Abstract
Introduction
Peripheral inhibition of tumor necrosis factor (TNF)‐α, outside of the central nervous system, may result in clinical improvement of Alzheimer's disease (AD) outcomes. TNF‐α inhibitors (TNFIs) are effective treatments for various autoimmune conditions and may be effective for preventing and/or treating AD. The objective of this study was to compare the risk of dementia and AD in patients initiating methotrexate versus those initiating TNFIs.
Methods
Insurance claims data from databases of commercially insured and Medicare‐eligible patients were used to estimate the risk of dementia and AD within patients with rheumatoid arthritis (RA) initiating a TNFI versus initiation of methotrexate. A sensitivity analysis included all patients without the RA diagnosis requirement. The at‐risk period spanned from the index date until a diagnosis of the outcome, loss‐to‐follow‐up, or receipt of the comparator drug. Patients were matched 1‐to‐1 using propensity scores. A Cox proportional hazards model was used to estimate the hazard ratio (HR). Negative controls were used to calibrate the results.
Results
A total of 11,092 new TNFI patients and 44,023 new methotrexate patients were identified, and 8925 from each group were matched. The outcome of dementia occurred in 1.4% of patients in both groups. The calibrated results from the Cox regression found no difference between the two groups (commercially insured database: calibrated HR = 0.69, 95% confidence interval = 0.45 to 1.05; Medicare‐only database: 1.14, 0.66 to 1.96). Results were similar in all sensitivity analyses: outcome of AD and including patients without RA.
Discussion
No significant difference for the risk of dementia or AD was seen between patients initiating a TNFI versus methotrexate. Although this study cannot conclude whether use of TNFIs is protective against dementia and AD compared with receiving no treatment, there was no evidence that it is more protective than the active comparator methotrexate.
Keywords: Alzheimer's disease, biologic therapy, claims data, dementia, methotrexate, TNF inhibitors
1. BACKGROUND
Alzheimer's disease (AD) affects nearly 6 million individuals in the United States and accounts for 60% to 80% of dementia cases.1 There are currently no available treatments that can slow or stop the progression of AD or the resulting neuronal damage, dementia, declining cognitive function, and ultimately death.
Inflammation is hypothesized to play a key role in the development of AD,2, 3, 4, 5, 6 and thus medications that may reduce inflammation could prevent the onset of AD or be effective in stopping or reversing its course. One mediator of inflammation is the production of tumor necrosis factor (TNF)‐α, a cytokine with proinflammatory and immunoregulatory functions, which was first found to play a role in the development of rheumatoid arthritis (RA) and later associated with other autoimmune conditions.7 The development of TNF‐α inhibitors (TNFIs) has revolutionized the treatment space for RA, psoriasis, ankylosing spondylitis, inflammatory bowel disease, and other inflammatory conditions.8
Some evidence suggests that TNF‐α levels in the brain are associated with AD pathophysiology and disease progression.9 It follows that the use of TNFI therapy may be effective in preventing and/or reversing the disease course. However, a limitation of current TNFI therapy for the treatment of inflammation occurring in the brain is the inability for the molecules to cross the blood‐brain barrier.10, 11 Although there is both clinical and pre‐clinical research suggesting the potential benefits of TNFI when bypassing the blood‐brain barrier via administration through intracerebroventricular and perispinal injections,12, 13, 14 these studies have been limited to mouse models of unproven value in prediction of human outcomes and human studies, which were either open‐label or too small to determine efficacy.
Furthermore, it has been hypothesized that peripheral inhibition of TNF‐α, outside of the central nervous system, may result in clinical improvement of AD outcomes without crossing the blood‐brain barrier, although evidence in support of such hypotheses is limited.9, 15 If it were true, however, then currently available TNFIs may be effective for the treatment and prevention of AD and related dementia without the necessity of crossing the blood‐brain barrier.
There is a large prior body of evidence suggesting that long‐term use of anti‐inflammatory medications reduces the risk of AD.16, 17, 18 Relatively few have, however, examined the use of TNFIs specifically. A retrospective cohort study that compared the effects of TNFIs to methotrexate on the incidence of AD found large protective effects associated with initiation of a TNFI.19 Other recent research has found similar results regarding the association between TNFI use and a lower risk of AD.20, 21 However, there are methodologic limitations to the prior research. In the Stacey et al. study, there may be an immortal time bias22 in the TNFI group due to many patients having previously received methotrexate—and must not have experienced the outcome during this time—whereas the opposite circumstance of receiving a TNFI prior to initiating methotrexate did not occur. The individuals who made it through methotrexate use and onto a TNFI may have been a subset of individuals least at risk to develop dementia or may have benefited from protective effects of the methotrexate prior to initiating the TNFI. The studies from Chou20 and Zhou21 are even more limited in their study design, as they relied on case‐control designs, which have been shown to have significant bias relative to retrospective cohort studies,23 and lacked robust control for confounding variables. A similarly designed, unpublished, case‐control study made headlines in 2019 when data suggested that there may be a protective effect of etanercept on the development of AD24; however, the signal was not pursued further by the drug maker for a number of potential reasons.25
This study aimed to improve on previous observational research by using a more appropriate study design to compare the risk of dementia and AD in patients initiating use of a TNFI versus those receiving methotrexate. This retrospective cohort study utilizes a new‐user design, large‐scale propensity score modeling, and negative control calibration to adjust for observed and unobserved biases.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources and meeting abstracts and presentations. Several recent publications examining the association between TNF‐α inhibitor (TNFI) use and Alzheimer's disease (AD) exist and have been cited; however, they have significant design limitations.
Interpretation: No significant difference for the risk of dementia or AD was seen between patients initiating TNFI versus methotrexate. These results contrast previous research, which may be due to confounding present in the design of previous studies.
Future directions: Additional observational research may be conducted in other patient populations and data sources to determine if the results are consistent with the data presented in this study. Databases with longer patient follow‐up may be useful for capturing more cases and for studying the long‐term comparative effectiveness of these therapies. Prospective clinical studies may be warranted if future observational studies show potential beneficial effects of TNFI for the prevention of AD and/or dementia.
2. METHODS
This retrospective observational study utilized administrative insurance claims data from two US‐based databases, which were analyzed independently. A schematic of the study design is shown in Figure 1.
FIGURE 1.
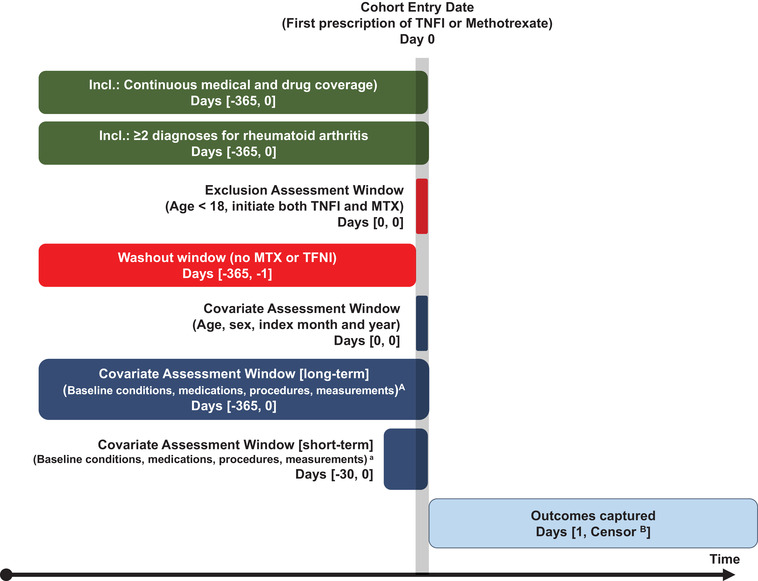
Study design schematic illustrating observation windows for inclusion and exclusion criteria, covariate assessment, and outcome identification in relation to the index date (Day 0). ABaseline covariates include all conditions, medications, procedures, measurements (eg, laboratory testing), and medical devices used; as well as variables specific to rheumatoid arthritis severity. BEarliest of: outcome of interest, filling comparator study drug, disenrollment, end of the study period
2.1. Data source
Each database contains data from adjudicated health insurance claims and health plan enrollment information.
Optum's Clinformatics® Data Mart de‐identified database. Includes 84 million members with private health insurance, who are fully insured in commercial plans or in administrative services only and Medicare Advantage. At the time of this study, data were available from May 31, 2000 through June 20, 2019.
IBM® MarketScan® Medicare Supplemental Database (MDCR): Includes data for > 9 million retirees with primary or Medicare supplemental coverage through privately insured fee‐for‐service, point‐of‐service, or capitated health plans. At the time of this study, data were available from January 1, 2000 through July 31, 2019.
Data elements included were outpatient pharmacy dispensing claims (coded with National Drug Codes) and inpatient and outpatient medical claims, which provide diagnosis codes (coded in ICD‐9‐CM or ICD‐10‐CM) associated with a visit. The use of the IBM MarketScan and Optum claims databases was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subjects research.
2.2. Exposures
Two exposure cohorts were identified. These included new users of a (1) TNFI (including infliximab, golimumab, etanercept, certolizumab, and adalimumab) or (2) methotrexate. Each exposure cohort was defined as the set of patients who had a first exposure for the cohort‐defining drug with no prior use of the comparator drug at any time (ie, TNFI patients had no prior methotrexate use, and vice versa). The date of the first exposure was considered the index date.
Patients were 18 years or older on the index date and were required to have ≥365 days of continuous pre‐index observation immediately prior to the index date. Patients with the outcome of interest prior to the index date were excluded. The primary analysis included RA patients, which included all patients with ≥2 visit dates with a diagnosis of RA within 365 days before and including the index date. A sensitivity analysis included all patients without the RA diagnosis requirement.
2.3. Outcomes
The primary outcome of interest was newly diagnosed dementia, which required a diagnosis of dementia on two distinct dates within 365 days of each other. The date of the first dementia diagnosis was considered the date of the event.
A secondary endpoint was newly diagnosed AD. Like dementia, the AD outcome definition required two diagnosis codes for AD within 365 days of each other, and the date of the first diagnosis was considered the date of the event.
AD tends to be under‐coded in claims data26 and thus we used the broader definition of “dementia” as our primary endpoint, which includes AD as well as generic conditions of “senility,” “degenerative brain disorder,” and others, but excludes Lewy body dementia, drug‐ and alcohol‐induced dementia, or dementia caused by concussions or syphilis, among others. A full list of the included concepts and mapped ICD‐9‐CM and ICD‐10‐CM codes can be found in Appendix Table 1.
The definition of dementia used in this study showed good sensitivity (85.5%), specificity (85.9%), and fair positive predictive value (77.6%) in a validation study comparing Medicare claims against clinical assessments from the Aging, Demographics, and Memory Study (ADAMS).26
2.4. Time at risk
Time‐at‐risk included all time starting from one day following the index date until the earliest date of (1) end of observation in the database; (2) end of the study period ( June 30, 2019 for Optum; July 31, 2019 for MDCR); (3) receiving the comparator study medication (ie, receipt of methotrexate for the TNFI cohort, or receipt of TNFI for the methotrexate cohort); (4) presence of the outcome.
2.5. Statistical analysis
Comparisons were made between new users of TFNI therapy versus new users of methotrexate for all combinations of the following variations: (1) population: RA patients and all patients; (2) outcome: dementia and AD; and (3) database: Optum and MDCR. This resulted in eight unique pairwise comparisons of TNFI versus methotrexate. The primary comparison was for the outcome of dementia within the subset of RA patients in each of the databases.
Propensity score matching was used to reduce potential confounding as the result of imbalance between the exposure cohorts (methotrexate and TNFI) on baseline covariates. The propensity score was estimated using the predicted probability from a regularized logistic regression model, fit with a Laplace prior (LASSO) and the regularization hyperparameter selected through cross‐validation. Covariates used in the propensity score model included demographics (gender, age, index year, and month), all previously diagnosed conditions, all previously received prescription drugs, all procedures received, all measurements taken, and the number of visits (total and by place of service), number of distinct prescription medications received, and number of distinct diagnosed conditions observed during the 365 days and 30 days prior to exposure, and the Charlson comorbidity index according to previously diagnosed conditions. Additional variables specific to RA severity were also used, including the number of RA‐specific visits (inpatient, emergency department, and outpatient), any corticosteroid use, disease‐modifying anti‐rheumatic drug use, opioid use, and joint surgeries observed in the 365 and 30 days preceding the index date.
The exposure cohorts were matched 1:1 using a caliper of 0.2 times the standard deviation of the logit of the propensity score distribution. Standardized mean difference was used to evaluate the performance of propensity score adjustment, where variables with standardized differences <0.10 were considered well‐balanced.27
The potential for residual systematic error was examined by plotting the distribution of estimates from negative control outcomes,28, 29 which can be found in Appendix Figure 1. The negative control outcomes showed slight positive residual bias, illustrated by the distribution of estimates centered just to the right of the null hypothesis of 1.0. Empirical calibration of effect estimates and confidence intervals (CIs) for the study endpoints was used to account for this small positive residual bias.30, 31
A Cox proportional hazard model conditioned on the matched sets32 was used as the final model. For each outcome model, we reported the empirically calibrated hazard ratio (HR) and 95% CIs. The methotrexate group served as the comparator; all risk estimates are in reference to methotrexate.
2.6. Registration
This study was registered on ClinicalTrials.gov (NCT04571697).
3. RESULTS
There were 44,023 methotrexate patients and 11,092 TNFI patients identified from the Optum database who were diagnosed with RA and met all inclusion criteria, of which 8925 in each group were matched on propensity scores. In the MDCR data, 2125 from each group were matched. Study flow and impact of each inclusion criteria are shown in Figure 2. Patients had between 2 and 3 years of follow‐up (2.2 years for TNFI and methotrexate patients in Optum; 2.7 and 2.9 years for TNFI and methotrexate patients, respectively, in MDCR).
FIGURE 2.
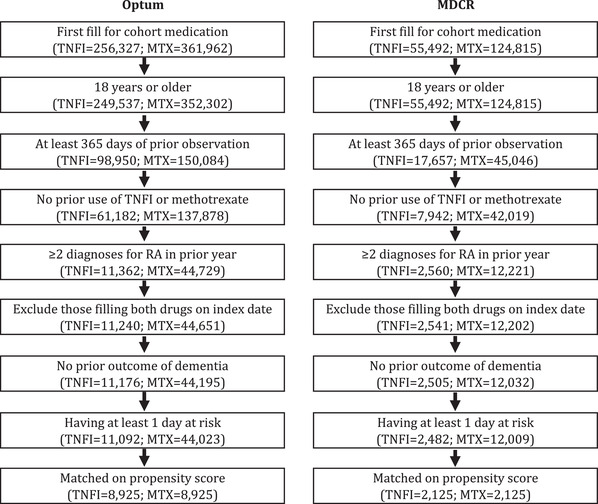
Study flow diagram of inclusion criteria within the cohorts of rheumatoid arthritis (RA) patients in the Optum and MDCR databases. TNFI, TNF‐α inhibitor; MTX, methotrexate
Baseline characteristics prior to and after matching, including patient demographics, healthcare utilization, and comorbid conditions are found in Table 1. The use of propensity scores to match the population resulted in well‐balanced cohorts for all patient characteristics in Table 1. In addition, all 51,982 covariates assessed in the Optum database were well balanced (standardized difference <0.10) and a single covariate in the MDCR database had a standardized difference of 0.10, whereas all others fell below the threshold (Appendix Figure 2). Distributions of the preference scores are displayed in Appendix Figure 3 and further illustrate well‐balanced cohorts after matching.
TABLE 1.
Patient characteristics and comorbid conditions (diagnosed during index date–365 days to index date, inclusive) before and after propensity score matching
| Optum | MDCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before matching | After matching | Before matching | After matching | |||||||||
| Characteristic | TNFI | MTX | Std. diff | TNFI | MTX | Std. diff | TNFI | MTX | Std. diff | TNFI | MTX | Std. diff |
| Number of patients | 11,092 | 44,023 | 8925 | 8925 | 28,482 | 12,009 | 2125 | 2125 | ||||
| Age, years (mean) | 54.7 | 59.4 | −0.33 | 55.3 | 54.7 | 0.04 | 72.7 | 74.2 | −0.23 | 72.9 | 73.0 | 0.01 |
| Gender: female (%) | 75.6% | 75.0% | 0.01 | 75.5% | 75.8% | −0.01 | 71.0% | 69.8% | 0.03 | 71.5% | 72.2% | −0.02 |
| Post‐index follow‐up, days (mean) | ||||||||||||
| Summary metrics | ||||||||||||
| Distinct medications used (ingredients) (mean) | 12.8 | 12.9 | −0.01 | 12.1 | 12.1 | 0.00 | 15.8 | 14.6 | 0.14 | 15.3 | 15.5 | −0.02 |
| Distinct conditions diagnosed (mean) | 25.0 | 25.3 | −0.02 | 23.8 | 23.8 | 0.00 | 23.7 | 23.1 | 0.04 | 23.2 | 23.2 | 0.00 |
| Charlson comorbidity index (mean) | 2.60 | 2.78 | −0.08 | 2.52 | 2.51 | 0.00 | 3.45 | 3.37 | 0.03 | 3.37 | 3.38 | 0.00 |
| 1‐year pre‐index utilization | ||||||||||||
| All‐cause | ||||||||||||
| Inpatient visits (mean) | 0.22 | 0.19 | 0.05 | 0.20 | 0.21 | −0.01 | 0.33 | 0.31 | 0.04 | 0.31 | 0.33 | −0.03 |
| ER visits (mean) | 1.19 | 1.01 | 0.06 | 1.12 | 1.06 | 0.02 | 0.67 | 0.64 | 0.03 | 0.64 | 0.62 | 0.02 |
| Outpatient visits (mean) | 31.2 | 28.6 | 0.10 | 29.5 | 29.0 | 0.02 | 31.9 | 27.9 | 0.19 | 30.8 | 30.1 | 0.03 |
| All visits (mean) | 32.6 | 29.8 | 0.11 | 30.8 | 30.3 | 0.02 | 32.9 | 28.8 | 0.18 | 31.8 | 31.1 | 0.03 |
| RA‐related | ||||||||||||
| RA outpatient visits (%) | 98.6% | 91.6% | 0.33 | 98.4% | 97.9% | 0.04 | 99.6% | 97.6% | 0.17 | 99.7% | 99.0% | 0.08 |
| RA ER visits (%) | 14.4% | 9.6% | 0.15 | 13.7% | 11.6% | 0.06 | 9.5% | 6.9% | 0.10 | 9.2% | 6.8% | 0.09 |
| RA inpatient visits (%) | 10.0% | 6.4% | 0.13 | 9.2% | 8.7% | 0.02 | 10.2% | 7.6% | 0.09 | 9.6% | 8.3% | 0.05 |
| Medication/surgery | ||||||||||||
| DMARD use (%) | 50.0% | 35.4% | 0.30 | 44.8% | 47.1% | −0.05 | 61.6% | 41.1% | 0.42 | 56.0% | 58.5% | −0.05 |
| Opioid use (%) | 51.8% | 55.1% | −0.07 | 49.9% | 50.1% | 0.00 | 64.3% | 61.3% | 0.06 | 63.3% | 63.2% | 0.00 |
| Corticosteroid use (%) | 27.2% | 26.9% | 0.01 | 26.2% | 25.4% | 0.02 | 26.8% | 26.8% | 0.00 | 26.7% | 28.6% | −0.04 |
| Joint surgeries (%) | 33.1% | 29.2% | 0.08 | 31.6% | 31.5% | 0.00 | 44.2% | 38.6% | 0.11 | 43.5% | 42.8% | 0.01 |
| Comorbid autoimmune conditions | ||||||||||||
| Psoriasis with arthropathy | 5.3% | 2.2% | 0.17 | 4.4% | 4.6% | −0.01 | 2.6% | 1.1% | 0.11 | 2.3% | 2.5% | −0.02 |
| Systemic lupus erythematosus | 4.7% | 5.2% | −0.02 | 4.8% | 5.2% | −0.02 | 0.0% | 0.1% | −0.01 | 0.0% | 0.0% | 0.00 |
| Psoriasis | 4.3% | 2.0% | 0.13 | 3.5% | 3.6% | 0.00 | 2.1% | 1.2% | 0.07 | 2.1% | 1.6% | 0.03 |
| Ankylosing spondylitis | 3.2% | 1.1% | 0.15 | 2.4% | 2.5% | −0.01 | 1.2% | 0.6% | 0.07 | 1.3% | 0.7% | 0.06 |
| Crohn's disease | 1.9% | 0.4% | 0.14 | 1.2% | 1.1% | 0.01 | 1.3% | 0.2% | 0.12 | 1.0% | 0.7% | 0.04 |
| Ulcerative colitis | 1.7% | 0.5% | 0.11 | 1.2% | 1.1% | 0.01 | 1.3% | 0.5% | 0.09 | 1.3% | 1.0% | 0.03 |
| Other notable common comorbidities | ||||||||||||
| Essential hypertension | 37.7% | 42.5% | −0.10 | 36.8% | 36.1% | 0.01 | 41.9% | 44.9% | −0.06 | 41.4% | 42.1% | −0.02 |
| Hyperlipidemia | 25.9% | 32.7% | −0.15 | 25.8% | 25.1% | 0.02 | 23.0% | 26.8% | −0.09 | 23.3% | 24.1% | −0.02 |
| Low back pain | 20.9% | 19.3% | 0.04 | 19.6% | 20.1% | −0.01 | 18.6% | 15.6% | 0.08 | 17.7% | 17.4% | 0.01 |
| Osteoarthritis | 15.7% | 20.8% | −0.13 | 15.6% | 15.4% | 0.01 | 16.3% | 19.0% | −0.07 | 16.4% | 16.7% | −0.01 |
| Anemia | 14.9% | 15.4% | −0.01 | 14.2% | 14.3% | 0.00 | 15.8% | 14.3% | 0.04 | 14.7% | 14.9% | −0.01 |
| Vitamin D deficiency | 14.8% | 15.1% | −0.01 | 13.9% | 13.8% | 0.00 | 7.8% | 7.0% | 0.03 | 7.7% | 7.1% | 0.02 |
| Osteoporosis | 14.0% | 13.5% | 0.02 | 13.7% | 13.2% | 0.01 | 17.2% | 15.2% | 0.05 | 17.1% | 17.7% | −0.01 |
| Acquired hypothyroidism | 13.6% | 14.6% | −0.03 | 13.5% | 13.3% | 0.00 | 11.2% | 12.4% | −0.04 | 11.3% | 12.5% | −0.04 |
| Gastroesophageal reflux disease | 12.3% | 11.8% | 0.01 | 11.9% | 12.0% | 0.00 | 0.2% | 0.2% | −0.01 | 0.1% | 0.1% | 0.00 |
| Diabetes mellitus without complication | 11.1% | 13.1% | −0.06 | 11.0% | 10.7% | 0.01 | 17.6% | 18.0% | −0.01 | 17.3% | 16.8% | 0.01 |
| Anxiety disorder | 9.8% | 9.7% | 0.01 | 9.2% | 9.6% | −0.01 | 0.0% | 0.1% | −0.02 | 0.0% | 0.0% | 0.00 |
| Edema | 8.9% | 10.0% | −0.04 | 8.6% | 8.2% | 0.01 | 9.4% | 10.6% | −0.04 | 9.4% | 10.2% | −0.03 |
| Obesity | 8.1% | 8.6% | −0.02 | 7.6% | 7.7% | 0.00 | 0.0% | 0.0% | 0.02 | 0.0% | NA | NA |
| Chronic obstructive lung disease | 7.9% | 9.4% | −0.05 | 7.8% | 7.6% | 0.01 | 14.6% | 12.6% | 0.06 | 13.6% | 14.9% | −0.03 |
| Depressive disorder | 7.3% | 7.2% | 0.00 | 6.8% | 7.1% | −0.01 | 3.7% | 4.3% | −0.03 | 3.5% | 4.0% | −0.03 |
| Asthma | 6.9% | 6.2% | 0.03 | 6.6% | 6.3% | 0.01 | 0.7% | 0.3% | 0.05 | 0.7% | 0.5% | 0.02 |
| Insomnia | 6.5% | 6.0% | 0.02 | 6.0% | 6.1% | 0.00 | 3.3% | 2.9% | 0.02 | 3.3% | 3.0% | 0.02 |
| Obstructive sleep apnea syndrome | 6.3% | 5.6% | 0.03 | 5.7% | 5.6% | 0.00 | 5.5% | 4.9% | 0.03 | 5.3% | 5.4% | 0.00 |
| Tobacco dependence syndrome | 5.5% | 5.6% | −0.01 | 5.3% | 5.5% | −0.01 | 2.4% | 2.4% | 0.01 | 2.4% | 2.1% | 0.02 |
Abbreviations: TNFI, TNF‐α inhibitor; MTX, methotrexate; Std. diff, standardized mean difference; DMARD, disease‐modifying antirheumatic drug.
Roughly three‐fourths of matched patients in each database were female, and the average age at index was 55‐years‐old in Optum and 73‐years‐old in MDCR. Common comorbid conditions included cardiovascular‐related conditions and diabetes (Table 1).
The primary analysis examined the outcome of dementia within patients having a prior diagnosis of RA. Within the Optum database, there were 124 individuals from each matched cohort who were first diagnosed with dementia during follow‐up, resulting in identical incidence rates of 6.3 per 1000 person‐years (Table 2). The calibrated HR from the Cox proportional hazards model was 0.69 (in favor of TNFI, 95% CI = 0.45 to 1.05), and did not reach statistical significance. Within the MDCR population incidence rates of dementia were higher than in the Optum database (12.9 and 13.7 per 1000 person‐years for TNFI and methotrexate, respectively). The calibrated HR indicated no significant difference between groups (HR = 1.14, 95% CI = 0.66 to 1.96). The variation between the unadjusted incidence rates and the HRs reflects adjustments from negative control calibration and, more significantly, the impact of differences in the timing of events between the two groups.
TABLE 2.
Outcome of primary objective examining risk of dementia within patients diagnosed with rheumatoid arthritis (RA) identified from the Optum and MDCR databases
| TNFI | Methotrexate | Calibrated results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Database | Sample | Outcome | N at risk | Person‐years | N with outcome | IR per 1000 py | N at risk | Person‐years | N with outcome | IR per 1000 py | HR | 95% CI Lower | 95% CI Upper |
| Optum | RA patients | Dementia | 8925 | 19,753 | 124 | 6.3 | 8925 | 19,542 | 124 | 6.3 | 0.69 | 0.45 | 1.05 |
| MDCR | RA patients | Dementia | 2125 | 5641 | 73 | 12.9 | 2125 | 6211 | 85 | 13.7 | 1.14 | 0.66 | 1.96 |
Abbreviations: RA, rheumatoid arthritis; TNFI, TNF‐α inhibitor; IR, incidence rate; py, person‐years; HR, hazard ratio; CI, confidence interval.
Similar results were observed across all other combinations of outcomes (AD), populations (not restricted to RA), and databases, with no statistically significant difference between TNFI and methotrexate groups (Table 3). Kaplan‐Meier curves from the Cox proportional hazards model are shown in Figure 3 and illustrate the absence of significant separation between the two groups. Uncalibrated estimates were similar in magnitude to the calibrated results, although slightly larger.
TABLE 3.
Results for all other combinations of outcomes (dementia and Alzheimer's disease), study samples (all patients and patients diagnosed with rheumatoid arthritis [RA]), within each database (optum and MDCR)
| TNFI | Methotrexate | Calibrated results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Database | Sample | Outcome | N at risk | Person‐years | N with outcome | IR per 1000 py | N at risk | Person‐years | N with outcome | IR per 1000 py | HR | 95% CI Lower | 95% CI Upper |
| Optum | All patients | Dementia | 29,079 | 72,981 | 318 | 4.4 | 29,079 | 64,424 | 322 | 5.0 | 0.91 | 0.69 | 1.19 |
| Optum | All patients | AD | 29,180 | 73,637 | 113 | 1.5 | 29,180 | 65,145 | 124 | 1.9 | 0.83 | 0.52 | 1.32 |
| Optum | RA patients | Dementia | 8925 | 19,753 | 124 | 6.3 | 8925 | 19,542 | 124 | 6.3 | 0.69 | 0.45 | 1.05 |
| Optum | RA patients | AD | 8958 | 19,979 | 42 | 2.1 | 8958 | 19,736 | 49 | 2.5 | 0.96 | 0.50 | 1.86 |
| MDCR | All patients | Dementia | 5414 | 15,971 | 190 | 11.9 | 5414 | 15,716 | 199 | 12.7 | 1.34 | 0.90 | 2.04 |
| MDCR | All patients | AD | 5467 | 16,340 | 82 | 5.0 | 5467 | 16,066 | 70 | 4.4 | 1.51 | 0.86 | 2.72 |
| MDCR | RA patients | Dementia | 2125 | 5641 | 73 | 12.9 | 2125 | 6211 | 85 | 13.7 | 1.14 | 0.66 | 1.96 |
| MDCR | RA patients | AD | 2143 | 5789 | 26 | 4.5 | 2143 | 6310 | 30 | 4.8 | 1.44 | 0.57 | 3.63 |
Abbreviations: RA, rheumatoid arthritis; TNFI, TNF‐α inhibitor; py, person‐years; HR, hazard ratio; CI, confidence interval; AD, Alzheimer's disease.
FIGURE 3.
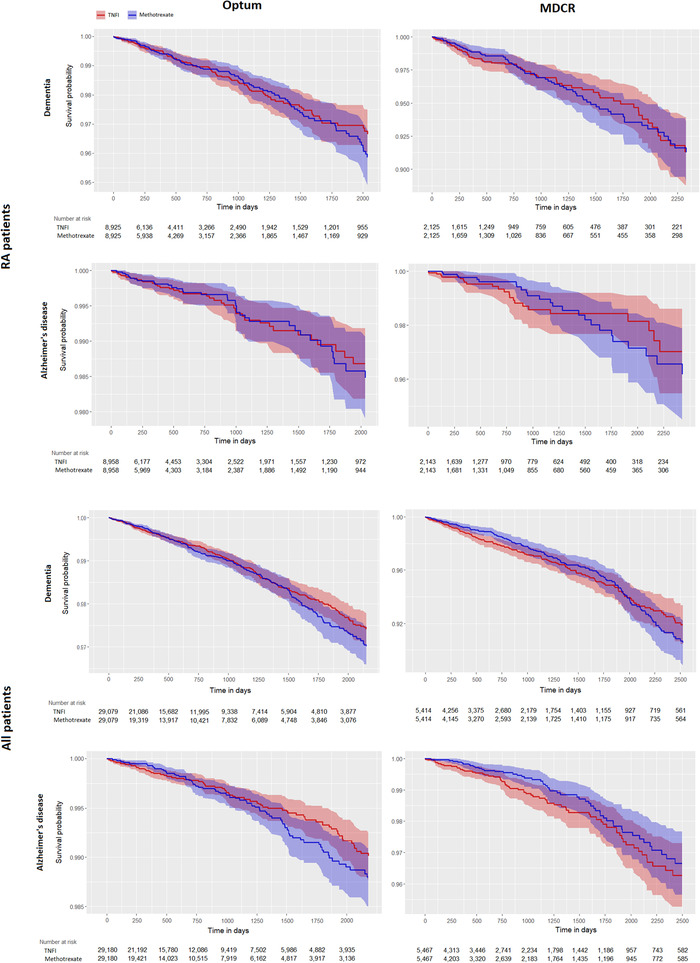
Kaplan‐Meier graphs for the outcomes of dementia (1st and 3rd rows) and Alzheimer's disease (2nd and 4th rows) for the population of patients diagnosed with rheumatoid arthritis (RA) (top two rows) and all patients (bottom two rows) in the Optum (left) and MDCR (right) databases
4. DISCUSSION
This study provides a robust assessment comparing the effectiveness of TNFI therapies and methotrexate for the incidence of dementia. The analysis leveraged two large US‐based administrative claims databases: one which included commercially insured individuals, including those with Medicare eligibility, and a second containing only Medicare beneficiaries. The analysis was replicated using various sensitivity analyses including the examination of dementia as an outcome as well as the more specific outcome of AD, and an examination of patients diagnosed with RA and a broad population of all patients regardless of diagnosed conditions. Across all comparisons, no significant difference in risk of dementia or AD was observed between patients initiating a TNFI versus those receiving methotrexate.
Results of this study contrast recent research that has shown TNFIs to be effective in preventing AD.19, 20, 21 There are significant differences in study design between prior work and the current study. Stacey et al.19 employed a retrospective cohort design and leveraged claims data, much like the current study, to compare users of methotrexate versus TNFI. However, that study allowed patients to have had prior exposure to the comparator drug. Because many patients using a TNFI first use methotrexate, and much less often do patients use a TNFI prior to methotrexate, this could present a bias. First, patients moving from methotrexate to TNFI may have experienced beneficial effects of the methotrexate prior to starting their TNFI. Second, those TNFI patients exposed to methotrexate did not have the outcome of AD prior to initiating TNFI and could represent a group of “healthy survivors” who are least at risk to develop AD; had they developed AD while on methotrexate and prior to initiating the TNFI they would not be eligible to enter the TNFI cohort by design, but had they started immediately with the TNFI rather than methotrexate they may have been diagnosed with AD while using TNFI. Thus the inclusion of TNFI patients with prior methotrexate exposure may have introduced immortal time bias.
The other two studies examining this question20, 21 were case‐control studies that have been shown to have significant bias,23 and these specific studies had minimal control for confounding factors, mainly limited to controlling for age, sex, race, and a handful of comorbidities. Conversely, the present study used techniques to control for all observed confounding using propensity score models and controlled for unmeasured confounding using negative control calibration. In addition, the case‐control studies aimed to estimate the association between TNFI use versus no TNFI use, which is a different research question then the one posed here, which examines the use of TNFI versus the use of methotrexate. Assessing the association between an outcome and a specific treatment using observational real‐world data without using an active comparator calls into question how interchangeable the exposed and unexposed groups are; that is, can we really expect patients who received a TNFI to be the same as patients who possibly received no disease‐modifying antirheumatic drug (DMARD) treatment for their RA?
This study followed best practices for conducting comparative effectiveness research using real‐world data,33 and there are notable strengths of this study. The use of multiple databases, multiple outcomes, and multiple target populations illustrates the robustness of the findings. Propensity score matching with the use of LASSO regression models allowed for the balance of all observed potential confounders in the claims data with > 50,000 variables assessed. In addition, the use of negative control outcomes to estimate the amount of residual bias inherent to the study design allowed for the calibration of the study results to account for this residual bias and unobserved confounding.
The comparative cohort study design allowed for the direct estimation of risk, as measured by hazard ratios, rather than relying on odds ratios obtained by case‐control designs. By implementing a new‐user design, this study captured events following treatment exposures while avoiding confounding from previous treatment effects.
There are limitations to this study. Outcomes of dementia and AD relied on ICD‐9‐CM and ICD‐10‐CM diagnosis codes, which are not perfect. The largest limitations are with the limited sensitivity of the AD definition likely resulting in a lower observed incidence of the condition than the truth. However, the primary analysis used the broader category of dementia and excluded many forms of dementia that were specifically not due to AD, such as Lewy body dementia, drug‐ and alcohol‐ induced dementia, or dementia caused by concussions or syphilis, among others. There is a tradeoff between using a highly specific measurement, such as the AD definition, versus using a more sensitive measure, such as the dementia definition34; by using both types of definitions and arriving at the same conclusion we gain confidence in the validity of our results.
Average observation time following initiation of one of the study drugs was < 3 years, which limits the ability to identify cases that were caused many years following initial exposure. In addition, dementia represents the last stage of AD pathophysiology35 ; therefore, identifying potential prevention of AD may be indicative of the identification of slowing or reversal of the AD pathway that has already begun.
The primary objective of this study included patients diagnosed with RA. The claims data do not have information on clinical RA disease severity indices such as the Rheumatoid Arthritis Severity Scale (RASS).36 Instead, the study used data on RA visits (outpatient, emergency room [ER], and inpatient), use of opioids, corticosteroids, and DMARDs, and the presence of joint surgeries to proxy disease severity. Furthermore, socioeconomic variables (such as race/ethnicity, education, income) and behavioral variables (such as diet and exercise) were not available.
Generalizability of the findings are limited to patients who have a moderate likelihood of receiving either of the treatment arms. Propensity score distributions show overlap between the two groups indicating reasonable generalizability. In addition, inclusion criteria required that patients had no prior exposure to either study therapy. In the RA population this resulted in an exclusion of 69% of TNFI patients in Optum and 74% of TNFI patients in MDCR. In the population of all patients, these criteria resulted in an exclusion of 38% and 55% of TNFI patients in Optum and MDCR, respectively. Although this appears to limit generalizability within the population of RA patients, the study design was chosen not to maximize generalizability in this population, but to minimize bias to obtain valid results. The high proportion of TNFI patients with prior methotrexate use was the driving factor for the decision to exclude these patients due to the bias it may introduce. Furthermore, the patient population included in this analysis may not be generalizable to the broader population of elderly patients at risk of dementia and AD; the databases used in this study comprise commercially insured individuals, including those who have purchased primary or supplementary Medicare through a Private Fee For Service plan (ie, Medicare Advantage).
Finally, this study examined the comparative effectiveness between TNFI use and newly diagnosed dementia and AD relative to methotrexate and the same outcomes. Therefore, this study does not rule out TNFI therapy as being protective against dementia and AD versus not receiving any treatment at all, but instead implies that any associations with the outcomes were no different than what was observed with the use of methotrexate. In fact, methotrexate has been found to be protective against dementia in previous observational research.37, 38 However, a recent study examining medications associated with a decreased risk of dementia found no protective effects of TNFI therapies when using a self‐controlled cohort design, comparing use of TNFI versus no TNFI use.39
5. CONCLUSIONS
No difference was found in the risk of being diagnosed with dementia or AD between patients initiating a TNFI versus those initiating methotrexate. Although this study cannot conclude whether use of TNFIs is protective against dementia and AD compared with receiving no treatment, there was no evidence that it is more protective than the active comparator methotrexate.
CONFLICTS OF INTEREST
All authors are employees of Janssen Research & Development, LLC and stockholders of Johnson & Johnson.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
All authors are employees of Janssen Research & Development, LLC.
Kern DM, Lovestone S, Cepeda MS. Treatment with TNF‐α inhibitors versus methotrexate and the association with dementia and Alzheimer's disease. Alzheimer's Dement. 2021;7:e12163. 10.1002/trc2.12163
REFERENCES
- 1.2020 Alzheimer's disease facts and figures. Alzheimer's Dement. 2020;16(3):391‐460. [DOI] [PubMed] [Google Scholar]
- 2.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement. 2018;4:575‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newcombe EA, Camats‐Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer's disease. J Neuroinflammation. 2018;15(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37(2):289‐305. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21(3):383‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers J, Webster S, Lue L‐F, et al. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17(5):681‐686. [DOI] [PubMed] [Google Scholar]
- 7.Willrich MAV, Murray DL, Snyder MR. Tumor necrosis factor inhibitors: clinical utility in autoimmune diseases. Transl Res. 2015;165(2):270‐282. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PC. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol. 2010;10(3):308‐315. [DOI] [PubMed] [Google Scholar]
- 9.Chang R, Yee K‐L, Sumbria RK. Tumor necrosis factor α inhibition for Alzheimer's disease. J Cent Nerv Syst Dis. 2017;9:1179573517709278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boado RJ, Hui EK‐W, Lu JZ, Zhou Q‐H, Pardridge WM. Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor‐specific IgG fusion protein. J Biotechnol. 2010;146(1‐2):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardridge WM. Biologic TNFα‐inhibitors that cross the human blood‐brain barrier. Bioeng Bugs. 2010;1(4):233‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobinick E, Gross H, Weinberger A, Cohen H. TNF‐alpha modulation for treatment of Alzheimer's disease: a 6‐month pilot study. MedGenMed. 2006;8(2):25. [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J‐Q, Shen W, Chen J, et al. Anti‐TNF‐α reduces amyloid plaques and tau phosphorylation and induces CD11c‐positive dendritic‐like cell in the APP/PS1 transgenic mouse brains. Brain Res. 2011;1368:239‐247. [DOI] [PubMed] [Google Scholar]
- 14.Butchart J, Brook L, Hopkins V, et al. Etanercept in Alzheimer disease: a randomized, placebo‐controlled, double‐blind, phase 2 trial. Neurology. 2015;84(21):2161‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detrait ER, Danis B, Lamberty Y, Foerch P. Peripheral administration of an anti‐TNF‐α receptor fusion protein counteracts the amyloid induced elevation of hippocampal TNF‐α levels and memory deficits in mice. Neurochem Int. 2014;72:10‐13. [DOI] [PubMed] [Google Scholar]
- 16.Etminan M, Gill S, Samii A. Effect of non‐steroidal anti‐inflammatory drugs on risk of Alzheimer's disease: systematic review and meta‐analysis of observational studies. BMJ. 2003;327(7407):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martyn C. Anti‐inflammatory drugs and Alzheimer's disease. BMJ. 2003;327(7411):353‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Wang Y, Wang D, Zhang J, Zhang F. NSAID exposure and risk of Alzheimer's disease: an updated meta‐analysis from cohort studies. Front Aging Neurosci. 2018;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacey J. Anti‐TNF‐α inhibitors in preventing Alzheimer's Disease (AD): a retrospective review using both EMR and claims data. Value Heal. 2019;22(Supp 3):S738‐S739. [Google Scholar]
- 20.Chou RC, Kane M, Ghimire S, Gautam S, Gui J. Treatment for rheumatoid arthritis and risk of Alzheimer's disease: a nested case‐control analysis. CNS Drugs. 2016;30(11):1111‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou M, Xu R, Kaelber DC, Gurney ME. Tumor Necrosis Factor (TNF) blocking agents are associated with lower risk for Alzheimer's disease in patients with rheumatoid arthritis and psoriasis. PLoS One. 2020;15(3):e0229819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492‐499. [DOI] [PubMed] [Google Scholar]
- 23.Ryan PB, Schuemie MJ, Madigan D. Empirical performance of the case‐control method: lessons for developing a risk identification and analysis system. Drug Saf. 2013;36:95‐106. [DOI] [PubMed] [Google Scholar]
- 24.Rowland C. Pfizer had clues its blockbuster drug could prevent Alzheimer's. Why didn't it tell the world?. The Washington Post. Wooster, OH: Bell & Howell Co; 2019. [Google Scholar]
- 25.Lowe D. A missed Alzheimer's opportunity? Not so much. Sci Transl Med. https://blogs.sciencemag.org/pipeline/archives/2019/06/06/a‐missed‐alzheimers‐opportunity‐not‐so‐much. Published online 2019. [Google Scholar]
- 26.Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28(25):3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p‐values. Stat Med. 2014;33(2):209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Robust empirical calibration of p‐values using observational data. Stat Med. 2016;35(22):3883‐3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population‐level effect estimation studies in observational healthcare data. Proc Natl Acad Sci U S A. 2018;115(11):2571‐2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal S, Madigan D, Burd RS, Suchard MA. High‐dimensional, massive sample‐size Cox proportional hazards regression for survival analysis. Biostatistics. 2014;15(2):207‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger ML, Sox H, Willke RJ, et al. Good practices for real‐world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR‐ISPE special task force on real‐world evidence in health care decision making. Value Heal. 2017;20(8):1003‐1008. [DOI] [PubMed] [Google Scholar]
- 34.Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol. 2012;65(3):343‐349.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampel H, Wilcock G, Andrieu S, et al. Biomarkers for Alzheimer's disease therapeutic trials. Prog Neurobiol. 2011;95(4):579‐593. [DOI] [PubMed] [Google Scholar]
- 36.Bardwell WA, Nicassio PM, Weisman MH, Gevirtz R, Bazzo D. Rheumatoid arthritis severity scale: a brief, physician‐completed scale not confounded by patient self‐report of psychological functioning. Rheumatology. 2002;41(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 37.Judge A, Garriga C, Arden NK, et al. Protective effect of antirheumatic drugs on dementia in rheumatoid arthritis patients. Alzheimer's Dement. 2017;3(4):612‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newby D, Prieto‐Alhambra D, Duarte‐Salles T, et al. Methotrexate and relative risk of dementia amongst patients with rheumatoid arthritis: a multi‐national multi‐database case‐control study. Alzheimers Res Ther. 2020;12(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kern DM, Cepeda MS, Lovestone S, Seabrook GR. Aiding the discovery of new treatments for dementia by uncovering unknown benefits of existing medications. Alzheimer's Dement Transl Res Clin Interv. 2019;5:862‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information


