Abstract
Near-infrared (NIR) light possesses many suitable optophysical properties for medical imaging including low autofluorescence, deep tissue penetration, and minimal light scattering, which together allow for high-resolution imaging of biological tissue. NIR imaging has proven to be a noninvasive and effective real-time imaging methodology that provides a high signal-to-background ratio compared to other potential optical imaging modalities. In response to this, the use of NIR imaging has been extensively explored in the field of immunotherapy. To date, NIR fluorescence imaging has successfully offered reliable monitoring of the localization, dynamics, and function of immune responses, which are vital in assessing not only the efficacy but also the safety of treatments to design immunotherapies optimally. This review aims to provide an overview of the current research on NIR imaging of the immune response. We expect that the use of NIR imaging will expand further in response to the recent success in cancer immunotherapy. We will also offer our insights on how this technology will meet rapidly growing expectations in the future.
Keywords: Near-infrared fluorophores, Immunoimaging, Immune responses, Immunotherapy
Graphical Abstract
1. Introduction
The optophysical parameters of near-infrared (NIR; 650-1,700 nm) light have been used extensively in the field of medicine for diagnostic and therapeutic purposes [1-4]. Amongst all the imaging modalities, NIR light is of particular importance due to its unique physical properties suitable for optical imaging of biological tissues [2,5]. As such, NIR fluorescence imaging in the clinical setting has improved therapeutic outcomes for patients [6,7]. NIR imaging could overcome the past challenges of conventional imaging methodologies including cytotoxicity to host cells, interference from background radiation, and high cost. It has been employed for real-time in vivo monitoring of the localization, dynamics, and function of immune responses, whereas conventional imaging methods fall short in these abilities. Since then, this imaging modality has provided valuable pieces of information on not only the efficacy, but also the safety of immunotherapy. In this review, we intend to provide an overview of the current research on the use of NIR imaging of the immune response.
2. Photophysical properties of NIR light
NIR light offers several clear advantages over other wavelengths for use in imaging of biological tissues. NIR light is nonionizing radiation and poses no risk of tissue damage or genotoxicity [6-9]. In addition, the preparation of molecular probes and detection hardware for NIR radiation is far less expensive than those for ionizing radiation [8,9]. The imager should be not only cost-efficient but also relatively simple to operate. NIR laser technology has been used in the field of medicine for the past three decades, and its safety and methodology are well established [10]. Imaging using NIR light is therefore low-cost and simple, making it readily applicable in clinics.
NIR-I (650-900 nm) (Fig. 1) has relatively low light scattering and absorption by biomolecules with deep tissue penetration compared to visible light (400-650 nm) (Fig. 2) [11-15]. In addition, due to the low autofluorescence of biologic tissues at these wavelengths, NIR-I imaging produces a high signal-to-background ratio (SBR) in living organisms compared to imaging using visible light. These characteristics together enable high sensitivity and resolution imaging in the NIR-I region. NIR imaging is therefore an attractive modality among potential optical imaging technologies. The second window of NIR (NIR-II), in the range of 1,000-1,700 nm (Fig. 1), shows similar characteristics to NIR-I but with less scattering in the neighboring tissue [11]. Use of this window has several advantageous properties, including extremely low autofluorescence, minimal tissue absorption and scattering allowing for deeper tissue penetration (Fig. 2), and high resolution and contrast in images compared to visible or NIR-I light [16-18]. Indeed, Antaris et al. synthesized small molecule fluorophores that emit photons in the NIR-II window and demonstrated them for molecular cancer imaging after conjugating with anti-EGFR affibody [19].
Fig. 1. NIR optical window.
The absorption spectrum of human skin showing the first (NIR-I) and second (NIR-II) biological windows. Note that NIR imaging allows low light absorption and scattering into tissue, favoring its application in biological tissues. Modified from Son et al. [5] with permission from Elsevier.
Fig. 2. NIR fluorescence imaging of biological tissue.
NIR light has deeper tissue penetration and lower background fluorescence than visible light. The detection depths with the concurrent optical imaging equipment typically range from millimeters with NIR fluorescence to micrometers with visible-range fluorescence. Modified from Owens et al. [11] with permission from John-Wiley.
3. Labeling tools for NIR imaging
To study the effect of immunotherapy, noninvasive and real-time visualization and subsequent analysis of responses from critical players in the immune system are often crucial. To this end, a variety of fluorescent probes from small molecules to nanoparticles have been developed for use in NIR imaging of cancer and immunotherapy (Fig. 3). The basic principle of molecular imaging probe development is to make them biocompatible and nonimmunogenic for use in biomedical applications suitable for clinical imaging of immune cells in order to emerge to meet this ever-increasing demand.
Fig. 3. NIR imaging in cancer immunotherapy.
Small-molecule fluorophores, nanoparticles, and targeted and activatable probes as labeling tools have been developed for the NIR window, which can directly label immune cells including T cells, B cells, macrophages, dendritic cells (DC), and natural killer (NK) cells, subsequently targeting the cancer cell and ultimately playing a critical role in cancer immunotherapy.
3.1. Small-molecule fluorophores
Small molecule fluorophores have several advantages over other forms of fluorescent probes. These agents have well-established routes of synthesis, well-defined molecular structures, high batch-to-batch consistency, and an easy means of maintaining purity that can be difficult to achieve in other probes [19-21].
For NIR-I imaging, small molecule fluorophores including polymethine cyanines, phthalocyanines, porphyrin derivatives, squaraine derivatives, BODIPY analogs, benzo[c]heterocycles, and xanthenes are commonly used as labeling probes [22]. The most representative NIR fluorophore is indocyanine green (ICG), despite of its undesirable optical properties for in vivo imaging including short blood circulation, serum instability, high liver uptake, and nontargetability [1]. Since ICG is the only NIR fluorophore approved by the US Food and Drug Administration (FDA) for clinical NIR imaging, it has been extensively used for monitoring cardiac output, hepatic function, and retinal angiography without appreciable adverse effects [23]. Tetrapyrrole-based compounds, such as porphyrins, chlorins, benzochlorins, phthalocyanines, and expanded porphyrins, have been widely used as photosensitizers for photodynamic therapy (PDT) [24]. These PDT agents are also used for diagnostic purposes in humans because of their fluorescent optical properties in both the visible and NIR regions [25].
The development of small organic molecule fluorophores for NIR-II imaging is an emerging technology [26]. Several types of fluorophores with relatively favorable toxicity profiles and excretion pharmacokinetics have been reported, but polymethine cyanines and donor-acceptor–donor (D-A-D) structures with a benzothiadiazole (BBTD) core have been mainly explored to date [26,27]. Additionally, long non-negligible emission tails of some NIR-I fluorophores reaching past 1,000 nm have been characterized [28], allowing the existing clinically approved fluorophore ICG to be suitable for NIR-II imaging of superficial organs including vessels and lymphatics [29]. Recently, the Chen group at Stanford tested ICG-based NIR-II imaging in the clinic and demonstrated its advantages, inferring that combining the NIR-I/II spectral windows could improve image-guided cancer therapy with higher accuracy and sensitivity of cancerous tissues [3]. This is the first demonstration of the integrated NIR-I/II multispectral imaging system in the clinical setting, which opens a new door for its broad future applications to many clinical disciplines in dermatology, ophthalmology, neurology, and gynecology, and oncology, along with its important impact to molecular imaging-based therapy [1].
3.2. Nanoprobes
Nanomaterials are another option for NIR fluorescence imaging [30]. There are a wide variety of nanoprobes and substances tuned to emit NIR fluorescence. These materials include, but are not limited to, semiconductor nanocrystals, quantum dots (QDs), metal nanoshells, and rare-earth-doped nanoparticles (RENPs) [31]. Small organic fluorophores can form a nanoprobe when they are self-assembled into a nanoparticle through nanoprecipitation [32]. Although this approach has several notable disadvantages including complexity of the design, high cost, difficulty in large-scale production, and, most importantly, the unknown long-term toxicity to biological systems [33], nanoparticles are generally more amenable to broader approaches for bioimaging and tissue targetability compared to small molecules.
Metal nanoshells including gold possess unique optophysical properties to produce NIR fluorescence depending on their geometry, a phenomenon known as surface plasmon resonance (SPR) [34]. However, there is a significant concern about their long-term toxicity which may be caused by inefficient clearance and accumulation in non-targeted organs. QDs, small semiconductor particles, show superior optical performance with exceptional stability [31]. However, the heavy metal components with a high propensity for toxicity present a large obstacle to the clinical application of this technology. RENPs consist of a core structure comprised of the host substance and dopants surrounded by a shell with the undoped host material [35]. RENPs show the outstanding optical properties of lanthanide ions, favorable biocompatibility, photostability and low cytotoxicity, and they are therefore promising candidates for NIR bioimaging [36]. Lanthanide nanostructures, such as Gd2O3: Er3+, and Yb3+, can serve as optomagnetic markers allowing the combination of optical imaging and magnetic resonance imaging (MRI).
New classes of emitters in the NIR-II range have been explored to improve SBR upon imaging. Carbon nanotubes, RENPs, and QDs have been developed with excellent photostability and relatively rapid clearance [37,38]. Semiconducting single-walled carbon nanotubes (SWNTs) are quasi-one-dimensional materials exhibiting photoluminescence in the NIR range [38,39]. SWNTs generally show poor solubility and biocompatibility, but surface modification has been explored to resolve these issues with some success toward clinical applications [38]. QDs are optically versatile and can be tuned to emit a wide range of fluorescence, which can extend into the NIR-II window [31,38]. RENPs show multicolor NIR-II emissions [36,38], offering a new promising tool for NIR-II bioimaging.
3.3. Targeted probes for NIR imaging
Targeted NIR imaging agents can generally be divided into the following categories: small molecule, antibodies and peptides, nanoparticles, and protein complex probes (Fig. 4) [40]. Antibodies and peptides are useful in designing targeted imaging probes due to their high selectivity for a specific target [41]. This characteristic is utilized to construct NIR fluorescent probes targeted to the desired biomarker by conjugating a fluorescent molecule to antibody fragments or small peptides [40,42]. This strategy gives NIR fluorescent molecules the ability to bind to a specific target. A small-molecule fluorophore is a popular choice for bioconjugation, but nanoparticles can also be attached to antibodies and peptides (Fig. 4).
Fig. 4. Building blocks for targeted NIR imaging probes.
Targeted fluorophores currently tested in clinical trials include small molecules, peptides, activatable probes, antibodies, and multimodal bioconjugates. Activatable fluorophores are initially quenched, but upon cleavage by enzymes, emit fluorescence. The relative sizes of these targeted fluorophores are displayed in the bottom panel. Reprinted from Zhang et al. [40] with permission from Springer-Nature.
Nanoparticle-based multifunctional agents encapsulating NIR fluorophores and ligands have been used for a theranostic approach in the context of cancer imaging and therapy [43,44]. Nanoparticles themselves can be delivered to tumor tissue by enhanced permeability and retention (EPR) [45], which is defined as passive targeting. Alternatively, they can be prepared to achieve active targeting to a specific target by coating them with antibodies or ligands such as poly(ethylene glycol) (PEG)-functionalized with cyclic RGD peptides (cRGD) [46].
3.4. Activatable or multimodal probes
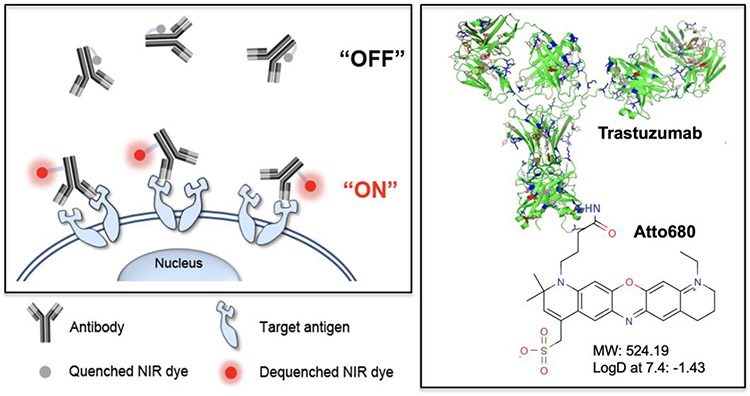
In order to improve the specificity or broaden their applications, activatable or multimodal probes have been also explored (Fig. 5). For example, upon interaction with their intended target, imaging agents undergo a chemical reaction including enzymatic cleavage and oxidation which switches the probe from a non- or weakly fluorescent form to a strongly fluorescent form [47]. Other NIR fluorescent constructs can be designed with quenched dyes and certain peptide sequences to image cells expressing specific proteases. The enzymes cleave the peptide linker, ceasing the quenching on the fluorescent label, and produce an NIR signal within the associated cells [48-50]. These strategies are proven to be highly sensitive due to the catalytic nature of signal generation upon encountering the target enzyme and especially effective for the development of NIR fluorophore-based multifunctional agents in areas of cancer targeting and imaging [47,51].
Fig. 5. Design of smart NIR fluorescence probes for improved specificity and SBR.
Antibody-fluorophore conjugates are designed for activatable fluorescence imaging of cancer cells. NIR fluorescence signals stay “off” at normal tissues but turn “on” after binding to the target antigens overexpressed on cancer cell. Shown are an example of an Ab-dye conjugate trastuzumab-ATTO680. Reprinted from Kim et al. [52] with permission from Ivyspring.
4. Imaging of Immune Components
There are two broad categories of fluorescent probes: genetic probes (fluorescent proteins) and chemical probes (fluorescent molecules). In this section, we focus on translatable chemical probes.
4.1. Antibodies
Antibodies are the proteins produced by B cells that show specific binding to antigens and subsequently activate other arms of the immune system to fight pathogen threats [53]. The biodistribution of antibodies usually depends on their targets. Antibodies are therefore frequently used to determine the biodistribution of biomarkers in vivo. NIR fluorophore-labeled antibodies are therefore most frequently used for NIR imaging of biological tissue (Table 1). Antibodies labeled with NIR small-molecule fluorophores or nanoparticles are commonly used for NIR imaging of biomarkers. In light of the rise of therapeutic approaches using monoclonal antibodies, it is critical to determine the biodistribution of therapeutic antibodies at tissue and cellular levels to understand their mode of action. Conventional bioanalytical techniques including ELISA and immunohistochemistry lack the direct detection and high spatial and temporal resolution of NIR imaging. As such, radiolabeling is still the standard method for bulk organ and tissue distributions [54]. NIR imaging overcame these challenges and enabled real-time high-resolution imaging. However, there is still an outstanding issue with the possible alteration of the binding ability as a result of the labeling procedure [55].
Table 1.
NIR fluorescence imaging of antibodies.
| Application (Target) | Contrast agent | Tracking time | Animal model | Ref |
|---|---|---|---|---|
| Trastuzumab, bevacizumab, Trastuzumab emtansine | IRDye800CW, Alexa Fluor 680 | 4-17 d | Xenograft tumor mice | [56] |
| Cetuximab | IRDye800CW | 1 d | Xenograft tumor mice | [57] |
| Cetuximab, bevacizumab | IRDye800CW | 3 d | Xenograft tumor mice | [58] |
| Bevacizumab, trastuzumab | IRDye800CW | 4 d | Xenograft tumor mice | [59] |
| MPDL3280A (PD-L1) | IRDye800CW | 3-5 d | Xenograft tumor mice | [60] |
| Cys-diabody A2 (PSCA) | sCy5 | 2-4 h | Xenograft tumor mice | [61] |
| Atezolizumab (PD-L1) | ErNPs | 3 d | Syngeneic and Xenograft tumor mice | [62] |
Cilliers et al. successfully labeled two clinical antibodies, Herceptin (trastuzumab) and Avastin (bevacizumab) with NIR fluorophores IRDye800CW or Alexa Fluor 680 at an optimum degree of labeling without altering antibody disposition for 4-17 days. This method was further proved to be useful for ex vivo determination of the biodistribution of Kadcyla (a conjugate of trastuzumab to a cytotoxic agent DM1) up to 7 days in a xenograft tumor model in mice [56]. Olivier et al. labeled a chimeric (mouse/human) monoclonal antibody against epidermal growth factor receptor (EGFR) cetuximab with IRDye800CW and 89Zr [57] The incremental uptake of fluorescence signals in xenograft tumor mice was observed 24 h post-injection of the NIR-antibody under NIR imaging. Both optical and positron emission tomography (PET) imaging gave consistent results in terms of tissue deposition of the antibody. However, Connor et al. found that IRDye800CW labeling via NHS chemistry significantly modified plasma pharmacokinetics and tissue deposition of the NIR fluorophore-labeled monoclonal antibody compared to the conventional radiolabeling [55]. Cohen et al. examined different labeling ratios of clinical monoclonal antibodies with IRDye800CW and concluded that alteration of biodistribution was observed when more than 1 equiv. of fluorophores were conjugated to antibodies [58]. Choi et al. confirmed this surface modulation using zwitterionic fluorophores conjugated on small peptides, proteins, and antibodies [63,64]. Considering the charge-to-mass ratio, when more than 2 small molecules are conjugated on the surface of a protein or an antibody, the fate of biodistribution and clearance could be changed significantly. Importantly, this method has been used to identify critical biomarkers in the field of cancer immunotherapy lately. Despite an improved overall survival with immune checkpoint inhibitors, the efficacy remains limited [65]. Programmed cell death-1 ligand-1 (PD-L1) expression by tumors has been shown to enrich for responses to anti-PD-L1 therapy for non-small cell lung carcinoma (NSCLC) and bladder cancer [66]. Chatterjee et al. conjugated IRDye800CW on a humanized, mouse and human cross-reactive PD-L1 antibody to detect the graded levels of PD-L1 expression in human tumor xenografts up to 120 h [60]. Zhong et al. further advanced this approach to conjugate anti-PD-L1 antibody with biocompatible rare-earth erbium-based nanoparticles (ErNPs) exhibiting downconversion luminescence at ~1,600 nm to realize NIR-IIb imaging with much improved SBR [62]. This group visualized T cells with lead sulfide (PbS) QDs to achieve noninvasive in vivo visualization of a critical biomarker and T cell population in the same NIR-IIb window in mouse models of breast and colon cancers at 3 d after injection. Remarkably, Zettlitz et al. established an universal dual-modality linker (DML) for conjugation of a fluorescent dye and an 18F-radioisotope to realize noninvasive, antibody-based dual-modality (PET/optical) imaging for noninvasive whole-body scanning and intraoperative fluorescence imaging [61]. Sequential immuno-PET and optical imaging 2-4 h after injection as a proof-of-principle experiment in prostate cancer xenograft model successfully showed feasibility of this strategy.
This approach can be used not only for diagnostic purposes but also as a therapeutic strategy. One of the remarkable advances in the use of antibodies with NIR fluorophores is photoimmunotherapy (PIT) (Fig. 6). PIT combines the cytotoxic effects of photodynamic therapy using a photo-sensitizer with antibodies specific to tumor antigens [67,68], which also allows for monitoring therapeutic effects under NIR imaging [69]. PIT which uses a photosensitizer activated in the NIR range (NIR-PIT) represents a new modality and has been shown to not only cause immediate cell death resulting in tumor shrinkage [70], but also induce host immune responses via the rapid release of immunogenic signals [71]. This approach has been tested in many preclinical settings and recently has been evaluated in clinical settings with promising results. In particular, monoclonal antibodies (mAb) targeting cancer antigen EGFRs conjugated to a photosensitizer such as IR700 or ICG successfully delayed tumor growth of breast [72], bladder [73], lung [74], pancreatic [75], prostate [76], and ovarian [77] cancer, as well as B-cell lymphoma [78,79] models in mice when activated with NIR light.
Fig. 6. Monitoring the effect of NIR-PIT.
Serial histology pictures show microdistribution of IR700 fluorescence in the tumor after repeated PIT. A) Only a few cells are fluorescent in the diffuse necrotic cells 1 h after the first PIT (top right). Scattered fluorescent micro-clusters of survived tumor cells (bottom; arrows) indicating proliferated cancer cells 1 d post PIT. B) Schematic drawing of a humanized antibody conjugate with IR700. Reprinted from Mitsunaga et al. [80] with permission from ACS Publications.
NIR-PIT is a highly flexible theranostic platform, as any antibody specific to a choice tumor antigen can be conjugated to an NIR photosensitizer. In addition, the fluorescence signal from the conjugate can be used for noninvasive imaging to monitor the effect of NIR-PIT. This approach is highly specific because antibody-targeted photosensitizers activate and induce cell death only in target cells, reducing some side effects of cancer treatment [80]. Phase 1 and 2 trials of NIR-PIT using the anti-EGFR antibody cetuximab, and the photo-absorber IR700, are ongoing in patients with recurrent head and neck cancer (https://clinicaltrials.gov/ct2/show/NCT02422979).
Targeting immune cells with PIT is another strategy to augment the anti-tumor immune response. It is well established that regulatory CD4+CD25+FOXP3+ regulatory T cells (Tregs) play a critical role in tumor immuno-evasion [81]. CD25-targeted NIR-PIT induced depletion of Tregs in tumors and enhanced anti-tumor response, resulting in tumor regression in a mouse model [82]. The same research group further demonstrated that combined CD44- and CD25-targeted NIR-PIT induced long-term antitumor immunity and prolonged survival compared with CD25-targeted NIR-PIT alone in both colon cancer and lung carcinoma models [83].
4.2. Vaccines
Vaccination is one of the most successful medical interventions to decrease morbidity and mortality caused by infectious diseases [84]. This modality administers bioactive molecules to challenge and enhance the immune response. To this end, efficient and timely delivery of the vaccine to the secondary lymphoid tissue, where the immune response is coordinated, is critical for optimal efficacy [85-87]. However, despite the clear advantages of NIR imaging in tracking biological molecules, there is a lack of methodology and a lack of tools to determine the biodistribution of the injected vaccines using NIR imaging (Table 2).
Table 2.
NIR fluorescence imaging of vaccines.
Lindsay et al. demonstrated that a dual radionuclide-NIR probe allows for quantitative, longitudinal, and noninvasive monitoring of the biodistribution of a model yellow fever prME mRNA vaccine labeled with 64Cu-DyLight 680 in the secondary lymphoid tissue by PET-CT and NIR imaging in monkeys [88]. In this method, the model vaccine was monitored up to 28 h by PET-CT while NIR imaging was used to extract relevant portions of organs that contained the RNA of interest. Future work could be done on whether NIR imaging could provide a noninvasive, low-cost, and high-resolution means of assessing translocation of the vaccine into lymph nodes. Katagiri et al. labeled model vaccines with a zwitterionic NIR fluorophore ZW800-1C, which showed minimal interaction with biological tissue and size-dependent uptake in the lymph nodes up to 72 h [89]. Panthani et al. used FDA-approved biocompatible poly(lactic-co-glycolic acid) (PLGA) microparticle-encapsulated QDs as a model mucosal vaccine. The QD vaccines were gavaged orally and imaged noninvasively in the gastrointestinal tract up to 48 h [90].
4.3. Immune cells
The recent clinical success of cancer immunotherapy highlights the importance of T cell imaging, in particular, noninvasive methods to assess the density and functions of tumor-infiltrating lymphocytes (TIL) to predict its therapeutic responses [91,92]. To date, ex vivo direct staining of isolated cells is a popular method for labeling lymphocytes (e.g., CAR-T cells) for NIR imaging [93]. However, this method has significant limitations due to unfavorable effects on lymphocytes during the isolation and labeling procedures. Therefore, a new methodology is highly desired to indirectly label T and B cells in vivo with an injectable probe.
4.3.1. T cells
T cells are major effector cells for combating foreign pathogens and cancer, as well as being responsible for peripheral tolerance to self- and non-harmful antigens [94]. T cell immunology gains more significant interest lately due to the success of cancer immunotherapy [91,92]. Tumor infiltrating T lymphocytes (TIL) density is a strong positive prognostic indicator for many types of tumors, and responses to immunotherapy preferentially occur in tumors with a preexisting antitumor T-cell response [91,92]. It is therefore critical to describe the in vivo behavior of T cells to understand the pathologic basis of diseases. NIR imaging has been extensively used for T cell imaging (Table 3).
Table 3.
NIR fluorescence imaging of T cells.
| Application (Target) | Contrast agent | Tracking time | Animal model | Ref |
|---|---|---|---|---|
| Tumor infiltrating T lymphocytes | DiR | 3 wk | Xenograft tumor mice | [95] |
| Model antigen vaccination | CIR38M | 7 d | C57BL/6 mice | [96] |
| Tumor infiltrating T lymphocytes | VivoTag 680 | 3 d | Xenograft tumor mice | [97] |
| Tumor infiltrating T lymphocytes | IRDye800CW | 3 d | Xenograft tumor mice | [98] |
| Antigen-specific T cells | NIR-797-isothiocyanate | 3 d | Syngeneic tumor model and bacterial infection in mice | [99] |
| Tumor infiltrating T lymphocytes | ErNP | 3 d | Xenograft tumor mice | [62] |
A cell-permeable lipophilic NIR fluorophore 1,1-dioctadecyltetramethyl indotricarbocyanine iodide (DiR) is frequently used for this purpose. Younis et al. used this approach and observed the fluorescence signal from ex vivo-stained T cells injected into a syngeneic mouse breast cancer model for 3 wk under a multi-spectral fluorescent imaging system [95]. No toxicity was detectable for up to 10 d after staining in vitro, suggesting that this approach could be durable and safe for the purpose of NIR imaging. Mellanby et al. used a tricarbocyanine N-triazole CIR38M with improved brightness and photostability compared to ICG or DiR to track post-transferred T cells responding to model vaccination in the secondary lymphoid tissues of mice for 7 d [96]. This method did not cause any significant functional alterations on T cells. CIR38M was also able to stain human T cells ex vivo without affecting their function for 24 h.
When diffused into cytotoxic T cells, an NHS ester of a (benz)indolium-derived far-red fluorescent probe VivoTag 680 (VT680) covalently bonded to cellular components which enabled longitudinal observation of T cells in xenografts of colon cancer for 3 d after ex vivo labeling with fluorescence-mediated molecular tomography (FMT) [97]. This labeling had no apparent toxicity or other adverse effects and elicited the same tumor growth delay as non-labeled control T cells with adoptive transfer therapy. Foster et al. used IRDye800CW for ex vivo T cell labeling, which also bears an NHS reactive group to couple with amino groups on proteins [98]. Human EBV-specific cytotoxic T cells stained with IRDye800CW showed normal viability, cytokine production and migration. The stained T cells also accumulated in a xenograft tumor model in mice that were imaged using in vivo NIR imaging up to 72 h. In order to compensate the limited penetration depth in NIR imaging, Zheng et al. applied a photoacoustic approach in bioimaging. The Nie group was able to observe gradual uptake of model cancer antigen (ovalbumin, OVA)-specific T cells labeled with NIR-797-isothiocyanate ex vivo up to 72 h into OVA-expressing breast cancer in mice with a peak signal at 12 h [99]. In fact, NIR imaging can be applied to deeply and noninvasively monitor the dynamic changes of immune cells in the lymph node and tumor.
For indirect labeling, the antibody-based approach has been extensively explored in the field of immuno-PET [100]. Similarly, Zhong et al. used anti-CD8α antibody targeting T cells conjugated with PbS QDs, which emits NIR-IIb fluorescence in murine breast and colon cancer models [62]. In this approach, the labeled antibody was intravenously injected and successfully detected tumor-infiltrating T cells by NIR-IIb imaging with high resolution and SBR at 3 d post-intravenous injection.
Recently, NIR imaging of T cell function has been increasingly studied. NIR macromolecular reporters, composed of a peptide-caged NIR signaling moiety linked with a hydrophilic PEG passivation chain, become fluorescent by specific granzyme B activation and can be used for real-time in vivo evaluation of the efficacy of immunotherapy. Especially, He et al. demonstrated that these probes passively targeted tumor and detected granzyme B with the NIR fluorescence at 707 nm in immunotherapeutics-treated breast tumor in mice [101]. Notably, the probes show high renal clearance which allowed noninvasive optical urinalysis and evaluations of immunoactivation with immunotherapy.
4.3.2. B cells
B cells and their progeny plasma cells produce antibodies responsible for humoral responses. These antibodies protect against infection, but also contribute to pathological tissue injury in autoimmunity [102]. B cell depletion has demonstrated successful treatment of non-Hodgkin's lymphoma and is beginning to see use in other B cell pathologies. Because of this, the ability to monitor B cells as they respond to an immune event is important for determining the efficacy and safety of B cells affecting drugs. Importantly, depletion of B cells is a clinically approved approach for the treatment of lymphoma [103]. However, the exploration of NIR imaging of B cells is relatively scarce compared to T cells (Table 4).
Table 4.
NIR fluorescence imaging of B cells.
Thorek et al. used an approach combining NIR imaging with superparamagnetic iron oxide (SPIO) nanoparticles to provide a means for whole body, longitudinal, and noninvasive imaging of B cells [104]. NIR fluorophore CellVue NIR815 (a lipophilic membrane dye) provided detectable signals from ex vivo-stained B cells injected into mice for up to 14 d using the whole body in vivo NIR imaging. In this study, a decrease of fluorescence signal was consistently observed following a model B cell depletion immunotherapy using an anti-CD79 antibody, confirming the specificity of this methodology. This method did not induce any detectable functional changes when tested in vitro.
Biologically inert semiconducting single-walled carbon nanotubes (SWNTs) with PEG functionalization conjugated to Rituxan (anti-CD20 antibody) have also been used for B cell imaging. Welsher et al. demonstrated that this method showed minimal non-specific binding and staining of non-targeted cells in vitro, but no in vivo imaging nor toxicity test was performed in this study [105].
4.3.3. Macrophages
Macrophages are immune cells that reside in local tissues and fulfill various functions in host defense, tissue homeostasis, and inflammatory response through phagocytosis and production of inflammatory cytokines [106]. Similar to T cells, tumor-associated macrophages (TAMs) have been gaining considerable attentions because of their predominant abundance in solid tumors and key roles in tumor progression [107,108]. Due to their significant roles in pathophysiology, macrophages are a frequent target to study.
NIR imaging has been extensively used to monitor the dynamics and function of macrophages (Table 5). A membrane-selective lipophilic carbocyanine dye (DiR) has been used for labeling macrophages ex vivo. Eisenblatter et al. demonstrated that the migration of ex vivo-labeled macrophages into inflammation induced by polyacrylamide gel pellets could be tracked up to 7 d after the injection of labeled cells using FMT [109]. Labeling did not induce appreciable changes in viability or the function of macrophages.
Table 5.
NIR fluorescence imaging of macrophages.
| Application (Target) | Contrast agent | Tracking time | Animal model | Ref |
|---|---|---|---|---|
| Migration into inflammation | DiR | 7 d | Polyacrylamide gel-induced inflammation | [109] |
| Brain cancer imaging | Silica iron oxide NPs + Cy5.5 | 1 d | Xenograft tumor mice | [110] |
| Accumulation in cardiac infarction | CLIO + VT750 | 1 d | Myocardial infarction or ischemia in mice | [111] |
| Migration into inflammation | F4/80 Ab + Cy5.5 | 3 d | Arthritis mouse model | [112] |
| Liver tumor imaging | TLR4 ab + ZW800-1C | 3 d | Syngeneic tumor mice | [113] |
| Mer imaging in colorectal cancer | UNC2025-SiR-COOH | 1 d | model of metastatic tumor in mice | [114] |
| Migration into inflammation | PNIPAM + IR750 + folate NPs | 1 d | LPS-induced tissue inflammation in mice | [115] |
| Migration into inflammation | CDnir7 | 18 h | LPS-induced tissue inflammation in mice | [116] |
Macrophages show strong phagocytic activities toward small particles; engulfing and digesting them in their phagosomes [117]. Taking advantage of this phenotype, macrophages have been labeled and visualized with fluorescent nanoparticles indirectly. Lee et al. synthesized NIR fluorescent (Cy5.5) silica-coated iron oxide nanoparticles (NF-SIONs) for this purpose [110]. NF-SIONs were about 37 nm in size and efficiently taken up by macrophages without apparent toxicity. Tumor-associated macrophages (TAMs) may occupy 30-50% of the whole cell population of a brain tumor [118,119], and can be used to identify the location of glioblastomas (GBM). Interestingly, NF-SIONs penetrated the blood-brain barrier after intravenous injection and specifically delineated an orthotopic xenograft model of GBM in mice up to 24 h under in vivo NIR imaging. Nahrendorf et al. intravenously injected a magneto-fluorescent iron oxide nanoparticle CLIO-VT750 for dual imaging in mice with cardiac infarction. This method detected an accumulation of inflammatory cells including macrophages and neutrophils in the infarction zone under FMT and MRI up to 24 h. The signal from simultaneously injected prosense-680, an activatable fluorescence sensor reporting on cathepsin activity colocalized with inflammatory cells, detected function of these inflammatory cells simultaneously in vivo under dual channel FMT [111].
Other indirect labeling methods have also been explored. NIR labeled fluorophore-antibody conjugates have been used for the monitoring of macrophage migration. Hansch et al. used a pan-macrophage marker F4/80 to visualize macrophages. Injection of anti-F4/80 monoclonal antibodies (mAb) labeled with Cy5.5 fluorophores revealed macrophage accumulation in the inflamed knee joints of a murine model of antigen- induced arthritis using in vivo NIR imaging up to 72 h [112]. Toll-like receptor 4 (TLR4) plays an important role in innate immunity and is highly expressed in macrophages [120]. Ji et al. labeled anti-TLR4 antibody with a zwitterionic NIR fluorophore with minimal interaction with biological tissue to avoid any possible interference from the fluorophore with the biodistribution of the mAb. Hepatocellular carcinoma is characterized by its inflammatory background and macrophages are closely related to its carcinogenesis and progression [121,122]. Upon intravenous injection of the conjugate, a strong signal was observed in TAM-enriched liver cancer grafts in a mouse model up to 72 h after injection (Fig. 7) [113].
Fig. 7. NIR fluorescence imaging of tumor-associated macrophages in hepatic tumor.
TLR4 antibody-conjugated ZW800-C was injected intravenously into mice of liver cancer model. Intraoperative tumor targeting and imaging were performed after a single intravenous injection of ZW800-conjugated TLR4 antibody under the real-time NIR fluorescence imaging system. Reprinted from Ji et al. [113] with permission from AME Publishing Company.
Proto-oncogene tyrosine-protein kinase Mer (MerTK) is a receptor tyrosine kinase influencing cell survival, migration, differentiation, and phagocytic activity. Importantly, Mer signaling influences tumor-associated leukocytes, including macrophages, to promote tumor progression [123]. Millar et al. developed a small molecule probe for NIR imaging of Mer based on its kinase inhibitor UNC2025 linked to silicon rhodamine carboxylate (SiR-COOH) fluorochrome [114]. An accumulation of a selective fluorescent molecular probe (MERi-SiR) in Mer+ TAMs in a model of metastatic murine colorectal cancer was confirmed by microscopic imaging, but not noninvasive NIR imaging.
Macrophages express elevated levels of the folate receptor (FR) when activated in inflamed tissues [124]. Low et al. synthesized an NIR fluorophore (IR750)-trapped poly(N-isopropyl acrylamide-co-styrene) nanoparticles and conjugated them with folate to target macrophages. The FR-targeting nanoprobes did not induce any toxicity in vitro and distinctively showed the fluorescence signal under NIR imaging in the LPS-induced inflamed tissues 24 h after intravenous administration [115]. Kang et al. took a diversity-oriented fluorescence library approach (DOFLA) [125] to find a macrophage-targeted NIR fluorophore CDnir7 with a heptamethine structure [116]. CDnir7 did not show any apparent toxicity in vitro, and upon intravenous injection, it detected infiltrating macrophages in a murine tissue inflammation model under FMT. The authors also demonstrated that CDnir7 could be used for photoacoustic imaging. However, compared to other labeling modalities such as 89Zr-labeled dextran nanoparticles [126], this method is rather short-lived (up to 18 h only).
4.3.4. Dendritic cells
Dendritic cells (DCs) play critical roles in both the innate and adaptive immune systems. In particular, they are known as the most versatile antigen-presenting cells, orchestrating immune responses against infectious diseases and cancer as well as maintaining tolerance to host and harmless antigens [127]. NIR imaging has been useful for analyzing the dynamics and function of DCs in vivo and informative for understanding DC biology in various settings including infection, allergy, autoimmune disease, transplantation, vaccination, and immunotherapy. To analyze DC migration, multiple imaging approaches have been used including MRI, PET and NIR imaging (Table 6).
Table 6.
NIR fluorescence imaging of dendritic cells.
Christian et al. conjugated a cell-permeable peptide Tat to NIR fluorescent polymersomes (Tat-NIR-emissive polymersomes) [134]. Ex vivo labeling of bone-marrow-derived DCs did not significantly affect the viability and function of the cells. Fluorescence lifetime imaging (FLIM) allowed a longitudinal observation of the accumulation of subcutaneously injected DCs into the popliteal lymph node over 33 d after the initial injection [128]. This approach was used in another study and found to have no significant effect on DC behavior compared to other labeling methods, including genetic or small fluorescence molecule approaches [135]. Noh et al. used CdSeTe/ZnS QD800 for ex vivo labeling of bone-marrow-derived DCs and tracked DC migration into lymph nodes up to 72 h after injection under in vivo NIR imaging. They similarly found that NIR cell labeling did not change the viability or antigen presentation capacity of DCs [129].
NIR imaging can be combined with other modes of imaging to create multimodal probe molecules for NIR imaging of DCs. Kim et al. combined gadolinium (Gd)-chelating MR probe and an NIR fluorophore, aza-BODIPY (boron-dipyrromethene) (AB-BCA) and successfully imaged migration of ex vivo-labeled bone marrow-derived DCs through the lymphatic vessels to the lymph nodes via MR and NIR imaging [130]. This labeling method did not influence obvious toxicity by in vitro testing. The fluorescence signal was observed under in vivo NIR imaging for 48 h after labeled cell injection. Chen et al. developed a dual-modal nanoprobe composed of superparamagnetic iron oxide (SPIO) and NIR fluorophore NIR797 to label bone-marrow derived DCs ex vivo. Migration of labeled DCs into lymph nodes was detected by both MRI and NIR imaging simultaneously for 5 d after cell injection [131]. Noh et al. further advanced this approach using multifunctional PLGA nanoparticles containing the MRI contrast agent iron oxide, NIR fluorophores (ICG), and the model antigen OVA [132]. Migration of ex vivo-labeled bone-marrow-derived DCs through lymph draining could be observed through NIR imaging, and accumulation of the DCs in the lymph nodes was detected by MRI imaging in response to specific antigen stimulation with OVA for 48 h. T cells isolated from treated mice showed high cytotoxicity against OVA-expressing EG7, indicating that the DCs loaded with the nanoparticles efficiently cross-presented. Heo et al. used PLGA nanoparticles containing both small interfering RNA (siRNA) for the knock-down of immune-suppressor gene (signal transducer and activator of transcription-3, STAT3) and an immune response modifier (imiquimod, R837) for the activation of DCs through the TLR7 along with OVA and ICG. DCs treated with the nanoparticles were similarly confirmed to prime CD8+T cells through cross-presentation, and migration of DCs to lymph nodes was detected by in vivo NIR imaging for 48 h [133].
4.3.5. Natural Killer Cells
Natural killer (NK) cells have gained attention as a promising candidate for future immunotherapeutic approaches due to their critical role in immune surveillance against viral infection [136] and cancer [137]. In response to this, a reliable imaging methodology to analyze their dynamics and function in vivo is desirable, and NIR imaging has been explored for this type of cell (Table 7).
Table 7.
NIR fluorescence imaging of natural killer cells.
Similar to DCs, ex vivo staining is a dominant method for this type of cell. Uong et al. stained ex vivo expanded NK cells from healthy donors with a small NIR fluorophore ESNF13 [138]. These NK cells were injected intravenously in human breast cancer xenograft mice and imaged using mini-FLARE NIR fluorescence imaging. The high fluorescent signal from the ESNF13-stained NK cells allowed for tracking of cells migrating to the tumor sites for 7 d. Lim et al. were able to observe the signal from NK92MI cells labeled with anti-human CD56 antibody-coated QD705 after intratumoral injection in a xenograft melanoma in mice under in vivo NIR imaging at least 24 h after injection [139]. Tavri et al. stained NK-92 cells with an NIR fluorophore DiD, which were engineered with a chimeric antigen receptor (CAR) for the epithelial cell adhesion molecule (EpCAM) expressed on prostate cancer, and intravenously injected them into a xenograft prostate cancer model in rats [140]. Progressive accumulation of DiD-labeled cells was observed up to 24 h after injection by NIR imaging. In these studies, the labeling procedures minimally affected the function of NK cells.
5. Perspectives
NIR fluorescence imaging not only displays high-sensitivity and specific real-time imaging of the biological system due to minimal autofluorescence, low light scattering, and low absorption in neighboring tissues, but also offers multispectral and multiplexing capability, making this technology suitable for bioimaging the inherently complicated immune system [113]. Accordingly, this imaging technique has been successfully used to track immune cells in vivo and contributed to improving our understanding of immune responses. Thus, monitoring the behavior and function of injected DCs via NIR imaging is critical to optimizing this cell-based therapeutic modality [141]. Since the first approval of DC-based cancer vaccines (i.e., Provenge) by the US FDA [142], DCs have played a pivotal role in cancer therapy due to their safety and immunogenicity. Consequently, detailed analyses of the dynamics and functions of DCs in vivo are desirable for studying infections, allergies, autoimmune diseases, transplantation, vaccination and immunotherapies. In particular, it has been shown that macrophages are highly associated with the initiation, progression, and metastasis of cancer [143]. Macrophages have also been implicated in the onset, progression, and symptoms of multifarious pathologic processes, such as the formation of malignant tumors and inflammatory diseases [106,144]. Due to its importance in many pathological conditions, the development of NIR imaging for these types of cells has been expanding. In response to the rise of cancer immunotherapy, the importance of an imaging methodology is expected to increase further. Similarly, considering the important roles of NK cells in immunosurveillance against cancer, this type of cells also represents a potentially ideal target for cancer immunotherapy. Further development of NIR imaging of NK cells is therefore warranted.
Nevertheless, there are two unsolved questions remined in the field: 1) Clinical imaging systems that can provide clinicians fast answers in the OR with high temporal and spatial resolution noninvasively for image-guided interventions, 2) clinical contrast agents that can be used in the human body with specificity and safety in the clinical setting [145,146]. To this end, novel imaging modalities are increasingly emerging, such as NIR-II fluorescence imaging [1,3], photoacoustic imaging [49,99], and afterglow imaging [147,148] to attack the first unsolved question. As discussed above, these imaging modalities have great promise as an advanced noninvasive molecular imaging tool with high sensitivity levels in the body. Additionally, due to the limited penetration of optical imaging, combination approaches including MRI-NIR and PET-NIR using multimodal probes would be necessary for noninvasive deep tissue imaging [5].
However, the latter is more serious. Since ICG is currently the only FDA-approved fluorophore in the NIR window, novel fluorophores in the NIR-II window showing superior resolution and sensitivity over the visible-NIR-I window are desperately needed to provide deep anatomical and functional features [11]. Additionally, we expect that more classes of multifunctional NIR fluorophores will emerge for the target-specific treatment of human diseases. However, the feasibility of applying this approach in the clinic, e.g. biocompatibility, nonimmunogenicity, and safety, needs to be fully explored, as many nanoparticle-based imaging probes used in this technology raise concerns about unknown long-term toxicity. Most works carefully examined the toxicity of probes in vitro, but safety assessment on long-term accumulation and toxicity upon in vivo application of nanoprobes is difficult using the current system. In order to move these candidate nanoprobes into clinical practice, a standardized guideline and reliable methodology for safety evaluation should be established. Despite the clear advantages of NIR imaging in tracking biological molecules, the methodology to determine the biodistribution of vaccines based on NIR imaging is surprisingly limited. Since the current gold standard radiologic method cannot reveal the detail of immune responses and immune cell dynamics, further exploration of this technology in this field is warranted. Noninvasive, high-resolution, real-time NIR imaging of vaccines and immunotherapeutics would provide a critical piece of information on how the immune system is primed and ultimately develops protective immune responses from vaccination or induces immunological tolerance with immunotherapy.
Acknowledgments
We thank Wesley Stiles for manuscript editing. This study was supported by the US NIH grants NIAID #R01AI105131 and #R21AI144103. This work was also supported by the Personnel Training Specialized Research Foundation of the Second Affiliated Hospital of Xi’an Jiaotong University #RC(GG)201803, Massachusetts General Hospital Executive Committee On Research (ECOR) Interim Support Funding, Natural Science Basic Research Program of Shaanxi #2020JZ-41, the Joint Research Project for Outstanding Research Institutions funded by the Gimhae Industry Promotion and Biomedical Foundation, and the Creative Materials Discovery Program through the National Research Foundation of Korea (2019M3D1A1078938).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Uncategorized References
- [1].Choi HS, Kim HK, Multispectral image-guided surgery in patients, Nat Biomed Eng, 4 (2020) 245–246. [DOI] [PubMed] [Google Scholar]
- [2].Owens EA, Henary M, El Fakhri G, Choi HS, Tissue-Specific Near-Infrared Fluorescence Imaging, Acc Chem Res, 49 (2016) 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, Su S, Sun X, Shi X, Li C, Zhou T, Zhang Y, Chi C, He P, Xia X, Chen Y, Gambhir SS, Cheng Z, Tian J, First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows, Nat Biomed Eng, 4 (2020) 259–271. [DOI] [PubMed] [Google Scholar]
- [4].Kang H, Hu S, Cho MH, Hong SH, Choi Y, Choi HS, Theranostic Nanosystems for Targeted Cancer Therapy, Nano Today, 23 (2018) 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Son J, Yi G, Yoo J, Park C, Koo H, Choi HS, Light-responsive nanomedicine for biophotonic imaging and targeted therapy, Adv Drug Deliv Rev, 138 (2019) 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Boer E, Harlaar NJ, Taruttis A, Nagengast WB, Rosenthal EL, Ntziachristos V, van Dam GM, Optical innovations in surgery, Br J Surg, 102 (2015) e56–72. [DOI] [PubMed] [Google Scholar]
- [7].Hussain T, Nguyen QT, Molecular imaging for cancer diagnosis and surgery, Adv Drug Deliv Rev, 66 (2014) 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He X, Gao J, Gambhir SS, Cheng Z, Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges, Trends Mol Med, 16 (2010) 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gioux S, Choi HS, Frangioni JV, Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation, Mol Imaging, 9 (2010) 237–255. [PMC free article] [PubMed] [Google Scholar]
- [10].Kashiwagi S, Brauns T, Gelfand J, Poznansky MC, Laser vaccine adjuvants. History, progress, and potential, Hum Vaccin Immunother, 10 (2014) 1892–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Owens EA, Lee S, Choi J, Henary M, Choi HS, NIR fluorescent small molecules for intraoperative imaging, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 7 (2015) 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Das P, Santos S, Park GK, Hoseok I, Choi HS, Real-Time Fluorescence Imaging in Thoracic Surgery, Korean J Thorac Cardiovasc Surg, 52 (2019) 205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim T, O'Brien C, Choi HS, Jeong MY, Fluorescence molecular imaging systems for intraoperative image-guided surgery, Appl Spectrosc Rev, 53 (2018) 349–359. [Google Scholar]
- [14].Park GK, Hoseok s., Kim GS, Hwang NS, Choi HS, Optical spectroscopic imaging for cell therapy and tissue engineering, Appl Spectrosc Rev, 53 (2018) 360–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sajedi S, Sabet H, Choi HS, Intraoperative biophotonic imaging systems for image-guided interventions, Nanophotonics, 8 (2019) 99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ding B, Xiao Y, Zhou H, Zhang X, Qu C, Xu F, Deng Z, Cheng Z, Hong X, Polymethine Thiopyrylium Fluorophores with Absorption beyond 1000 nm for Biological Imaging in the Second Near-Infrared Subwindow, J Med Chem, 62 (2019) 2049–2059. [DOI] [PubMed] [Google Scholar]
- [17].Zhou B, Hu Z, Jiang Y, Zhong C, Sun Z, Sun H, Theoretical exploitation of acceptors based on benzobis(thiadiazole) and derivatives for organic NIR-II fluorophores, Phys Chem Chem Phys, 20 (2018) 19759–19767. [DOI] [PubMed] [Google Scholar]
- [18].Huang J, Xie C, Zhang X, Jiang Y, Li J, Fan Q, Pu K, Renal-clearable Molecular Semiconductor for Second Near-Infrared Fluorescence Imaging of Kidney Dysfunction, Angew Chem Int Ed Engl, 58 (2019) 15120–15127. [DOI] [PubMed] [Google Scholar]
- [19].Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, Zhang X, Yaghi OK, Alamparambil ZR, Hong X, Cheng Z, Dai H, A small-molecule dye for NIR-II imaging, Nat Mater, 15 (2016) 235–242. [DOI] [PubMed] [Google Scholar]
- [20].Guo Z, Park S, Yoon J, Shin I, Recent progress in the development of near-infrared fluorescent probes for bioimaging applications, Chem Soc Rev, 43 (2014) 16–29. [DOI] [PubMed] [Google Scholar]
- [21].Qian K, Qu C, Ma X, Chen H, Kandawa-Schulz M, Song W, Miao W, Wang Y, Cheng Z, Tuning the near infrared II emitting wavelength of small molecule dyes by single atom alteration, Chem Commun (Camb), 56 (2020) 523–526. [DOI] [PubMed] [Google Scholar]
- [22].Escobedo JO, Rusin O, Lim S, Strongin RM, NIR dyes for bioimaging applications, Curr Opin Chem Biol, 14 (2010) 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alander JT, Kaartinen I, Laakso A, Patila T, Spillmann T, Tuchin VV, Venermo M, Valisuo P, A review of indocyanine green fluorescent imaging in surgery, Int J Biomed Imaging, 2012 (2012) 940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ochsner M, Photophysical and photobiological processes in the photodynamic therapy of tumours, Journal of Photochemistry and Photobiology B: Biology, 39 (1997) 1–18. [DOI] [PubMed] [Google Scholar]
- [25].Zhu H, Cheng P, Chen P, Pu K, Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics, Biomater Sci, 6 (2018) 746–765. [DOI] [PubMed] [Google Scholar]
- [26].Zhu S, Tian R, Antaris AL, Chen X, Dai H, Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery, Adv Mater, (2019) e1900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang S, Fan Y, Li D, Sun C, Lei Z, Lu L, Wang T, Zhang F, Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing, Nat Commun, 10 (2019) 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhu S, Yung BC, Chandra S, Niu G, Antaris AL, Chen X, Near-Infrared-II (NIR-II) Bioimaging via Off-Peak NIR-I Fluorescence Emission, Theranostics, 8 (2018) 4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].He S, Song J, Qu J, Cheng Z, Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics, Chem Soc Rev, 47 (2018) 4258–4278. [DOI] [PubMed] [Google Scholar]
- [30].Li J, Pu K, Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer, Acc Chem Res, 53 (2020) 752–762. [DOI] [PubMed] [Google Scholar]
- [31].Altinoglu EI, Adair JH, Near infrared imaging with nanoparticles, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2 (2010) 461–477. [DOI] [PubMed] [Google Scholar]
- [32].Caponetti V, Trzcinski JW, Cantelli A, Tavano R, Papini E, Mancin F, Montalti M, Self-Assembled Biocompatible Fluorescent Nanoparticles for Bioimaging, Front Chem, 7 (2019) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Soares S, Sousa J, Pais A, Vitorino C, Nanomedicine: Principles, Properties, and Regulatory Issues, Front Chem, 6 (2018) 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zheng YB, Kiraly B, Weiss PS, Huang TJ, Molecular plasmonics for biology and nanomedicine, Nanomedicine (Lond), 7 (2012) 751–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Labrador-Paez L, Ximendes EC, Rodriguez-Sevilla P, Ortgies DH, Rocha U, Jacinto C, Martin Rodriguez E, Haro-Gonzalez P, Jaque D, Core-shell rare-earth-doped nanostructures in biomedicine, Nanoscale, 10 (2018) 12935–12956. [DOI] [PubMed] [Google Scholar]
- [36].Hemmer E, Venkatachalam N, Hyodo H, Hattori A, Ebina Y, Kishimoto H, Soga K, Upconverting and NIR emitting rare earth based nanostructures for NIR-bioimaging, Nanoscale, 5 (2013) 11339–11361. [DOI] [PubMed] [Google Scholar]
- [37].Cai Y, Wei Z, Song C, Tang C, Han W, Dong X, Optical nano-agents in the second near-infrared window for biomedical applications, Chem Soc Rev, 48 (2019) 22–37. [DOI] [PubMed] [Google Scholar]
- [38].Ding F, Fan Y, Sun Y, Zhang F Beyond 1000 nm Emission Wavelength: Recent Advances in Organic and Inorganic Emitters for Deep-Tissue Molecular Imaging, Adv Healthc Mater, (2019) e1900260. [DOI] [PubMed] [Google Scholar]
- [39].Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, Cooke JP, Dai H, Multifunctional in vivo vascular imaging using near-infrared II fluorescence, Nat Med, 18 (2012) 1841–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang RR, Schroeder AB, Grudzinski JJ, Rosenthal EL, Warram JM, Pinchuk AN, Eliceiri KW, Kuo JS, Weichert JP, Beyond the margins: real-time detection of cancer using targeted fluorophores, Nat Rev Clin Oncol, 14 (2017) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaur S, Venktaraman G, Jain M, Senapati S, Garg PK, Batra SK, Recent trends in antibody-based oncologic imaging, Cancer letters, 315 (2012) 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rao J, Dragulescu-Andrasi A, Yao H, Fluorescence imaging in vivo: recent advances, Curr Opin Biotechnol 18 (2007) 17–25. [DOI] [PubMed] [Google Scholar]
- [43].Vats M, Mishra SK, Baghini MS, Chauhan DS, Srivastava R, De A, Near infrared fluorescence imaging in nano-therapeutics and photo-thermal evaluation, Int J Mol Sci, 18 (2017) 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K, Nanomaterials for theranostics: recent advances and future challenges, Chem Rev, 115 (2015) 327–394. [DOI] [PubMed] [Google Scholar]
- [45].Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, Lammers T, Tumor targeting via EPR: Strategies to enhance patient responses, Adv Drug Deliv Rev, 130 (2018) 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV, Design considerations for tumour-targeted nanoparticles, Nat Nanotechnol, 5 (2010) 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Luo S, Zhang E, Su Y, Cheng T, Shi C, A review of NIR dyes in cancer targeting and imaging, Biomaterials, 32 (2011) 7127–7138. [DOI] [PubMed] [Google Scholar]
- [48].Weissleder R, Tung C-H, Mahmood U, Bogdanov A Jr, In vivo imaging of tumors with protease-activated near-infrared fluorescent probes, Nature biotechnology, 17 (1999) 375. [DOI] [PubMed] [Google Scholar]
- [49].Huang J, Pu K, Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging, Angew Chem Int Ed Engl, (2020) in press. [DOI] [PubMed] [Google Scholar]
- [50].Li J, Cui D, Jiang Y, Huang J, Cheng P, Pu K, Near-Infrared Photoactivatable Semiconducting Polymer Nanoblockaders for Metastasis-Inhibited Combination Cancer Therapy, Adv Mater, 31 (2019) e1905091. [DOI] [PubMed] [Google Scholar]
- [51].Tung CH, Fluorescent peptide probes for in vivo diagnostic imaging, Peptide Science, 76 (2004) 391–403. [DOI] [PubMed] [Google Scholar]
- [52].Kim H, Choi HS, Kim SK, Lee BI, Choi Y, Antigen-responsive molecular sensor enables real-time tumor-specific imaging, Theranostics, 7 (2017) 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sela-Culang I, Kunik V, Ofran Y, The structural basis of antibody-antigen recognition, Front Immunol, 4 (2013) 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Eckelman WC, Paik CH, Reba RC, Radiolabeling of antibodies, Cancer Res, 40 (1980) 3036–3042. [PubMed] [Google Scholar]
- [55].Conner KP, Rock BM, Kwon GK, Balthasar JP, Abuqayyas L, Wienkers LC, Rock DA, Evaluation of near infrared fluorescent labeling of monoclonal antibodies as a tool for tissue distribution, Drug Metab Dispos, 42 (2014) 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cilliers C, Nessler I, Christodolu N, Thurber GM, Tracking Antibody Distribution with Near-Infrared Fluorescent Dyes: Impact of Dye Structure and Degree of Labeling on Plasma Clearance, Mol Pharm, 14 (2017) 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Oliveira S, Cohen R, Walsum MS, van Dongen GA, Elias SG, van Diest PJ, Mali W, van Bergen En Henegouwen PM, A novel method to quantify IRDye800CW fluorescent antibody probes ex vivo in tissue distribution studies, EJNMMI Res, 2 (2012) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cohen R, Stammes MA, de Roos IH, Stigter-van Walsum M, Visser GW, van Dongen GA, Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets, EJNMMI Res, 1 (2011) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL, Schroder CP, Kosterink JG, Lub-de Hoog MN, de Vries EG, Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies, J Nucl Med, 52 (2011) 1778–1785. [DOI] [PubMed] [Google Scholar]
- [60].Chatterjee S, Lesniak WG, Gabrielson M, Lisok A, Wharram B, Sysa-Shah P, Azad BB, Pomper MG, Nimmagadda S, A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors, Oncotarget, 7 (2016) 10215–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zettlitz KA, Waldmann CM, Tsai WK, Tavare R, Collins J, Murphy JM, Wu AM, A Dual-Modality Linker Enables Site-Specific Conjugation of Antibody Fragments for (18)F-Immuno-PET and Fluorescence Imaging, J Nucl Med, 60 (2019) 1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhong Y, Ma Z, Wang F, Wang X, Yang Y, Liu Y, Zhao X, Li J, Du H, Zhang M, Cui Q, Zhu S, Sun Q, Wan H, Tian Y, Liu Q, Wang W, Garcia KC, Dai H, In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles, Nat Biotechnol, 37 (2019) 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Choi HS, Nanoparticle assembly: building blocks for tumour delivery, Nature nanotechnology, 9 (2014) 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV, Targeted zwitterionic near-infrared fluorophores for improved optical imaging, Nature biotechnology, 31 (2013) 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nakamura Y, Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events, Front Med (Lausanne), 6 (2019) 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tunger A, Sommer U, Wehner R, Kubasch AS, Grimm MO, Bachmann MP, Platzbecker U, Bornhauser M, Baretton G, Schmitz M, The Evolving Landscape of Biomarkers for Anti-PD-1 or Anti-PD-L1 Therapy, J Clin Med, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kobayashi H, Choyke PL, Super enhanced permeability and retention (SUPR) effects in tumors following near infrared photoimmunotherapy, Nanoscale, 8 (2016) 12504–12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H, Cancer cell–selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules, Nature medicine, 17 (2011) 1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Maruoka Y, Nagaya T, Nakamura Y, Sato K, Ogata F, Okuyama S, Choyke PL, Kobayashi H, Evaluation of Early Therapeutic Effects after Near-Infrared Photoimmunotherapy (NIR-PIT) Using Luciferase-Luciferin Photon-Counting and Fluorescence Imaging, Mol Pharm, 14 (2017) 4628–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mitsunaga M, Nakajima T, Sano K, Kramer-Marek G, Choyke PL, Kobayashi H, Immediate in vivo target-specific cancer cell death after near infrared photoimmunotherapy, BMC Cancer, 12 (2012) 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ogawa M, Tomita Y, Nakamura Y, Lee MJ, Lee S, Tomita S, Nagaya T, Sato K, Yamauchi T, Iwai H, Kumar A, Haystead T, Shroff H, Choyke PL, Trepel JB, Kobayashi H, Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity, Oncotarget, 8 (2017) 10425–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nagaya T, Sato K, Harada T, Nakamura Y, Choyke PL, Kobayashi H, Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen, PLoS One, 10 (2015) e0136829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Nagaya T, Okuyama S, Ogata F, Maruoka Y, Knapp DW, Karagiannis SN, Fazekas-Singer J, Choyke PL, LeBlanc AK, Jensen-Jarolim E, Kobayashi H, Near infrared photoimmunotherapy targeting bladder cancer with a canine anti-epidermal growth factor receptor (EGFR) antibody, Oncotarget, 9 (2018) 19026–19038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nakamura Y, Ohler ZW, Householder D, Nagaya T, Sato K, Okuyama S, Ogata F, Daar D, Hoa T, Choyke PL, Kobayashi H, Near Infrared Photoimmunotherapy in a Transgenic Mouse Model of Spontaneous Epidermal Growth Factor Receptor (EGFR)-expressing Lung Cancer, Mol Cancer Ther, 16 (2017) 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Aung W, Tsuji AB, Sugyo A, Takashima H, Yasunaga M, Matsumura Y, Higashi T, Near-infrared photoimmunotherapy of pancreatic cancer using an indocyanine green-labeled anti-tissue factor antibody, World J Gastroenterol, 24 (2018) 5491–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Kobayashi H, Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) Antibody, Mol Cancer Res, 15 (2017) 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sato K, Hanaoka H, Watanabe R, Nakajima T, Choyke PL, Kobayashi H, Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer, Mol Cancer Ther, 14 (2015) 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Kobayashi H, Near infrared photoimmunotherapy of B-cell lymphoma, Mol Oncol, 10 (2016) 1404–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Heryanto YD, Hanaoka H, Nakajima T, Yamaguchi A, Tsushima Y, Applying near-infrared photoimmunotherapy to B-cell lymphoma: comparative evaluation with radioimmunotherapy in tumor xenografts, Ann Nucl Med, 31 (2017) 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H, Near-infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate, Bioconjug Chem, 23 (2012) 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sakaguchi S, Miyara M, Costantino CM, Hafler DA, FOXP3+ regulatory T cells in the human immune system, Nat Rev Immunol, 10 (2010) 490–500. [DOI] [PubMed] [Google Scholar]
- [82].Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, Hasegawa Y, Kobayashi H, Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy, Sci Transl Med, 8 (2016) 352ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Maruoka Y, Furusawa A, Okada R, Inagaki F, Fujimura D, Wakiyama H, Kato T, Nagaya T, Choyke PL, Kobayashi H, Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models, Cancer Immunol Res, 8 (2020) 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rappuoli R, Pizza M, Del Giudice G, De Gregorio E, Vaccines, new opportunities for a new society, Proc Natl Acad Sci U S A, 111 (2014) 12288–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tozuka M, Oka T, Jounai N, Egawa G, Ishii KJ, Kabashima K, Takeshita F, Efficient antigen delivery to the draining lymph nodes is a key component in the immunogenic pathway of the intradermal vaccine, J Dermatol Sci, 82 (2016) 38–45. [DOI] [PubMed] [Google Scholar]
- [86].Finco O, Rappuoli R, Designing vaccines for the twenty-first century society, Front Immunol, 5 (2014) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rappuoli R, Mandl CW, Black S, De Gregorio E, Vaccines for the twenty-first century society, Nat Rev Immunol, 11 (2011) 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lindsay KE, Bhosle SM, Zurla C, Beyersdorf J, Rogers KA, Vanover D, Xiao P, Arainga M, Shirreff LM, Pitard B, Baumhof P, Villinger F, Santangelo PJ, Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging, Nat Biomed Eng, 3 (2019) 371–380. [DOI] [PubMed] [Google Scholar]
- [89].Katagiri W, Lee JH, Tetrault MA, Kang H, Jeong S, Evans CL, Yokomizo S, Santos S, Jones C, Hu S, Fakhri GE, Tsukada K, Choi HS, Kashiwagi S, Real-Time Imaging of Vaccine Biodistribution Using Zwitterionic NIR Nanoparticles, Adv Healthc Mater, (2019) e1900035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Panthani MG, Khan TA, Reid DK, Hellebusch DJ, Rasch MR, Maynard JA, Korgel BA, In vivo whole animal fluorescence imaging of a microparticle-based oral vaccine containing (CuInSe(x)S(2-x))/ZnS core/shell quantum dots, Nano Lett, 13 (2013) 4294–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Trujillo JA, Sweis RF, Bao R, Luke JJ, T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection, Cancer Immunol Res, 6 (2018) 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Havel JJ, Chowell D, Chan TA, The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy, Nat Rev Cancer, 19 (2019) 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Brown CE, Mackall CL, CAR T cell therapy: inroads to response and resistance, Nat Rev Immunol, 19 (2019) 73–74. [DOI] [PubMed] [Google Scholar]
- [94].Kumar BV, Connors TJ, Farber DL, Human T Cell Development, Localization, and Function throughout Life, Immunity, 48 (2018) 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Youniss FM, Sundaresan G, Graham LJ, Wang L, Berry CR, Dewkar GK, Jose P, Bear HD, Zweit J, Near-infrared imaging of adoptive immune cell therapy in breast cancer model using cell membrane labeling, PLoS One, 9 (2014) e109162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mellanby RJ, Scott JI, Mair I, Fernandez A, Saul L, Arlt J, Moral M, Vendrell M, Tricarbocyanine N-triazoles: the scaffold-of-choice for long-term near-infrared imaging of immune cells in vivo, Chem Sci, 9 (2018) 7261–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Swirski FK, Berger CR, Figueiredo JL, Mempel TR, von Andrian UH, Pittet MJ, Weissleder R, A near-infrared cell tracker reagent for multiscopic in vivo imaging and quantification of leukocyte immune responses, PLoS One, 2 (2007) e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Foster AE, Kwon S, Ke S, Lu A, Eldin K, Sevick-Muraca E, Rooney CM, In vivo fluorescent optical imaging of cytotoxic T lymphocyte migration using IRDye800CW near-infrared dye, Appl Opt, 47 (2008) 5944–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zheng S, Li H, Lai K, Chen M, Fu G, Liu WH, Fu G, Nie L, Noninvasive photoacoustic and fluorescent tracking of optical dye labeled T cellular activities of diseased sites at new depth, J Biophotonics, 11 (2018) e201800073. [DOI] [PubMed] [Google Scholar]
- [100].Tavare R, Escuin-Ordinas H, Mok S, McCracken MN, Zettlitz KA, Salazar FB, Witte ON, Ribas A, Wu AM, An Effective Immuno-PET Imaging Method to Monitor CD8-Dependent Responses to Immunotherapy, Cancer Res, 76 (2016) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].He S, Li J, Lyu Y, Huang J, Pu K, Near-Infrared Fluorescent Macromolecular Reporters for Real-Time Imaging and Urinalysis of Cancer Immunotherapy, J Am Chem Soc, 142 (2020) 7075–7082. [DOI] [PubMed] [Google Scholar]
- [102].Tarlinton D, B cells still front and centre in immunology, Nat Rev Immunol, 19 (2019) 85–86. [DOI] [PubMed] [Google Scholar]
- [103].June CH, Sadelain M, Chimeric Antigen Receptor Therapy, N Engl J Med, 379 (2018) 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Thorek DL, Tsao PY, Arora V, Zhou L, Eisenberg RA, Tsourkas A, In vivo, multimodal imaging of B cell distribution and response to antibody immunotherapy in mice, PLoS One, 5 (2010) e10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Welsher K, Liu Z, Daranciang D, Dai H, Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules, Nano Lett, 8 (2008) 586–590. [DOI] [PubMed] [Google Scholar]
- [106].Varol C, Mildner A, Jung S Macrophages: development and tissue specialization, Annu Rev Immunol, 33 (2015) 643–675. [DOI] [PubMed] [Google Scholar]
- [107].Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS, N. Cancer Genome Atlas Research, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I, The Immune Landscape of Cancer, Immunity, 48 (2018) 812–830 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Engblom C, Pfirschke C, Pittet MJ, The role of myeloid cells in cancer therapies, Nat Rev Cancer, 16 (2016) 447–462. [DOI] [PubMed] [Google Scholar]
- [109].Eisenblatter M, Ehrchen J, Varga G, Sunderkotter C, Heindel W, Roth J, Bremer C, Wall A, In vivo optical imaging of cellular inflammatory response in granuloma formation using fluorescence-labeled macrophages, J Nucl Med, 50 (2009) 1676–1682. [DOI] [PubMed] [Google Scholar]
- [110].Lee C, Kim GR, Yoon J, Kim SE, Yoo JS, Piao Y, In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance, Sci Rep, 8 (2018) 11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, Figueiredo JL, Pittet MJ, Weissleder R, Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct, Circ Res, 100 (2007) 1218–1225. [DOI] [PubMed] [Google Scholar]
- [112].Hansch A, Frey O, Sauner D, Hilger I, Haas M, Malich A, Bräuer R, Kaiser WA, In vivo imaging of experimental arthritis with near- infrared fluorescence, Arthritis & Rheumatology, 50 (2004) 961–967. [DOI] [PubMed] [Google Scholar]
- [113].Ji Y, Wang Z, Bao K, Park GK, Kang H, Hu S, McDonald E, Kim MS, Kashiwagi S, Choi HS, Targeted molecular imaging of TLR4 in hepatocellular carcinoma using zwitterionic near-infrared fluorophores, Quant Imaging Med Surg, 9 (2019) 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Miller MA, Kim E, Cuccarese MF, Plotkin AL, Prytyskach M, Kohler RH, Pittet MJ, Weissleder R, Near infrared imaging of Mer tyrosine kinase (MERTK) using MERi-SiR reveals tumor associated macrophage uptake in metastatic disease, Chem Commun (Camb), 54 (2017) 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhou J, Tsai Y-T, Weng H, Baker DW, Tang L, Real time monitoring of biomaterial-mediated inflammatory responses via macrophage-targeting NIR nanoprobes, Biomaterials, 32 (2011) 9383–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kang NY, Park SJ, Ang XW, Samanta A, Driessen WH, Ntziachristos V, Vasquez KO, Peterson JD, Yun SW, Chang YT, A macrophage uptaking near-infrared chemical probe CDnir7 for in vivo imaging of inflammation, Chem Commun (Camb), 50 (2014) 6589–6591. [DOI] [PubMed] [Google Scholar]
- [117].Johnston RB Jr, Monocytes and macrophages, New England Journal of Medicine, 318 (1988) 747–752. [DOI] [PubMed] [Google Scholar]
- [118].Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H, The brain tumor microenvironment, Glia, 59 (2011) 1169–1180. [DOI] [PubMed] [Google Scholar]
- [119].Hambardzumyan D, Gutmann DH, Kettenmann H, The role of microglia and macrophages in glioma maintenance and progression, Nat Neurosci, 19 (2016) 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Vaure C, Liu Y, A comparative review of toll-like receptor 4 expression and functionality in different animal species, Front Immunol, 5 (2014) 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A, Morita M, Tsujitani S, Sakaguchi Y, Maehara Y, Role of tumor-associated macrophages in the progression of hepatocellular carcinoma, Surg Today, 42 (2012) 1–7. [DOI] [PubMed] [Google Scholar]
- [122].Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L, Preclinical and Clinical Therapeutic Strategies Affecting Tumor-Associated Macrophages in Hepatocellular Carcinoma, J Immunol Res, 2018 (2018) 7819520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vouri M, Hafizi S, TAM Receptor Tyrosine Kinases in Cancer Drug Resistance, Cancer Res, 77 (2017) 2775–2778. [DOI] [PubMed] [Google Scholar]
- [124].Low PS, Henne WA, Doorneweerd DD, Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases, Acc Chem Res, 41 (2008) 120–129. [DOI] [PubMed] [Google Scholar]
- [125].Kang NY, Ha HH, Yun SW, Yu YH, Chang YT, Diversity-driven chemical probe development for biomolecules: beyond hypothesis-driven approach, Chem Soc Rev, 40 (2011) 3613–3626. [DOI] [PubMed] [Google Scholar]
- [126].Keliher EJ, Yoo J, Nahrendorf M, Lewis JS, Marinelli B, Newton A, Pittet MJ, Weissleder R, 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging, Bioconjug Chem, 22 (2011) 2383–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Merad M, Sathe P, Helft J, Miller J, Mortha A, The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting, Annu Rev Immunol, 31 (2013) 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Christian NA, Benencia F, Milone MC, Li G, Frail PR, Therien MJ, Coukos G, Hammer DA, In vivo dendritic cell tracking using fluorescence lifetime imaging and near-infrared-emissive polymersomes, Mol Imaging Biol, 11 (2009) 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Noh YW, Lim YT, Chung BH, Noninvasive imaging of dendritic cell migration into lymph nodes using near-infrared fluorescent semiconductor nanocrystals, FASEB J, 22 (2008) 3908–3918. [DOI] [PubMed] [Google Scholar]
- [130].Kim E-J, Bhuniya S, Lee H, Kim HM, Shin WS, Kim JS, Hong KS, In vivo tracking of phagocytic immune cells using a dual imaging probe with gadolinium-enhanced MRI and near-infrared fluorescence, ACS Appl Mater Interfaces, 8 (2016) 10266–10273. [DOI] [PubMed] [Google Scholar]
- [131].Chen YC, Wen S, Shang SA, Cui Y, Luo B, Teng GJ, Magnetic resonance and near-infrared imaging using a novel dual-modality nano-probe for dendritic cell tracking in vivo, Cytotherapy, 16 (2014) 699–710. [DOI] [PubMed] [Google Scholar]
- [132].Noh Y-W, Jang Y-S, Ahn K-J, Lim YT, Chung BH, Simultaneous in vivo tracking of dendritic cells and priming of an antigen-specific immune response, Biomaterials, 32 (2011) 6254–6263. [DOI] [PubMed] [Google Scholar]
- [133].Heo MB, Lim YT, Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells, Biomaterials, 35 (2014) 590–600. [DOI] [PubMed] [Google Scholar]
- [134].Christian NA, Milone MC, Ranka SS, Li G, Frail PR, Davis KP, Bates FS, Therien MJ, Ghoroghchian PP, June CH, Hammer DA, Tat-functionalized near-infrared emissive polymersomes for dendritic cell labeling, Bioconjug Chem, 18 (2007) 31–40. [DOI] [PubMed] [Google Scholar]
- [135].John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, Gregg B, De Almeida DM, Weninger W, Hammer DA, Hunter CA, Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis, PLoS Pathog, 7 (2011) e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hammer Q, Ruckert T, Romagnani C, Natural killer cell specificity for viral infections, Nat Immunol, 19 (2018) 800–808. [DOI] [PubMed] [Google Scholar]
- [137].Habif G, Crinier A, Andre P, Vivier E, Narni-Mancinelli E, Targeting natural killer cells in solid tumors, Cell Mol Immunol, 16 (2019) 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Uong TNT, Lee KH, Ahn SJ, Kim KW, Min JJ, Hyun H, Yoon MS, Real-Time Tracking of Ex Vivo-Expanded Natural Killer Cells Toward Human Triple-Negative Breast Cancers, Front Immunol, 9 (2018) 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lim YT, Cho MY, Noh YW, Chung JW, Chung BH, Near-infrared emitting fluorescent nanocrystals-labeled natural killer cells as a platform technology for the optical imaging of immunotherapeutic cells-based cancer therapy, Nanotechnology, 20 (2009) 475102. [DOI] [PubMed] [Google Scholar]
- [140].Tavri S, Jha P, Meier R, Henning TD, Muller T, Hostetter D, Knopp C, Johansson M, Reinhart V, Boddington S, Sista A, Wels WS, Daldrup-Link HE, Optical imaging of cellular immunotherapy against prostate cancer, Mol Imaging, 8 (2009) 15–26. [PubMed] [Google Scholar]
- [141].Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, Fromm PD, Hart DN, Van Tendeloo VF, Berneman ZN, Dendritic cells as pharmacological tools for cancer immunotherapy, Pharmacol Rev, 67 (2015) 731–753. [DOI] [PubMed] [Google Scholar]
- [142].Santos PM, Butterfield LH, Dendritic Cell-Based Cancer Vaccines, J Immunol, 200 (2018) 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Qian B-Z, Pollard JW, Macrophage diversity enhances tumor progression and metastasis, Cell, 141 (2010) 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Na YR, Je S, Seok SH, Metabolic features of macrophages in inflammatory diseases and cancer, Cancer Lett, 413 (2018) 46–58. [DOI] [PubMed] [Google Scholar]
- [145].Choi HS, Frangioni JV, Nanoparticles for biomedical imaging: fundamentals of clinical translation, Molecular imaging, 9 (2010) 291–310. [PMC free article] [PubMed] [Google Scholar]
- [146].Gioux S, Choi HS, Frangioni JV, Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation, Molecular imaging, 9 (2010) 237–255. [PMC free article] [PubMed] [Google Scholar]
- [147].Miao Q, Xie C, Zhen X, Lyu Y, Duan H, Liu X, Jokerst JV, Pu K, Molecular afterglow imaging with bright, biodegradable polymer nanoparticles, Nat Biotechnol, 35 (2017) 1102–1110. [DOI] [PubMed] [Google Scholar]
- [148].Jiang Y, Huang J, Zhen X, Zeng Z, Li J, Xie C, Miao Q, Chen J, Chen P, Pu K, A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging, Nat Commun, 10 (2019) 2064. [DOI] [PMC free article] [PubMed] [Google Scholar]