Abstract
Opioid use among pregnant women is a growing public health concern in the US. Infants exposed to opioids in utero are at risk of exhibiting Neonatal Opioid Withdrawal Syndrome (NOWS). The biological mechanisms underlying short and long-term consequences of in utero opioid exposure and NOWS are unknown. A potential genetic factor is a single nucleotide polymorphism (SNP) in the mu-opioid receptor gene (OPRM1 A118G). Opioid exposed infants with the G-allele spend less time in hospitals after birth. To determine whether this SNP modulates the neurobehavioral effects of neonatal opioid exposure and withdrawal we used mice possessing the equivalent Oprm1 SNP (A112G). Pups were treated chronically with saline or morphine from postnatal day (PND) 1-14, a developmental period equivalent to the third trimester of a human pregnancy and a sensitive period for opioid exposure in rodents. Morphine treatment produced significant developmental delays regardless of genotype and increased total ultrasonic vocalizations in males during spontaneous withdrawal. Animals were aged and tested for anxiety and drug response during adolescence and adulthood, respectively. AA morphine treated animals showed reduced activity in the marble burying task compared to saline controls, however this effect was absent in AG and GG animals. As adults, AA males exposed to morphine from PND 1-14 exhibited enhanced development of locomotor sensitization to morphine, whereas females showed reduced locomotor sensitization. These data suggest the involvement of the Oprm1 SNP for certain outcomes of neonatal opioid exposure and highlight the importance of considering sex and genetic variability for the prognosis of NOWS.
Keywords: Mouse, Neonatal, Opioid, Oprm1, Withdrawal
Introduction
Infants exposed to opioids in utero are at high risk of exhibiting Neonatal Opioid Withdrawal Syndrome (NOWS), a combination of somatic withdrawal symptoms including high pitched crying, sleeplessness, irritability, gastrointestinal distress, and in the worst cases, seizures. In the United States alone, the incidence of NOWS has increased more than fivefold between 2004 and 2014, creating a surge in hospital and other costs 1. In addition to the acute withdrawal syndrome, opioid-exposed infants may be at risk of adverse neurodevelopmental outcomes 2. Thus, the financial and emotional burdens of NOWS are clear and dramatically on the rise.
Not all infants exposed to opioids in utero develop clinical withdrawal symptoms requiring treatment. While the mechanisms underlying resilience in these infants remain obscure, genetic factors are likely to contribute. One gene of interest is OPRM1, which encodes the mu-opioid receptor (MOR) and is the target for both endogenous and licit and illicit opioids. Varying with ethnicity, approximately 25% of humans carry a single nucleotide polymorphism (SNP) in OPRM1 3. This SNP results in an adenine-to-guanine substitution (A118G), exchanging an asparagine for an aspartic acid in exon 1 of the gene. The A118G SNP has been implicated in a wide variety of disorders, including addiction stress response, and pain perception 3. Interestingly, recent clinical evidence suggests that the A118G SNP may also influence NOWS severity and course. Opioid exposed infants harboring the OPRM1 G-allele have shorter length of stay in the hospital and reduced likelihood of requiring treatment for withdrawal compared to infants with the A-allele 4. However, in these and most clinical studies, the small number of subjects and potential covariates of NOWS severity, such as maternal poly-pharmacy, parental rearing or household environment, make it impossible to isolate the effects of genotype on withdrawal and long-term outcome.
Animal models provide a means of creating controlled environments to examine the consequences of opioid exposure and withdrawal in the absence of confounding variables. Prolonged exposure to opioids can result in MOR-mediated neuroadaptive processes that are thought to contribute to opioid dependence and withdrawal in adults 5. However, our understanding of these phenomena in neonatal models is limited. Our laboratory previously created a targeted mutation in the Oprm1 gene (Oprm1 A112G) possessing the equivalent nucleotide and amino acid substitution to the human OPRM1 A118G variant. This line recapitulates many of the molecular and behavioral phenotypes previously identified in humans, such as reduced mRNA expression in the brain, reduced analgesic response to opiates, and altered behavioral response to drugs of abuse 6-8. Moreover, basal expression of genes associated with addiction and stress-response systems are altered in GG animals, suggesting that this SNP may also impact MOR-mediated signaling cascades 9.
The goal of the current study was to assess the impact of the A112G SNP on the short and long-term behavioral consequences of neonatal opioid exposure in a mouse model. Notably, in utero maturation of a rodent fetus occurs on a different time scale compared to humans. Although developmental equivalencies vary depending on the specific brain region being examined, there is general consensus that the third trimester of a human pregnancy is most analogous to the first two postnatal weeks in rodents in terms of CNS development 10-12. In rodents, opioid exposure only during the early postnatal period can induce a withdrawal state following abstinence or treatment with an opioid receptor antagonist 13 and is sufficient to produce long-lasting changes in behavior 14,15. While examining the impact of opioid exposure throughout gestation is critical, peak brain growth occurs during the third trimester in humans 10. The higher permeability of the placental barrier during the third trimester may result in increased levels of fetal exposure nearing delivery compared to earlier in pregnancy 16. In addition, maternal opioid use in the third trimester is associated with higher risk of NOWS 17. To investigate the impact of opioid exposure during the last trimester equivalent period, we exposed mice to morphine from PND 1-14 and examined the impact of the A112G SNP on withdrawal and adolescent and adult behaviors. During the neonatal period we determined the effects of morphine on weight gain, emergence of developmental milestones, and ultrasonic vocalizations, which have been associated with negative affect during drug withdrawal 18. We then tested animals in a battery of affective behavioral tests during late adolescence. Finally, as a proxy for drug sensitivity, we examined locomotor sensitization to morphine in adulthood.
Materials and Method
Animals
All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Animals were maintained on a 12-h/12-h light/dark cycle (lights on at 6:00 A.M.) in a temperature (20-22°C) and humidity (44-60%) controlled environment with food and water available ad libitum. Oprm1 A112G mice were generated on a C57BL/6 mouse background using site-directed mutagenesis to replace an adenine (A) nucleotide at position 112 with a guanine (G) nucleotide in exon 1 of the Oprm1 gene as described earlier 6. AG females were mated with AA, AG, or GG males to generate litters of mixed genotypes. Pregnant dams were individually housed and left undisturbed until parturition. Cages were checked daily at 9:00 A.M. for new births and pups discovered at that time were considered postnatal day (PND) 1. Litters were randomly assigned to saline or morphine treatment, with all pups in a given litter receiving the same treatment. Animals from a total of 44 injected litters (19 saline treated and 25 morphine treated) were used for behavioral analyses (see Table 1 for details). All experimental testing sessions were conducted between 9:00 A.M. and 5:00 P.M. An overview of the experimental timeline is shown in Figure 1A.
Table 1.
Litter characteristics and distribution across behaviors.
| Saline |
Morphine |
|
|---|---|---|
| Total Litters Injected | 19 | 25 |
| Development/USVs | 19 | 25 |
| Affective Behavior | 18 | 20 |
| Sensitization | 15 | 20 |
| Litter Size | 6.42 ± 0.41 | 5.88 ± 0.21 |
| % Male | 43.09 ± 5.73 | 44.54 ± 3.32 |
| % Female | 56.91 ± 5.73 | 55.46 ± 3.32 |
Figure 1. Experimental Timeline and Developmental Milestones.
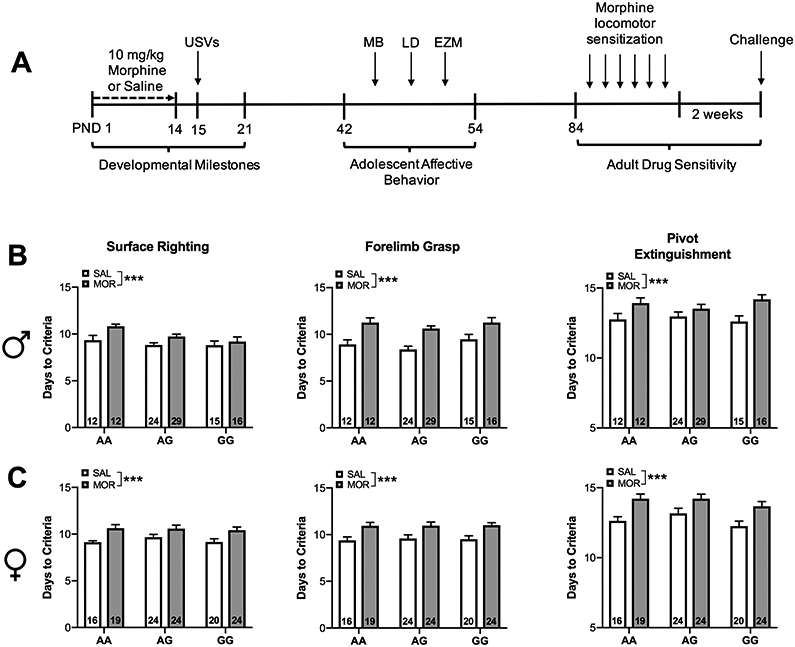
(A) Schematic describing experimental timeline for neonatal morphine exposure and behavioral testing. Male and female mouse pups were treated with morphine from postnatal day (PND) 1 to 14 and tested for isolation-induced ultrasonic vocalizations (USVs) after 24hrs of abstinence. A subset of these animals were aged and tested for baseline adolescent affective behavior in the marble burying task (MB), light dark (LD) box, and elevated zero maze (EZM). Beginning at 12 weeks of age animals were tested for behavioral sensitization to morphine. (B) Male and (C) female morphine treated pups exhibited significant delays in reaching developmental milestones independent of genotype. Data are expressed as mean ± SEM. *** p < 0.001 compared to saline treated animals.
Drugs
Morphine sulfate was obtained from NIDA Drug Supply (Research Triangle Park, NC), dissolved in 0.9% saline and administered subcutaneously (s.c.) in a volume of 10 ml/kg.
Chronic Morphine Exposure Paradigm
Morphine or saline treatment began on the evening of PND 1 and continued until the morning of PND 14. Morphine sulfate was delivered at a dose of 10 mg/kg. Controls received saline in an equal injection volume. Neonatal mouse plasma morphine concentrations have been shown to peak rapidly in minutes and have a short half-life 14, therefore all animals were injected twice daily at 9:00 A.M. and 5:00 P.M. This exposure paradigm was chosen based on findings from preliminary studies and relevant previous literature 15. Pups remained with the dam until weaning (PND 24), at which point they were genotyped and housed with same-sex/treatment mice.
Experiment 1: Developmental Milestones and Ultrasonic vocalizations
Mice were examined daily throughout the first 21 days of life to track weight gain. Beginning on PND 3, individual pups were identified using a non-toxic felt-tip marker and examined daily for the emergence of reflexes and coordinated movements 19. Assayed behaviors tested included surface righting (ability to flip over from its back to abdomen in 1 second or less), forelimb grasp (ability to grip a rod suspended over bedding for 1 second or more), and extinguishment of pivoting behavior (ability to ambulate outside a 5 cm diameter circle within 30 seconds). Data were collected as days to criteria, with criteria defined as meeting a given milestone for two consecutive days.
On PND 15, 24 hours after the final morphine or saline injection, pups were tested for separation-induced ultrasonic vocalization. Individual pups were separated from the dam and littermates and placed in a shallow plastic dish with fresh bedding. The dish was placed into a recording chamber maintained at 28-30°C. This temperature was chosen to avoid the confound of vocalization caused by heat loss 20. An Echo Meter Touch bat detector (Wild Life Acoustics, Maynard, MA) attached to an iPad Mini (Apple, Cupertino, CA) was situated 10 cm above the pup and recorded vocalizations for 5 minutes. Spectrographic analysis of USVs was performed using RavenPro software (Cornell Laboratory of Ornithology; Ithaca, NY). Vocalizations occurring in the range of 40-125 kHz were included in the analysis. USV categories were adapted from previously described waveform patterns 21 and included complex calls (two or more directional changes in pitch), composite calls (two harmonically independent components emitted simultaneously), two-syllable calls (a main call with an additional component towards the end), upward-modulated calls (a continuous increase in pitch of ≥ 3 kHz), downward-modulated calls (a continuous decrease in pitch of ≥ 3 kHz), chevron calls (resembling an 'inverted-U'), flat calls (≤ 3 kHz change in frequency between the beginning and the ending of the call), frequency steps (frequency changes appearing as a vertically discontinuous "step"), and short calls (shorter than 10 ms).
Experiment 2: Adolescent Affective Behavior
A subset of animals from Experiment 1 were allowed to age and examined for affective behavior during adolescence and drug sensitivity in adulthood. Animals aged PND 42-54 (considered late adolescence) were evaluated in the marble burying task, the light-dark box, and the elevated zero maze, in that order. These behavioral tests were administered at inter-test intervals of at least 24 hours. Prior to all behavioral assessments, mice were acclimated to the testing rooms for 1 hour.
Marble burying (MB)
Mice were placed individually in test cages resembling the home cage (26×20×14 cm), in which twenty marbles were distributed evenly on top of mouse bedding (5-cm deep), and a clear lid placed on top of the cage. Mice were left undisturbed for 15 minutes, and number of buried marbles (3/4 submerged in bedding) were counted by a trained blinded observer.
Light-Dark Box (LD)
Mice were placed in a testing apparatus which consisted of a two-chambered box (17 × 20 cm for each side) made of Plexiglas with an opening (5 x 5 cm) connecting both chambers. One side was colored black and covered to limit room light entry, while the other side was white. Mice were placed into the dark side and then allowed to freely explore either the light or dark side for 300 seconds. Mice were video recorded for the duration of testing. Latency to emerge from the dark side, time spent exploring the light side, and transitions between compartments was quantified by a trained blinded observer.
Elevated Zero Maze (EZM)
Mice were placed onto the maze (elevated 24 inches) facing a closed arm and allowed to freely explore undisturbed for 300 seconds. Mice were video recorded for the duration of testing. Latency to emerge from the closed arm, the amount of time spent in the open arms, and transitions between arms was quantified by a trained blinded observer.
Experiment 3: Adult Morphine Locomotor Sensitization
Beginning at PND 84, adult animals were assayed for morphine locomotor sensitization. Mice were placed in a test cage (26 x 20 x 14 cm) containing a small layer of fresh bedding and surrounded by a photo beam frame (30 x 24 x 8 cm) with sensors arranged in an eight-beam array strip. Locomotor activity, recorded as beam breaks using MedAssociates personal computer-designed software (MedAssociates, St. Albans, VT), was measured for 120 minutes immediately following an injection of either saline or morphine (20 mg/kg, s.c). On the first two treatment days all animals were administered saline. Locomotor activity from the second day of saline treatment was considered baseline for statistical analyses. For the following four treatment days all animals received morphine (20 mg/kg). Development of behavioral sensitization to morphine was defined as a significant increase in locomotor activity between the first and fourth day of morphine treatment. Two weeks following the last morphine injection, all animals were treated with a challenge dose of 10 mg/kg morphine and monitored for 120 minutes.
Statistical Analysis
Weight, developmental milestones, individual USV call types, anxiety behaviors, and sensitization data were analyzed by linear mixed-effects models. Total USVs were analyzed by generalized linear mixed-effects model with a Poisson mass probability function and a log link, an approach well-suited for modeling non-normal count data 22,23. Call type probability was calculated for each subject as the number of calls within each category/total number of calls, and probability values were transformed by angular transformation. All models included fixed effects of sex (male and female), genotype (AA, AG, and GG), drug exposure (morphine and saline), and their interactions, and random effects included by-litter slopes and intercepts. The model used to analyze repeated measures sensitization data also included fixed effects of day (baseline, day 1 of morphine, and day 4 of morphine), and random by-subject slopes and intercepts. Mixed model analyses were performed using package ‘lme4’ 24 within R 25 and post-hoc analyses were performed using package ‘phia’ 26 with the Holm-Bonferroni correction for multiple comparisons. A p<0.05 was considered significant throughout.
Results
Neonatal morphine exposure blunts weight gain
Neonatal morphine exposure reduced weight gain over the first three weeks of life (Table 2). Morphine treatment significantly reduced body weight in male and female mice of each genotype relative to saline treatment on PND 7 [χ2 (1, N = 223) = 10.19, p<0.01], PND 14 [χ2 (1, N = 223) = 15.92, p<0.0001], and PND 21 [χ2 (1, N = 223) = 6.94, p<0.01].
Table 2.
The effect of neonatal morphine exposure on weight.
| PND 1 | PND 7 | PND 14 | PND 21 | |||||
|---|---|---|---|---|---|---|---|---|
| Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
|
| Male | ||||||||
| AA | 1.39 ± 0.03 | 1.34 ± 0.04 | 3.04 ± 0.15 | 2.73 ± 0.09** | 5.71 ± 0.18 | 4.83 ± 0.2*** | 7.95 ± 0.26 | 6.93 ± 0.28** |
| AG | 1.37 ± 0.04 | 1.37 ± 0.02 | 3.46 ± 0.13 | 2.81 ± 0.08** | 6.04 ± 0.18 | 5.04 ± 0.12*** | 8.30 ± 0.24 | 7.38 ± 0.19** |
| GG | 1.37 ± 0.03 | 1.34 ± 0.03 | 3.08 ± 0.18 | 2.55 ± 0.14** | 5.71 ± 0.26 | 4.76 ± 0.19*** | 7.56 ± 0.40 | 6.83 ± 0.28** |
| Female | ||||||||
| AA | 1.32 ± 0.02 | 1.38 ± 0.02 | 2.96 ± 0.07 | 2.56 ± 0.06** | 5.35 ± 0.13 | 4.94 ± 0.19*** | 7.07 ± 0.16 | 6.73 ± 0.23** |
| AG | 1.35 ± 0.03 | 1.37 ± 0.02 | 3.25 ± 0.16 | 2.63 ± 0.09** | 6.07 ± 0.22 | 4.82 ± 0.14*** | 7.99 ± 0.29 | 6.99 ± 0.21** |
| GG | 1.39 ± 0.03 | 1.40 ± 0.02 | 3.27 ± 0.13 | 2.68 ± 0.09** | 5.85 ± 0.18 | 5.10 ± 0.16*** | 7.87 ± 0.23 | 7.22 ± 0.20** |
p < 0.001
p < 0.01 compared to saline treated.
Neonatal morphine exposure delays emergence of developmental milestones
In comparison to saline-treated animals, morphine-treated mice took significantly longer to reach criteria on forelimb grasp χ2 (1, N = 223) = 31.72, p<0.0001], surface righting [χ2 (1, N = 220) = 12.73, p<0.0001], and extinguishment of pivoting behavior [χ2 (1, N = 223) = 23.85, p<0.0001]. A significant main effect of sex was observed on surface righting [χ2 (1, N = 220) = 6.49, p<0.05] wherein females took longer to reach this milestone compared to males. (Figure 1B-C).
Spontaneous withdrawal from neonatal morphine treatment increases isolation-induced ultrasonic vocalization in male, but not female pups
There is considerable variability in both the timing and presentation of withdrawal symptoms in infants suffering from NOWS. This variability may be due to differences in opioid metabolism, placental transfer, and other pharmacogenetic variables. On average, NOWS symptoms arising from heroin exposure (which rapidly metabolizes into morphine once entering the central nervous system) typically manifest 24-48 hours after birth 27. Therefore, we chose to evaluate USVs 24 hours into spontaneous withdrawal. Analysis of ultrasonic vocalizations during spontaneous withdrawal revealed a significant Sex × Genotype × Drug interaction [χ2 (2, N = 200) = 76.33, p<0.0001]. A post-hoc analysis revealed that morphine-treated AA (p<0.05) and GG males (p<0.001) vocalized significantly more compared to saline-treated animals. A non-significant trend was observed in AG males (p=0.067) (Figure 2A). There was no significant effect of morphine treatment on total ultrasonic vocalizations in female mice (Figure 2B).
Figure 2. Withdrawal from neonatal morphine exposure increases total ultrasonic vocalizations in males.
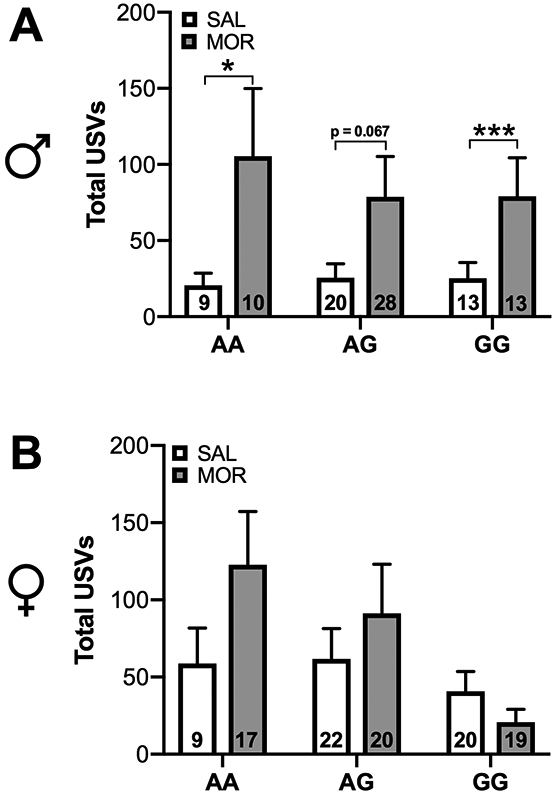
Figures depict total ultrasonic vocalizations occurring in the range of 40-125 kHz. (A) Male AA and GG morphine-exposed pups exhibited increased vocalization compared to saline-exposed pups 24 hours after cessation of drug treatment. (B) There was no effect of morphine exposure on ultrasonic vocalization in females. Data are expressed as mean ± SEM. * p < 0.05, *** p < 0.001 compared to saline treated animals.
Following analysis of each USV call type separately, a main effect of drug was found on the probability of producing two-syllable calls, with morphine treated animals overall more likely to emit this call type compared to saline treated animals [χ2 (1, N = 200) = 4, p<0.05] (Table 3). A significant Genotype x Drug interaction was observed on probability to emit a chevron call [χ2 (2, N = 200) = 6.51, p<0.05]. Morphine treated GG mice were more likely to emit a chevron call compared to saline treated mice (p<0.05). Additionally, a significant Sex x Drug interaction was observed for probability to emit a composite call [χ2 (1, N = 200) = 4.13, p<0.05], however post-hoc analyses failed to detect a significant difference between males and females.
Table 3.
Probability of ultrasonic vocalization call type by sex, genotype, and drug treatment.
| Chevron | Inverted Chevron | Two Syllables | Upward | Downward | Frequency Step | Harmonic | Short | Complex | Flat | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
Saline |
Morphine |
|
| Male | ||||||||||||||||||||
| AA | 0.24 ± 0.08 | 0.23 ± 0.06 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.05 ± 0.04 | 0.25 ± 0.06* | 0.17 ± 0.06 | 0.19 ± 0.06 | 0.13 ± 0.06 | 0.28 ± 0.06 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.00 ± 0.00 | 0.08 ± 0.03 | 0.27 ± 0.11 | 0.48 ± 0.13 | 0.30 ± 0.10 | 0.32 ± 0.06 | 0.07 ± 0.05 | 0.09 ± 0.03 |
| AG | 0.30 ± 0.09 | 0.29 ± 0.05 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.07 ± 0.03 | 0.13 ± 0.03* | 0.17 ± 0.05 | 0.21 ± 0.04 | 0.12 ± 0.05 | 0.30 ± 0.06 | 0.01 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.09 ± 0.03 | 0.27 ± 0.09 | 0.43 ± 0.07 | 0.34 ± 0.11 | 0.29 ± 0.05 | 0.05 ± 0.02 | 0.08 ± 0.02 |
| GG | 0.19 ± 0.08 | 0.54 ± 0.14* | 0.00 ± 0.00 | 0.04 ± 0.02 | 0.19 ± 0.06 | 0.17 ± 0.04* | 0.07 ± 0.04 | 0.33 ± 0.11 | 0.27 ± 0.10 | 0.25 ± 0.06 | 0.04 ± 0.02 | 0.06 ± 0.03 | 0.05 ± 0.02 | 0.07 ± 0.03 | 0.43 ± 0.13 | 0.33 ± 0.07 | 0.17 ± 0.07 | 0.32 ± 0.07 | 0.09 ± 0.04 | 0.14 ± 0.03 |
| Female | ||||||||||||||||||||
| AA | 0.48 ± 0.14 | 0.39 ± 0.09 | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.08 ± 0.03 | 0.16 ± 0.04* | 0.16 ± 0.05 | 0.16 ± 0.04 | 0.36 ± 0.07 | 0.35 ± 0.1 | 0.07 ± 0.04 | 0.06 ± 0.02 | 0.09 ± 0.05 | 0.07 ± 0.02 | 0.37 ± 0.08 | 0.41 ± 0.09 | 0.39 ± 0.08 | 0.29 ± 0.06 | 0.16 ± 0.04 | 0.10 ± 0.02 |
| AG | 0.27 ± 0.06 | 0.37 ± 0.06 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.19 ± 0.05* | 0.19 ± 0.04 | 0.20 ± 0.04 | 0.20 ± 0.05 | 0.23 ± 0.05 | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.09 ± 0.03 | 0.06 ± 0.02 | 0.30 ± 0.08 | 0.39 ± 0.06 | 0.33 ± 0.08 | 0.38 ± 0.08 | 0.07 ± 0.02 | 0.12 ± 0.03 |
| GG | 0.20 ± 0.05 | 0.31 ± 0.10* | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.12 ± 0.03 | 0.06 ± 0.03* | 0.16 ± 0.04 | 0.17 ± 0.06 | 0.28 ± 0.09 | 0.31 ± 0.1 | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.38 ± 0.11 | 0.18 ± 0.07 | 0.22 ± 0.05 | 0.27 ± 0.09 | 0.07 ± 0.02 | 0.07 ± 0.03 |
p < 0.05 compared to saline treated.
Morphine-treated animals overall emitted more chevron [χ2 (1, N = 200) = 5.97, p<0.05], inverted chevron [χ2 (1, N = 200) = 4.93, p<0.05], complex [χ2 (1, N = 200) = 5.2, p<0.05], upward [χ2 (1, N = 200) = 5.39, p<0.05], frequency step [χ2 (1, N = 200) = 4.98, p<0.05], flat [χ2 (1, N = 200) = 4.08, p<0.05], and short [χ2 (1, N = 200) = 7.65, p<0.01] calls compared to saline-treated animals (Figure 3). Morphine treatment increased emission of two-syllable calls [χ2 (2, N = 200) = 7.84, p<0.05] in AA (p < 0.01) and AG animals (p < 0.05). Additionally, a significant Sex x Drug interaction was detected for production of composite calls [χ2 (1, N = 200) = 4.95, p<0.05]. Post-hoc analyses revealed a non-significant trend towards morphine treatment increasing production of composite calls in males only (p = 0.06).
Figure 3. Production of ultrasonic vocalizations by call type.
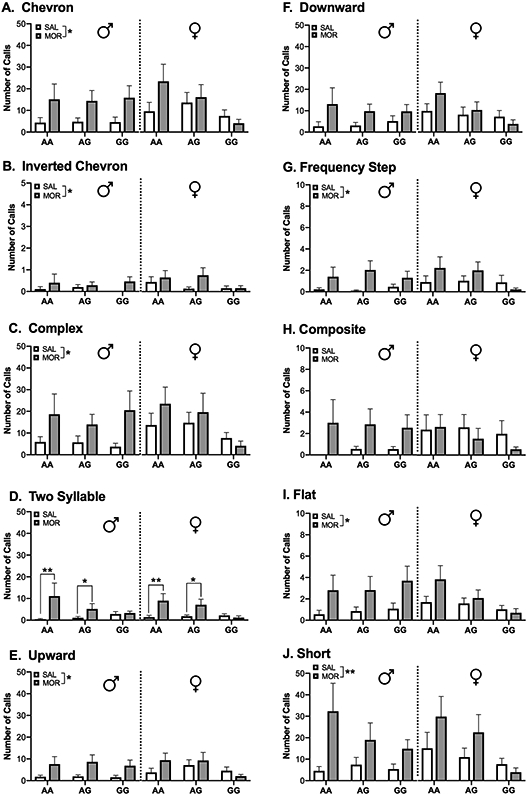
Morphine exposure and withdrawal increased production of most of the observed call types. Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01 compared to saline treated animals.
Neonatal morphine exposure alters adolescent behavior in the marble burying task in a genotype-dependent manner, but has no effect on behavior in the light dark test and elevated zero maze
A linear mixed-effects model revealed a significant Genotype × Drug interaction on behavior in the marble burying task [χ2 (2, N = 143) = 6.41, p<0.05]. AA morphine-treated mice buried significantly fewer marbles compared to AA saline-exposed animals (p < 0.05), however, a morphine effect was not observed in AG or GG animals (Figure 5).
Figure 5. Neonatal morphine exposure alters adolescent behavior in the marble burying task in a genotype-dependent manner.
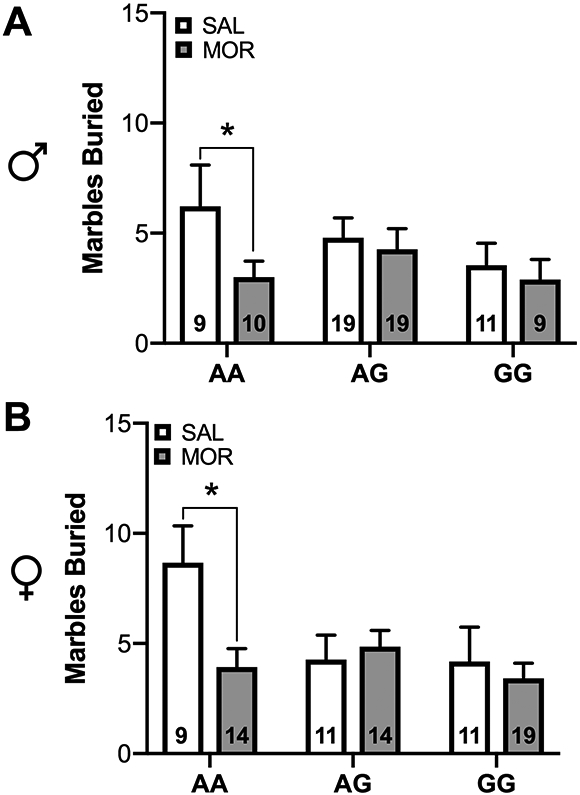
(A) Wild-type males and (B) females exposed to morphine as pups exhibited reduced burying in the marble burying task compared to saline-treated animals. This effect was not seen in AG and GG animals. Data are expressed as mean ± SEM. * p < 0.05
In the light dark test, a main effect of sex was observed for time spent in the light compartment [χ2 (1, N = 115) = 7.41, p<0.001], latency to emerge into the light compartment [χ2 (1, N = 114) = 12.75, p<0.001], and number of transitions between the light and dark compartment [χ2 (1, N = 115) = 11.54, p<0.001]. Independent of genotype and drug treatment, female mice spent more time in the light compartment, took less time to emerge, and made more transitions between the light and dark compartments (Figure 6A-B).
Figure 6. Neonatal morphine exposure does not impact adolescent behavior in the light-dark box test or elevated zero maze.
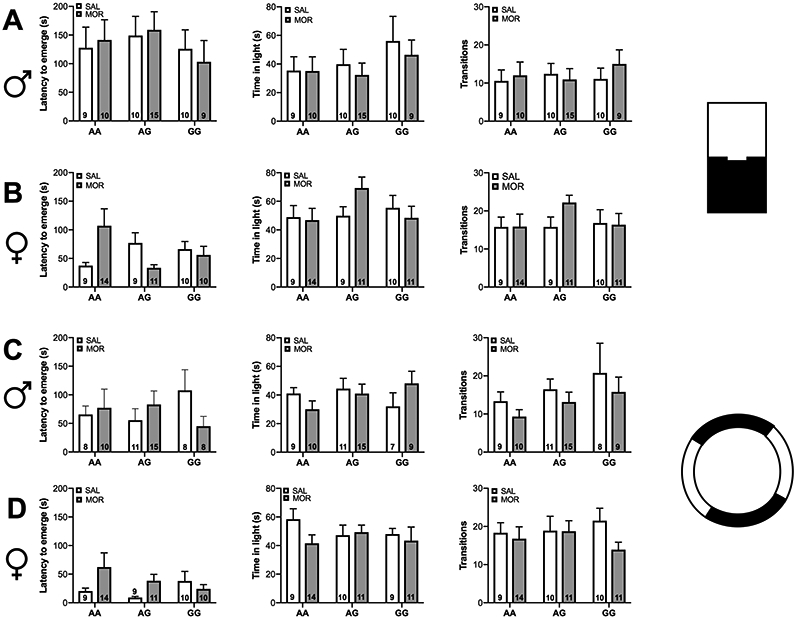
(A-B) In the light-dark box test female mice spent more time in the light compartment, had a lower latency to emerge, and made more transitions between the light and dark compartments compared to males. (C-D) In the elevated zero maze females spent more time in the open arm and had a lower latency to emerge compared to males. No effect of genotype or drug exposure was observed in the light dark box test or elevated zero maze. Data are expressed as mean ± SEM.
In the elevated zero maze there was a significant main effect of sex on time spent in the open compartment [χ2 (1, N = 113) = 4.78, p<0.05] and latency to emerge to the open compartment [χ2 (1, N = 111) = 12.44, p<0.001]. Overall, females spent more time in the open compartment and took less time to emerge (Figure 6C-D). A significant Genotype × Drug interaction was observed [χ2 (2, N = 111) = 7.1, p<0.05], but post-hoc analyses revealed no significant changes.
Neonatal morphine exposure alters development of adult morphine locomotor sensitization in a sex- and genotype-dependent manner
Analysis of the development of locomotor sensitization by linear mixed-effects model revealed a significant Day × Sex × Genotype × Drug interaction [χ2 (4, N = 92) = 17.01, p<0.001]. In male animals, a significant increase in locomotor activity between morphine day 1 and morphine day 4 (development of sensitization) was observed in AA saline- (p < 0.0001) and morphine-exposed animals (p<0.0001) and AG saline- (p<0.0001] and morphine-exposed animals (p<0.001) (Figure 7A). However, on day 4 neonatal morphine exposure resulted in augmented locomotor activity in AA males (p<0.05), but not in AG males (Figure 7A; significant Sex × Genotype × Drug interaction [χ2 (2, N = 92) = 10.49, p<0.01]). Development of sensitization was not observed in GG saline-treated mice as previously shown 6 and prior exposure to morphine did not alter this genotype effect.
Figure 7. Neonatal morphine exposure alters development of adult morphine locomotor sensitization in a sex- and genotype-dependent manner.
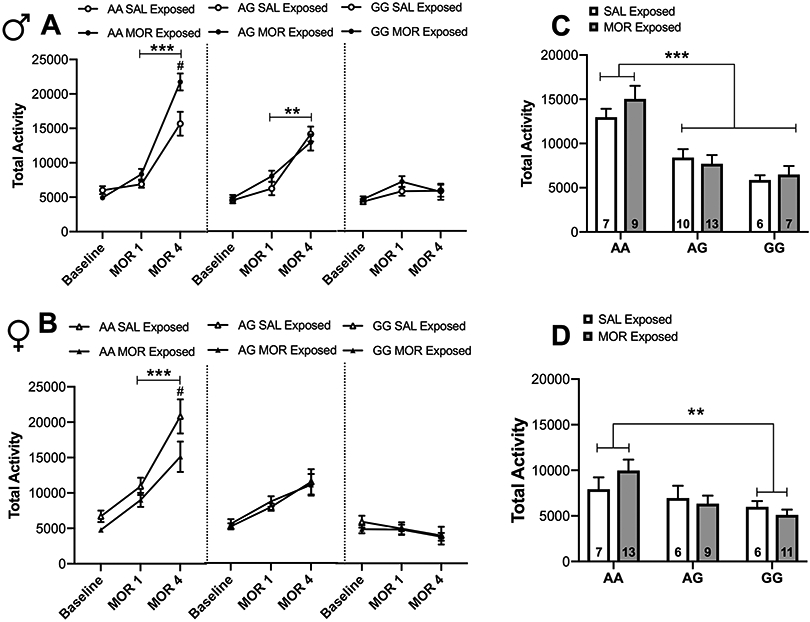
(A) Male wild-type mice exposed to morphine as pups exhibited augmented morphine locomotor sensitization in adulthood compared to those that were exposed to saline as pups. Both saline and morphine pre-exposed AG males expressed sensitization to morphine as adults, whereas GG males showed no evidence of sensitization ***p < 0.001, **p < 0.01 between MOR1 and MOR4. #p <0.05 compared to saline exposed. (B) Within females, both saline and morphine pre-exposed AA animals exhibited morphine sensitization, however, AG and GG females did not sensitize to morphine. ***p < 0.001 between MOR1 and MOR4. #p <0.05 compared to saline exposed. (C) Following 2 weeks of abstinence and a subsequent challenge dose of morphine, a genotype effect was observed in which AG and GG males exhibited reduced activity compared to AA males, although there was no impact of pre-exposure at this point. *** p < 0.001 compared to AA animals. (D) In females, GG animals exhibited significantly less activity compared to AA animals. ** p < 0.01 compared to AA and AG animals. Data are expressed as mean ± SEM.
In females, development of morphine sensitization was only observed for AA saline- (p<0.0001) and morphine-exposed animals (p<0.0001) (Figure 7B). In contrast to the effects observed in male AA mice, neonatal morphine exposure in female AA mice significantly reduced locomotor activity on morphine day 4 compared to saline-exposed pups (p<0.05) (Figure 7B). Development of sensitization did not occur in AG or GG females regardless of drug exposure. The expression of sensitization, measured after a two-week drug-free period, was elicited by injecting half the dose of morphine used to develop sensitization (10mg/kg). In this case, neonatal exposure to morphine did not alter locomotor response regardless of sex or genotype. However, there was a significant Sex × Genotype interaction [χ2 (2, N = 92) = 9.59, p<0.01] wherein AG (p<0.0001) and GG (p<0.0001) males show reduced locomotor activity on challenge day compared to AA males (Figure 7C) and GG females exhibited significantly reduced locomotor activity compared to AA females (p<0.01) (Figure 7D).
Discussion
These studies are the first to examine the impact of the Oprm1 SNP on the short and long-term consequences of neonatal opioid exposure and withdrawal in a mouse model. Opioid exposure during the third trimester equivalent period in mice significantly reduced weight gain and delayed expression of several developmental milestones in male and female pups, regardless of genotype. During spontaneous withdrawal, morphine-treated males, but not females, exhibited increased ultrasonic vocalization independent of genotype. However, during late adolescence and adulthood, the Oprm1 SNP attenuated the behavioral effects of early morphine exposure in the marble burying task and morphine locomotor sensitization. Altogether, these findings indicate that the Oprm1 SNP differentially affects certain phenotypes that arise later in life without significantly altering morphine's effect on neurodevelopment or opioid withdrawal early in life.
It is not known if opioid exposure restricted to the in utero period in humans results in long-term developmental disabilities, however the accumulation of exogenous opioids in fetal brain tissue clearly appears to be detrimental to the development of the nervous system 28,29. There are key differences in the timing of brain maturation events occurring in human versus rodent development that may influence the impact of opioid exposure on neurodevelopment. In the current study, mice received morphine treatment for the first fourteen days of life, a period of rapid brain development similar to that seen in the last trimester of a human pregnancy. This time frame is longer than the "rule of thumb" use of PND 1-10 as the third-trimester equivalent of a human pregnancy, which is primarily based on early comparative neuroanatomy studies and does not account for variability in the timing of regional maturation events within the brain 10,11. For example, rapid axonal and dendritic growth occurs up to PND 10 in the rat cerebellum and cortex, however this period of rapid growth extends up to PND 20 in the hippocampus and corpus callosum 30. Thus, exposure throughout the first two postnatal weeks allows for the inclusion of important brain development events that may be altered in human infants exposed to opioids in utero.
We found that morphine treatment throughout PND 1-14 resulted in blunted weight gain and delays in reaching several developmental milestones. Similar phenotypes have been observed following prenatal opioid exposure models 31, but variability among the studies in both timing and extent of opioid exposure have made it challenging to determine exactly what periods during gestation are critical for these effects. An advantage of the PND 1-14 exposure paradigm is that it eliminates the potential confound of differential drug distribution in utero 32 and allows for equal and consistent drug dosing between pups. The major limitation of this model is that it does not reflect the typical pattern of exposure in human opioid-exposed infants. However, a recent study examining Medicaid data collected from 46 states revealed that at-risk infants who were exposed to opioids in the third trimester were more likely to exhibit withdrawal symptoms compared to those exposed only in the first two trimesters, suggesting that late gestation is a sensitive period for expression of NOWS 17.
When isolated or in distress , mouse pups emit ultrasonic vocalizations (USVs) consisting of a large repertoire of calls that can vary widely in frequency range, duration, and acoustic structure 33. Several studies point to a role of the endogenous opioid system in modulating USVs. Neonatal mice lacking the mu-opioid receptor emit less USVs compared to wildtype controls 34. In neonatal rats, morphine treatment has been shown to suppress isolation-induced USVs 35, while precipitation of withdrawal with a mu-opioid receptor (MOR) antagonist, such as naloxone or naltrexone, potentiates USVs 36. Thus, increased ultrasonic vocalizations have been used as a behavioral indicator of withdrawal 18. Moreover, significant differences in the repertoire of USVs have been observed between inbred mouse strains, suggesting that there is a genetic component to these variations 21. Overall, we observed modest differences in the vocal repertoire of morphine treated pups in withdrawal compared to animals treated with saline, and genotype-specific alterations in the probability or rate of USV production was only seen in two of the ten call types analyzed. Thus, our findings indicate that morphine exposure and withdrawal primarily affects rate of USV production as opposed to distribution of USV call types.
We detected a significant increase in total USVs in AA and GG male morphine-treated during spontaneous withdrawal. The lack of genotype effect is consistent with data from adult animals showing that physical dependence in morphine treated animals, as measured by precipitated somatic withdrawal, does not differ between AA and GG mice 6. We do detect differences in both quality and quantity of USVs in males and females following morphine exposure. Sexual differentiation in the developing brain may underlie differing responses to morphine withdrawal in males versus females. For example, norepinephrine (NE) transmission is known to play an important role in opioid withdrawal 37, and prenatal morphine exposure has been shown to increase hypothalamic NE transmission in male, but not female, rats 38.Thus, sex-specific alterations in these systems may preferentially affect males compared to females. However, a potential caveat of using USV frequency as a measure of neonatal withdrawal is that the frequency and acoustics of pup USVs changes throughout development. Vocalization typically peaks around PND 7 and extinguishes around two weeks of age 39. Thus, increased vocalizing post drug cessation may, in and of itself, be a neurodevelopmental deficit and reflect a morphine-induced temporal shift in vocalizing patterns as opposed to a symptom of withdrawal. Future studies should incorporate additional behavioral measures of withdrawal, such as mechanical and thermal nociception 40,41 with traditional somatic signs (e.g. tremors, wall climbing, jumping) at multiple time points following abstinence to determine the influence of this genotype on development and the time course of neonatal opioid withdrawal. Of note, adult AA and GG Oprm1 mice show similar somatic signs of naloxone precipitated opioid withdrawal, but significant differences in conditioned place aversion to withdrawal 6. Therefore, it will be of interest to compare and contrast various withdrawal behavior in these genotypes in the neonates.
Mood and anxiety disorders are closely linked to early life adversity 42. There is conflicting preclinical evidence as to whether or not early opioid exposure impacts affective behavior later in life. Some previous studies show that gestational exposure to opioids increases anxiety- and depressive-like behavior in adolescence and adulthood 43, while others have reported the opposite or no effect 15. A goal of the current study was to determine whether opioid exposure during the mouse equivalent of human third trimester modifies baseline affective behavior during adolescence, a period of synaptic pruning and remodeling of neural circuits 44. AA animals exposed to morphine exhibited a significant reduction in burying activity compared to saline treated AA in the marble burying (MB) task, a behavioral assay rooted in the mouse's natural inclination to dig or bury in response to novel objects. Reduced marble burying in this task is indicative of low anxiety-like behavior, as administration of anxiolytic drugs has been shown to inhibit marble burying behavior 45. However, neither morphine exposure nor genotype had an effect on behavior in the elevated zero maze (EZM) or light dark (LD) box, two widely used measures for assaying anxiety-like behavior in rodents 46. Thus, it is unclear whether our observations in the marble burying task is suggestive of a global change in baseline anxiety-like behavior. Alternatively, low burying activity in this task may be reflective of reduced object exploration or responsiveness to a new environment 47. Of interest, we found that morphine's effects in the MB task was absent in animals harboring either one or two copies of the G-allele. Morphine treatment from PND 5-9 has been shown to significantly reduce adult basal corticosterone levels 14. The endogenous opioid system is an important regulator of the hypothalamic-pituitary-adrenal (HPA) axis, and persistent changes in the functioning of the neuroendocrine system resulting from neonatal morphine exposure may ultimately impact general arousal and/or sensitivity to environmental cues 48. In humans, individuals with the A118G SNP exhibit elevated cortisol levels at baseline 49. Thus, future studies should measure HPA axis activity in animals harboring the G-allele to determine if these are correlated with responsiveness to novelty in these animals and if this is altered following opioid exposure.
It remains controversial as to whether individuals exposed to opioids in utero are at increased risk of drug use and addiction later in life. In animal studies, prenatal and early postnatal opioid exposure has been shown to enhance conditioned place preference for morphine 50, increase self-administration of heroin 51, and increase behavioral sensitization to morphine 52. In the current study, we observed a robust increase in the development of locomotor sensitization in AA male animals exposed to morphine from PND 1-14. Strikingly, this effect was reversed in females, with AA morphine exposed females exhibiting significantly reduced sensitization compared to saline controls. Similar to previously reported findings, GG males and females overall failed to develop locomotor sensitization to morphine 6. Although AG males exhibited development of sensitization, the augmented effect of neonatal morphine exposure seen in AA males was not observed. AG females, on the other hand, did not show a significant sensitization effect regardless of neonatal treatment.
The locomotor-stimulating effects of morphine are mediated by activation of MORs expressed by GABAergic interneurons in the ventral tegmental area, which facilitate mesolimbic dopamine release in key reward processing neural substrates 53,54. Previous studies have established a fundamental role for both the dopaminergic 55 and glutamatergic system 56 in the development of locomotor sensitization to opioids. Dysregulation of these systems by exogenous opioids early in development may contribute to alterations in the development of sensitization in adulthood. Indeed, prenatal exposure to morphine has been shown to increase dopamine and serotonin turnover rates in the nucleus accumbens of male adult rats, which is associated with enhanced locomotor response to morphine 52. Neonatal opioid exposure may also alter expression or function of other opioid receptors involved in the induction of sensitization. Pharmacological blockade of either the delta 57 or kappa 58 opioid receptor results in increased locomotor sensitization to morphine.
The dimorphic effects of neonatal morphine exposure on adult locomotor sensitization to morphine are likely due to sex- and genotype-specific differences in the neuromodulatory systems underlying the sensitization process. There is evidence suggesting that estrogen modulates MOR gene expression 59, and differences in opioid receptor availability have been shown to underlie sex differences in analgesic response to morphine 60. Previous work from our lab demonstrate that both male and female GG animals exhibit decreased MOR mRNA expression and protein levels compared to AA animals, and this is associated with reduced locomotor response to opioids 6,8. A recent study investigating baseline mRNA expression levels of drug- and stress-related genes in A112G mice found that hypothalamic expression of the neuropeptides arginine vasopressin (Avp) and galanin (Gal), and hippocampal expression of the opioid-related nociception receptor (Oprl1) and cannabinoid receptor 1 (Cnr1) were significantly reduced in animals with the G allele (Collins 2018). Thus, the influence of the Oprm1 SNP in these studies may be due to differential expression of opioid receptors and/or an attenuation of MOR-mediated transcriptional regulation.
Clinical findings suggest that genomic variation in opioid related genes, including the A118G SNP, may influence NOWS severity, with the OPRM1 G-allele specifically associated with shorter length of stay in the hospital and reduced likelihood of receiving any treatment compared to infants with an AA genotype 4. Thus, we predicted that animals with the Oprm1 variant would have fewer neurodevelopmental deficits and show reduced withdrawal. In contrast, we found only a main effect of opioid exposure, with no significant differences between genotypes. However, it is important to note that in the current study , mice were not exposed to opioids throughout gestational development. Murine Oprm1 mRNA is detected at embryonic day 11.5 in basal ganglia. This expression later increases during mid- and late-gestation in many of the brain areas that show high expression in the adult 61. Thus, it is possible that the Oprm1 SNP may influence early withdrawal when a full 3-trimester equivalency model is used in which we extend the duration of opioid exposure to cover the equivalent length of human pregnancy (gestation plus PND 1-14).
Reduced hospital stay may also reflect better response to opioid pharmacotherapy in infants with the OPRM1 SNP. Currently, the first-line therapy for infants with NOWS is neonatal morphine solution or methadone. Recently, treatment with buprenorphine, an opioid drug with mixed activity at opioid receptors, has been shown to be more effective than morphine in reducing NOWS-related hospital length of stay 62. In adult mice, we see that the Oprm1 SNP attenuates the analgesic, anxiolytic and hyperlocomotor effects of buprenorphine 8. Buprenorphine is an FDA-approved drug for the treatment of opioid use disorder, and our data suggest that the Oprm1 SNP may impact its therapeutic efficacy. Thus, additional studies are needed to determine what role this SNP may play in treatment efficacy.
In conclusion, we developed a mouse model of NOWS that recapitulates clinically relevant behaviors and enables the study of genetic contributions to NOWS. Our data supports the early postnatal period in rodents as a particularly sensitive period for developmental and long-term consequences of opioid exposure and further expanded on the current literature by demonstrating that the Oprm1 SNP may be important in modulating morphine-induced behavioral deficits that manifest later in life. Further use of this model will allow for more in depth investigation into the influence of genetic variability in NOWS prognosis and treatment, as well as provide insights into the underlying neurobiology of this syndrome.
Figure 4. Distribution of ultrasonic vocalization call types.
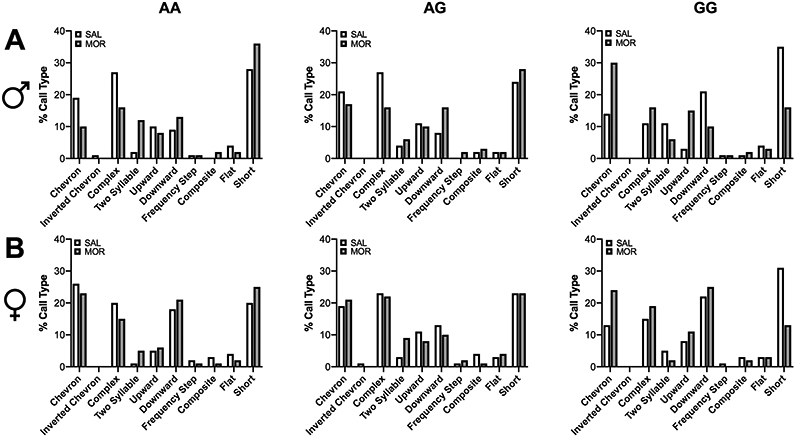
Bar graphs depict the percentages of the different call types emitted by A) males and B) females in each experimental group.
Acknowledgements
This work was supported by grants from R21 DA044017 (JAB), K12 GM081259 (SAR) and T32 DA028874 (JKB). The authors have no conflicts of interest to report.
References
- 1.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and Costs of Neonatal Abstinence Syndrome Among Infants With Medicaid: 2004–2014. Pediatrics 2018;141(4):e20173520. 10.1542/peds.2017-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conradt E, Crowell SE, Lester BM. Early life stress and environmental influences on the neurodevelopment of children with prenatal opioid exposure. Neurobiol Stress 2018;9:48–54. 10.1016/j.ynstr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mague SD, Blendy JA. OPRM1 SNP (A118G): Involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 2010;108(3):172–82. 10.1016/j.neuroimage.2013.08.045.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wachman E, Hayes M, Brown M, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 2013;309(17):1821–7. 10.1001/jama.2013.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Merrer J, Becker JAJ, Befort K, Kieffer BL. Reward Processing by the Opioid System in the Brain. Physiol Rev 2009;89:1379–412. 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mague SD, Isiegas C, Huang P, Liu-Chen L-Y, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci U S A 2009;106(26):10847–52. 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Picetti R, Butelman ER, Ho A, Blendy JA, Kreek MJ. Mouse Model of the OPRM1 (A118G) Polymorphism: Differential Heroin Self-Administration Behavior Compared with Wild-Type Mice. Neuropsychopharmacology 2015;40(5):1091–100. 10.1038/npp.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne CA, Erickson RL, Blendy JA, Lucki I. Genetic variation in the behavioral effects of buprenorphine in female mice derived from a murine model of the OPRM1 A118G polymorphism. Neuropharmacology 2017;117:401–7. 10.1016/j.neuropharm.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins D, Randesi M, da Rosa JC, Zhang Y, Kreek MJ. Oprm1 A112G, a single nucleotide polymorphism, alters expression of stress-responsive genes in multiple brain regions in male and female mice. Psychopharmacology (Berl) 2018;235(9):2703–11. 10.1007/s00213-018-4965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013;106–107:1–16. 10.1016/j.immuni.2010.12.017.Two-stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to human. Neurotoxicology 2007;28(5):931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev 1991;26(1):61–7. [DOI] [PubMed] [Google Scholar]
- 13.Jones KL, Barr G a. Ontogeny of morphine withdrawal in the rat. Behav Neurosci 1995;109(6):1189–98. [DOI] [PubMed] [Google Scholar]
- 14.Boasen JF, McPherson RJ, Hays SL, Juul SE, Gleason CA. Neonatal stress or morphine treatment alters adult mouse conditioned place preference. Neonatology 2009;95(3):230–9. 10.1159/000165379. [DOI] [PubMed] [Google Scholar]
- 15.Craig MM, Bajic D. Long-term behavioral effects in a rat model of prolonged postnatal morphine exposure. Behav Neurosci 2015;129(5):643–55. 10.1037/bne0000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seligman NS, Salva N, Hayes EJ, Dysart KC, Pequignot EC, Baxter JK. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol 2008;199(4). 10.1016/j.ajog.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 17.Desai R, Huybrechts K, Hernandez-Diaz S, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ 2015;350:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr GA, McPhie-Lalmansingh A, Perez J, Riley M. Changing mechanisms of opiate tolerance and withdrawal during early development: Animal models of the human experience. ILAR J 2011;52(3):329–41. 10.1093/ilar.52.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill JM, Lim MA, Stone MM. Developmental Milestones in the Newborn Mouse. Neuropept Tech 2008;39(1):131–49. 10.1007/978-1-60327-099-1_10. [DOI] [Google Scholar]
- 20.Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev 2001;108(1):83–95. 10.1037//0033-295X. [DOI] [PubMed] [Google Scholar]
- 21.Scattoni M, Gandhy S, Ricceri L, Crawley J. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One 2008;3(8):e3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolker B, Brooks M, Clark C, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 2009;24:127–35. [DOI] [PubMed] [Google Scholar]
- 23.O’Hara R, Kotze D. Do not log-transform count data. Methods Ecol Evol 2010;1:118–22. [Google Scholar]
- 24.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 2015;67(1):1–48. [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. 2017. [Google Scholar]
- 26.De Rosario-Martinez H phia: Post-Hoc Interaction Analysis. R package version 0.2-1. 2015. [Google Scholar]
- 27.Kocherlakota P Neonatal Abstinence Syndrome. Pediatrics 2014;134(2):e547–61. 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
- 28.Walhovd KB, Watts R, Amlien I, Woodward LJ. Neural tract development of infants born to methadone-maintained mothers. Pediatr Neurol 2012;47(1):1–6. 10.1016/j.pediatrneurol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Monnelly VJ, Anblagan D, Quigley A, et al. Prenatal methadone exposure is associated with altered neonatal brain development. NeuroImage Clin 2018;18:9–14. 10.1016/j.nicl.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baloch S, Verma R, Huang H, et al. Quantification of brain maturation and growth patterns in C57BL/6J mice via computational neuroanatomy of diffusion tensor images. Cereb Cortex 2009;19(3):675–87. 10.1093/cercor/bhn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrnes EM, Vassoler FM. Modeling prenatal opioid exposure in animals: Current findings and future directions. Front Neuroendocrinol 2018;51(August 2017):1–13. 10.1016/j.yfrne.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton JW, Robie HC, Ling Z, Weese-Mayer DE, Carvey PM. The magnitude of brain dopamine depletion from prenatal cocaine exposure is a function of uterine position. Neurotoxicol Teratol 1998;20(4):373–82. 10.1016/S0892-0362(97)00143-8. [DOI] [PubMed] [Google Scholar]
- 33.Ehret G Infant rodent ultrasounds - A gate to the understanding of sound communication. Behav Genet 2005;35(1):19–29. 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- 34.Moles A, Kieffer BL, Amato FRD. Deficit in Attachment Behavior in Mice Lacking the μ -Opioid Receptor Gene. Science (80- ) 2004;304(June):1983–7. [DOI] [PubMed] [Google Scholar]
- 35.Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Dev Brain Res 1991;62:17–22. [DOI] [PubMed] [Google Scholar]
- 36.Barr GA, Wang S. Tolerance and withdrawal to chronic morphine treatment in the week-old rat pup. Eur J Pharmacol 1992;215(1):35–42. 10.1016/0014-2999(92)90605-4. [DOI] [PubMed] [Google Scholar]
- 37.Aston-Jones G, Kalivas PW. Brain Norepinephrine Rediscovered in Addiction Research. Biol Psychiatry 2008;63(11):1005–6. 10.1016/j.biopsych.2008.03.016.BRAIN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vathy I, Rimanoczy A, Eaton RC, Katay L. Modulation of catecholamine turnover rate in brain regions of rats exposed prenatally to morphine. Brain Res 1994;662(1–2):209–15. 10.1016/0006-8993(94)90814-1. [DOI] [PubMed] [Google Scholar]
- 39.Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol 1982;15(3):221–7. 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- 40.Zhang GH, Sweitzer SM. Neonatal morphine enhances nociception and decreases analgesia in young rats. Brain Res 2008;1199:82–90. 10.1016/j.brainres.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 41.Balter RE, Dykstra LA. Thermal sensitivity as a measure of spontaneous morphine withdrawal in mice. J Pharmacol Toxicol Methods 2013;67(3):162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas K, Chan G, Gelernter J, Arias A. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict Behav 2010;35(1):7–13. 10.1016/j.addbeh.2009.07.004.Adverse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmadalipour A, Sadeghzadeh J, Vafaei AA, Bandegi AR, Mohammadkhani R, Rashidy-Pour A. Effects of environmental enrichment on behavioral deficits and alterations in hippocampal BDNF induced by prenatal exposure to morphine in juvenile rats. Neuroscience 2015;305:372–83. 10.1016/j.neuroscience.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000;24(4):417–63. 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 45.Nicolas LB, Kolb Y, Prinssen EPM. A combined marble burying-locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol 2006;547(1–3):106–15. 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Belzung C The genetic basis of the pharmacological effects of anxiolytics: A review based on rodent models. Behav Pharmacol 2001;12(6–7):451–60. 10.1097/00008877-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Balemans MCM, Huibers MMH, Eikelenboom NWD, et al. Reduced exploration, increased anxiety, and altered social behavior: Autistic-like features of euchromatin histone methyltransferase 1 heterozygous knockout mice. Behav Brain Res 2010;208(1):47–55. 10.1016/j.bbr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. Int J Psychophysiol 2009;72(1):67–73. 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez-Avila C, Covault J, Wand G, Zhang H, Gelernter J, Kranzler H. Population-specific effects of the Asn40Asp polymorphism at the mu-opioid receptor gene (OPRM1) on HPA-axis activation. Pharmacogenet Genomics 2007;17(12):1031–8. [DOI] [PubMed] [Google Scholar]
- 50.Gagin R, Kook N, Cohen E, Shavit Y. Prenatal morphine enhances morphine-conditioned place preference in adult rats. Pharmacol Biochem Behav 1997;58(2):525–8. 10.1016/S0091-3057(97)00281-5. [DOI] [PubMed] [Google Scholar]
- 51.Ramsey NF, Niesink RJM, Van Ree JM. Prenatal exposure to morphine enhances cocaine and heroin self-administration in drug-naive rats. Drug Alcohol Depend 1993;33(1):41–51. 10.1016/0376-8716(93)90032-L. [DOI] [PubMed] [Google Scholar]
- 52.Wu L, Chen J, Tao P, Huang EY. Attenuation by dextromethorphan on the higher liability to morphine-induced reward, caused by prenatal exposure of morphine in rat offspring. J Biomed Sci 2009;16:106. 10.1186/1423-0127-16-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci 1992;89(6):2046–50. 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 1992;12(2):483–8. 10.1016/j.brainres.2007.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serrano A, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Miarro J. Effects of DA D1 and D2 antagonists on the sensitisation to the motor effects of morphine in mice. Prog Neuro-Psychopharmacology Biol Psychiatry 2002;26(7–8):1263–71. 10.1016/S0278-5846(02)00265-8. [DOI] [PubMed] [Google Scholar]
- 56.Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res 1993;613(2):291–4. 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- 57.Chefer V, Shippenberg TS. Augmentation of Morphine-Induced Sensitization but Reduction in Morphine Tolerance and Reward in Delta-Opioid Receptor Knockout Mice. Neuropsychopharmacology 2009;34(4):887–98. 10.1016/j.dcn.2011.01.002.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spanagel R, Shippenberg TS. Modulation of morphine-induced sensitization by endogenous κ opioid systems in the rat. Neurosci Lett 1993;153(2):232–6. 10.1016/0304-3940(93)90329-J. [DOI] [PubMed] [Google Scholar]
- 59.Zubieta J, Smith YR, Bueller JA, et al. Mu-Opioid Receptor-Mediated Antinociceptive Responses Differ in Men and Women. J Neurosci 2002;22(12):5100–7. https://doi.org/22/12/5100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craft RM. Sex Differences in Analgesic, Reinforcing, Discriminative, and Motoric Effects of Opioids. Exp Clin Psychopharmacol 2008;16(5):376–85. 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Hsu M-S, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci 1998;18(7):2538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kraft WK, Adeniyi-Jones SC, Chervoneva I, et al. Buprenorphine for the Treatment of the Neonatal Abstinence Syndrome. N Engl J Med 2017;376(24):2341–8. 10.1056/NEJMoa1614835. [DOI] [PMC free article] [PubMed] [Google Scholar]


