be normally exposed (18). Moreover, Sakariassen KS et al noted that platelets adhered in the normal range at shear rates up to 1 000 s. At higher shear rates, platelets had a lesser contact spread over the subendothelium and there was no platelet aggregation (19). Girolami A et al have also described manifestations of severe arterial and venous thrombosis in patients with Glanzmann thrombasthenia (20). Hence, physicians should be cautious when treating Covid-19 patients with pre-existing Glanzmann thrombasthenia, given that the underlying disorder is associated with a bleeding tendency and the rare occurrence of thrombosis in those patients can prove to be life threatening. Also, physicians should ensure that Glanzmann thrombasthenia is diagnosed via light transmission aggregometry test for platelet aggregation; molecular analytical studies and platelet glycoprotein expression studies are carried out. Moreover, the thrombotic nature of Covid-19 exposes infected patients with pre-existing Glanzmann thrombasthenia to a high risk for thrombosis.
Management
The management of Covid-19 patients with preexisting diseases such as sticky platelet syndrome, sickle cell disease and Glanzmann syndrome needs to be carefully monitored and conducted. The coagulation profile including complete blood count, platelet count, prothrombin time, activated partial thromboplastin time as well as light transmission aggregometry test for platelet aggregation to rule out the sticky platelet syndrome, Hb electrophoresis for diagnosing sickle cell disease, molecular and platelet glycoprotein expression studies for diagnosis of Glanzmann syndrome are all needed. Starting an anti-coagulant therapy in critically ill Covid-19 patients with pre-existing sticky platelet syndrome, sickle cell disease and Glanzmann syndrome with evidence of thrombosis is also a challenge.
The International Society on Thrombosis and Haemostasis (ISTH) and the American Society of Hematology (ASH) have recommended the use of low-molecular-weight heparin as anticoagulant for the treatment of thrombosis in Covid-19 patients, but the best effective dosage is yet to be defined and needs further exploration (21). Aspirin and anticoagulants such as warfarin and clopidogrel have been given to patients with sticky platelet syndrome (22, 23) and non-steroidal anti-inflammatory drugs have been used for managing vaso-occlusive crises along with intravenous fluids in patients with sickle cell disease (24-26). If patients with those diseases contract Covid-19 infection and get critically ill, researchers should first explore which anti-coagulants are to be started and at what doses, and then obtain a relevant scientific body’s certification. The real challenge will be to care for patients with Glanzmann syndrome, whose routine management consists of hemostatic agents, recombinant activated factor VII, platelet transfusions and antifibrinolytic drugs. If those patients develop Covid-19, the anti-thrombotic nature of the pre-existing disease might prevent the occurrence of thrombosis; but in a different scenario, patients suffering from Glanzmann thrombasthenia with severe proximal deep vein thrombosis were effectively managed with a low molecular weight heparin (18). Moreover, given the manifestation of pulmonary thrombosis as well as severe arterial and venous thrombosis in patients with Glanzmann syndrome, physicians need to be alert while addressing such situations. Administering warfarin in Glanzmann syndrome with complicated pulmonary thrombosis was found to be an effective way of resolving thrombosis symptoms (26).
CONCLUSION
In conclusion, patients with sticky platelet syndrome and sickle cell disease may have an increased risk of developing severe thrombosis if infected with Covid-19. While due to the dual nature of Glanzmann syndrome manifestations with typical bleeding tendencies and rare occurrence of severe venous and arterial thrombosis, physicians have to be alert and administering the adequate treatment as per individual manifestations. More studies are needed for a better understanding of the clinical manifestations and investigations as well as for designing standard management protocols in patients with sticky platelet syndrome, sickle cell disease or Glanzmann syndrome who contract Covid-19 infection.
Conflict of interests: none declared.
Financial support: none declared.
Acknowledgments: Authors would like to thank Dr Maj Gen (Dr) Vibha Dutta, SM Director and CEO, All India Institute of Medical Sciences, Nagpur, India, for her constant encouragement, guidance and support for writing the manuscript, as well as Dr Vikas Bhatia, Director, All India Institute of Medical Sciences, Bibinagar, Telangana, India, for encouraging us in writing this review article.
FIGURE 1.
Causes of thrombosis in Covid-19, sticky platelet syndrome, sickle cell disease and Glanzmann syndrome
Contributor Information
Nitin Ashok JOHN, Department of Physiology, All India Institute of Medical Sciences, Bibinagar, Telangana, India.
Jyoti JOHN, Department of Biochemistry, All India Institute of Medical Sciences, Nagpur, Maharashtra, India.
Praful KAMBLEC, Department of Physiology, All India Institute of Medical Sciences, Bibinagar, Telangana, India.
Anish SINGHAL, Department of Physiology, All India Institute of Medical Sciences, Bibinagar, Telangana, India.
Vandana DAULATABAD, Department of Physiology, Dr. V. M. Government Medical College, Solapur, Maharashtra, India.
I. S. VAMSHIDHAR, Department of Physiology, All India Institute of Medical Sciences, Bibinagar, Telangana, India
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, comorbidities, and outcome among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . WHO Dashboard. https://covid19.who.int/. 25th April 2021. 2021.
- 3.Kubisz P, Stasko J, Holly P. Sticky platelet syndrome. Semin Thromb Hemost. 2013;6:674–683. doi: 10.1055/s-0033-1353394. [DOI] [PubMed] [Google Scholar]
- 4.B. Aygun, I. Odame. A global perspective on sickle cell disease. Pediatric Blood & Cancer. 2012;2:386–390. doi: 10.1002/pbc.24175. [DOI] [PubMed] [Google Scholar]
- 5.Toogeh G, Sharifian R, Lak M, et al. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004;2:198–199. doi: 10.1002/ajh.20159. [DOI] [PubMed] [Google Scholar]
- 6.Klok FA, Kruip MJHA, Van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;7:1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isabela Bispo Santos da Silva Costa, Cristina Salvadori Bittar, Antônio Everaldo de Araújo Filho, et al. The Heart and COVID-19: What Cardiologists Need to Know. Arq Bras Cardiol. 2020;5:805–816. doi: 10.36660/abc.20200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factor for mortality of adult in patients with COVID-19 in Wuhan China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J, Tacquard C, Severac F, et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;6:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubisz P, Stanciakova L, Stasko J, et al. Sticky platelet syndrome: an important cause of life-threatening thrombotic complications. Expert Rev Hematol. 2016;1:21–35. doi: 10.1586/17474086.2016.1121095. [DOI] [PubMed] [Google Scholar]
- 12.Azamar-Solis B, Cantero-Fortiz Y, Olivares-Gazca JC, et al. Primary Thrombophilia in Mexico XIII: Localization of the Thrombotic Events in Mexican Mestizos With the Sticky Platelet Syndrome. Clin Appl Thromb Hemost. 2019;25:1076029619841700. doi: 10.1177/1076029619841700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldenborg PA. CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol. 2013;2013:614–619. doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissinger R, Petkova-Kirova P, Mykhailova O, et al. Thrombospondin-1/CD47 signaling modulates transmembrane cation conductance, survival, and deformability of human red blood cells. Cell Commun Signal. 2020;1:155. doi: 10.1186/s12964-020-00651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballas SK, Mohandas N. Sickle red cell microrheology and sickle blood rheology. Microcirculation. 2004;2:209–225. doi: 10.1080/10739680490279410. [DOI] [PubMed] [Google Scholar]
- 16.Toogeh G, Sharifian R, Lak M, et al. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004;2:198–199. doi: 10.1002/ajh.20159. [DOI] [PubMed] [Google Scholar]
- 17.George JN, Caen JP, Nurden AT. Glanzmann’s thrombasthenia: the spectrum of clinical disease. Blood. 1990;7:1383–1395. [PubMed] [Google Scholar]
- 18.Gruel Y, Pacouret G, Bellucci S, Caen J. Severe proximal deep vein thrombosis in a Glanzmann thrombasthenia variant successfully treated with a low molecular weight heparin. Blood. 1997;2:888–890. [PubMed] [Google Scholar]
- 19.Sakariassen KS, Nievelstein PF, Coller BS, Sixma JJ. The role of platelet membrane glycoproteins Ib and IIb-IIIa in platelet adherence to human artery subendothelium. Br J Haematol. 1986;4:681–691. doi: 10.1111/j.1365-2141.1986.tb07552.x. [DOI] [PubMed] [Google Scholar]
- 20.Girolami A, Sambado L, Bonamigo E, et al. Occurrence of thrombosis in congenital thrombocytopenic disorders: a critical annotation of the literature. Blood Coagul Fibrinolysis. 2013;1:18–22. doi: 10.1097/MBC.0b013e3283597634. [DOI] [PubMed] [Google Scholar]
- 21.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;5:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammen EF. Sticky platelet syndrome. Semin. Thromb. Hemost. 1999;4:361–365. doi: 10.1055/s-2007-994939. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, Poon MC. Inherited platelet functional disorders: General principles and practical aspects of management. Transfus Apher Sci. 2018;4:494–501. doi: 10.1016/j.transci.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Carroll CP. Opioid treatment for acute and chronic pain in patients with sickle cell disease. Neuroscience Letters. Elsevier BV. 2020;714:134534. doi: 10.1016/j.neulet.2019.134534. [DOI] [PubMed] [Google Scholar]
- 25.Okomo U, Meremikwu MM. Fluid replacement therapy for acute episodes of pain in people with sickle cell disease. Cochrane Database Syst Rev. 2017. [DOI] [PMC free article] [PubMed]
- 26.Seretny M, Senadheera N, Miller E, Keeling D. Pulmonary embolus in Glanzmann's thrombasthenia treated with warfarin. Haemophilia. 2008;5:1138–1139. doi: 10.1111/j.1365-2516.2008.01804.x. [DOI] [PubMed] [Google Scholar]