Abstract
In skin and wound research the instrumental measurement of skin function is established. Despite the widespread use, empirical evidence about measurement errors is widely lacking. The aim of this study was to measure reliability and agreement of skin temperature, transepidermal water loss, epidermal hydration, and erythema at the heel and sacral skin. Four experienced researchers performed skin measurements in 15 subjects. Lowest reliability was observed for transepidermal water loss at the sacral skin (ICC (1) 0.46 (95% CI 0.00‐0.78)) and highest for skin temperature at the heel skin (ICC (1) 0.99 (95% CI 0.99‐1.00)). Lowest Standard Errors of Measurement were calculated for skin temperature measurements at the heels (0.11°C) and highest for erythema measurements at the sacral skin (26.7 arbitrary units). There was a clear association between variability of estimates and reliability coefficients. Single measurements of skin temperature, stratum corneum, and epidermal hydration at the sacral and heel skin areas can be used in clinical research and practice. Means of at least two measurements should be used for estimating transepidermal water loss and erythema. Evidence is needed to inform researchers about relative and absolute measurement errors of commonly applied instruments and measurements in skin and wound research.
Keywords: Epidermis, erythema, hydration, reliability, stratum corneum
1. INTRODUCTION
The skin is often considered the largest organ of the human body and it fulfils a variety of important functions. One of the most important tasks is protection against environmental influences such as physical or chemical irritants. At the same time, the skin prevents uncontrolled loss of water from the inside and protects the human body from drying out thus enabling survival on land.1, 2
In the fields of skin and wound research and dermatology the measurement of selected aspects of skin function such as skin temperature, transepidermal water loss (TEWL), or skin hydration is established for decades. For example, skin temperature is considered an important variable associated with pressure ulcer (PU) development and detection,3, 4 TEWL is proposed as a parameter to measure maceration in venous leg ulcers5 or scar quality,6 and stratum corneum hydration (SCH) is used to measure the occlusivity of wound dressings.7, 8
Today, skin barrier measurements are also increasingly used in the context of skin microclimate and PU prevention research,9 because mechanical loading is not only associated with skin and soft tissue deformation but also with occlusion.10, 11 In addition to the parameters listed above, erythema measurements play an important role, because the degree of erythema is associated with (reactive) hyperaemia and vasodilation during and after loading.12, 13
Despite the widespread use and availability of various measurement devices from various manufacturers, the accurate estimation of skin barrier properties is not easy and prone to error.14, 15 Therefore, numerous documents and standards have been published to provide guidance how to plan, to conduct, to report, and how to interpret obtained values appropriately.16, 17, 18, 19, 20 However, transparent and comprehensive reporting is often missing21, 22 and interpretation of skin barrier estimates sometimes surprising.15, 23
Fundamental properties of all clinical and instrumental measurements in clinical practice and research are reliability quantifying the relative measurement error and agreement quantifying the absolute measurement error.24, 25 Evidence about both types of measurement errors is important, because they directly affect the power of statistical tests of between and within group comparisons and the usefulness of the measurements for clinical decision‐making.26, 27
Currently, the evidence about common skin barrier measurement properties in different clinical and research settings is rare. In an environmentally controlled research setting intraclass correlation coefficients (ICC) of TEWL ranged between 0.85 and 0.94 with rather high absolute limits of agreement ranging from 4 to 12 g/m2/h when measuring different skin areas.6 High ICCs of approximately 0.9 of TEWL measurements were also observed in geriatric care settings with limits of agreement ranging from approximately 2 to 5 g/m2/h.28, 29 The high reported ICCs of TEWL estimates indicate that this measurement seems to be useful in clinical research for group comparisons,25 but the absolute measurement errors of single readings are high. Evidence about the absolute and relative measurement errors of other relevant skin barrier measurements in clinical skin and wound research is missing completely, in particular for skin areas prone to pressure ulceration. Therefore, the aim of this study was to measure reliability and agreement of skin temperature, TEWL, SCH, epidermal hydration, and erythema at the heel and sacral skin before and after loading.
2. MATERIALS AND METHODS
2.1. Study design
This is a secondary data analysis of a randomised controlled exploratory clinical trial comparing the effects of support surfaces on the skin function of pressure areas when lying supine.30 After written informed consent, 15 female subjects were invited to consecutively lie on three different support surfaces in supine position for 2 hours. Main eligibility criteria were being non‐smoker, absence of any skin diseases or conditions that may influence the skin measurements. The use of topical leave‐on products on the investigational skin areas was not allowed.30
Skin temperature, TEWL, SCH, epidermal hydration, and erythema were measured on the sacral and heel skin areas before and after loading. In order to reduce measurement errors, all skin measurements were conducted twice per measurement time and means were used for results presentation and calculation. Differences between baseline and 2 hours were calculated and compared between support surface types. The study design and procedures were approved by the responsible ethics committee (EA1/270/15) and results have been published in clinicaltrials.gov (NCT02930590) and in this journal.30
2.2. Measurement devices and process
All measurements were conducted by experienced and educated researchers according to standard operating procedures for controlled clinical skin research settings. Before baseline measurements, the subjects acclimatised to standard room conditions of 40% to 60% relative humidity and 20°C to 22°C temperature with the sacral and heel skin uncovered for 30 minutes. All skin measurements were conducted twice per investigational skin area and per subject by the same researcher. During the prone position after acclimatisation at baseline and after loading one researcher performed the sacral and another researcher the heel measurements. The reason was that all skin measurements needed to be conducted in parallel as fast as possible to capture immediately the cutaneous response due to loading and occlusion. After the first readings were taken, the probes were removed and immediately placed again on the same skin areas and second measurements were conducted. This method resembles a test‐retest design but not all subjects were measured by all raters. The researchers were not blinded to the two readings per skin area.
Skin surface temperature (°C) was measured with a skin thermometer based on the infrared technique (Courage & Khazaka electronic GmbH, Cologne, Germany). TEWL was measured with the Tewemeter TM 300 (Courage & Khazaka electronic GmbH, Cologne, Germany). This is an open chamber device and readings are expressed in in g/m2/h. According to the applied standard operating procedures, the TEWL probe was placed on the skin surface and readings started with a frequency of 1 Hz. The readings stopped automatically after the standard deviation (SD) of the average of the last 20 readings was lower than 1 g/m2/h. The mean of these 20 readings was then used. According to the manufacturer, the device has an accuracy of ±0.5 g/m2/h under normal room conditions (10°C‐30°C) with TEWL‐values lower than 70 g/m2/h. SCH was measured with the Corneometer CM 825 (Courage & Khazaka electronic GmbH, Cologne, Germany) in arbitrary units (AU) (range, 0‐120 AU). This device measures the SCH from the skin surface to and a depth of approximately 20 μm. According to the manufacturer, the accuracy is ±3%. The MoistureMeterEpiD (Delfin Technologies Ltd.) was used to measure hydration of deeper epidermal and dermal skin layers up to a depth 0.5 mm. The values are expressed in percent of local tissue water (0%‐100%). Erythema was measured with the Mexameter MX 18 (Courage & Khazaka electronic GmbH, Cologne, Germany). This is a narrow‐band reflectance spectrophotometer. An erythema index was expressed in AU (range, 0‐999). The values are the decimal logarithm of the ratio between the intensity of the reflected red (λ = 660 nm) and green (λ = 568 nm) lights (accuracy ±5%).
2.3. Statistical analyses
For this secondary data analysis, the obtained values of the standard mattress group were used only because it is well‐known that reliability and agreement are affected by the population values and within this trial the baseline and post‐loading values were most different. The support surface was a Basic Foam mattress (Stryker Medical, Portage, Michigan) with an depth of 12 cm.30
The means and SDs were calculated per time point and for sacral and heel skin areas. One researcher conducted the repeat measurements per subject and skin area, but different researchers measured different subjects and different skin areas. Therefore, a one‐way model of the ICC was selected to estimate reliability. The ICC was calculated for one reading (ICC (1) and for the means of both readings (ICC (1, 2)) according to the terminology by Shrout and Fleiss.31 According to widely adopted criteria reliability coefficients of 0.8 and higher are considered ‘almost perfect’.25 The standard errors of measurement (SEM)24 and lower and upper limits of agreement32 were calculated to estimate agreement. Bland‐Altman plots33 were created for all skin areas and measurements at baseline.
3. RESULTS
In total, four experienced researchers performed the skin measurements in 15 female subjects, whereas different researchers measured different subjects and skin areas. All researchers passed the standardised education programme for skin measurements according to the standard operating procedures of the study centre. They were female and actively conducted skin measurements for at least 5 years.
The median age of the subjects was 66 (interquartile range 63‐69) years and the median BMI 24.5 (interquartile range 23.7‐26.0) kg/m2.30 Nine subjects hat a Fitzpatrick skin phototype II, and three subjects each had phototypes I and III.30, 34 The reliability and agreement estimates per skin area and time point are shown in Table 1. Bland‐Altman plots are shown in Figures 1, 2, 3, 4, 5, 6, 7, 8, 9, 10.
TABLE 1.
Intraclass correlation, standard errors of measurement, and limits of agreement of skin barrier measurements (n = 15)
| Baseline | After 2 h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | ICC (1) (95% CI) | ICC (1, 2) (95% CI) | Standard error of measurement | Lower limit of agreement | Upper limit of agreement | Mean (SD) | ICC (1) (95% CI) | ICC (1, 2) (95% CI) | Standard error of measurement | Lower limit of agreement | Upper limit of agreement | |
| Skin temperature in °C | ||||||||||||
| Sacrum | 28.8 (1.4) | 0.95 (0.87 to 0.98) | 0.98 (0.93 to 0.99) | 0.31 | −0.78 | 0.90 | 31.8 (0.6) | 0.70 (0.32 to 0.89 | 0.82 (0.48 to 0.94) | 0.29 | −0.50 | 1.03 |
| Heel | 24.8 (1.6) | 0.99 (0.99 to 1.00 | 0.99 (0.99 to 1.00) | 0.11 | −0.27 | 0.34 | 26.8 (1.5) | 0.98 (0.95 to 0.99) | 0.99 (0.98 to 1.00) | 0.18 | −0.35 | 0.62 |
| Transepidermal water loss in g/m2/h | ||||||||||||
| Sacrum | 7.7 (1.7) | 0.46 (0.00 to 0.78) | 0.63 (0.00 to 0.88) | 0.57 | 0.39 | 3.53 | 15.5 (1.5) | 0.66 (0.25 to 0.87) | 0.79 (0.40 to 0.93) | 2.61 | −1.75 | 12.51 |
| Heel | 9.9 (3.6) | 0.89 (0.71 to 0.96) | 0.94 (0.83 to 0.98) | 0.99 | −1.60 | 3.81 | 25.9 (11.3) | 0.85 (0.62 to 0.95) | 0.92 (0.76 to 0.97) | 3.87 | −6.90 | 14.26 |
| Stratum corneum hydration in a.u. | ||||||||||||
| Sacrum | 29.2 (8.5) | 0.96 (0.89 to 0.99) | 0.98 (0.94 to 0.99) | 1.74 | −4.34 | 5.19 | 34.0 (12.3) | 0.96 (0.88 to 0.99) | 0.98 (0.94 to 0.99) | 2.69 | −7.69 | 7.04 |
| Heel | 14.7 (8.1) | 0.98 (0.93 to 0.99) | 0.99 (0.97 to 0.99) | 1.27 | −3.25 | 3.71 | 20.6 (8.4) | 0.92 (0.78 to 0.97) | 0.96 (0.88 to 0.99) | 2.06 | −3.67 | 7.58 |
| Epidermal hydration in a.u. | ||||||||||||
| Sacrum | 42.7 (5.1) | 0.88 (0.69 to 0.96) | 0.94 (0.81 to 0.98) | 1.9 | −5.08 | 5.34 | 45.0 (5.5) | 0.86 (0.63 to 0.95) | 0.92 (0.78 to 0.97) | 2.2 | −6.38 | 5.72 |
| Heel | 24.8 (5.3) | 0.90 (0.74 to 0.97) | 0.95 (0.85 to 0.98) | 1.8 | −4.97 | 4.70 | 29.1 (5.6) | 0.87 (0.66 to 0.95) | 0.93 (0.79 to 0.98) | 2.15 | −5.28 | 6.48 |
| Erythema in a.u. | ||||||||||||
| Sacrum | 180.0 (48.8) | 0.75 (0.41 to 0.91) | 0.86 (0.58 to 0.95) | 26.7 | −66.49 | 79.36 | 241.9 (72.3) | 0.96 (0.90 to 0.99) | 0.98 (0.95 to 0.99) | 14.5 | −37.29 | 42.09 |
| Heel | 182.3 (57.4) | 0.92 (0.78 to 0.97) | 0.96 (0.88 to 0.99) | 17.2 | −50.49 | 43.42 | 240.7 (75.7) | 0.92 (0.79 to 0.97) | 0.96 (0.88 to 0.99) | 22.5 | −58.50 | 64.26 |
FIGURE 1.
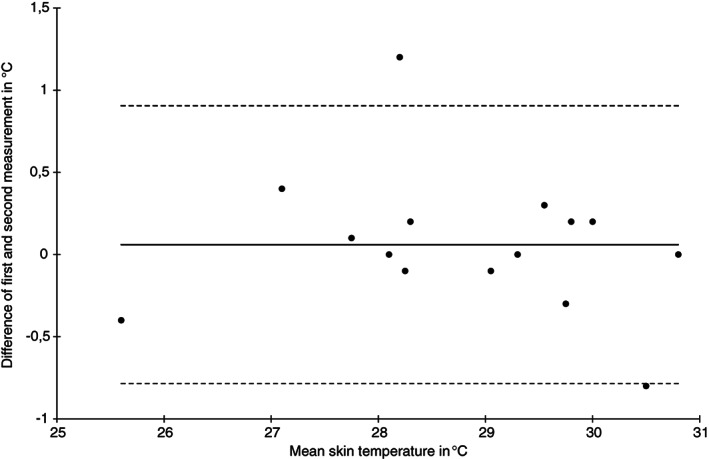
Bland‐Altman plot of skin temperature water loss measurements at the sacral skin area at baseline (n = 15)
FIGURE 2.
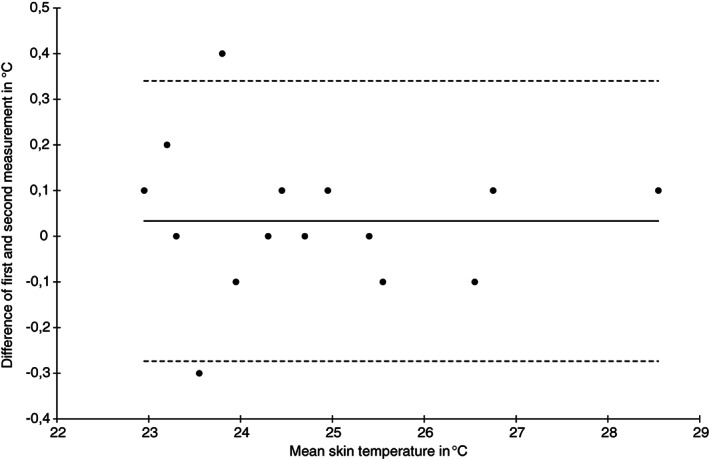
Bland‐Altman plot of skin temperature measurements at the heel skin area at baseline (n = 15)
FIGURE 3.
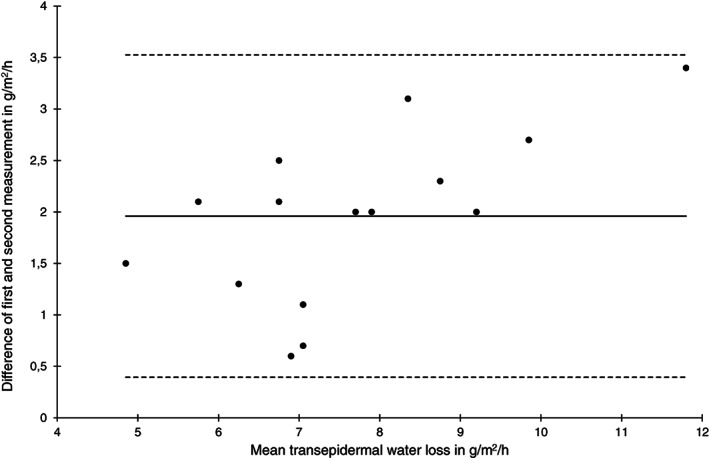
Bland‐Altman plot of transepidermal water loss measurements at the sacral skin area at baseline (n = 15)
FIGURE 4.
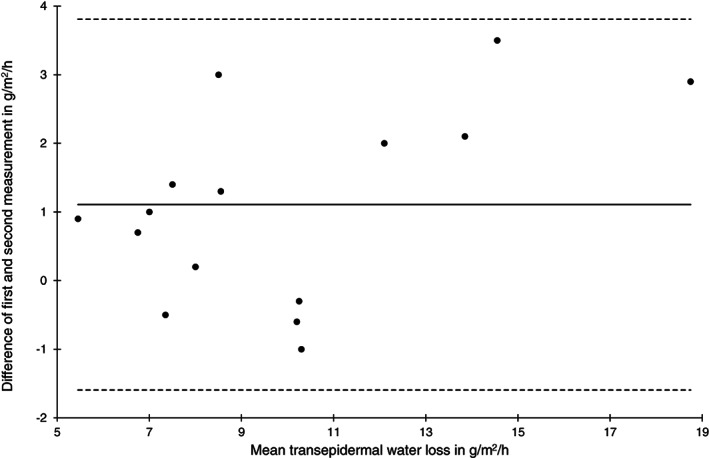
Bland‐Altman plot of transepidermal water loss measurements at the heel skin area at baseline (n = 15)
FIGURE 5.
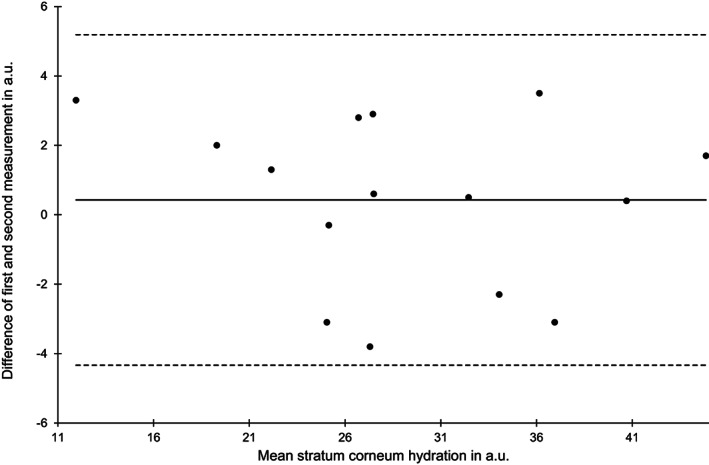
Bland‐Altman plot of stratum corneum hydration measurements at the sacral skin area at baseline (n = 15)
FIGURE 6.
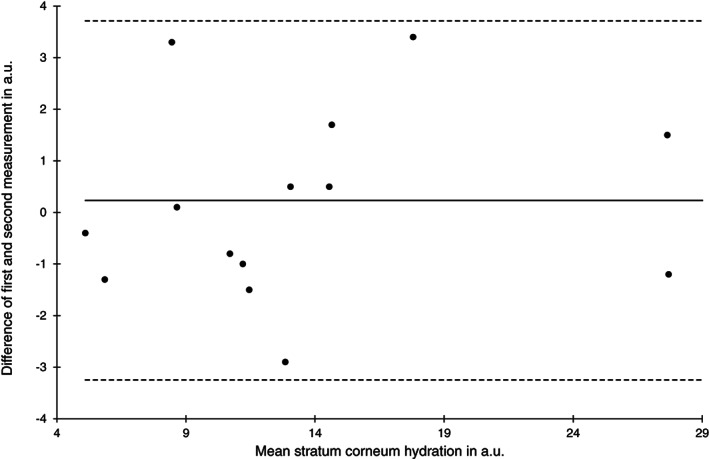
Bland‐Altman plot of stratum corneum hydration measurements at the heel skin area at baseline (n = 15)
FIGURE 7.
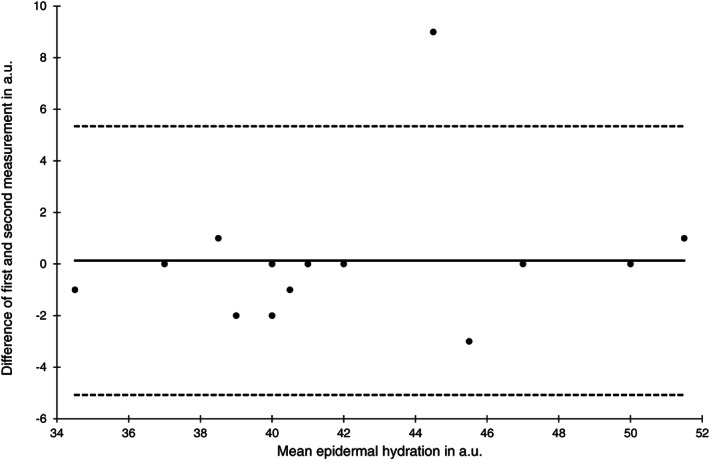
Bland‐Altman plot of epidermal hydration measurements at the sacral skin area at baseline (n = 15)
FIGURE 8.
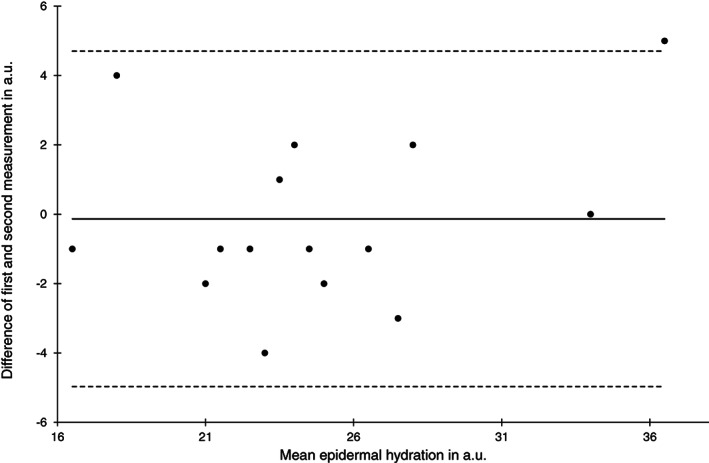
Bland‐Altman plot of epidermal hydration measurements at the heel skin area at baseline (n = 15)
FIGURE 9.
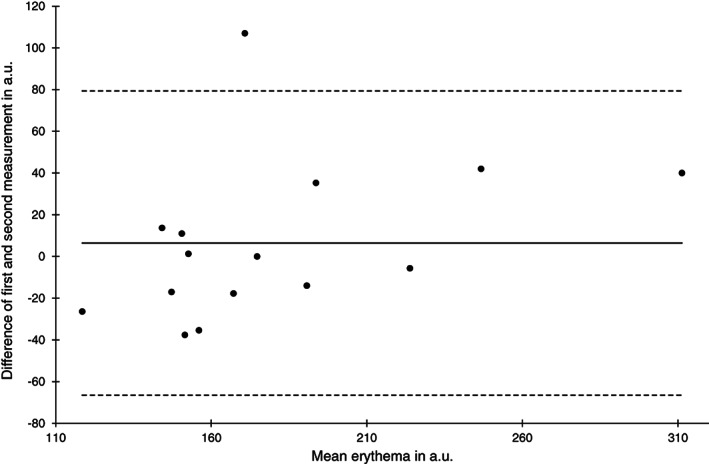
Bland‐Altman plot of erythema measurements at the sacral skin area at baseline (n = 15)
FIGURE 10.
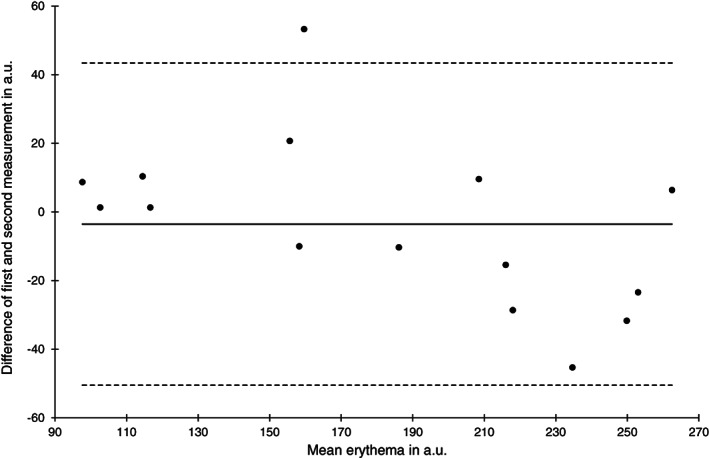
Bland‐Altman plot of erythema measurements at the heel skin area at baseline (n = 15)
The ICC (1) for skin temperature was highest for the heel skin at baseline (0.99, 95% CI 0.99‐1.00) and lowest at the sacral skin (0.70, 95% CI 0.32‐0.89) after 2 hours. The SEM was approximately 0.3°C for the sacrum, and substantially lower for the heel. The limits of agreement were wider for the sacrum (−0.8 to 1.0) and narrower for the heels (−0.3 to 0.6) and the Bland‐Altman plots indicate higher variability for sacral (Figure 1) compared with the heel area temperature measurements (Figure 2).
Reliability of TEWL at the sacral area at baseline and after 2 hours was low (ICC (1) 0.46 and 0.66) and high for the heels. On the contrary, the SEM was higher and the limits of agreement were wider for the heel skin compared with the sacral skin at both time points. Bland‐Altman plots indicate that the absolute measurement errors for the sacral (Figure 3) and the heel area (Figure 4) were comparable.
The reliability of the SCH estimates was high for both skin areas and time points (ICC (1) 0.92‐0.98). Limits of agreement and SEM were similar between all four estimates (Figures 5 and 6).
The reliability of the epidermal hydration measurements was lower compared with the SCH with ICC (1) ranging from 0.86 to 0.90. Limits of agreement and SEM were comparable to the SCH measurements and nearly identical between skin areas and time points (Figures 7 and 8).
The lowest reliability was observed for erythema measurements at the sacral skin area at baseline (ICC (1) 0.75) but it was higher than 0.9 at the heel skin and after 2 hours. Compared with all other skin barrier measurements the SEM was highest ranging from 14.5 to 26.7 a.u. and the limits of agreement where widest. The high absolute measurement errors can be also seen in the Bland‐Altman Plots (Figures 9 and 10).
4. DISCUSSION
Temperature, TEWL, SCH, and erythema values at baseline and after 2 hours loading were very well comparable with previous study results and reference values.10, 11, 21 Therefore, the observed skin barrier measurements seem to be realistic estimates for the sacral and heel skin in healthy aged women. In addition, comparing baseline with post loading values indicates that 2 hours loading on a basic foam mattress leads to an increase of skin temperature, TEWL, SCH, epidermal hydration, and erythema.30 Similar observations were described previously and indicate that mechanical loading of heel and sacral skin on a basic foam mattress leads to occlusion and to a variety of structural skin and soft tissue changes.9, 10, 11, 35, 36
Overall, the SCH and the epidermal hydration single measurements (ICC (1)) showed nearly perfect reliability at both skin areas and time points. Therefore, the reliability increase of the means of both repeat measurements (ICC (1, 2)) was small. Similar high ICC of SCH for the forearm and leg skin was previously reported.29 Therefore, single SCH estimates using the abovementioned device and measurement procedures seem to contain low relative measurement errors and seem to be a useful parameter in clinical skin and PU prevention research for group comparisons. The same may apply for epidermal hydration measurements, but because this is the first published reliability estimate of this device, it is difficult to be generalised. The SEM of approximately two for both hydration measurements and the range of limits of agreement of approximately 10 indicate rather high absolute measurement errors. Therefore, single SCH and epidermal hydration estimates at the heels and sacrum should not be used to make inferences about individual skin conditions and or changes during an intervention.
Except for the sacrum after 2 hours, there was a nearly perfect reliability for skin temperature measurements, which is also supported by previous research.29 Using different infrared thermal imaging systems Liu et al reported an ICC of 0.82 for back skin37 and Chatchawan et al reported ICCs for foot skin temperatures higher than 0.9.38 This indicates that skin temperature measurements using infrared methods are reliable. Limits of agreement and the SEM also indicate small absolute measurement errors not exceeding 1°C. The low ICC (1) of 0.7 might be explained by the low variability of the mean sacral temperature of 31.8°C. The SD of 0.6 was substantially lower compared with the other three temperature values.
At the sacral skin the reliability of the single (ICC (1)) and the mean of both TEWL measurements (ICC (1, 2)) was low, but high at the heel skin. Similar to the skin temperature, the SD indicates a higher variability of the heel measurements, which may explain the lower relative error. On the contrary, the absolute measurements errors of TEWL at the heels were higher compared with the sacrum. Interestingly, the SEM and limits of agreement at baseline are very well comparable to previous estimates.29 After 2 hours the absolute measurement errors of TEWL increased substantially at heels and sacrum but our results are very similar to SEMs and limits of agreement in scar research.6 It is well‐known that compared with other skin barrier measurements, TEWL readings may be affected by various internal and external influences causing variability.17, 19 Our results seem to support this biological variability of TEWL in PU prevention research. Increasing the number of repeated measurements and/or using closed or condenser chamber39 instead of open chamber devices might decrease measurement error in this setting. Because TEWL is associated with skin temperature, adjustment to a standard reference temperature is also recommended to reduce variability in clinical research.30, 40, 41
Except for the sacrum at baseline, the erythema measurements were nearly perfectly reliable. ICC estimates of 0.87 for Mexameter MX 18 values in 258 newborns42 and near 1 in approximately 400 women were reported recently.43 This indicates that erythema values using this device are reliable in different populations. The SEM and the limits of agreement were highest compared with all other skin parameters indicating high absolute measurement errors. However, related to the rather wide range of possible readings produced by this device the errors seem to be comparable.
Results further indicate a clear association between variability in terms of SD and reliability coefficients, which in turn is not necessarily related to absolute measurement errors. Therefore, our results support the methodological recommendation that always both, reliability and agreement must be reported to characterise the performance of empirical measurements.24, 25, 26 At the same time results indicate that measurement errors seem to be independent from the observed values and there were no substantial differences between baseline and post loading values.
In wound and PU prevention research skin barrier measurements are increasingly being used.9 One major advantage is, that functional cutaneous changes occur long before obvious physical alterations become visible.30, 44 However, our results indicate that obtained readings of skin barrier are prone to measurement error. This is not usual. Measurement errors occur in nearly every discipline.45 Because it cannot be avoided completely, we recommend (a) that more empirical evidence is generated to obtain an understanding of the measurement instrument properties of different skin measurement devices and instruments in the skin and wound care field; (b) to select those skin instruments and measurements that contain low measurement errors; (c) to develop strategies to reduce known sources of variability by strictly developing and following standard operating procedures in clinical research and practice.
5. LIMITATIONS
This study was not planned as a reliability and agreement study, but existing data from a clinical trial was used. Therefore, the sample size of n = 15 was low and variability of skin measurements was investigated, but not possible examiner variability. The analysis followed an exploratory descriptive approach and it was not intended to demonstrate certain degrees of reliability or agreement. Female subjects were included only, to reduce possible additional biological variability due to sex. The presented measurement properties of the sacral and heel skin values are not generalisable to other skin areas and skin measurement instruments.
6. CONCLUSION
Single measurements of skin temperature, SCH, and epidermal hydration using the applied methods at the sacral and heel skin areas are nearly perfectly reliable and can be used for group comparisons in clinical research and practice. Means of at least two measurements should be used for estimating TEWL and erythema. Evidence is needed to inform researchers about relative and absolute measurement errors of commonly applied instruments and measurements in skin and wound research and the provided estimates are to be used for future confirmatory study designs.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENTS
The authors highly appreciate the support of the team of the Clinical Research Centre for Hair and Skin Science at the Department of Dermatology and the participation of the healthy volunteers. We thank Prof. Dr. Michael Clark (Welsh Wound Network, UK) for correcting the English. This investigator‐initiated study was supported by Stryker European Operations BV, Amsterdam, Netherlands.
Kottner J, Blume‐Peytavi U. Reliability and agreement of instrumental skin barrier measurements in clinical pressure ulcer prevention research. Int Wound J. 2021;18:716–727. 10.1111/iwj.13574
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1.Akdeniz M, Tomova‐Simitchieva T, Dobos G, Blume‐Peytavi U, Kottner J. Does dietary fluid intake affect skin hydration in healthy humans? A systematic literature review. Skin Res Technol. 2018;24(3):459‐465. [DOI] [PubMed] [Google Scholar]
- 2.Chuong CM, Nickoloff BJ, Elias PM, et al. What is the 'true' function of skin? Exp Dermatol. 2002;11(2):159‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner A, Kottner J, Coleman S, et al. Outcomes for pressure ulcer trials (OUTPUTs) project: review and classification of outcomes reported in pressure ulcer prevention research. Br J Dermatol. 2020. [DOI] [PubMed] [Google Scholar]
- 4.Farid KJ, Winkelman C, Rizkala A, Jones K. Using temperature of pressure‐related intact discolored areas of skin to detect deep tissue injury: an observational, retrospective, correlational study. Ostomy Wound Manage. 2012;58(8):20‐31. [PubMed] [Google Scholar]
- 5.Dini V, Barbanera S, Romanelli M. Quantitative evaluation of maceration in venous leg ulcers by transepidermal water loss (TEWL) measurement. Int J Low Extrem Wounds. 2014;13(2):116‐119. [DOI] [PubMed] [Google Scholar]
- 6.Gardien KL, Baas DC, de Vet HC, Middelkoop E. Transepidermal water loss measured with the Tewameter TM300 in burn scars. Burns. 2016;42(7):1455‐1462. [DOI] [PubMed] [Google Scholar]
- 7.Berardesca E, Vignoli GP, Fideli D, Maibach H. Effect of occlusive dressings on the stratum corneum water holding capacity. Am J Med Sci. 1992;304(1):25‐28. [DOI] [PubMed] [Google Scholar]
- 8.Cavallini M, Gazzola R, Vaienti L. Effects of adhesive dressings on stratum corneum conductance. Skin Res Technol. 2012;18(2):241‐244. [DOI] [PubMed] [Google Scholar]
- 9.Bader DL, Worsley PR. Technologies to monitor the health of loaded skin tissues. Biomed Eng Online. 2018;17(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfannes EKB, Blume‐Peytavi U, Kottner J. Patterns and associations of structural and functional cutaneous responses during loading at heel and sacral skin in aged females: a reanalysis of clinical study data. J Tissue Viability. 2018;27(3):123‐129. [DOI] [PubMed] [Google Scholar]
- 11.Kottner J, Black J, Call E, Gefen A, Santamaria N. Microclimate: a critical review in the context of pressure ulcer prevention. Clin Biomech (Bristol, Avon). 2018;59:62‐70. [DOI] [PubMed] [Google Scholar]
- 12.Scheel‐Sailer A, Frotzler A, Mueller G, Annaheim S, Rossi RM, Derler S. Biophysical skin properties of grade 1 pressure ulcers and unaffected skin in spinal cord injured and able‐bodied persons in the unloaded sacral region. J Tissue Viability. 2017;26(2):89‐94. [DOI] [PubMed] [Google Scholar]
- 13.Yapp JH, Raja Ahmad RMK, Mahmud R, et al. Determining weight‐bearing tissue condition using peak reactive hyperemia response trend and ultrasonographic features: implications for pressure ulcer prevention. Wound Repair Regen. 2019;27(3):225‐234. [DOI] [PubMed] [Google Scholar]
- 14.Kottner J, Ludriksone L, Garcia Bartels N, Blume‐Peytavi U. Do repeated skin barrier measurements influence each other's results? An explorative study. Skin Pharmacol Physiol. 2014;27(2):90‐96. [DOI] [PubMed] [Google Scholar]
- 15.Kottner J, Vogt A. Transepidermal water loss. In: Baran R, Maibach HI, eds. Textbook of Cosmetic Dermatology. Boca Raton, FL: CRC Press; 2017. [Google Scholar]
- 16.Berardesca E. European Group for Efficacy Measurements on C, other topical P. EEMCO guidance for the assessment of stratum corneum hydration: electrical methods. Skin Res Technol. 1997;3(2):126‐132. [DOI] [PubMed] [Google Scholar]
- 17.du Plessis J, Stefaniak A, Eloff F, et al. International guidelines for the in vivo assessment of skin properties in non‐clinical settings: part 2. Transepidermal water loss and skin hydration. Skin Res Technol. 2013;19(3):265‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefaniak AB, Plessis J, John SM, et al. International guidelines for the in vivo assessment of skin properties in non‐clinical settings: part 1. pH. Skin Res Technol. 2013;19(2):59‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogiers V, Group E . EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001;14(2):117‐128. [DOI] [PubMed] [Google Scholar]
- 20.Pierard GE. EEMCO guidance for the assessment of skin colour. J Eur Acad Dermatol Venereol. 1998;10(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 21.Akdeniz M, Gabriel S, Lichterfeld‐Kottner A, Blume‐Peytavi U, Kottner J. Transepidermal water loss in healthy adults: a systematic review and meta‐analysis update. Br J Dermatol. 2018;179(5):1049‐1055. [DOI] [PubMed] [Google Scholar]
- 22.Ludriksone L, Garcia Bartels N, Kanti V, Blume‐Peytavi U, Kottner J. Skin barrier function in infancy: a systematic review. Arch Dermatol Res. 2014;306(7):591‐599. [DOI] [PubMed] [Google Scholar]
- 23.Kottner J, Vogt A, Pfannes EB, et al. Letter to the editor. Clin Biomech (Bristol, Avon). 2016;33:84. [DOI] [PubMed] [Google Scholar]
- 24.de Vet HC, Terwee CB, Mokkink LB, KD L. Measurement in Medicine. New York, NY: Cambridge University Press; 2011. [Google Scholar]
- 25.Kottner J, Audige L, Brorson S, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. Int J Nurs Stud. 2011;48(6):661‐671. [DOI] [PubMed] [Google Scholar]
- 26.Streiner DL, Norman GR, Cairney J. Health Measurement Scales. Oxford: Oxford University Press; 2015. [Google Scholar]
- 27.Kottner J, Cuddigan J, Carville K, et al. Pressure ulcer/injury classification today: an international perspective. J Tissue Viability. 2020;29(3):197‐203. [DOI] [PubMed] [Google Scholar]
- 28.Rayner R, Carville K, Leslie G, Dhaliwal SS. Measurement of morphological and physiological skin properties in aged care residents: a test‐retest reliability pilot study. Int Wound J. 2017;14(2):420‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elban F, Hahnel E, Blume‐Peytavi U, Kottner J. Reliability and agreement of skin barrier measurements in a geriatric care setting. J Tissue Viability. 2020;29:269‐276. [DOI] [PubMed] [Google Scholar]
- 30.Tomova‐Simitchieva T, Lichterfeld‐Kottner A, Blume‐Peytavi U, Kottner J. Comparing the effects of 3 different pressure ulcer prevention support surfaces on the structure and function of heel and sacral skin: an exploratory cross‐over trial. Int Wound J. 2018;15(3):429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420‐428. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135‐160. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307‐310. [PubMed] [Google Scholar]
- 34.Roberts WE. Skin type classification systems old and new. Dermatol Clin. 2009;27(4):529‐533. viii. [DOI] [PubMed] [Google Scholar]
- 35.Lechner A, Rancan F, Hadam S, Vogt A, Blume‐Peytavi U, Kottner J. Comparing the effects of three different multilayer dressings for pressure ulcer prevention on sacral skin after prolonged loading: an exploratory crossover trial. Wound Repair Regen. 2020. [DOI] [PubMed] [Google Scholar]
- 36.Kottner J, Dobos G, Andruck A, et al. Skin response to sustained loading: a clinical explorative study. J Tissue Viability. 2015;24(3):114‐122. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Duan Z, Chen L, et al. Short‐term effect of different taping methods on local skin temperature in healthy adults. Front Physiol. 2020;11:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatchawan U, Narkto P, Damri T, Yamauchi J. An exploration of the relationship between foot skin temperature and blood flow in type 2 diabetes mellitus patients: a cross‐sectional study. J Phys Ther Sci. 2018;30(11):1359‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imhof RE, De Jesus ME, Xiao P, Ciortea LI, Berg EP. Closed‐chamber transepidermal water loss measurement: microclimate, calibration and performance. Int J Cosmet Sci. 2009;31(2):97‐118. [DOI] [PubMed] [Google Scholar]
- 40.Hahnel E, Blume‐Peytavi U, Trojahn C, et al. The effectiveness of standardized skin care regimens on skin dryness in nursing home residents: a randomized controlled parallel‐group pragmatic trial. Int J Nurs Stud. 2017;70:1‐10. [DOI] [PubMed] [Google Scholar]
- 41.Mathias CG, Wilson DM, Maibach HI. Transepidermal water loss as a function of skin surface temperature. J Invest Dermatol. 1981;77(2):219‐220. [DOI] [PubMed] [Google Scholar]
- 42.Maya‐Enero S, Candel‐Pau J, Garcia‐Garcia J, Gimenez‐Arnau AM, Lopez‐Vilchez MA. Validation of a neonatal skin color scale. Eur J Pediatr. 2020;179(9):1403‐1411. [DOI] [PubMed] [Google Scholar]
- 43.Isa ZM, Shamsuddin K, Bukhari NBI, et al. The reliability of Fitzpatrick skin type chart comparing to Mexameter (MX 18) in measuring skin color among first trimester pregnant mothers in Petaling district, Malaysia. Malays J Public Health Med. 2016;16(3):59‐65. [Google Scholar]
- 44.Marks R, Black D. Methodologies to produce and assess standardized trauma to the skin. Am J Ind Med. 1985;8(4‐5):491‐498. [DOI] [PubMed] [Google Scholar]
- 45.Buonaccorsi JP. Measurement Error: Models, Methods and Applications. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


