Abstract
Introduction: Coronavirus disease 2019 (COVID-19) is an emerging viral infection without any approved treatment. Investigational therapies for COVID-19 may cause clinically important drug-drug interactions (DDIs). We aimed to study drug-drug interactions (DDIs) and their risk factors in hospitalised COVID-19 patients.
Methods: We conducted a retrospective study in a tertiary care hospital dedicated to COVID-19 patients. The Lexi-Interact database was used to investigate clinically important DDIs. The database output, including interacting drug pairs, risk rating, reliability rating, mechanism, and management, was evaluated.
Results: Medical records of 200 COVID-19 patients were analysed. All patients had at least one clinically important DDI. More than half of interactions were associated with hydroxychloroquine and azithromycin, the most commonly prescribed medications for the management of COVID-19. Concomitant drugs for comorbid conditions leading to polypharmacy were significantly associated with the occurrence of this.
Conclusion: There is a higher chance of DDI, which necessitates ongoing care evaluation and therapy adjustment. Drugs used to treat COVID-19 should be carefully selected.
Keywords:drug-drug interactions (DDIs), COVID-19 patients, polypharmacy, adverse drug reactions (ADRs).
INTRODUCTION
The novel coronavirus pneumonia (COVID-19) is caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) infection (1). SARS-CoV-2 emerged in Wuhan, China, in December 2019, and has rapidly spread across the world due to its high transmissibility and pathogenicity (2). On the 30th of January 2020, the World Health Organization (WHO) declared it a public health emergency of international concern and a global pandemic was declared on the 11th of March 2020.
Drug-drug interaction (DDI) is defined as a modification of the effects of one drug (the object drug) by the prior or concomitant administration of another drug (the precipitant drug) (3). DDIs are one of the causes of adverse drug reactions (ADRs) and sometimes therapeutic failure following multiple drug therapies (4, 5).
The patient population most affected by severe forms of COVID-19 includes not only the elderly (> 65 years), but also younger people living with co-morbidities such as obesity, hypertension and type 2 diabetes (6–8). Most of these particularly fragile patients benefit from chronic treatment, often combining several drugs. The worsening of COVID-19 sometimes requires treatment in intensive care units (ICUs), combining sedation and mechanical ventilation. In the most serious forms, multi-organ failure can be observed (e.g. cardiac, renal, and hepatic, along with thrombotic issues) (9-11), which requires poly-pharmacy (benzodiazepines, opioids, anticoagulants, calcium channel blockers, glucocorticoids, etc.).
Several therapeutic strategies (hydroxychloroquine, azithromycin, remdesivir, corticosteroids, etc.) have been tried for COVID-19 treatment (12-15). These drugs are given in combination, or along, with drug used for associated co-morbidities in several COVID-19 patients. Some of the drugs being tested are likely to interact with chronic treatment as well as the treatment used in patient resuscitation. Such DDIs largely result from their pharmacokinetic properties (e.g., induction or inhibition of cytochrome P450 (CYP) isoenzymes, competition in renal elimination) as well as their pharmacodynamics properties (e.g., QT prolongation). In addition to these interactions, there is a large inflammatory component in COVID-19 patients, which can modify the pharmacokinetic behaviour of the drugs used (e.g., down-regulation of CYP isoenzymes, organ failure, modification of plasma protein concentrations) (16, 17).
These interactions can lead to the development of various side effects and therapeutic failure in COVID-19 patients. There is a lack of prescribing pattern on the basic of theoretical knowledge of drug interaction in the management of COVID-19 infection. Therefore, we designed this study to assess the clinically relevant DDIs of COVID-19 candidate drugs and concomitant drugs used for comorbidities. This study will provide an insight into a safe and rational use of medicines among COVID-19 patients.
MATERIALS AND METHODS
This retrospective observational study was carried out in All India Institute of Medical Science (AIIMS), Patna, (Bihar) India on COVID-19 patients admitted to AIIMS Patna from March 2020 to August 2020, after obtaining approval from Institutional Ethics Committee, AIIMS Patna.
Two hundred (n=200) medical records of COVID-19 patients confirmed by RTPCR were evaluated from the Medical Record Department (MRD) of AIIMS Patna. Data were recorded on a standardised format on Microsoft Excel summarising information about age, sex, presenting complaints and comorbidities. Data regarding the number of drugs prescribed for COVID-19 and comorbid conditions, hospital stay, and ICU admission were gathered. Patients with daily prescriptions of two or more drugs were considered for analysis, while those who were receiving more than two drugs, intravenous fluids, multivitamins (Vit. C, Vit. D), minerals (Zn), and blood products were excluded.
DDI in each prescription was assessed using Lexicomp drug interaction software (18) and then categorised for severity, risk rating, mechanism, and reliability rating for drug interactions.
Severity rating indicates the reported or possible magnitude of an interaction outcome and it is classified as minor (causes minimal effects that are usually tolerable — do not require medical intervention), moderate (potential for significant interaction, but do not meet the criteria for major severity . generally requires monitoring of therapy and in few cases, medical intervention may be needed), major (potential for serious interaction — typically requires medical intervention and/or close monitoring) and contraindicated (drugs which should never be used together because of severe life-threatening interactions) (19).
Risk rating reflects both the level of urgency and the nature of actions necessary to respond to an interaction (18). Based on risk rating, the interactions are classified into five categories, including A (no known interaction), B (no action needed), C (monitor therapy), D (modify regimen), and X (avoid combination) (20). The DDIs were also categorized as pharmacokinetic and pharmacodynamics interactions based on their underlying mechanisms.
Reliability rating indicates the quantity and nature of documentation for an interaction and is scaled as excellent (E), good (G), or fair (F) (18).
Statistical analysis
Data were capture on Microsoft Excel. The drug interactions detected by the software were documented. Data were analysed as mean, frequency, percentage and interquartile range using SPSS software version 16.0.
RESULTS
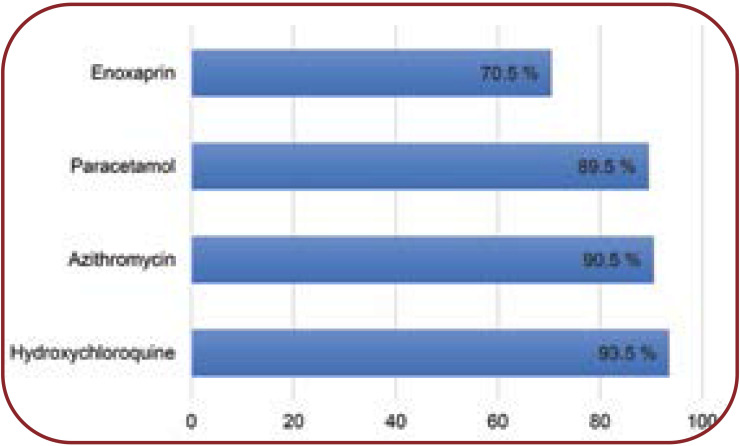
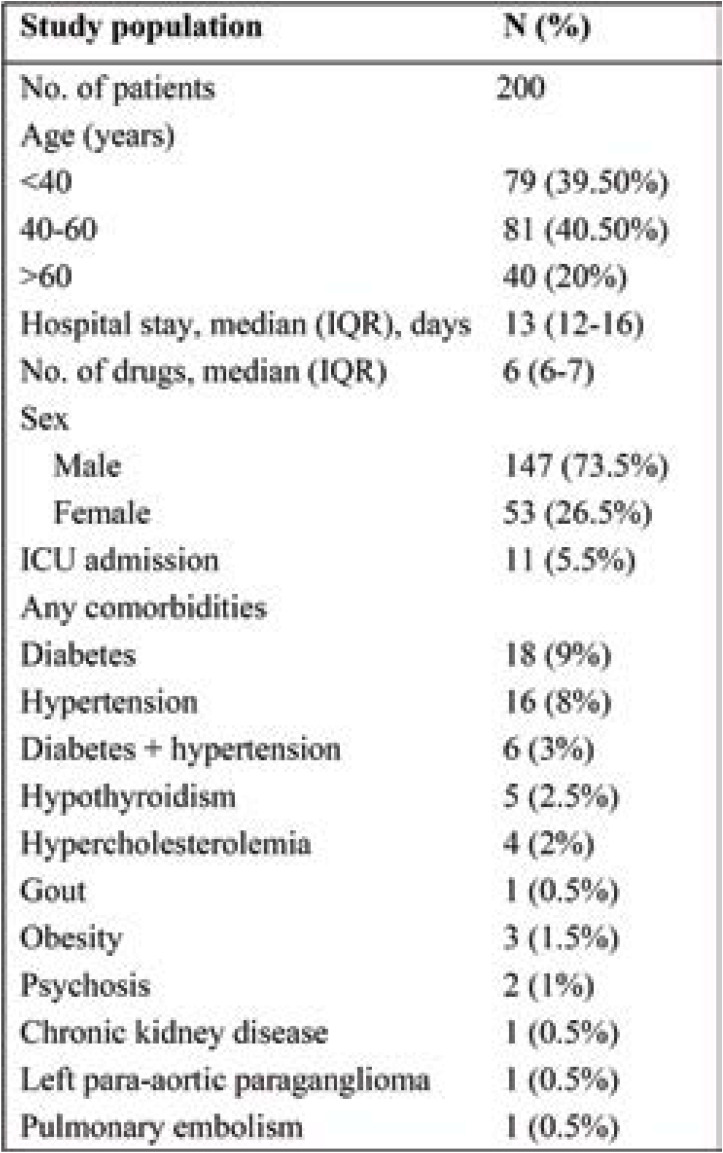
Two hundred medical records of patients with confirmed COVID-19 were evaluated. Table 1 shows the patients’ demographic and clinical characteristics. Of the total study population, 73.5% were male. A median number of six medications (IQR, 6–7) were administered to patients. Diabetes (9%) was the most common comorbidity, followed by hypertension (8%), hypothyroidism (2.5%) and hypercholesterolemia (2%). The most commonly prescribed drugs for COVID-19 patients include hydroxychloroquine (93.5%), followed by azithromycin (90.5%), paracetamol (89.5%) and enoxaparin (70.5%) (Figure 1).
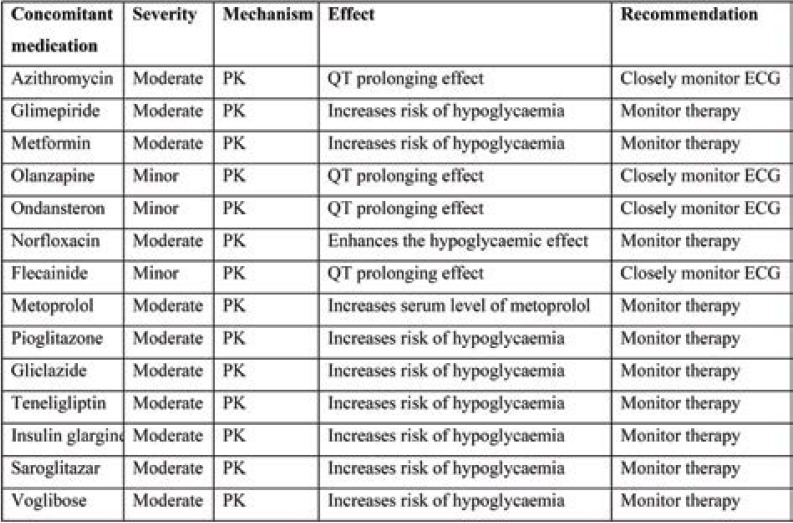
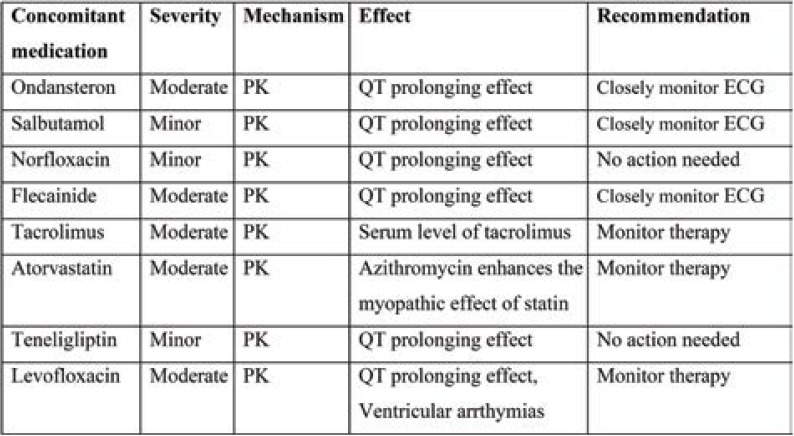
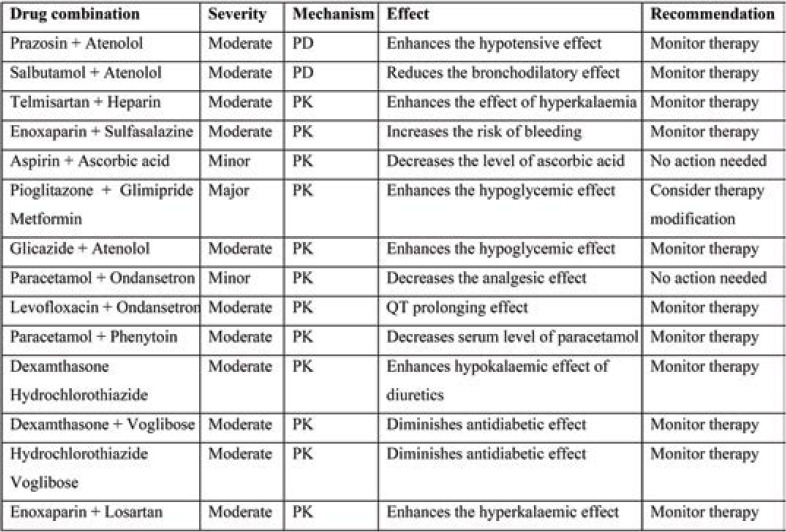
Tables 2 and 3 show the interactions between hydroxychloroquine and azithromycin with concomitant medications. Pharmacokinetic interaction is common with moderate severity. QT prolongation and increased risk of hypoglycaemia requires close monitoring of ECG and therapy.
Pharmacokinetic interaction is common with moderate severity for comorbid medications. The DDI between Pioglitazone+ Glimipride+ Metformin is of major severity, leading to hypoglycaemic effect, and therapy modification may be required. Other interactions require therapy monitoring (Table 4).
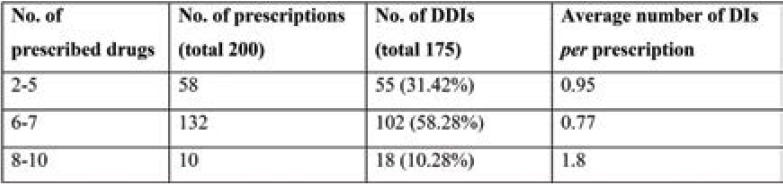
The highest number of DDIs were found in prescriptions of 6–7 drugs (n=102; 58.28%); however, the average number of DDIs per prescription was highest in those with 8-10 drugs (1.8) (Table 5).
The global usage rate of OAC at discharge (either as monotherapy or in combination with at least one antiplatelet), including patients with newly diagnosed AF, was 74.3% (130/175). Among patients receiving OAC, NOAC usage was reported in 57.7% (75/130) of cases, and in 92% (69/75) of them factor Xa inhibitors were recommended.
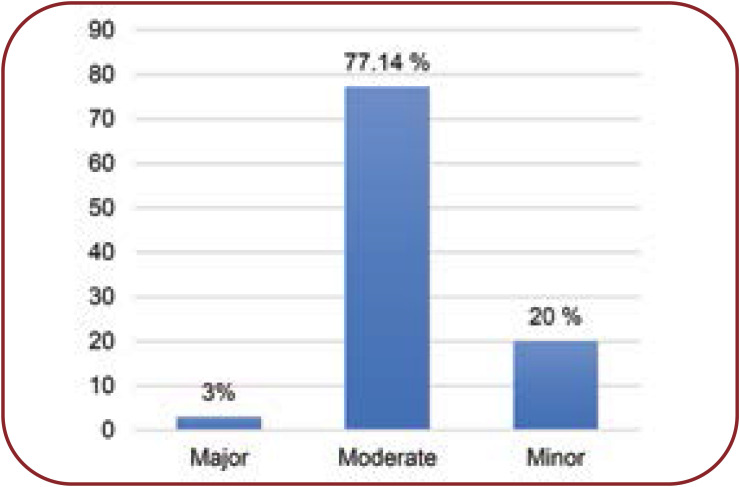
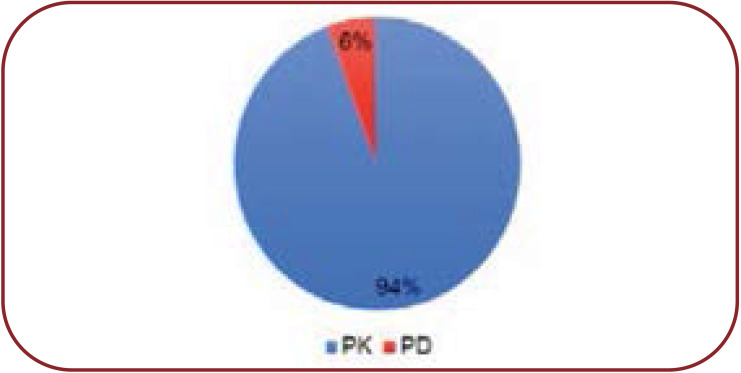
In our study we noted 35 types of DDIs, of which 27 (77.14%) were “moderate” in severity, seven (20%) minor and one (3%) major, totalizing 175 interactions (Figure 2). There were 94% pharmacokinetic interactions and 6% pharmacodynamics interactions (Figure 3).
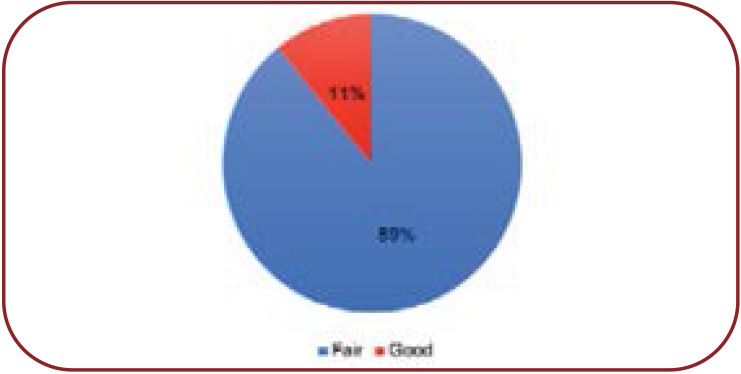
Reliability rating indicates the quantity and nature of documentation for an interaction. Reliability rating in our study was fair [33 (89.19%)] and good [4 (10.81%)] (Figure 4).
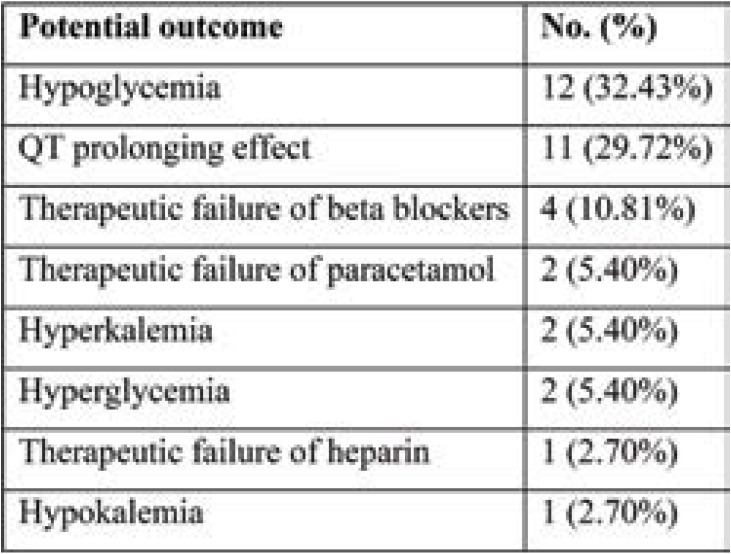
The most common potential outcomes included hypoglycaemia (n=12, 32.43%), QT prolonging effect (n=11, 29.72%), and therapeutic failure of beta blockers (n=4, 10.81%). Hyperglycemia, therapeutic failure of heparin, hyperkalemia as well as hypokalemia were other potential outcome noted by us (Table 6).
DISCUSSION
The concept of DDI, which has been studied in pharmacology, poses a challenge to clinicians, being often difficult to identify and diagnose, especially in clinical conditions which are associated with comorbidities. Given that there is no specific treatment for COVID-19, attempts have been made to use polypharmacy for the management of infected patients, which was acknowledged to carry a significant risk of DDIs. Therefore, we conducted this retrospective study to identify the severity, risk rating, frequency, reliability rating, mechanism and recommendation of DDIs in hospitalized patients with COVID-19. We assessed 200 medical records using Lexicomp drug interaction software.
The severity assessment of DDIs (using Lexicomp drug interaction software) showed that most of them were of moderate severity (77.14%), followed by minor (20 %) and major (3%) interactions. This sequence is comparable to a study conducted by Amir Ali Mahboobipour and Shadi Baniasadi (23).
We noted a high prevalence (6-7 drugs) of polypharmacy in our study population. Many studies have considered polypharmacy to be the concurrent use of five or more drugs (21, 22), while extensive polypharmacy is deemed to be the use of 10 or more in adults (24). The high level of DDI prevalence in our study could be due to several factors such as comorbidities, presence of extensive polypharmacy, and long duration of hospital stay, and many others (25, 26). An important finding in this study was the relationship between the number of drugs prescribed to a patient and the incidence of DDIs. Although the interactions were more numerous in prescriptions of 6–7 drugs, the average DDIs per prescription were the highest in those that had 8-10 drugs (1.8 per prescription). Similar findings were reported by Sherin and Udaykumar (27), who found that the average number of PDDI per prescription increased with an increase in the number of concomitant medications. This suggests that an increase in polypharmacy is linked to an increase in the number of DDIs, which is an established fact in several other studies as well (28). Other studies also convey a clear and strong evidence that the risk of DDI is proportional to the number of medications (29- 31). We found that 94% were pharmacodynamic interactions and 6% pharmacokinetic interactions, which is in contrast to another study in a paediatric population (65% pharmacodynamic vs. 35% pharmacokinetic interactions) (32).
Hypoglycaemia was found to be the most common potential outcome in our study (32.43%), which contributed mainly by an interaction between hydroxychloroquine and oral hypoglycaemic agents. As earlier mentioned, it is generally of moderate severity and requires monitoring therapy. In our study, we have also found potential instances of QTc prolongation (29.72%). In a study conducted by Baniasadi et al, prolongation of QTc interval was found to be the most common potential outcome (19).
The strength of our study lies in the fact that DDI research is minimal among COVID-19 patients. Hence, information provided by our research can both improve the understanding of prescription pattern and encourage physicians to carefully prescribe certain medications to these patients. Apart from this, our study can provide a framework for future pharmacotherapeutic studies.
This was a single.center study with a small sample size. Hence, our findings cannot be generalized. Interactions identified by using Lexicomp software were DDIs, and we did not assess whether they manifested in patients or not, which is a major limitation. Although Lexicomp drug interaction checker is a very reliable (supported by Wolters Kluwer Health) and commonly used system for identifying PDDIs, discrepancies have been found in the identification and grading of DDIs severity between different systems (33, 34). Hence, the results of this study may not correlate well with those of other similar studies that have used different DDI.checking systems. Furthermore, discrepancies have been reported between the number of PDDIs detected with electronic systems and those evaluated by doctors as clinically relevant (35), which could be a potential limitation to our study.
CONCLUSION
There is no definitive treatment of COVID-19 to date. Therefore, there is a trend towards the use of polypharmacy in the management of COVID-19. Our findings reveal an increased risk of DDI, which needs continuous monitoring of treatment and therapy modification. Therefore, drugs should be chosen carefully in the management of COVID-19. This study will be helpful to provide an insight for safe and rational use of medicine among COVID-19 patients. Our study has few limitations, including small sample size and discrepancies in identification and grading of DDI severity between different system. Therefore, further research with a large sample size is required.
Conflict of interests: none declared
Financial support of the study: none declared.
Acknowledgement: We thank all corona warriors of AIIMS Patna working on the front line against COVID-19. We are grateful to AIIMS Patna central library for providing free access to Lexicomp Drug Interaction checker through UpTodate. We also appreciate the cooperation of staff working in the medical record department of the hospital, who provided access to medical records.
Ethical approval and informed consent: The study was approved by the Institutional Ethics Committee, AIIMS Patna (Ref. No. AIIMS/Pat/IEC/2020/559 Dated 08/09/2020). Waiver of informed consent was granted as patient details were anonymized and only medical records of hospitalized COVID-19 patients were analysed.
FIGURE 1.
Commonly prescribed drugs for COVID-19
TABLE 1.
Patients’ demographic and clinical characteristics
TABLE 2.
DDIs between hydroxychloroquine and concomitant medication their severity, mechanism, effect and recommendation based on Lexicomp drug interaction software
TABLE 3.
DDIs between azithromycin and concomitant medication their severity, mechanism, effect and recommendation based on Lexicomp drug interaction software
TABLE 4.
DDIs between concomitant medication their severity, mechanism, effect and recommendation based on Lexicomp drug interaction software
TABLE 5.
Number of drug-drug interactions and number of drugs per prescription
FIGURE 2.
Types of DDIs based on severity
FIGURE 3.
Types of DDIs based on mechanism
FIGURE 4.
Reliability rating of DDIs
TABLE 6.
Common potential outcome of DDIs
Contributor Information
Pramod Kumar MANJHI, Department of Pharmacology, All India Institute of Medical Sciences, Patna, Bihar, India.
Rajesh KUMAR, Department of Pharmacology, All India Institute of Medical Sciences, Patna, Bihar, India.
Aakanksha PRIYA, Department of Pharmacology, All India Institute of Medical Sciences, Patna, Bihar, India.
Insha RAB, Department of Pharmacology, All India Institute of Medical Sciences, Patna, Bihar, India.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;10223:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;8:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatro DS. Drug interactions. In: Herfindal ET, Gourley DR, editors. Textbook of Therapeutics, Drug and Disease Management. 2000;7th ed., Philadelphia: Lippincott:35–49. [Google Scholar]
- 4.Abarca J, Malone DC, Armstrong EP, et al. Concordance of Severity Ratings Provided in Four Drug Interaction Compendia. J Am Pharm Assoc. 2004;2:136–141. doi: 10.1331/154434504773062582. [DOI] [PubMed] [Google Scholar]
- 5.Bjerrum L, Andersen M, Petersen G, Kragstrup J. Exposure to potential drug interactions in primary health care. Scand J Prim Health Care. 2003;3:153–158. doi: 10.1080/02813430310001806. [DOI] [PubMed] [Google Scholar]
- 6.Simonnet A, Chetboun M, Poissy J, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity. 2020;7:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji D, Zhang D, Xu J, et al. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin Infect Dis. 2020;6:1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;10229:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020;1 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried JA, Ramasubbu K, Bhatt R, et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020;23:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zheng L, Liu L, et al. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;9:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;1:1–3. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;25:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020;24:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;19:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan ET. Impact of Infectious and Inflammatory Disease on Cytochrome P450–Mediated Drug Metabolism and Pharmacokinetics. Clin Pharmacol Ther. 2009;4:434–438. doi: 10.1038/clpt.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ofotokun I, Lennox JL, Eaton ME, et al. Immune Activation Mediated Change in Alpha-1-Acid Glycoprotein: Impact on Total and Free Lopinavir Plasma Exposure. J Clin Pharmacol. 2011;11:1539–1548. doi: 10.1177/0091270010385118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baniasadi S, Hassanzad M, Alehashem M. Potential drug-drug interactions in the pediatric intensive care unit of a pulmonary teaching hospital. Eur Respir J. 2021;8 Suppl 60:PA1301. [Google Scholar]
- 19.Hovstadius B, Petersson G. Factors leading to excessive polypharmacy. Clin Geriatr Med. 2012;28:159–172. doi: 10.1016/j.cger.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63:187–195. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amir Ali Mahboobipour, Shadi Baniasadi. Clinically important drug–drug interactions in patients admitted to hospital with COVID-19: drug pairs, risk factors, and management. Drug Metabol Pers Ther. 2020;20200145 doi: 10.1515/dmpt-2020-0145. [DOI] [PubMed] [Google Scholar]
- 24.Haider SI, Johnell K, Weitoft GR, et al. The influence of educational level on polypharmacy and inappropriate drug use: A register-based study of more than 600,000 older people. J Am Geriatr Soc. 2009;57:62–69. doi: 10.1111/j.1532-5415.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 25.Bjerrum L, Gonzalez Lopez-Valcarcel B, Petersen G. Risk factors for potential drug interactions in general practice. Eur J Gen Pract. 2008;14:23–29. doi: 10.1080/13814780701815116. [DOI] [PubMed] [Google Scholar]
- 26.Womer J, Zhong W, Kraemer FW, et al. Variation of opioid use in pediatric inpatients across hospitals in the U.S. J Pain Symptom Manage. 2014;48:903–914. doi: 10.1016/j.jpainsymman.2013.12.241. [DOI] [PubMed] [Google Scholar]
- 27.Sherin R, Udaykumar P. Assessment of possible drug interactions in patients with psoriasis and associated comorbid medical conditions: An observational study. Rev Recent Clin Trials. 2016;11:128–134. doi: 10.2174/1574887111666160201122532. [DOI] [PubMed] [Google Scholar]
- 28.Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- 29.Kulkarni V, Bora SS, Sirisha S, et al. A study on drug-drug interactions through prescription analysis in a South Indian teaching hospital. Ther Adv Drug Saf. 2013;4:141–146. doi: 10.1177/2042098613490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mrowietz U, Elder JT, Barker J. The importance of disease associations and concomitant therapy for the long-term management of psoriasis patients. Arch Dermatol Res. 2006;298:309–319. doi: 10.1007/s00403-006-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel PS, Rana DA, Suthar JV, et al. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J Basic Clin Pharm. 2014;5:44–48. doi: 10.4103/0976-0105.134983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santibáñez C, Roque J, Morales G, Corrales R. Characteristics of drug interactions in a pediatric intensive care unit. Rev Chil Pediatr. 2014;85:546–553. doi: 10.4067/S0370-41062014000500004. [DOI] [PubMed] [Google Scholar]
- 33.Barrons R. Evaluation of personal digital assistant software for drug interactions. Am J Health Syst Pharm. 2004;61:380–385. doi: 10.1093/ajhp/61.4.380. [DOI] [PubMed] [Google Scholar]
- 34.Vonbach P, Dubied A, Krähenbühl S, Beer JH. Evaluation of frequently used drug interaction screening programs. Pharm World Sci. 2008;30:367–374. doi: 10.1007/s11096-008-9191-x. [DOI] [PubMed] [Google Scholar]
- 35.Roblek T, Vaupotic T, Mrhar A, Lainscak M. Drug-drug interaction software in clinical practice: A systematic review. Eur J Clin Pharmacol. 2015;71:131–142. doi: 10.1007/s00228-014-1786-7. [DOI] [PubMed] [Google Scholar]