Abstract
Patients with arteriolosclerosis have impaired microvascular perfusion leading to impaired wound healing. Aged garlic extract has shown to have a positive impact on vascular elasticity. The present study aimed to assess the effect of long‐term treatment with AGE on peripheral tissue perfusion in patients with confirmed atherosclerosis. Ninety three patients with a CT‐scan confirmed coronary artery arteriolosclerosis were randomised in a double‐blind manner to placebo or 2400 mg AGE daily for 1 year. Peripheral tissue perfusion was evaluated at 0‐ and 12‐months using Laser Speckle Contrast Imaging. Measurement of post occlusive reactive hyperemia (PORH) and cutaneous vascular conductance (CVC) using acetylcholine iontophoresis (Ach) was conducted. After 12 months a significant increase of 21.6% (95% CI 3.2%‐40.0%, P < .05) was seen in the relative change of PORH in the AGE compared with the placebo group. The same response was seen for CVC and Ach with an increase of 21.4% (95% CI 3.4%‐39.4%, P < .05) in the AGE group compared with the placebo group. Aged garlic extract regenerated peripheral tissue perfusion and increase microcirculation in patients with arteriolosclerosis. Adequate peripheral tissue perfusion and tissue oxygen tension are important prerequisites for successful tissue repair. Restored microcirculation in patients could hypothetically facilitate wound healing.
Keywords: aged garlic extract, atherosclerosis, double blinded, laser speckle contrast imaging, peripheral tissue perfusion
1. INTRODUCTION
Complications secondary to arteriosclerosis are the most common causes of death in the Western countries.1, 2 Atherosclerosis involves inflammation and endothelial dysfunction leading to structural change to the intima and media of the arteries and aterioloies.3 These changes lead to not only secondary manifestations such as hypertension and coronary artery disease but also impaired tissue perfusion and impaired microcirculation.4 Inadequate perfusion may underlie much of the organ and tissue dysfunction associated with chronic conditions, why regeneration or restoration of tissue perfusion is of importance. Alterations in microcirculation are correlated to low‐grade systemic inflammatory response secondary to oxidative stress, endothelial dysfunction, altered capillary proliferation, and vasomotor dysfunction.5, 6, 7 Reduced microcirculation has been suggested to be an early marker for cardiovascular diseases4, 8, 9 and is also involved in impaired wound healing, especially in persons with diabetes mellitus.
Nitric oxide (NO) is a crucial modulator of atherosclerosis and peripheral tissue perfusion and has several intracellular effects that lead to endothelial regeneration, vasorelaxation, and inhibition of leukocyte chemotaxis. Atherosclerosis induced damage to the endothelium leads to a reduction in endothelial NO synthase (eNOS) with subsequent impaired release of NO. Which leads to a local enhanced degradation of NO by increased generation of reactive oxygen species with subsequent cascade of oxidation‐sensitive mechanisms in the arterial wall. Previously, studies have shown that NO production in the endothelium interferes with crucial mechanisms in the atherosclerotic process and inflammation.10 NO is therefore a commonly therapeutic targeted of action.
Aged garlic extract (AGE) has been shown to have a positive effect on endothelial function and atherosclerosis,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 and exerts its effects, through NO dependent pathways.17, 24, 25, 26, 27 AGE has therefore been suggested to have a positive effect on vascular elasticity and endothelial function.17, 26, 27, 28
In the present study, patients with cardiac computed tomography (CT) scan confirmed arteriosclerosis were randomised to an intake of capsules of 2400 mg AGE daily (two capsules of 600 mg twice daily) or two placebo capsules twice daily for 12 months using a double‐blinded placebo‐controlled trial design. Peripheral tissue perfusion was measured at 0‐ and 12‐months using Laser Speckle Contrast imaging (LSCI), a well‐known technology for estimation of perfusion and use in functional testing of endothelium‐dependent microvascular reactivity.29, 30, 31, 32, 33, 34, 35, 36 LSCI provides a continuous record of the microvascular blood flow and is therefore an excellent method to measure microvascular blood flow changes and microvascular perfusion.29, 30, 31, 32, 33, 34, 35, 36
The primary outcome of this study was to assess the effectiveness of 12 months of AGE treatment on peripheral tissue perfusion measured using LSCI in patients with confirmed coronary artery disease. To our knowledge no such study has been performed previously.
2. MATERIAL AND METHODS
The study was designed as a single‐centre, parallel, double‐blind placebo‐controlled randomised study to determine whether AGE can influence the rate of atherosclerosis plaque burden and CAC and inflammatory biomarkers in a European cohort. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by the local ethical committee DNR 2016/745 (Lund, Sweden). All participants signed a written consent form before entering the study. The study protocol was registered at (https://clinicaltrials.gov/ct2/show/NCT03860350?term=NCT03860350&rank=1) with ClinicalTrials.gov Identifier: NCT03860350. The study was monitored externally by Preventia AB, Sweden, https://www.preventia.se/en/startsida/. The study was conducted according to the CONSORT (Consolidate Standards of Reporting Trials) guidelines and statement.37 The study was conducted at Skåne University hospital in Lund, Sweden between October 2016 and October 2018.
2.1. Study outcomes
The primary outcome was changes in peripheral tissue perfusion and microcirculation after 1 year of placebo or AGE intake measured using LSCI. Inclusion and exclusion criteria are listed in Table 1.
TABLE 1.
Inclusion and exclusion criteria for patients in the study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age 40‐75 | Myocardial infarction |
| Framingham risk score >10 | Ischemic heart disease |
| Subjects with diabetes had to have a glycated haemoglobin (HbA1c) <8.0 and stable HbA1c level (variation range within 0.5%) for 6 months | Resting hypotension (systolic <90 mmHg) or hypertension (resting blood pressure >170/110) |
| Stable medications for 4 months prior to randomization | History of malignancy within the last 5 years or evidence of active cancer |
| CAC score (>10) on cardiac CT scan | Serum creatinine >140 μmol/L |
| Prior life‐threatening arrhythmia | |
| Unstable medical disorder | |
| Heart failure NYHA class III or IV | |
| Hypersensitivity to AGE | |
| Diabetic subjects with HbA1c >8.0 | |
| Triglycerides >4.0 mmol/L baseline visit | |
| Bleeding disorder or stroke or drug abuse | |
| Proximal CAC or CAC score >1000 units | |
| Hypersensitivity to AGE therapy |
2.2. CAC measurements
Patients underwent cardiac CT with a 128‐multidetector computed tomography scanner, SOMATOM Definition AS+ with Stellar detector by Siemens. Electrocardiographic triggering was performed at 70% of the R‐R interval. The coronary arteries were imaged in sequential mode with 3.0 mm (Acq. 32 × 1.2 mm) axial slices. Measurement of Agatston calcium score was performed with software, syngo.via, by Siemens. CAC score measurements were performed on non‐contrast studies by an experienced reader blinded to the patient and clinical information. CAC was defined as a plaque of at least three contiguous pixels (area 1.02 mm2) with a density of >130 Hounsfield units. The lesion score was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield unit within this area, as described by Agatston et al.10 The density factor was recorded in the following manner: 1 for lesions with peak attenuation of 130 to 199, two for lesions with peak attenuation of 200 to 299, three for lesions with peak attenuation of 300 to 399, and four for lesions with peak attenuation of >400. Total calcium score was determined by totalling individual lesion scores from each of the four main coronary arteries (left main coronary, left anterior descending coronary, left circumflex coronary, and right coronary arteries). Cardiac CT was performed before randomization.
2.3. Randomization
One hundred and four patients met the inclusion criteria and were randomised in a double‐blind manner, using numbered containers assigned to a computer‐generated randomization chart. The patients were randomised to an intake of capsules with 2400 mg AGE daily (two capsules of 600 mg twice daily, Kyolic Reserve formula; Wakunaga of America Co Ltd) or two placebo capsules twice daily.
2.4. Clinical evaluation
Medical evaluation including medical history, cardiovascular risk factors, prescribed medications, smoking, and alcohol intake was performed at 0, 4, 8, and 12 months. In addition, assessment of patients' compliance with medication were carried out.
2.5. Microvascular blood flow measured using laser speckle contrast imaging (LSCI)
Blood perfusion was monitored using a LSCI instrument (PeriCam PSI NR System, Perimed AB, Stockholm, Sweden). The forearm skin was illuminated by an infrared 785 nm laser light. Interference pattern of the backscattered light with dark and bright areas, creating a speckled pattern incident to the moving particles in the illuminated area. Alterations in this speckle pattern caused by perfusion changes, recorded in real time by a CCD‐camera, result in motion blurring and changes in the standard deviation of the signal. Speckle contrast is defined as the ratio ration between the standard deviation and the mean of the intensity in the signal. The system captures a two‐dimensional perfusion map with up to 100 images per second, and at a spatial resolution down to 100 μm/pixel. Perfusion is automatically calculated by the system by analysing the variations in the speckle pattern and presented in arbitrary units called perfusion units (PU).
2.6. Measurements of post occlusive reactive hyperemia (PORH) response
Measurements were performed in a quiet and temperature‐controlled room. The study was conducted with ≥10 hours of abstinence from tobacco, alcohol, caffeine or exercise. The patients had 30 minutes of acclimatisation time in a temperature‐controlled study room before the measurements were made.
The measurements were made on the forearm before partial occlusion, during partial occlusion and during post occlusive reactive hyperemia (PORH) using a manual blood pressure cuff (Boso Varius, AB Henry Eriksson, Bandhagen, Sweden) inflated at 250 mmHg for 3 minutes. The LSCI image was framed to the forearm and, when analysed, a region of interest (ROI) was placed 10 cm bellow the elbow joint on the ventral side of the forearm exactly in the study setup is shown in Figure 1.
FIGURE 1.
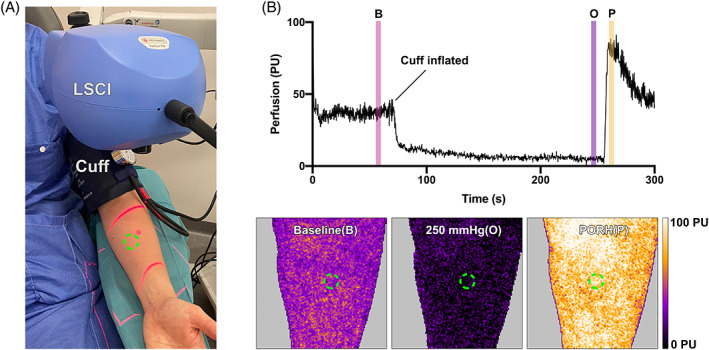
Illustration of the clinical Laser speckle contrast imaging (LSCI) setup for post occlusive reactive hyperemia (PORH) analysis (A). The LSCI camera was framed at the forearm of the study subjects, the red aiming beams are visualised. A blood pressure cuff was inflated to cause occlusion of the blood flow. The measurements, here illustrated with a representative example (B), were made on the forearm before before (B), during (O) and after (P) blood flow occlusion, to estimate the PORH. The time of interest for the measurements are illustrated with the colour bars in the in (B) and the region of interest are illustrated with the green circles in both (A) and the LSCI images in (B)
2.7. Measurements cutaneous vascular conductance (CVC)
To further study the endothelial function, LSCI was also used in conjunction with an iontophoresis system, PeriIont Micro Pharmacology System (Perimed AB, Stockholm, Sweden) that deliver minute volumes of drugs non‐invasively in a controlled manner. Each iontophoresis test requires one drug delivery electrode (LI 611 Drug Delivery Electrode (Perimed AB, Stockholm, Sweden)) together with one counter‐electrode (PF 384 Dispersive Electrode (Perimed AB, Stockholm, Sweden)). Acetylcholine (Miochol‐E, Bausch & Lomb Nordic AB, Bagsværd, Danmark) was used in the drug delivery electrode to study the microcirculatory response.38 The setup was similar to the PORH setup but with the acetylcholine iontophoresis electrodes coupled on the forearm during LSCI‐measurement and whiteout occlusion from a blood pressure cuff.
3. ETHICAL CONSIDERATIONS
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by the local ethical committee DNR 2016/745. All participants signed a written consent form before entering the study. The study protocol was registered at (https://clinicaltrials.gov/ct2/show/NCT03860350?term=NCT03860350&rank=1) with ClinicalTrials.gov Identifier: NCT03860350. The study was monitored externally by Preventia AB, Sweden, https://www.preventia.se/en/startsida/.
4. STATISTICS
A power analysis was based on previously published studies evaluating the effect of garlic and supplements on coronary atherosclerosis, blood pressure, cholesterol, and inflammatory biomarkers.18, 39 All continuous data are presented as a mean value ± SEM or mean value with 95% confidence interval. A repeated measures mixed‐effect model ANOVA with a Greenhouse‐Geisser correction was performed to test for differences between groups between time points 0‐ and 12 months. Post hoc tests using Šídák's multiple comparisons test were used to make correction for multiple measurements. Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA). Significance was defined as: P < .05, and P > .05 (not significant).
5. RESULTS
5.1. Patients demographics
A total of 175 patients with a Framingham risk score ≥ 10 were assessed for the study and underwent a cardiac CT scan. Seventy‐one patients were excluded after the initial cardiac CT scan: of these 59 did not have a positive CAC score and the remaining had a heavy CAC burden and were sent for further assessment at the cardiology department. In total 104 patients were enrolled and randomised in the study, 11 participants were excluded during the study and consequently 93 patients, 46 in the AGE group and 47 in the placebo group were analysed, see CONSORT (Consolidate Standards of Reporting Trials) outlined in Figure 2. No patient in the study had any adverse reaction of the active therapy that indicated removal from the study. At baseline there were no significant differences in cardiovascular risk factors calculated using Framingham risk score. Patient demographics are shown in Table 2.
FIGURE 2.
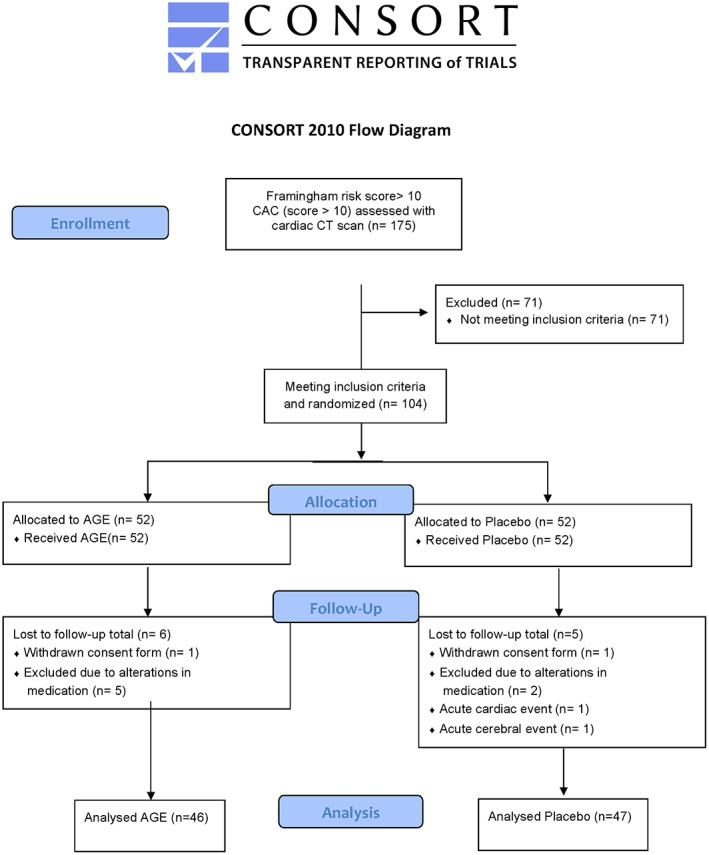
CONSORT statement (consolidated standards of reporting trials) flow chart. Showing demographics and baseline clinical information of the study cohort
TABLE 2.
Patient demographics at baseline
| Variable | AGE | Placebo | P | ||
|---|---|---|---|---|---|
| n = 47 | % | n = 46 | % | ||
| Age (years) (SD) | 63 | (SD 6) | 64 | (SD 6) | .95 |
| Gender (male) | 30 | 65 | 31 | 66 | .94 |
| Hypertension | 37 | 80 | 41 | 82 | .38 |
| Hypercholesterolemia | 24 | 52 | 32 | 68 | .12 |
| Diabetes mellitus | 5 | 11 | 12 | 26 | .07 |
| Current smoker | 5 | 11 | 3 | 6 | .57 |
| Family history of CVD | 33 | 72 | 25 | 53 | .09 |
| Framingham risk score | 23 | (SD 7) | 21 | (SD 7) | .27 |
Abbreviations: CVD, cardiovascular disease; SD, standard deviation.
5.2. Increased post occlusive reactive hyperemia (PORH) response in patients treated with AGE
Peripheral tissue perfusion was measured using LSCI at 0‐ and 12‐months. Measurements were taken before, during, and after partial occlusion using a blood pressure cuff creating a post occlusive reactive hyperemia (PORH) response. The relative change in PORH in the AGE‐group was 21.3% (95% CI 6.6%‐36.0%) compared with −0.3% (95% CI −8.5% to 7.9%) in the placebo group, giving a relative treatment response of 21.6% (95% CI 3.2%‐40.0%, P < .05) (Figure 3).
FIGURE 3.
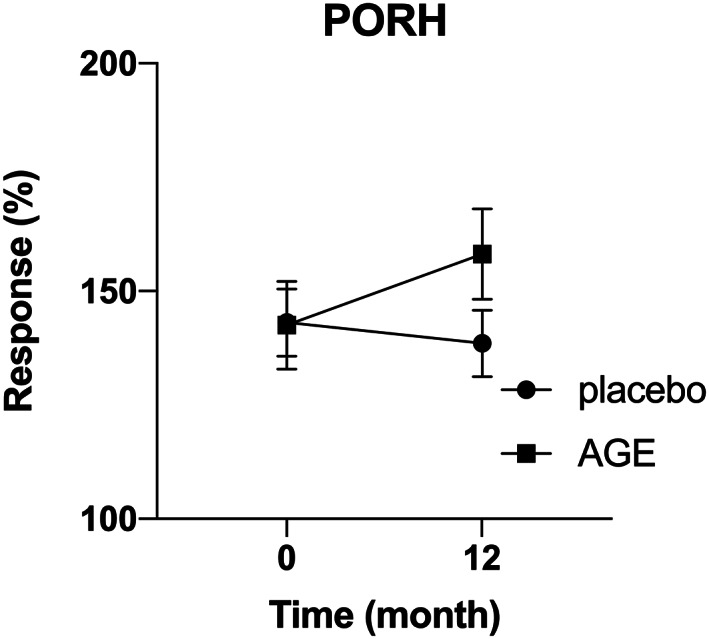
Increased post occlusive reactive hyperemia (PORH) response in patients treated with AGE. Peripheral tissue perfusion was measured at 0‐ and 12‐months. Measurements were done before, during and after partial occlusion using a blood pressure cuff creating a post occlusive reactive hyperemia (PORH) response. A significant increase in relative change in PORH was seen in the AGE group compared with the placebo group (P < .05)
5.3. Improved endothelial function in patients treated with AGE
Endothelial function and vascular response were measured at 0‐ and 12‐months using iontophoresis and acetylcholine (Ach) provocation. Measurements, CVC was calculated at rest and at peak vasodilatation in the first 5 minutes following ACh iontophoresis. The relative change in Ach‐response in the AGE‐group was 22.3% (95% CI 7.6%‐37.0%) compared with 0.9% (95% CI −7.5% to 9.3%) in the placebo group, giving a relative treatment response of 21.4% (95% CI 3.4 %‐39.4%, P < .05) (Figure 4).
FIGURE 4.
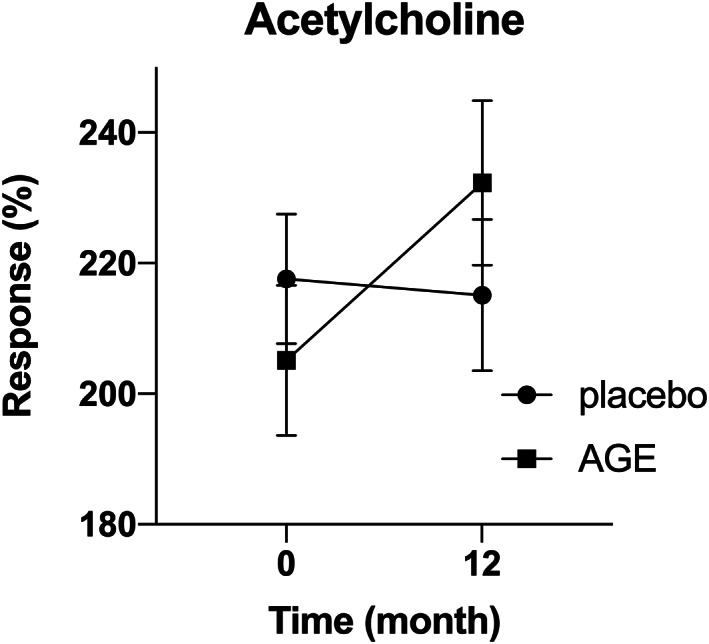
Improved endothelial function in patients treated with AGE. Endothelial function and vascular response were measured at 0‐ and 12‐months using iontophoresis and acetylcholine (Ach) provocation. Measurements, cutaneous vascular conductance (CVC) was calculated at rest and at peak vasodilatation in the first 5 minutes following ACh iontophoresis. A significant increase in relative change in CVC was seen in the AGE group compared with the placebo group (P < .05)
6. DISCUSSION
Adequate peripheral tissue perfusion and tissue oxygen tension are important prerequisites for successful tissue repair. The efficacy of tissue repair decreases with age and is linked to decrease peripheral tissue perfusion.40 Atherosclerosis progresses with age and involves inflammation and endothelial dysfunction with secondary manifestations such as impaired tissue perfusion.3, 4 Inadequate perfusion may underlie much of the organ and tissue dysfunction associated with chronic conditions, why regeneration and restoration of tissue perfusion are of importance.
In the present study, we did selected patients with confirmed atherosclerosis with secondary manifestations show as coronary artery calcifications using cardiac CT scan, in addition 80% of the patients had confirmed hypertension with concomitant medication. Patients with confirmed atherosclerosis are interesting to study regarding peripheral tissue perfusion because their underlining progressive disease causes a deterioration over time in the same. Restoration of the lost tissue perfusion might improve both tissue and organ dysfunction. The present study demonstrates that 12 months of AGE supplement improves peripheral tissue perfusion measured by LSCI compared with the placebo group.
In the present study, we used the laser speckle contrast analysis technology, also known as LSCI. The method visualises tissue blood perfusion in real time. LSCI combines dynamic response and high spatial resolution in one instrument, providing both real‐time graphs and video recordings of the tissue being studied. Compared with laser doppler velocimetry (LVD) measurement, LSCI provides a more stable and easier reproducible measurements.41, 42, 43 LSCI is nowadays commonly used in studies of endothelial function often by combining imaging with iontophoresis and CVC or/and PORH. In the present study, we used both tests for comparison.
Vascular function can be assessed by observing the response to reactive hyperemia. Reactive hyperemia is an increase in blood flow because of a temporary occlusion of an arterial blood supply leading to an oxygen deficit. In PORH a partial arterial occlusion is performed, as in the present study by using a blood pressure cuff inflated to 250 mmHg for 3 minutes. After the partial occlusion, the pressure is released, resulting in a large inflow of blood into the previously occluded tissue. The perfusion is measured before the occlusion, during the occlusion and after the occlusion. Parameters related to both magnitude, occlusion and baseline measurements before occlusion give information about the vascular health of the patient. PORH measurements have been shown to be highly reproducible,44 and the mechanisms behind are thought to be a combination of myogenic relaxation, release of local mediators and metabolites and involvement of sensory nerves.45, 46, 47 The PORH response to ischemia has also been shown to be associated with increased cardiovascular risk.48, 49In the present study, we did see a significant increase in PORH in patients receiving AGE supplement, indicating that AGE does improve peripheral tissue perfusion.
Tests of the vascular effect of acetylcholine (Ach), an endothelium‐dependent vasodilator mediated by the effect of ACh on NO and prostaglandines,50 are often performed to evaluate the endothelium‐dependent microvascular response.51, 52 In the present study iontophoresis with Ach provocation was used, to further investigate endothelial function. Iontophoresis is a technique to transport charged molecules or drugs across a tissue barrier. Combined with the laser speckle techniques, iontophoresis is a valuable tool for diagnosis and studying endothelial dysfunction.38, 50 The iontophoresis system delivers minute volumes of drugs non‐invasively in a controlled manner. It is a versatile accessory to study microcirculatory responses to non‐invasively delivered drugs. The method is considered safe and painless that enables transdermal drug delivery. It acts by the application a low intensity electric current, over two electrodes, one of which is an active electrode containing the drug that follows the current transdermal when the other electrode closes the circuit.38 In the present study we did see a significant increase in CVC in patients receiving AGE supplement compared with patients receiving placebo, indicating that AGE does improve peripheral tissue perfusion.
Endothelium covers the inside wall of the vessels and has a major influence on blood pressure regulation, microvascular blood flow and peripheral tissue perfusion. NO is synthesised in the endothelial cells by the nitric oxide synthase (NOS) and causes vasodilation by relaxation of the smooth muscle cells of the vascular walls and is a crucial modulator of atherosclerosis and peripheral tissue perfusion. NO has wide‐ranging biological properties and plays a crucial role in the normal endothelial function. Atherosclerosis induced damage to the endothelium and leads to an impaired release of NO, and endothelial dysfunction. NO is a common therapeutic targeted of action in preventing or delaying the arteriolosclerosis process.
AGE with the active ingredient S‐allylcysteine (SAC) has been shown to have a positive effect on atherosclerosis.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23AGE exerts its effects, through NO dependent pathways. S‐1‐propenylcysteine (S1PC) and SAC are the two predominant sulphur‐containing amino acids present in AGE.24 S1PC modulates antioxidant gene expression via the NO/heme (heme oxygenase‐1)/BACH1 (BTB domain and CNC homologue 1) signalling pathway by the property of downregulating BACH1 in a NO‐dependent manner and in the same time enhancing the expression of antioxidant genes reciprocally regulated by nuclear factor erythroid 2‐related factor 2 (NRF2) and BACH1.25 AGE has therefore been suggested to have a positive effect on vascular elasticity and endothelial function.17, 26, 27
In the present study, we use two different setups PORH and CVC, to evaluate endothelial function and peripheral tissue perfusion in 93 patients with confirmed atherosclerosis and coronary artery disease. Both evaluations methods show convincing evidence that 12 months of AGE supplement does lead to an improved peripheral tissue perfusion. PORH and CVC was used in conjunction with LSCI. LSCI has many advantages compared with earlier and less robust microvascular blood flow measurements such as LDV. We have earlier reported PORH measurements in using LDV in 122 patients with an increased risk developing cardiovascular events.21 Even if it is challenging to compare results from two different studies using two different techniques (LSCI, and LDV), the results from the current study seem to be in line with the previous studies by us and by others.21, 26, 27, 53 Restoring, by regeneration and secondary increasing the microvascular blood flow might be due to an inhibition of the atherosclerosis process by restoring natural compliance of vessels. However, it might also be due to increased angiogenesis, vascular neovascularization, and by potentially affecting vascular tone and endothelial function, meaning restored vascular health and thereby improving wound healing.
6.1. Limitations
The present study has both strengths and limitations. LSCI is easily implemented and has a high reproducible. LSCI is less is sensitive to movement artefacts compared with LDV. LSCI is sensitive to external factors such as changes in room temperature, stress, caffeine intake, nicotine intake, and exercise. However, this was compensated for using standardised measuring techniques, controlled environment and meticulous application of the measuring probe, and repeated measures on patients. The study was monitored externally by a study monitor during the whole study period, who carefully secured that all values were correct and were adequate.
6.2. Conclusions
The present study demonstrates that 12 months of aged garlic extract supplement regenerated peripheral tissue perfusion and increase microcirculation in patients with arteriolosclerosis and coronary artery disease. The effect might be due to an inhibition of the atherosclerosis process, but also by potentially affecting vascular tone and endothelial function. Adequate peripheral tissue perfusion and tissue oxygen tension are important prerequisites for successful tissue repair. Restored microcirculation in patients could hypothetically facilitate wound healing.
Abbreviations
- Ach
acetylcholine
- AGE
aged garlic extract
- BACH1
BTB domain and CNC homologue 1
- CAC
coronary artery calcification
- CT
computed tomography
- CVC
cutaneous vascular conductance
- LDV
laser Doppler velocemetry
- LSCI
laser speckle contrast imaging
- NO
nitric oxide
- NRF2
nuclear factor erythroid 2‐related factor 2
- S1PC
S‐1‐propenylcysteine
- SAC
S‐allylcysteine
CONFLICT OF INTERESTS
Dr Lindstedt has received a grant to support this research from Wakunaga of America LTD. None of the other authors has conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Martiné Wlosinska, Rafi Sheikh, Joanna Hlebowicz and Sandra Lindstedt participated in the design of the study. Sandra Lindstedt wrote the application for the ethical approval. Martiné Wlosinska, Ann‐Christin Nilsson, Mohammed Fakhro, Rafi Sheikh and Sandra Lindstedt carried out all clinical appointments. Ann‐Christin Nilsson and Martiné Wlosinska carried out the monitoring of the study together with Preventia AB. Rafi Sheikh, Martiné Wlosinska, Mohammed Fakhro and Sandra Lindstedt analysed the study results. Rafi Sheikh and Sandra Lindstedt drafted the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT
The study was approved by the ethical committee in Lund, Sweden, Lund University, and Skane University Hospital, Lund, Sweden, with reference number DNR 2016/745. The study protocol was registered at (https://clinicaltrials.gov/ct2/show/NCT03860350?term=NCT03860350&rank=1) with ClinicalTrials.gov Identifier: NCT03860350. All participants signed a written consent form before entering the study.
ACKNOWLEDGEMENTS
Vera Celander for the help with advertising and recruitment of study patients. The study was funded by ALF foundation and SUS foundation. The study was also funded by a research grant to Dr Lindstedt from Wakunaga of America LTD. Wakunaga of America LTD also provided the placebo and the AGE capsules used in this study free of charge. Wakunaga of America LTD had no role in the design, analysis or writing of this article.
Lindstedt S, Wlosinska M, Nilsson A‐C, Hlebowicz J, Fakhro M, Sheikh R. Successful improved peripheral tissue perfusion was seen in patients with atherosclerosis after 12 months of treatment with aged garlic extract. Int Wound J. 2021;18:681–691. 10.1111/iwj.13570
Funding information ALF Foundation; SUS Foundation; Wakunaga of America LTD
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Leys D. Atherothrombosis: a major health burden. Cerebrovasc Dis. 2001;11(Suppl 2):1‐4. [DOI] [PubMed] [Google Scholar]
- 2.Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol. 2002;7(1):40‐53. [PMC free article] [PubMed] [Google Scholar]
- 3.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104(4):503‐516. [DOI] [PubMed] [Google Scholar]
- 4.Levy BI, Schiffrin EL, Mourad JJ, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118(9):968‐976. [DOI] [PubMed] [Google Scholar]
- 5.Bottino DA, Lopes FG, de Oliveira FJ, Mecenas Ade S, Clapauch R, Bouskela E. Relationship between biomarkers of inflammation, oxidative stress and endothelial/microcirculatory function in successful aging versus healthy youth: a transversal study. BMC Geriatr. 2015;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin‐6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22(3):147‐151. [DOI] [PubMed] [Google Scholar]
- 7.Machin DR, Phuong TT, Donato AJ. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr Opin Pharmacol. 2019;45:66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strain WD, Paldanius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauten A, Ferrari M, Goebel B, et al. Microvascular tissue perfusion is impaired in acutely decompensated heart failure and improves following standard treatment. Eur J Heart Fail. 2011;13(7):711‐717. [DOI] [PubMed] [Google Scholar]
- 10.Bult H. Nitric oxide and atherosclerosis: possible implications for therapy. Mol Med Today. 1996;2(12):510‐518. [DOI] [PubMed] [Google Scholar]
- 11.Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor‐kappa b activation. J Nutr. 2001;131(3s):1020S‐1026S. [DOI] [PubMed] [Google Scholar]
- 12.Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60(5):417‐420. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi N, Nabavi V, Hajsadeghi F, et al. Aged garlic extract with supplement is associated with increase in brown adipose, decrease in white adipose tissue and predict lack of progression in coronary atherosclerosis. Int J Cardiol. 2013;168(3):2310‐2314. [DOI] [PubMed] [Google Scholar]
- 14.Allison GL, Lowe GM, Rahman K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012;91(25–26):1275‐1280. [DOI] [PubMed] [Google Scholar]
- 15.Budoff M. Aged garlic extract retards progression of coronary artery calcification. J Nutr. 2006;136(3 Suppl):741S‐744S. [DOI] [PubMed] [Google Scholar]
- 16.Zeb I, Ahmadi N, Flores F, Budoff MJ. Randomized trial evaluating the effect of aged garlic extract with supplements versus placebo on adipose tissue surrogates for coronary atherosclerosis progression. Coron Artery Dis. 2018;29(4):325‐328. [DOI] [PubMed] [Google Scholar]
- 17.Zeb I, Ahmadi N, Nasir K, et al. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: a randomized clinical trial. J Cardiovasc Dis Res. 2012;3(3):185‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budoff MJ, Ahmadi N, Gul KM, et al. Aged garlic extract supplemented with B vitamins, folic acid and L‐arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49(2–3):101‐107. [DOI] [PubMed] [Google Scholar]
- 19.Ried K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: a review and meta‐analysis. Exp Ther Med. 2020;19(2):1472‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wlosinska M, Nilsson AC, Hlebowicz J, et al. The effect of aged garlic extract on the atherosclerotic process—a randomized double‐blind placebo‐controlled trial. BMC Complement Med Ther. 2020;20(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wlosinska M, Nilsson AC, Hlebowicz J, Malmsjo M, Fakhro M, Lindstedt S. Aged garlic extract preserves cutaneous microcirculation in patients with increased risk for cardiovascular diseases: a double‐blinded placebo‐controlled study. Int Wound J. 2019;16(6):1487‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchins E, Shaikh K, Kinninger A, et al. Aged garlic extract reduces left ventricular myocardial mass in patients with diabetes: a prospective randomized controlled double‐blind study. Exp Ther Med. 2020;19(2):1468‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamal S, Cherukuri L, Birudaraju D, et al. Short‐term impact of aged garlic extract on endothelial function in diabetes: a randomized, double‐blind, placebo‐controlled trial. Exp Ther Med. 2020;19(2):1485‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodera Y, Kurita M, Nakamoto M, Matsutomo T. Chemistry of aged garlic: diversity of constituents in aged garlic extract and their production mechanisms via the combination of chemical and enzymatic reactions. Exp Ther Med. 2020;19(2):1574‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuneyoshi T. BACH1 mediates the antioxidant properties of aged garlic extract. Exp Ther Med. 2020;19(2):1500‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi N, Tsimikas S, Hajsadeghi F, et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol. 2010;105(4):459‐466. [DOI] [PubMed] [Google Scholar]
- 27.Weiss N, Ide N, Abahji T, Nill L, Keller C, Hoffmann U. Aged garlic extract improves homocysteine‐induced endothelial dysfunction in macro‐ and microcirculation. J Nutr. 2006;136(3 Suppl):750S‐754S. [DOI] [PubMed] [Google Scholar]
- 28.Kim KM, Chun SB, Koo MS, et al. Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S‐allyl cysteine. Free Radic Biol Med. 2001;30(7):747‐756. [DOI] [PubMed] [Google Scholar]
- 29.Zografos GC, Martis K, Morris DL. Laser Doppler flowmetry in evaluation of cutaneous wound blood flow using various suturing techniques. Ann Surg. 1992;215(3):266‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schabauer AM, Rooke TW. Cutaneous laser Doppler flowmetry: applications and findings. Mayo Clin Proc. 1994;69(6):564‐574. [DOI] [PubMed] [Google Scholar]
- 31.Anesater E, Borgquist O, Torbrand C, et al. The use of a rigid disc to protect exposed structures in wounds treated with negative pressure wound therapy: effects on wound bed pressure and microvascular blood flow. Wound Repair Regen. 2012;20(4):611‐616. [DOI] [PubMed] [Google Scholar]
- 32.Lindstedt S, Malmsjo M, Hansson J, Hlebowicz J, Ingemansson R. Microvascular blood flow changes in the small intestinal wall during conventional negative pressure wound therapy and negative pressure wound therapy using a protective disc over the intestines in laparostomy. Ann Surg. 2012;255(1):171‐175. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh R, Memarzadeh K, Torbrand C, Blohme J, Lindstedt S, Malmsjo M. Blood perfusion in a full‐thickness eyelid flap, investigated by laser Doppler Velocimetry, laser speckle contrast imaging and thermography. Eplasty. 2018;18:e9. [PMC free article] [PubMed] [Google Scholar]
- 34.Lindstedt S, Hlebowicz J. Blood flow response in small intestinal loops at different depths during negative pressure wound therapy of the open abdomen. Int Wound J. 2013;10(4):411‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmsjo M, Ingemansson R, Lindstedt S, Gustafsson L. Comparison of bacteria and fungus‐binding mesh, foam and gauze as fillers in negative pressure wound therapy—pressure transduction, wound edge contraction, microvascular blood flow and fluid retention. Int Wound J. 2013;10(5):597‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindstedt S, Malmsjo M, Hlebowicz J, Ingemansson R. Comparative study of the microvascular blood flow in the intestinal wall, wound contraction and fluid evacuation during negative pressure wound therapy in laparostomy using the V.a.C. abdominal dressing and the ABThera open abdomen negative pressure therapy system. Int Wound J. 2015;12(1):83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D, Group C . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puissant C, Abraham P, Durand S, et al. Assessment of endothelial function by acetylcholine iontophoresis: impact of inter‐electrode distance and electrical cutaneous resistance. Microvasc Res. 2014;93:114‐118. [DOI] [PubMed] [Google Scholar]
- 39.Budoff MJ, Takasu J, Flores FR, et al. Inhibiting progression of coronary calcification using aged garlic extract in patients receiving statin therapy: a preliminary study. Prev Med. 2004;39(5):985‐991. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchida Y. Age‐related changes in skin blood flow at four anatomic sites of the body in males studied by xenon‐133. Plast Reconstr Surg. 1990;85(4):556‐561. [DOI] [PubMed] [Google Scholar]
- 41.Roustit M, Blaise S, Millet C, Cracowski JL. Reproducibility and methodological issues of skin post‐occlusive and thermal hyperemia assessed by single‐point laser Doppler flowmetry. Microvasc Res. 2010;79(2):102‐108. [DOI] [PubMed] [Google Scholar]
- 42.Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013;34(7):373‐384. [DOI] [PubMed] [Google Scholar]
- 43.Puissant C, Abraham P, Durand S, et al. Reproducibility of non‐invasive assessment of skin endothelial function using laser Doppler flowmetry and laser speckle contrast imaging. PLoS One. 2013;8(4):e61320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yvonne‐Tee GB, Rasool AH, Halim AS, Rahman AR. Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods. 2005;52(2):286‐292. [DOI] [PubMed] [Google Scholar]
- 45.Patterson GC. The role of intravascular pressure in the causation of reactive hyperaemia in the human forearm. Clin Sci. 1956;15(1):17‐25. [PubMed] [Google Scholar]
- 46.Kontos HA, Mauck HP Jr, Patterson JL Jr. Mechanism of reactive hyperemia in limbs of anesthetized dogs. Am J Physiol. 1965;209(6):1106‐1114. [DOI] [PubMed] [Google Scholar]
- 47.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol. 2007;585(Pt 1):295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strain WD, Chaturvedi N, Bulpitt CJ, Rajkumar C, Shore AC. Albumin excretion rate and cardiovascular risk: could the association be explained by early microvascular dysfunction? Diabetes. 2005;54(6):1816‐1822. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto‐Suganuma R, Aso Y. Relationship between post‐occlusive forearm skin reactive hyperaemia and vascular disease in patients with type 2 diabetes—a novel index for detecting micro‐ and macrovascular dysfunction using laser Doppler flowmetry. Diabet Med. 2009;26(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 50.Durand S, Tartas M, Bouye P, Koitka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004;561(Pt 3):811‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz A, Ekberg K, Johansson BL, Wahren J. Diminished skin blood flow in type I diabetes: evidence for non‐endothelium‐dependent dysfunction. Clin Sci (Lond). 2001;101(1):59‐64. [PubMed] [Google Scholar]
- 52.Jagren C, Gazelius B, Ihrman‐Sandal C, Lindblad LE, Ostergren J. Skin microvascular dilatation response to acetylcholine and sodium nitroprusside in peripheral arterial disease. Clin Physiol Funct Imaging. 2002;22(6):370‐374. [DOI] [PubMed] [Google Scholar]
- 53.Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T. Effect of garlic on blood pressure: a systematic review and meta‐analysis. BMC Cardiovasc Disord. 2008;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


