Abstract
Recent reviews suggest that amniotic membrane products may accelerate healing of diabetic foot ulcers. A new dried human amniotic membrane (dHAM) has been used for ocular ulcers but not for diabetic foot ulcers. This was a multi‐centre, prospective, patient and observer blind, randomised controlled pilot trial, to investigate whether 2 weekly addition of the dHAM to standard care versus standard care alone increased the proportion of healed participants' index foot ulcers within 12 weeks. Thirty‐one people (mean age 59.8 years, 81% male, 87% type 2 diabetes) were randomised (15 dHAM, 16 usual care). Within 12 weeks, healing occurred in 4 (27%) ulcers in the dHAM group versus 1 (6.3%) usual care group (P = .1). Percentage wound area reduction was higher in the dHAM versus control group. (P = .0057). There was no difference in AEs between the two groups. Six participants allocated to dHAM correctly identified their treatment group, although 5 in usual care incorrectly thought they were in the intervention arm. This pilot trial result is encouraging showing that this dHAM preparation is safe and promising treatment. These results will be used to design a statistically powered, definitive double blind randomised controlled trial.
Keywords: amniotic membrane, amputation, diabetic foot ulcer, wound healing
1. BACKGROUND
Diabetic foot ulcers (DFUs) are common and present a major source of disability, distress, and cost. Healing is often delayed and both major and minor amputations are common outcomes.1, 2 Standard treatment strategies should—when indicated—include antimicrobial treatment, vascular surgical intervention, offloading, and regular debridement.3 If these treatment strategies are properly applied a majority of chronic non‐healing ulcers will heal, although healing times may be prolonged.
There are many dressing products available for the clinician to choose from many of which purport to accelerate healing, and improve outcomes such as avoidance of amputations. The published literature to support these interventions is poor however,4 many of the studies being of insufficient quality for clinicians to have confidence in any apparent positive benefits.5
A number of products derived from different components of amniotic membrane have been developed to enhance healing; cryopreserved preparations contain living cells as well as growth factors, whereas dehydrated products, which are easier to store and handle, contain growth factors but no living cells. Although the quality of the evidence was graded as low in a recent systematic review independently performed by the International Working Group of the Diabetic Foot (IWGDF), the associated guidance suggested that amnion derived products may have a beneficial effect on ulcer healing. The evidence was insufficient however to support the superiority of one product above another, and it was emphasised that the use of amniotic membrane should be considered only when usual best quality care alone was insufficient to heal the ulcer.3
A new UK‐based product Omnigen (NuVision Biotherapies Ltd, Nottingham, UK), is a delicately dried human amniotic membrane derived off‐the shelf (i.e., can be stored at room temperature) therapy. It has been used in ophthalmology and has demonstrated healing of ocular surface chronic ulcers and ocular surface disease.6 It has not, to date however, been used as an adjunctive therapy to aid healing of non‐healing diabetic foot ulcers.
The aim of this pilot study was therefore to investigate whether the addition of this dHAM product to standard care versus standard care alone led to a greater increase in the proportion of participants achieving healing of their index diabetic foot ulcer within 12 weeks, with a view to using the data to design a future, statistically powered, definitive trial.
2. METHODS
2.1. Trial design
This was a multi‐centre, prospective, patient and observer blind, randomised controlled pilot trial, recruiting patients from two specialist diabetic foot clinic in the United Kingdom; University Hospitals of Derby and Burton NHS Foundation Trust (UHBD) and Nottingham University Hospitals NHS Trust (NUH).
The study was performed in compliance with all UK regulatory requirements and in accordance with the ethical principles of the Declaration of Helsinki and recommendations for Good Clinical Practice. The study sponsor was University Hospitals of Derby and Burton NHS Foundation Trust. The study was approved by the National Health Research Authority and East Midlands‐Derby Research Ethics Committee; IRAS Project ID: 235573, and was registered with Clinicaltrials.gov NCT03483467.
2.2. Study setting and participants
Participants were people aged 18 years and over who had diabetes according to WHO criteria complicated by one or more foot ulcers and an HbA1c≤108 mmol/mol at baseline. If the participant had more than one foot ulcer, one was chosen as the index ulcer, usually the largest or most clinically significant. Index ulcers were situated below the level of the malleolus and had a minimum ulcer diameter of 5 mm and maximum ulcer diameter of 20 mm. All ulcers had been present for 4 weeks or more. Either the ankle‐brachial index (ABPI) of the affected limb was >0.9 or the dorsalis pedis pulse and/or the tibialis posterior pulse was palpable. Full inclusion and exclusion criteria are detailed in Table 1.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion criteria |
Patients were eligible for inclusion only if ALL of the following criteria applied:
|
| Exclusion criteria |
The participant was not eligible to enter the trial if ANY of the following applied:
|
2.3. Randomisation and blinding
Eligible participants were randomly assigned to usual care plus intervention or usual care alone. Participants were assigned to treatment groups using an online randomisation system maintained by the Derby Clinical Trial Support Unit (DCTSU). Access to the system was granted by the DCTSU in accordance with the responsibilities on the delegation log. Block randomisation was used, with varying sized blocks to reduce predictability including stratification by size of wound big or small (defined as <1 cm2 versus ≥1 cm2) to guarantee an even distribution across the two study arms. Participants and clinical investigators assessing outcomes were masked to group assignment throughout the study duration, as was the study statistician until the primary analysis had been completed and reported to the Sponsor and Funder. Site care givers were not blind to the treatment allocation.
2.4. Procedures
Eligible patients were approached by their usual clinical carers to determine whether they were interested in participating in the study. Written informed consent was taken prior to any trial procedures.
All eligible ulcers were managed with the best available standard of usual care, including offloading, according to International Working Group of the Diabetic Foot (IWGDF) guidelines3 either alone or in addition to the intervention. Basic demographics, medical history, and eligibility criteria were assessed at baseline following informed consent. Thereafter, assessment of wound characteristics, active medication including antibiotic prescriptions, adverse effects, serious adverse events were recorded at every visit. Participant visits were scheduled every 2 weeks.
2.5. The study intervention
The active intervention was the application of the dHAM product directly to the index ulcer following any sharp debridement of callus required. The dHAM was placed epithelial side up on the wound. The index foot was draped so that participants could not see how the wound was being dressed and dummy packaging used to keep participants blind to the intervention group. The dummy packaging was developed to look identical to the dHAM packaging including dummy serial numbers, but contained no product. Treatment protocols were identical in both groups including opening the dummy packaging. The same inert non‐adherent primary dressing was used whether placed over the dHAM or directly onto the index ulcer, and participants and their carers advised not to disturb the primary dressing. This was then covered with an inert secondary dressing or retention layer. Participants were advised they could replace the secondary dressing and/or retention layer as necessary.
2.6. Outcomes
The primary outcome was the number (%) of ulcers that healed within 12 weeks following baseline visit. Healing was assessed following any necessary debridement of callus and was defined as complete epithelialisation without drainage, and which was maintained for 2 weeks. Healing was confirmed both at the time of healing and 2 weeks thereafter by an observer who was blind to randomisation group. The date of healing was defined as that at which the ulcer was first noted by the clinical researcher and confirmed by the observer blind to the randomisation group.
Secondary ulcer‐related outcomes included time to healing in those that healed within the 12 weeks active intervention period, the number (%) of ulcers healed within 6 weeks, the percentage change in ulcer area from baseline assessed from digital images of acetate tracings using Image J,7 the incidence of secondary infection of the index ulcer, and pain in the area of the ulcer assessed by a 100 mm Visual analogue scale (VAS).
Secondary patient‐related outcomes also included adverse and serious adverse events, the incidence of major (above ankle) and minor (below ankle) amputation.
Trial related outcomes included recruitment rate (randomisations/month/centre), and reasons for patients or investigators not randomising (from screening logs). We also assessed the proportion of patients who were able to determine treatment allocation as judged by a patient questionnaire administered at the time of confirmed healing or week 12, whichever was the soonest. The questionnaire asked the patient whether or not they thought they knew which arm of the trial they were in and if so why.
2.7. Sample size
As this was a pilot study formal power calculations were not undertaken. A sample size target of 30 participants was chosen as an acceptable sample size for pilot studies.8, 9
2.8. Statistical methods
All analyses were conducted using an Intention To Treat (ITT) approach, unless otherwise specified as Per Protocol (PP) approach. Under the ITT approach participants were included in the analysis group that they were originally randomised. Under the PP approach, participants, who completed the study and did not have any major protocol violations that affected the outcomes, were included in the analysis under the treatment group they were receiving when their index wound healed or at 12 weeks, if their index wound did not heal.
Standard descriptive statistics summarised the distribution of baseline and secondary variables across each of the randomisation groups. Continuous baseline variables were reported with means and 95% confidence intervals (95% CIs), if shown to be normally distributed using a combined skewness and kurtosis test (as described by D'Agostino et al10 but with the adjustment made by Royston,11 otherwise were reported with medians and Interquartile Ranges (IQRs). Categorical variables were reported with frequencies and percentages.
The primary analysis was the proportions and 95% CIs of participants whose index wound healed within 12 weeks of study treatment in the two treatment arms, along with the difference in proportions and 95% CI between treatment arms, and a comparison using a Fisher's Exact test. This was also repeated using a Per Protocol approach instead of ITT as a sensitivity analysis.
Wound area reduction was compared between treatment groups using a Kruskal‐Wallis test. Time to healing, number of secondary infections, number of Serious Adverse Reactions (SAR), and number of Serious Adverse Events (SAE) were compared between treatment groups using t‐tests, if shown to be normally distributed, otherwise using Mann U Whitney tests.
Fisher's Exact tests were used to compare the proportion of index wounds healed at 6 weeks in each treatment group and to compare the proportion of secondary infections in index wounds.
All feasibility endpoints were reported with frequencies and percentages. Missing data were not imputed and cases with complete data were included in the analyses, except for the wound area reduction where the 'last observation carried forward' method was used for imputing any missing values. This method was applied for the wound area reduction as it is clinically assumed that if a measurement is not taken then no change in wound size has occurred since the last visit. As this was an external pilot study, any P‐values were presented but not compared with a predefined cut‐off value and were interpreted with caution.
2.9. Role of the funders and sponsor
The study was sponsored by University Hospitals of Derby and Burton NHS Foundation Trust, and funded by NuVision Biotherapies Ltd and an unrestricted grant from Medilink East Midlands Ltd and East Midlands Academic Health Science Network. The funders had no role in study performance, data collection, data analyses or data interpretation. The trial statisticians (MJ and AF) had access only to anonymised data until the primary analysis of the study had been completed. The chief investigator (FG,) had full access to all data after the primary analysis had been performed and had final responsibility for the decision to submit the results for publication.
3. RESULTS
The first patient was consented in April 2018 and the last in May 2019. Altogether 31 people and randomised, (Figure 1). Sixteen participants were randomised to usual care and 15 participants to dHAM in addition to usual care.
FIGURE 1.
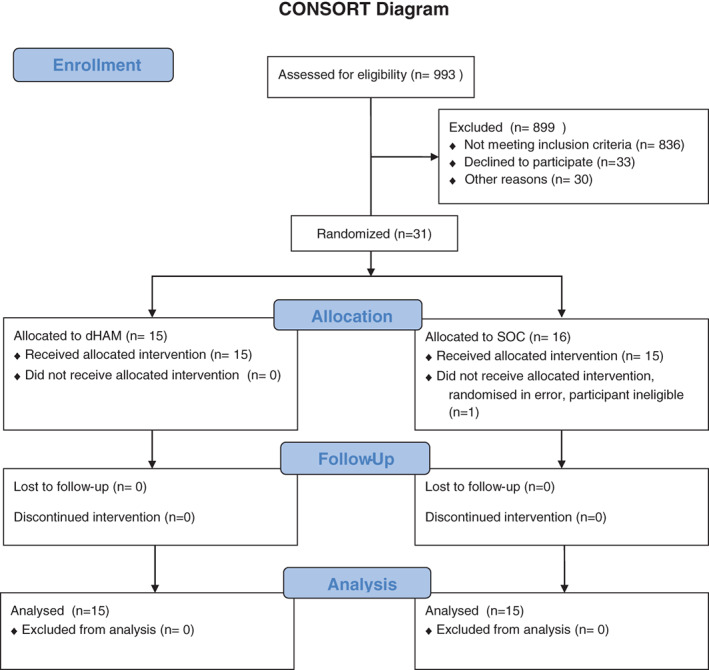
Consort diagram
3.1. Baseline data
The baseline characteristics were well balanced between treatment groups (Table 2). The mean age of participants was 59.8 (SD = 10) years, 25 (81%) male, and 27 (87%) had type 2 diabetes. The median HbA1c was 61 (IQR 51‐84) mmol/mol. The median baseline ulcer area was 0.62 (IQR 0.35‐1.30) cm2 and 2 (6.5%) had mild infection by IDSA criteria at baseline. Twenty eight (88%) of participants had palpable pulses with only 1 participant (3.1%) having an ABPI <0.8 of the index limb.
TABLE 2.
Baseline clinical characteristics
| Summary of baseline data | dHAM + standard care (n = 15) | Standard care (n = 16) |
|---|---|---|
|
Age (years) Mean (SD) |
62.8 (9.21) | 57 (10.39) |
| Male sex n (%) | 12 (80) | 13 (81) |
|
Type 2 diabetes n (%) |
15 (100) | 12 (75) |
|
Index wound area (cm2) Median (25th‐75th IQR) |
0.62 (0.38‐1.15) | 0.68 (0.34‐1.28) |
|
HbA1c (mmol/mol) mean (95% CI) |
65 (54‐77) | 72 (63‐80) |
| Type of offloading, n (%) | ||
| Fitted footwear/custom made shoes/orthoses | 5 (33) | 3 (19) |
| Non‐removeable cast/device for foot | 0 (0) | 1 (6) |
| Non‐removeable cast/device for lower leg | 1 (7) | 0 (0) |
| Normal footwear | 0 (0) | 3 (19) |
| Padded slipper or shoe | 1 (7) | 1 (6) |
| Removeable cast/device for foot | 6 (60) | 4 (25) |
| Removeable cast/device for lower leg | 2 (13) | 4 (25) |
| Index wound infected (defined by IDSA) n (%) | 0 (0) | 2 (13) |
| Palpable pulses n (%) | 13 (87) | 15 (94) |
| ABPI n (%) | ||
| 0.5‐0.79 | 1 (7) | 0 (0) |
| 0.8‐0.99 | 4 (27) | 1 (6) |
| 1.0‐1.39 | 7 (47) | 9 (56) |
| ≥1.4 | 1 (7) | 2 (13) |
| Not recorded/unable to measure | 2 (13) | 4 (25) |
| eGFR n (%) | ||
| 31‐45 mL/min/1.73 m2 | 3 (20) | 3 (19) |
| 46‐60 mL/min/1.73 m2 | 2 (13) | 1 (6) |
| Greater than 60 mL/min/1.73 m2 | 10 (67) | 12 (75) |
Note: Data are given as median (IQR) or number of participants (%).
The two groups were well matched in terms of the types of offloading used throughout the study.
3.2. Numbers analysed
One participant was withdrawn immediately post randomisation as they were on antibiotics for suspected osteomyelitis, they had no trial treatments and were thus not included in the intention to treat (ITT) population. They had been randomised to standard care, leaving the ITT population of 15 participants allocated to dHAM plus usual care and 15 participants in standard care alone.
Under the PP population, 12 participants were included in the dHAM group and 13 in the standard care group.
3.3. Primary outcome
Within 12 weeks 4 (27%) of index ulcers in the dHAM group had healed versus 1 (6.3%) in the standard care group [proportion difference = 20% (−4.9%, 46%), P = .172] in the ITT population. In the PP population healing occurred in four participants (33%) in the intervention group versus 1 (8%) in the usual care group [proportion difference = 25% (−5.6%, 47%), P = .160].
3.4. Secondary and feasibility outcomes
The change in ulcer area from baseline is given in Figure 2. The percentage wound area reduction was higher in the dHAM group compared with the standard care group [Chi‐squared with ties = 10.231, P = .0014].
FIGURE 2.
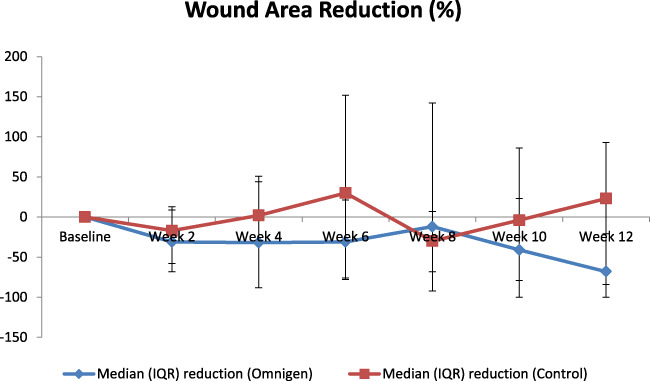
Median (IQR) percentage wound area reduction from randomisation
Other secondary outcomes are shown in Table 3.
TABLE 3.
Primary secondary and safety study outcomes
| dHAM (n = 15) | Standard care (n = 16) | Difference between treatments | P value | |
|---|---|---|---|---|
| Index wound healed within 12 weeks, n (%) [95% CI] | 4 (27%) [1.3‐52] |
1 (6%) [0.01a‐19.6] |
20% (−4.9% to 46%) | .172 |
|
Time to healing of those that healed Mean number weeks (95% CI) |
5 (−1 to 11) (n = 4) |
6 (‐a) (n = 1) |
−1 (‐a) | ‐a |
|
Index wound healed within 6 weeks % (95% CI) |
20% (0.01b‐43) | 6.3% (0.01b‐20) | 14 (0.01b‐37) | .3 |
|
Number of SAEs Median (25th‐75th IQR) |
0 (0‐1) (n = 9) |
0 (0‐1) (n = 7) |
0 (0‐1) (n = 16) |
.457 |
|
Number of AEs Median (25th‐75th IQR) |
1 (1‐2) (n = 9) |
1 (1‐2) (n = 7) |
0 (0‐1) (n = 16) |
.846 |
Note: Data are given for important primary and secondary outcomes for patients allocated to intention‐to‐treat population in people randomised to standard care alone (N = 15) or standard care and dHAM plus standard care (N = 15). For the pre protocol analyses the population was standard care alone (N = 13) and dHAM plus standard care (N = 12).
Not enough data available to calculate.
Unable to calculate an accurate non‐negative 95% CI lower boundary due to the small sample size.
Of the 29 participants who completed an end of treatment questionnaire 13 (45%) said that they thought they knew which treatment arm they were allocated to. Of these, eight correctly identified their treatment arm; six allocated to dHAM and two allocated to standard care alone. An almost equal number of those allocated to standard care alone, however, incorrectly perceived they were in the treatment arm (n = 5). The most common reason for participants perceiving that they were in the intervention arm was that their ulcer improved quicker than they expected (n = 7).
3.5. Harms
There were no differences in any of the safety outcomes within 12 weeks from randomisation between the two groups (Table 3).
4. DISCUSSION
The recent review and guidelines from the IWGDF3, 5 noted a large increase in the number of trials with placental derived products and although they gave a recommendation that health care professionals should consider the use of these as an adjunctive treatment in addition to best standard of care when the latter alone had failed to reduce the size of the wound, this was a weak recommendation based on low quality evidence. The main reason for this is that none of the controlled studies identified were patient and outcome blind.5 Indeed of the six randomised trials identified, only three reported any form of blinded outcome assessment.12, 13, 14 Usual care was poorly described in most3 meaning that it would be uncertain as to whether any positive effects on healing were definitely due to the intervention.
The intervention utilised here, a gently dried amniotic membrane product, has shown promising effects in healing of chronic ocular surface ulcers, but has not yet be utilised in wound healing of diabetic foot ulcers. The main strength of the study was that the design and conduct fulfilled the exacting requirements specified for work in this field.4 All participants received good standard care using pre‐specified criteria, including the use of appropriate off‐loading devices. Crucially, the primary outcome of healing was assessed by someone who was blind to the allocation group. In addition, through the use of dummy packaging and foot draping, it was possible to blind the patients to their allocation group with few correctly guessing their treatment allocation group. Future outcome and patient blind studies are therefore possible, considerably reducing the risk of bias and improving confidence in any positive outcome.
As a pilot trial, this study was not designed to have sufficient statistical power to show a difference in healing within 12 weeks, however the results are encouraging and can be used to estimate the sample size for an appropriately powered trial. The apparent difference in healing between the two groups, albeit statistically insignificant, was similar to that seen in two other large RCTs published in the last few years, both of which reported an absolute 18% difference.15, 16 The secondary outcome of change in ulcer area from baseline, was also assessed blind, did show a difference between the two groups in favour of the intervention. Whilst again encouraging, it is important to note that this was a secondary outcome.
There was no difference in any safety outcome measure between the two groups in either major or minor amputation or the incidence of any adverse events or serious adverse events.
4.1. Limitations
The main weakness regarding study design and conduct was that it was not possible to blind the clinical researchers, and it was not possible to use a placebo for the amniotic membrane. Using a limited number of centres with experienced investigators, the use of foot draping and dummy packaging meant however that we were able to maintain the patient blind. A future large multi‐centre trial means that a robust training package for sites will be needed to ensure blinding is maintained to this standard.
5. CONCLUSION
In summary, although this this pilot trial was not powered to show a significant difference in healing by 12 weeks, these encouraging results show that this dHAM product is safe, and patient blinding is possible. As far as we are aware, this is the first study of amniotic membrane therapies to be published that is both patient and observer blind.
These results will be used to design a statistically powered, definitive double blind randomised controlled trial.
ACKNOWLEDGEMENTS
Funding was provided by an unrestricted grant from NuVision Biotherapies Ltd, Medilink East Midlands Ltd and East Midlands Academic Health Science Network (part funded by European Regional Development Fund (ERDF).
Game F, Gray K, Davis D, et al. The effectiveness of a new dried human amnion derived membrane in addition to standard care in treating diabetic foot ulcers: A patient and assessor blind, randomised controlled pilot study. Int Wound J. 2021;18:692–700. 10.1111/iwj.13571
The study was sponsored by University Hospitals of Derby and Burton NHS Foundation Trust.
Funding information East Midlands Academic Health Science Network; Medilink East Midlands Ltd; NuVision Biotherapies Ltd
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719‐1724. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and Management of Diabetic Foot Ulcers. Diabetes Care. 2018;41(4):645‐652. [DOI] [PubMed] [Google Scholar]
- 3.Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3283. [DOI] [PubMed] [Google Scholar]
- 4.Jeffcoate WJ, Bus SA, Game FL, et al. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality. Lancet Diabetes Endocrinol. 2016;4(9):781‐788. [DOI] [PubMed] [Google Scholar]
- 5.Vas P, Rayman G, Dhatariya K, et al. Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3284. [DOI] [PubMed] [Google Scholar]
- 6.Elalfy M, Maqsood S, Elsawwah K, Dhillon N, Hamada S, Lake D. The use of OmniLenz in treating persistent corneal epithelial defects https://www.escrs.org/amsterdam2020/programme/posters-details.asp?id=35714 (accessed 15th November 2020).
- 7.Jeffcoate WJ, Musgrove AJ, Lincoln NB. Using image J to document healing in ulcers of the foot in diabetes. Int Wound J. 2017;14(6):1137‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14:1933‐1940. [DOI] [PubMed] [Google Scholar]
- 9.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180‐191. [DOI] [PubMed] [Google Scholar]
- 10.D'Agostino RB, Belanger AJ, D'Agostino RB Jr. A suggestion for using powerful and informative tests of normality. Am Stat. 1990;44:316‐321. [Google Scholar]
- 11.Royston P. sg3.5: Comment on sg3.4 and an improved D'Agostino test. Stat Tech Bull. 1991;3:23‐24.reprinted in Stata Technical Bulletin Reprints, vol. 1, pp. 110–112. College Station, TX: Stata Press. [Google Scholar]
- 12.Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix([R]) for the treatment of chronic diabetic foot ulcers: results of a multi‐Centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Game F, Jeffcoate W, Tarnow L, et al. LeucoPatch system for the management of hard‐to‐heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer‐masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018. Nov;6(11):870‐878. [DOI] [PubMed] [Google Scholar]
- 16.Edmonds M, Lazaro‐Martinez JL, Alfayate‐Garcia JM, et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (explorer): an international, multicentre, double‐blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):186‐196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


