Abstract
Background:
Reactive case detection (RCD) is a commonly used strategy for malaria surveillance and response in elimination settings. Many approaches to RCD assume detectable infections are clustered within and around homes of passively detected cases (index households), which has been evaluated in a number of settings with disparate results.
Methods:
Household questionnaires and diagnostic testing were conducted following RCD investigations in Zanzibar, Tanzania, including the index household and up to 9 additional neighboring households.
Results:
Of 12,487 participants tested by malaria rapid diagnostic test (RDT), 3·2% of those residing in index households and 0·4% of those residing in non-index households tested positive (OR = 8·4; 95%CI: 5·7, 12·5). Of 6,281 participants tested by quantitative polymerase chain reaction (qPCR), 8·4% of those residing in index households and 1·3% of those residing in non-index households tested positive (OR = 7·1; 95%CI: 6·1, 10·9). Within households of index cases defined as imported, odds of qPCR-positivity amongst members reporting recent travel were 1·4 times higher than among those without travel history (95%CI: 0·2, 4·4). Amongst non-index households, odds of qPCR-detectable infection were no different between households located within 50 m of the index household as compared with those located farther away (OR = 0·8, 95%CI: 0·5, 1·4). Sensitivity of RDT to detect qPCR-detectable infections was 34% (95%CI: 26·4, 42·3).
Conclusions:
Malaria prevalence in index households in Zanzibar is much higher than in non-index households, in which prevalence is very low. Travelers represent a high-risk population. Low sensitivity of RDTs due to a high prevalence of low-density infections results in an RCD system missing a large proportion of the parasite reservoir.
Keywords: malaria, surveillance, elimination, reactive case detection, focal mass drug administration, RCD, fMDA
Introduction
In low-transmission and elimination settings, strategies for prevention and control of malaria are often both reactive and focal. One such strategy is reactive case detection (RCD), whereby an active search for cases is performed in response to the passive detection of a case (index case) seeking care for febrile illness at a health facility. This search is conducted within the household of the index case (index household) and sometimes within nearby neighboring households. The search is followed by distribution of antimalarials to parasitologically confirmed cases.
RCD makes use of an assumption that detectable malaria infections are clustered within and around index households. Published data on RCD generally support the hypothesis that index household members are at increased risk of malaria infection as compared to members of surrounding households (Aidoo et al., 2018; Bjorkman et al., 2017; Fontoura et al., 2016; Hsiang et al., 2019; Littrell et al., 2013; Stresman et al., 2010; Sturrock et al., 2013). These studies examined differences in rapid diagnostic test (RDT) and/or polymerase chain reaction (PCR) prevalence between index household members and the household members of neighbors of varying distances from the index household. A few studies presented evidence of elevated risk within surrounding households when compared with control households (Aidoo et al., 2018; Fontoura et al., 2016; Hsiang et al., 2019).
While these studies suggest that index household members are at higher risk of infection, a number of similarly structured studies showed no evidence of such elevated risk in or around the index household (Hustedt et al., 2016; Rossi et al., 2018; van Eijk et al., 2016). This suggests that the risk of infection within an index household relative to risk in non-index households may be dependent on more factors than simply the residence of a passively detected case. Previous research has suggested that age, travel history, and occupation may modify infection risk in low transmission settings (Rossi et al., 2018; Lynch et al., 2015; Yukich et al., 2013). Other factors might include coverage of indoor residual spraying, bed-net use, or characteristics of the locality such as vicinity to breeding sites, housing density, or transmission season.
Regardless of surveillance strategy, infections must be detectable by the diagnostic test applied during RCD. RDTs, which are used widely by malaria programs, have been shown to have a low sensitivity to detect a large proportion of PCR-detectable infections (Kobayashi et al., 2019; Wu et al., 2015). A recent study from Papua New Guinea showed that gametocytes were detectable in 44% of Plasmodium falciparum infections detected by quantitative PCR (qPCR) but undetected by highly-sensitive RDT (Hofmann et al., 2018). These infections might have gone untreated in a RCD program using RDTs despite the presence of gametocytes, thus failing to interrupt the potential for onward transmission.
Another important aspect of malaria elimination with implications for RCD is case-importation (Le Menach et al., 2011). In low-transmission settings, individuals with confirmed malaria also reporting recent travel are often suspected to have imported their infection. Because the suspected source of their infection is external, the distribution of risk surrounding their residence may differ, leading to different manifestations of infection prevalence surrounding their household as compared with locally acquired infections.
This study aims to characterize the prevalence of malaria infection and the sensitivity of the RDT to detect these infections within and surrounding households of passively detected cases of malaria diagnosed at a health facility in order to better inform policy decisions around focal strategies to control malaria. In particular, this study will investigate the difference in malaria prevalence between index households, surrounding households, and background prevalence as well as the effect modification of case importation and the sensitivity of RDTs in detecting the prevalent infections.
Methods
Study Setting
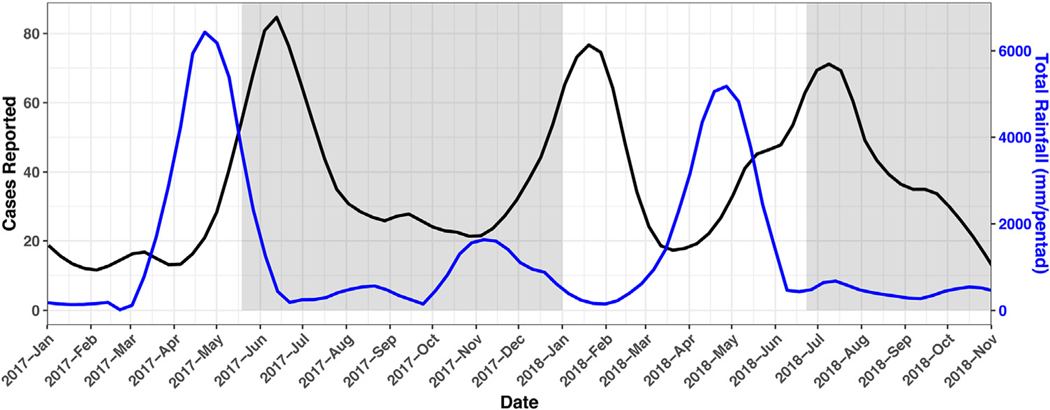
Zanzibar is a semiautonomous archipelago in the United Republic of Tanzania with two main islands, Unguja and Pemba (Fig. 1). In 2012 the population of Zanzibar was approximately 1·3 million, of which approximately 700,000 were living in the 5 districts included in this study: Chake Chake, Kusini, Magharibi, Michiweni, and Mkoani (Statistics TNBo, Finance TMo, 2012). The districts were selected such that there would be at least one of each of high and low transmission districts from each island included in the study. Zanzibar has two rainy seasons: the longer and heavier occurring March through May and the shorter occurring October through December (Fig. 2). Pemba has slightly higher precipitation and is more forested than Unguja. The dominant malaria species is P. falciparum, although a previous study found up to 43.2% of infections to include P. malaria (Morris et al., 2015). The dominant mosquito vector in Zanzibar is Anopheles arabiensis (Jones et al., 2013).
Fig. 1.
Location of Zanzibar and study districts. Left: Tanzania and location of the Zanzibar archipelago. Right: Zanzibar archipelago with the two Islands Pemba (top) and Unguja (bottom)and locations of study districts (shown in white).
Fig. 2.
Smoothed rainfall and number of weekly notifications from health facilities within study districts for 2017 and 2018. Shaded areas represent data collection periods for current study.
Parasite prevalence in Zanzibar has been historically as high as 68% among children in the mid-1920’s and 35% in 198 (Mansfield-Aders, 1927; Programme, 2009). Incidence dropped substantially after renewed control efforts beginning in the early 2000’s (Bhattarai et al., 2007). These gains motivated the Zanzibar Malaria Elimination Programme (ZAMEP) to shift focus from control to elimination (Programme, 2009). further declines were achieved after scaling up of vector control in 2006, but further progress toward elimination has been elusive. (Ashton et al., 2019).
When an individual is diagnosed at any of Zanzibar’s 154 public health facilities or 50 private facilities via RDT, a staff member sends a SMS-based notification to a central server. A District Malaria Surveillance Officer (DMSO) is then notified and tasked with visiting the individual’s household. All cohabitants and visitors present are tested for malaria by RDT and those positive receive free treatment with artesunate-amodiaquine (van der Horst et al., 2019).
Data Collection
A rolling cross-sectional survey was conducted in which study staff accompanied DMSOs on a sample of their investigations, typically the DMSO’s first investigation of the day. Household members 3 months and older were asked for consent or parental consent and tested for malaria by RDT (SD BIOLINE Malaria Ag Pf [histidine-rich protein II (HRP2)]/Pan [lactose dehydrogenase (pLHD)]). Dried blood spots (DBS) from finger-prick blood were also collected for P. falciparum detection by qPCR. If any members of the index household were not present during the first visit, the household was revisited up to two additional times. After completion of the index household, the DMSO departed and the study staff remained to survey the four nearest neighboring households and five households selected along a 200 m transect drawn in a random direction away from the index household. Neighboring and transect households were aggregated in these analyses, as prevalence by both RDT and qPCR was similar between them.
At each household, geographic coordinates were recorded, and a questionnaire was administered in Kiswahili to collect data on a number of characteristics including demographics, household asset ownership, physical characteristics of the household, bed-net ownership and use, and detailed travel history within the past 60 days. Responses to questions were recorded on tablets using ODK Collect (Hartung et al., 2010).
Data collection occurred over two periods, the first from May 19, 2017 to January 1, 2018 and the second from June 23 to October 31, 2018. The first period was the original intended duration of the study. The second period was added to increase the sample size.
Laboratory testing
P. falciparum DNA was detected by quantitative polymerase chain reaction (qPCR). Primers targeted the conserved C-terminal region of the multi-copy var gene family (varATS) (Hofmann et al., 2015). The varATS amplicon was pre-amplified directly from pooled or individual DBS punches using the Phusion Blood Direct PCR Kit (Thermo Fisher Scientific) (Grossenbacher et al., 2020). Pre-amplified PCR products were diluted 1:50 and used as template in varATS qPCR. VarATS qPCR was performed on 4 μl of diluted pre-amplification product using the Applied Biosystems StepOne System and GoTaq Probe qPCR Mastermix (Promega).
Parasite density was determined using a 10-fold dilution row of the WHO 1st international standard for P. falciparum DNA Nucleic Amplification Techniques. This DNA standard was included in qPCR starting from the pre-amplification step. A positivity cutoff based on a limit of detection (LOD) of 0.13 parasites per microliter (parasites/μL) was introduced, permitting the detection of parasite infection with 95% sensitivity.
Not all blood samples were processed by qPCR. Organization of blood samples was improved prior to the second period of data collection and were prioritized during qPCR analysis in order to maximize the number of samples analyzed before the close of the study. Prioritization of samples occurred at the cluster level and qPCR analysis for all individuals with in prioritized clusters was completed.
Data analysis
All quantitative statistical analyses were conducted in R statistical software version 3.5.1 and the lme4 package (Team, 2015; Douglas Bates et al., 2015).
Prevalence and density
Prevalence of infection as measured by qPCR and RDT was summarized as proportion positive of tested, disaggregated by household type and, for qPCR, parasite density. Estimates are further disaggregated by travel outside of Zanzibar by the index case and co-travel with the index case by household members. Index cases reporting travel outside of Zanzibar in the past 60 days are considered ‘suspected imported’. ‘Suspected imported’ and ‘suspected locally acquired’ cases will be referred to as ‘imported’ and ‘locally acquired’ cases, respectively. Co-habitants of imported index cases that joined the index case on their recent travel are suspected to be at different risk of exposure than other co-habitants or travelers. Logistic regression was used to estimate the difference in infection prevalence between index households and non-index households, adjusting for index case source and co-travel with imported index cases.
Individual- and household-level risk factors
The regression model above was expanded to identify and estimate the effect of individual and household risk factors on prevalence. Suspected risk factors including non-index case related travel, distance of residence from index household, testing within 3 days of index case diagnosis, age group, sex, high-risk job classification (public bus drivers, construction workers, factory workers, and farmers), population density, household electricity ownership, closed eaves, and bednet use the previous night were added to the regression. Stratification of the models by specific risk groups was not possible to the limited number of outcome events in the data. A final model was arrived at by a backwards variable 2 selection process. Population density estimates in the 100 m2 surrounding index households used in this analysis were estimated using 2015 WorldPop-estimated population density (Tatem, 2017).
Diagnostic sensitivity
The results of those tested by both RDT and qPCR were used to estimate sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of RDT in the detection of qPCR detectable infections. A sensitivity analysis was conducted to predict the performance of diagnostic tests with LODs of 10 and 1 parasites/mL, loosely corresponding to LODs of highly-sensitive RDT (hsRDT) and loop-mediated isothermal amplification (LAMP), respectively (Polley et al., 2013; Organization, 2018).
Malaria indicator survey comparison
The recently published 2017 malaria indicator survey (MIS) for Tanzania included RDT prevalence for 540 children aged 6–59mo for both Unguja and Pemba and was conducted during the current study. The MIS is a nation-wide cross-sectional survey of households selected in a stratified random sample, meant to provide regional level estimates of malaria-related outcomes (Ministry of Health, 2020a). Children aged 6–59mo were tested for malaria by RDTs of the same brand used in this study. The MIS was conducted between October and December 2017. MIS estimates and their margins of error were compared with RDT results from among the same age group in the study population.
Results
The sample consisted of 409 investigated household clusters (Fig. 3) with 3,380 households interviewed including 406 index cases (Table 1). These households housed 17,458 individuals that were not index cases, 12,780 of which gave, or were given, permission for malaria testing and DBS collection. Of these, 12,478 (97·6%) were tested for malaria by RDT and 12,434 (97·3%) had a DBS collected of which 6,281 (50·5%) were processed by qPCR.
Fig. 3.
Locations of study districts and investigated household clusters (green diamonds) on Unguja Island (left) and Pemba Island (right). Some investigations occurred outside of study districts as not all individuals live in the same district in which they sought care.
Table 1.
Characteristics of visited households and participants by index household status. Participant characteristics do not include index cases.
| Households | Participants | |||
|---|---|---|---|---|
| Index Household | Non-Index Household | Index Household | Non-Index Household | |
| Households Visited (n) | 409 | 3064 | ·· | ·· |
| Present and consented to questionnaire (n (% of visited)) | 405 (99·0) | 2,975 (97·1) | 2,284 | 15,174 |
| Unguja (n (% of present and consented)) | 226 (55·8) | 1,694 (56·9) | 1,190 (52·1) | 8,276 (54·5) |
| Pemba (n (% of present and consented)) | 179 (44·2) | 1,281 (43·1) | 1,094 (47·9) | 6,898 (45·5) |
| Phase 1 (n (% of present and consented)) | 279 (68·9) | 2,116 (71·1) | 1,625 (71·1) | 11,087 (73·1) |
| Phase 2 (n (% of present and consented)) | 126 (31·1) | 859 (28·9) | 659 (28·9) | 4,087 (26·9) |
| Visit within 2 days of notification (n (% of present and consented)) | 304 (75·1) | 2,199 (73·9) | 1,686 (73·8) | 10,321 (68·0) |
| Visit within 5 days of notification (n (% of present and consented)) | 392 (96·8) | 2,877 (96·7) | 2,217 (97·1) | 14,513 (95·6) |
| Distance within 50 m of index household (n (% of present and consented)) | ·· | 1,491 (50·1) | ·· | 7,706 (50·8) |
| Distance within 200 m of index household (n (% of present and consented)) | ·· | 2,912 (97·9) | ·· | 14,895 (98·2) |
| Household has electricity (n (% of present and consented)) | 182 (44·9) | 1,334 (44·8) | 1,112 (48·7) | 7,252 (47·8) |
| Household Size (Mean) | 6·7 | 5·1 | ·· | ·· |
| Age (Mean) | ·· | ·· | 20·2 | 21·8 |
| Male (n (% of present and consented)) | ·· | ·· | 1,098 (48·1) | 6,989 (46·1) |
| Eligible for testing and bloodwork (n (% of present and consented)) | ·· | ·· | 2,265 (99·2) | 15,039 (99·1) |
| Present during 1 st visit (n (% of eligible for testing)) | ·· | ·· | 1,782 (78·7) | 11,188 (74·4) |
| Present during any visit (n (% of eligible for testing)) | ·· | ·· | 2,026 (89·4) | 12,327 (82) |
| Permission granted for testing and bloodwork (n (% of eligible for testing)) | ·· | ·· | 1,952 (96·3) | 10,828 (87·8) |
| Tested by RDT (n (% of permission granted for testing)) | ·· | ·· | 1,917 (98·2) | 10,561 (97·5) |
| RDT Positives (n (% of tested)) | ·· | ·· | 62 (3·2) | 42 (0·4) |
| Collected DBS (n (% of permission granted for testing)) | ·· | ·· | 1,913 (98) | 10,521 (97·2) |
| DBS processed by qPCR (n (% of collected DBS)) | ·· | ·· | 953 (49·8) | 5,328 (50·6) |
| qPCR Positives (n (% of tested)) | ·· | ·· | 80 (8·4) | 68 (1·3) |
Prevalence and density
Among the 12,478 non-index cases tested by RDT, 104 tested positive for malaria. In index households, 3·2% of household members tested positive by RDT compared with 0·4% in non-index households (Table 2& Fig. 4). Overall, odds of RDT-detectable infection were 7·4 times higher in index households (95%CI: 4·7, 11·5).
Table 2.
Demographic characteristics and test results of participants stratified by importation of the index case, index household status, and co-travel with the index case.
| Index case locally acquired | Index case suspected imported | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index household | Non-Index Household | Index household | Non-index household | ||||||
| Index cases | Non-index members | All members | All members | Index cases | Co-travelers | Non-index non-cotraveling members | All members | All members | |
| Participants | 281 | 1,619 | 1,900 | 10,305 | 137 | 340 | 325 | 802 | 4,869 |
| Female (% of participants) | 120 (42·7) | 825 (51) | 945 (49·7) | 5,576 (54·1) | 59 (43·1) | 193 (56·8) | 168 (51·7) | 420 (52·4) | 2,609 (53·6) |
| Age under 3mo (% of participants) | 1 (0·4) | 11 (0·7) | 12 (0·6) | 89 (0·9) | 0 (0) | 3 (0·9) | 5 (1·5) | 8 (1) | 40 (0·8) |
| Age 3–59mo (% of participants) | 37 (13·2) | 265 (16·4) | 302 (15·9) | 1,611 (15·6) | 21 (15·3) | 65 (19·1) | 39 (12) | 125 (15·6) | 731 (15) |
| Age 5–17yr (% of participants) | 130 (46·3) | 604 (37·3) | 734 (38·6) | 3,703 (35·9) | 23 (16·8) | 98 (28·8) | 125 (38·5) | 246 (30·7) | 1,636 (33·6) |
| Age 18–35 yr (% of participants) | 85 (30·2) | 435 (26·9) | 520 (27·4) | 2,719 (26·4) | 63 (46) | 123 (36·2) | 112 (34·5) | 298 (37·2) | 1,439 (29·6) |
| Age over 35 yr (% of participants) | 28 (10) | 304 (18·8) | 332 (17·5) | 2,177 (21·1) | 30 (21·9) | 51 (15) | 44 (13·5) | 125 (15·6) | 1,023 (21) |
| Lives in household with Electricity (% of participants) | 116 (41·3) | 729 (45) | 845 (44·5) | 4,272 (41·5) | 71 (51·8) | 204 (60) | 179 (55·1) | 454 (56·6) | 2,980 (61·2) |
| Recent Travel (% of participants) | 0 (0) | 14 (0·9) | 14 (0·7) | 148 (1·4) | 136 (99·3) | 340 (100) | 2 (0·6) | 478 (59·6) | 178 (3·7) |
| RDT Tests performed (% of participants) | ·· | 1,375 (84·9) | 1,375 (72·4) | 7,204 (69·9) | ·· | 284 (83·5) | 258 (79·4) | 542 (67·6) | 3,357 (68·9) |
| RDT Positives (% of RDT Tests) | ·· | 35 (2·5) | 35 (2·5) | 34 (0·5) | ·· | 22 (7·7) | 5 (1·9) | 27 (5) | 8 (0·2) |
| qPCR Tests performed (% of participants) | ·· | 637 (39·3) | 637 (33·5) | 3,420 (33·2) | ·· | 183 (53·8) | 133 (40·9) | 316 (39·4) | 1,908 (39·2) |
| qPCR Positives (% of PCR Tests) | ·· | 40 (6·3) | 40 (6·3) | 34 (1) | ·· | 30 (16·4) | 10 (7·5) | 40 (12·7) | 34 (1·8) |
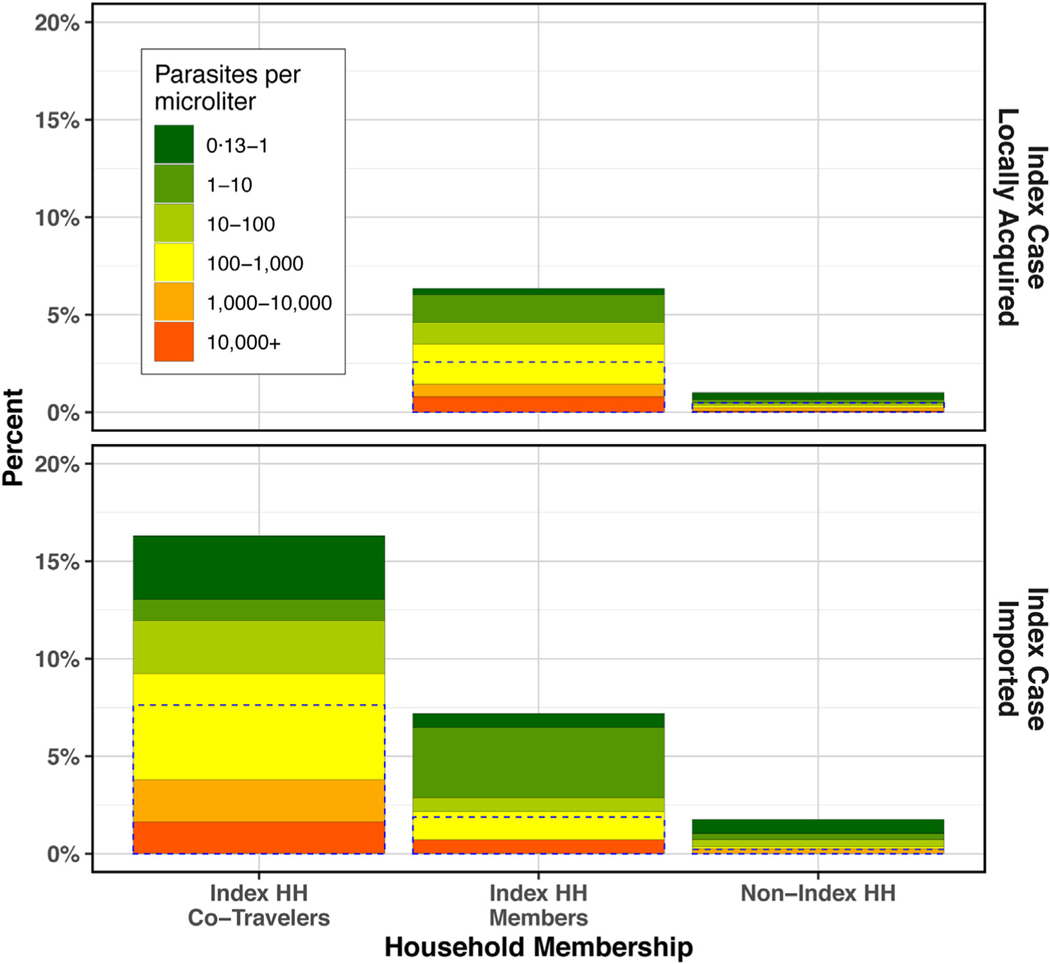
Fig. 4.
qPCR prevalence and density in clusters of imported versus locally acquired index cases by co-travel and household category. Dotted lines represent RDT positivity among all RDT-tested individuals. Empty slot in upper left due to lack of co-travel with index case when index case did not travel.
Among all 6,281 non-index cases tested by qPCR, 148 tested positive (8·4% positive in index households and 1·3% in non-index households). Odds of qPCR-detectable infection were 6·1 times higher in index households as compared to non-index households (95%CI: 5·1, 9·9).
From multivariable logistic regression (Table 3, model a), non-co-traveling members of index households exhibited 5·8 times the odds of qPCR-positivity of members of non-index households (95% CI: 5·4, 6·2). Odds of positivity in clusters with imported index cases was 1·4 times that of clusters with locally acquired index cases (95%CI: 1·0, 1·8). Within index households with imported index cases, odds of qPCR-positivity among co-traveling members was 2·5 times that of non-co-traveling members (95%CI: 1·9, 3·1).
Table 3:
Results of logistic regression on qPCR-positivity. Coefficient estimates are reported as odds ratios with their 95% confidence intervals in brackets below.
| Outcome: qPCR positive | |||
|---|---|---|---|
| (a) Base | (b) Full | (c) Final | |
| Index Household | 5·8*** [5·4, 6·2] | 5·9*** [5·4, 6·4] | 5·5*** [5·1, 5·9] |
| Index Case Imported | 1·4 [1·0, 1·8] | 1·4 [1·0, 1·8] | 1·4 [1·0, 1·8] |
| Cotravel with Index Case | 2·5** [1·9, 3·1] | 2·8** [2·2, 3·4] | 2·8** [2·2, 3·4] |
| Recent travel only within Zanzibar | ·· | 1 [−0·4, 2·4] | ·· |
| Recent travel out of Zanzibar (not with index case) | ·· | 4·8** [3·8, 5·8] | 4·6** [3·6, 5·6] |
| Residence within 50 m of index household | ·· | 0·9 [0·4, 1·4] | ·· |
| Tested within 3 days of index case diagnosis | ·· | 4·5** [3·5, 5·5] | 4·5** [3·5, 5·5] |
| Age 5–16 | ·· | 1·5 [0·9, 2·1] | ·· |
| Age 17–34 | ·· | 2·3** [1·7, 2·9] | 1·9*** [1·6, 2·2] |
| Age 35–59 | ·· | 1·4 [0·7, 2·1] | ·· |
| Age 60+ | ·· | 0·4 [−11, 1·9] | ·· |
| Male | ·· | 0·7 [0·3, 1·1] | ·· |
| High-risk job | ·· | 2·3*** [1·8, 2·8] | 2·2*** [1·8, 2·6] |
| Population density surrounding index household (log) | ·· | 0·9 [0·7, 11] | ·· |
| Electricity in household | ·· | 1·1 [0·7, 1·5] | ·· |
| Closed eaves | ·· | 0·9 [0·5, 1·3] | ·· |
| Slept under net previous night | ·· | 0·6* [0·2, 1·0] | 0·7* [0·3, 1·1] |
| N | 6281 | 6281 | 6281 |
| AIC | 1263.739 | 1224.873 | 1216.627 |
| BIC | 1290.721 | 1346.288 | 1277.334 |
| Pseudo R Squared | 0.115 | 0.167 | 0.159 |
p < 0·001;
p < 0·01;
p < 0·05·
AIC = Akaike information criterion
BIC = Bayesian information criterion
Individual and household risk factors
The full model including all suspected risk factors (Table 3, model b) was reduced to a final model including terms for risk factors with significant associations with positivity (Table 3, model c). Reduction from the full model had minimal impact on magnitudes of effect. Recent travel outside of Zanzibar, testing within 3 days of index case diagnosis, being of ages 17–34 (relative to all other age groups), and high-risk occupation were all significantly associated with increased positivity. Bednet use was protective against positivity.
Diagnostic sensitivity
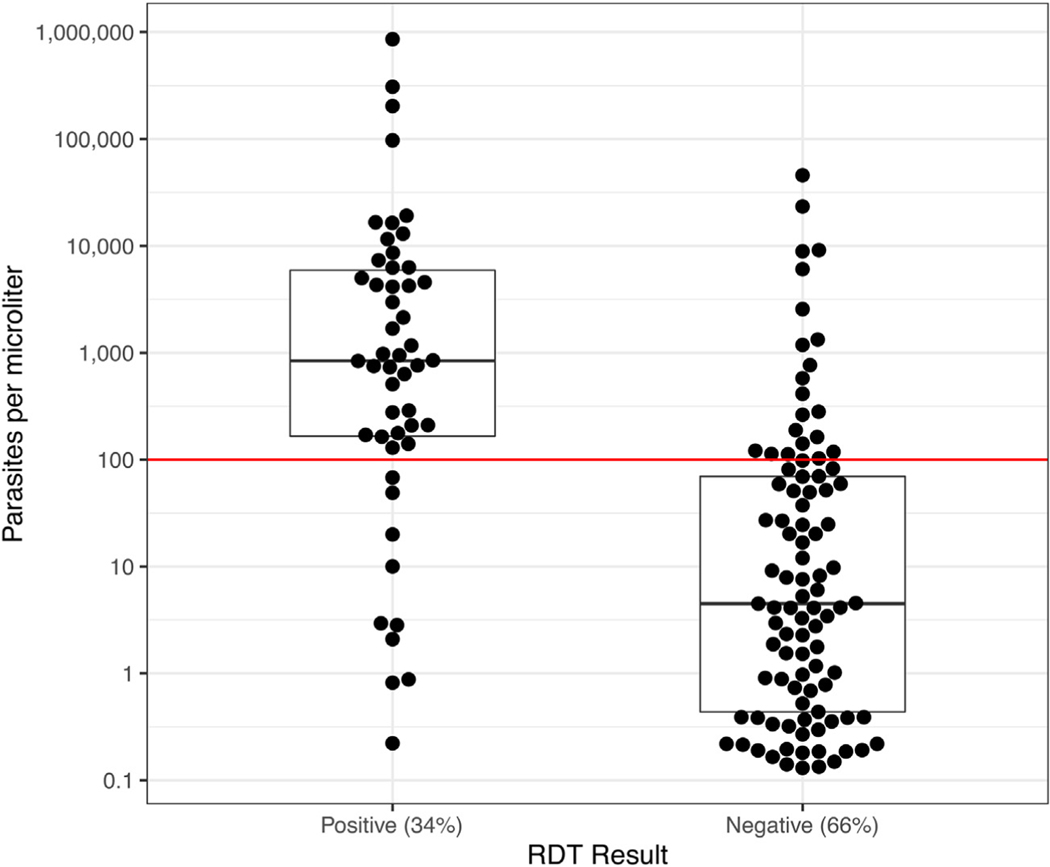
The RDT used in the study had a sensitivity of 34·0% to detect qPCR detectable infections overall (95%CI: 26·4, 42·3). RDT was 39·2% sensitive in index households (95%CI:28·4, 50·9) and 27·9·% sensitive in non-index households (95%CI: 17·7, 40·1). On average, using a RDT yielded 152 and 13 positive tests per 1,000 index and non-index households investigated, respectively (Fig. 5 & Table 4). Assuming a diagnostic had lower detection limits of 10 and 1 parasites/μL would have had sensitivities of 57·1% and 76·2%, respectively.
Fig. 5.
Bee-swarm and box plot of qPCR estimated parasite density among qPCR positives stratified by RDT result (Grossenbacher et al., 2020) The horizontal line provides a reference at 100 parasites/μL, representing the assumed detection limit of RDT.
Table 4:
Summary of RDT and qPCR test results in those tested by both methods. RDT sensitivity, specificity, PPV, and NPV assume qPCR as the gold standard.
| All Households | Index Households | Non-Index Households | |
|---|---|---|---|
| Tested by both qPCR and RDT | 6279 | 952 | 5327 |
| qPCR Positive & RDT Positive | 50 | 31 | 19 |
| qPCR Positive & RDT Negative | 97 | 48 | 49 |
| qPCR Negative & RDT Positive | 6 | 5 | 1 |
| qPCR Negative & RDT Negative | 6126 | 868 | 5258 |
| qPCR > 100 parasites/μL & RDT Negative | 21 | 15 | 6 |
| RDT Sensitivity | 0·34(0·26 – 0·42) | 0·39(0·28 – 0·51) | 0·28(0·18 – 0·40) |
| RDT Specificity | 1·00(1·00 – 1·00) | 0·99(0·99 – 1·00) | 1·00(1·00 – 1·00) |
| RDT PPV | 0·89(0·78 – 0·96) | 0·86(0·71 – 0·95) | 0·95(0·75 – 1·00) |
| RDT NPV | 0·98(0·98 – 0·99) | 0·95(0·93 – 0·96) | 0·99(0·99 – 0·99) |
| Infections detected per 1000 Households using RDT | 29 | 152 | 13 |
| Hypothetical Diagnostic with LOD of 10 parasites/μL - Sensitivity | 0·57(0·49 – 0·65) | 0·68(0·57 – 0·78) | 0·44(0·32 – 0·57) |
| Hypothetical Diagnostic with LOD of 1 parasites/μL - Sensitivity | 0·76(0·68 – 0·83) | 0·89(0·79 – 0·95) | 0·62(0·49 – 0·73) |
The median infection densities amongst qPCR-positives/RDT-negatives was 4.5 parasites/μL (IQR: 0.4, 69.7) and 842.5 parasites/μL (IQR: 165.8, 5,951.3) amongst qPCR-positives/RDT-positives. Of 97 qPCR-positives/RDT-negatives, 21 (21·6%) were of densities greater than 100 parasites/μL.
MIS comparison
Children tested in Zanzibar in the 2017 MIS were similar to children tested in the current study in terms of age, sex, and electricity in the household (Table 5) (Ministry of Health, 2020b). On average, children tested in the 2017 MIS were significantly less likely than children from the current study to have slept under a net the night before the survey.
Table 5:
Comparison of 3–59mo old children tested in 2017 MIS and current study. P-value is the result of chi-square or ANOVA test.
| Current Study | 2017 MIS | |||
|---|---|---|---|---|
| Index Households | Non-Index Households | p for difference | ||
| Participants (n) | 333 | 2242 | 540 | ·· |
| Age (mean (SD)) | 2·39 (1·28) | 2·41 (1·30) | 2·29 (1·32) | 0·182 |
| Male (n (%)) | 163 (48·9) | 1108 (49·4) | 264 (48·9) | 0·968 |
| Lives in household with electricity (n (%)) | 140 (42·8) | 995 (45·6) | 222 (41·1) | 0·144 |
| Slept under a net the previous night (n (%)) | 254 (76·3) | 1729 (77·1) | 367 (68·0) | <0·001 |
| RDT positive (n (%)) | 5(1·5) | 5(0·2) | 1(0·2) | 0·001 |
The 2017 MIS estimated RDT-based prevalence among 3–59mo old children in Zanzibar at 0·23% (95%CI: 0·03, 1·82). In the current study, 1·50% (95%CI: 0·49, 3·47) of children in the same age group were RDT-positive in index households and 0·22% (95%CI: 0·08, 1·82) in non-index households. Unadjusted logistic regression on the pooled data returned a statistically significant difference between RDT-positivity amongst index household members and MIS participants (OR = 9·9, 95%CI: 1·6, 191·0, p = 0·036) but no difference between the MIS and non-index household members (OR=1·5, 95%CI: 0·2, 285, p = 0·717).
Discussion
Malaria infection prevalence in Zanzibar is much higher among cohabitants in household of passively detected index case as compared to members of nearby households. No relationship was found between infection prevalence in members of non-index households and distance from the index household. Additionally, no evidence was found that infection prevalence was any higher in households surrounding the index household than the background infection prevalence measured in the MIS. These results clearly show that the current practice of only searching index households may be a more efficient way of finding additional cases than searching nearby households or random households in the general population.
The higher prevalence in index households is particularly pronounced in households in which the index case is suspected to be imported. This heightened prevalence in these households is driven by infections in the large number of index household members reporting co-travel with the index case. The high-risk co-travelers represent a clear target for intervention.
The risk factor analysis revealed that individuals who travel outside of Zanzibar and working adults whose jobs require them to work outdoors are at increased risk of positivity, and thus may be targets for intervention. It also showed that individuals tested within 3 days of the diagnosis of the index case are more likely to test positive, suggesting that investigations conducted sooner are more likely to detect additional cases. Bednet use the previous night was protective, reinforcing the effectiveness of bednets even in elimination settings. No association was found between positivity and distance to the index household within the same cluster, suggesting that clustering of cases occurs primarily within the index household and that there is no gradient of decreasing risk with increasing distance.
A high proportion of infections detected during RCD were of very low parasite density resulting in very low sensitivity of RDTs in this setting and application. Only 34% of all qPCR-detected infections were detected by RDT. Previous surveys conducted on Zanzibar following trends in infection from 2005–2013 already revealed increasingly low infection densities as prevalence decreased (Morris et al., 2015). Given that gametocytes have previously been detected in a large proportion of RDT-negative but qPCR-positive P. falciparum infections, (Kobayashi et al., 2019; Hofmann et al., 2018). the lack of sensitivity offered by RDT is problematic as the majority of opportunities to treat infections are missed, even though the individuals were tested. As a consequence, RCD based on a test-and-treat procedure with the current type of RDT is unlikely to prevent the majority of infections from being transmitted onwards and may therefore fail to contribute to further transmission reduction in Zanzibar. A new hsRDT has been shown to offer improved sensitivity over RDT and could offer some gains (Organization, 2018; Das et al., 2017; Mwesigwa et al., 2019). Molecular diagnosis by LAMP is more sensitive than by RDT and could be beneficial but has some implications in logistics and cos. (Cook et al., 2015).
Nearly 22% of qPCR-positives/RDT-negatives were of parasite densities greater than 100 parasites/μL. Considering that the qPCR method was P. falciparum-specific and that the RDT detected P. falciparum using HRP2, this may allude to the presence of parasites with HRP2 gene deletions in Zanzibar. While this has not been evaluated in Zanzibar, this has been documented in nearby Tanzania and Uganda (Kozycki et al., 2017; Thomson et al., 2019). This finding raises further concern with the use of RDTs in this setting.
An alternative to RCD is reactive focal mass drug administration (rfMDA), whereby passive case detection triggers presumptive treatment of all individuals living in index case clusters, forgoing an active search for cases and removing the reliance on diagnostic tests (Hsiang et al., 2020). rfMDA would ensure that a larger number of potentially transmissible infections are treated (Bjorkman et al., 2017). rfMDA might also prevent any onward transmission from the index case, co-travelers, or other source via prophylactic effect in household members uninfected at the time of treatment.
Limitations
PCR positivity was higher in the second year of data collection than in the first. This could have been due to degradation of DNA in DBS samples as samples from 2018 were analyzed within 2–3 months after sampling while samples from 2017 were stored for close to a year until analysis (Schwartz et al., 2015).
The definition of an imported case as an infection in any individual reporting travel outside Zanzibar in the past 60 days is a potential limitation. Nonetheless, such a ‘high sensitivity-low specificity’ definition may be appropriate in low-transmission settings in which importation risk is of high concern.
The window of time within which most of the data were collected was limited to within 5 days of index case notification. We cannot know if prevalence would have been higher or lower in households if investigations had occurred later, as 5 days may not be sufficient time for detectable infections originating from the index case to manifest, depending on a multitude of factors at the level of the human host, the parasite, and the vector.
Conclusions
In this study in Zanzibar, prevalence of malaria infections was primarily clustered in households of passively detected malaria cases. RCD found only few additional infections in neighboring households within a 200-meter radius in which prevalence was comparable to the general population. Index cases and household members with a travel history were at particularly high risk of infection and may benefit from higher prioritization in elimination efforts. RCD based on RDT-testing fails to identify and clear a substantial number of low-density malaria infections in this setting. Better diagnostics and/or alternative strategies must be sought to further reduce transmission and accelerate efforts of malaria elimination in Zanzibar.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the important contributions of the following individuals, without whose tireless efforts and contributions this research would not have been possible: the data collection team, all the DMSOs in the study districts, and the Shehia leaders in the study districts.
Funding Statement
Funding for this study was provided by the Swiss Tropical and Public Health Institute and the US President’s Malaria Initiative via the US Agency for International Development/Tanzania under the terms of an inter-agency agreement with Centers for Disease Control and Prevention (CDC) and the US Agency for International Development/Tanzania through a cooperative agreement with the MEASURE Evaluation consortium, under the associate cooperative agreement No. AID-621-LA-14-00001 titled ‘Measure Phase III—Strengthening the monitoring, evaluation and research capacity of the community health and social service programmes in the United Republic of Tanzania’. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the President’s Malaria Initiative via the US Agency for International Development, or other employing organizations or sources of funding.
Footnotes
Ethics
Ethical approval for this study was obtained from the Zanzibar Medical Research Ethics Committee, the Institutional Review Boards of Tulane University and of the Ifakara Health Institute as well as the Ethics Commission of North-western and Central Switzerland.
Declaration of interests
No conflicts of interest exist.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.06.017.
References
- Aidoo EK, Afrane YA, Machani MG, Chebore W, Lawson BW, Atieli H, et al. Reactive case detection of Plasmodium falciparum in western Kenya highlands: effective in identifying additional cases, yet limited effect on transmission. Malar J 2018;17(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton RA, Bennett A, Al-Mafazy AW, Abass AK, Msellem MI, McElroy P, et al. Use of Routine Health Information System Data to Evaluate Impact of Malaria Control Interventions in Zanzibar, Tanzania from 2000 to 2015. EClinicalMedicine 2019; (12):11–9. [DOI] [PMC free article] [PubMed]
- Bhattarai A, Ali AS, Kachur SP, Abbas AK, Khatib R, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med 2007;4(11):e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman A, Cook J, Sturrock H, Msellem M, Ali A, Xu W, et al. Spatial Distribution of Falciparum Malaria Infections in Zanzibar: Implications for Focal Drug Administration Strategies Targeting Asymptomatic Parasite Carriers. Clin Infect Dis 2017;64(9):1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Aydin-Schmidt B, Gonzalez IJ, Bell D, Edlund E, Nassor MH, et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J 2015;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Jang IK, Barney B, Peck R, Rek JC, Arinaitwe E, et al. Performance of a High-Sensitivity Rapid Diagnostic Test for Plasmodium falciparum Malaria in Asymptomatic Individuals from Uganda and Myanmar and Naive Human Challenge Infections. Am J Trop Med Hyg 2017;97(5):1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas Bates MM, Ben Bolker, Steve Walker. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 2015;67(1):1–48. [Google Scholar]
- Fontoura PS, Finco BF, Lima NF, de Carvalho JF Jr., Vinetz JM, Castro MC, et al. Reactive Case Detection for Plasmodium vivax Malaria Elimination in Rural Amazonia. PLoS Negl Trop Dis 2016;10(12)e0005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossenbacher B, Holzschuh A, Hofmann NE, Omar KA, Stuck L, Fakih BS, et al. Molecular methods for tracking residual Plasmodium falciparum transmission in a close-to-elimination setting in Zanzibar. Malar J 2020;19(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G. Open data kit: tools to build information services for developing regions. New York, New York, USA: ACM; 2010;. [Google Scholar]
- Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultrasensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 2015;12(3)e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann NE, Gruenberg M, Nate E, Ura A, Rodriguez-Rodriguez D, Salib M, et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect Dis 2018;18(10):1108–16. [DOI] [PubMed] [Google Scholar]
- Hsiang MS, Ntshalintshali N, Kang Dufour MS, Dlamini N, Nhlabathi N, Vilakati S, et al. Active case-finding for malaria: A three-year national evaluation of optimal approaches to detect infections and hotspots through reactive case detection in the low transmission setting of Eswatini. Clin Infect Dis 2019;. [DOI] [PMC free article] [PubMed]
- Hustedt J, Canavati SE, Rang C, Ashton RA, Khim N, Berne L, et al. Reactive case-detection of malaria in Pailin Province, Western Cambodia: lessons from a year-long evaluation in a pre-elimination setting. Malar J 2016;15(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang MS, Ntuku H, Roberts KW, Dufour MK, Whittemore B, Tambo M, et al. Effectiveness of reactive focal mass drug administration and reactive focal vector control to reduce malaria transmission in the low malaria-endemic setting of Namibia: a cluster-randomised controlled, open-label, two-by-two factorial design trial. Lancet 2020;395(10233):1361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Haji KA, Khatib BO, Bagi J, Mcha J, Devine GJ, et al. The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit Vectors 2013;6(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kanyangarara M, Laban NM, Phiri M, Hamapumbu H, Searle KM, et al. Characteristics of Subpatent Malaria in a Pre-Elimination Setting in Southern Zambia. Am J Trop Med Hyg 2019;100(2):280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozycki CT, Umulisa N, Rulisa S, Mwikarago EI, Musabyimana JP, Habimana JP, et al. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J 2017;16(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Menach A, Tatem AJ, Cohen JM, Hay SI, Randell H, Patil AP, et al. Travel risk, malaria importation and malaria transmission in Zanzibar. Sci Rep 2011;1:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littrell M, Sow GD, Ngom A, Ba M, Mboup BM, Dieye Y, et al. Case investigation and reactive case detection for malaria elimination in northern Senegal. Malar J 2013;12(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CA, Bruce J, Bhasin A, Roper C, Cox J, Abeku TA. Association between recent internal travel and malaria in Ugandan highland and highland fringe areas. Trop Med Int Health 2015;20(6):773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield-Aders W. Notes on malaria and filariasis in the Zanzibar protectorate. Transactions of the Royal Society of Tropical Medicine and Hygiene 1927;21 (3):207–14. [Google Scholar]
- Ministry of Health CD, Gender, Elderly and Children (MoHCDGEC), (MoH) MoH, (NBS) NBoS, (OCGS) OotCGS, ICF. Tanzania Malaria Indicator Survey 2017. Dar es Salaam, Tanzania and Rockville, Maryland, USA, 2017. [Google Scholar]
- Ministry of Health CD, Gender, Elderly and Children (MoHCDGEC), (MoH) MoH, (NBS) NBoS, (OCGS) OotCGS, ICF. Tanzania Malaria Indicator Survey. 2017. [Google Scholar]
- Morris U, Xu W, Msellem MI, Schwartz A, Abass A, Shakely D, et al. Characterising temporal trends in asymptomatic Plasmodium infections and transporter polymorphisms during transition from high to low transmission in Zanzibar, 2005–2013. Infect Genet Evol 2015;33:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwesigwa J, Slater H, Bradley J, Saidy B, Ceesay F, Whittaker C, et al. Field performance of the malaria highly sensitive rapid diagnostic test in a setting of varying malaria transmission. Malar J 2019;18(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. WHO technical consultation on research requirements to support policy recommendations on highly sensitive point-of- care diagnostics for. 2018;1–34.
- Polley SD, Gonzalez IJ, Mohamed D, Daly R, Bowers K, Watson J, et al. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis 2013;208(4):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Programme ZMC. Malaria Elimination in Zanzibar: A feasibility assessment 2009;.
- Rossi G, Van den Bergh R, Nguon C, Debackere M, Vernaeve L, Khim N, et al. Adapting Reactive Case Detection Strategies for falciparum Malaria in a Low-Transmission Area in Cambodia. Clin Infect Dis 2018;66(2):296–8. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Baidjoe A, Rosenthal PJ, Dorsey G, Bousema T, Greenhouse B. The Effect of Storage and Extraction Methods on Amplification of Plasmodium falciparum DNA from Dried Blood Spots. Am J Trop Med Hyg 2015;92(5):922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics TNBo, Finance TMo. Tanzania Population and Housing Census 2012;2014. [Google Scholar]
- Stresman GH, Kamanga A, Moono P, Hamapumbu H, Mharakurwa S, Kobayashi T, et al. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in Southern Province, Zambia. Malar J 2010;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock HJ, Novotny JM, Kunene S, Dlamini S, Zulu Z, Cohen JM, et al. Reactive case detection for malaria elimination: real-life experience from an ongoing program in Swaziland. PLoS One 2013;8(5)e63830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ. WorldPop, open data for spatial demography. Sci Data 2017;4(1)170004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RC Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2015.
- Thomson R, Beshir KB, Cunningham J, Baiden F, Bharmal J, Bruxvoort KJ, et al. pfhrp2 and pfhrp3 Gene Deletions That Affect Malaria Rapid Diagnostic Tests for Plasmodium falciparum: Analysis of Archived Blood Samples From 3 African Countries. The Journal of Infectious Diseases 2019;220(9):1444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst T, Al-Mafazy AW, Fakih BS, Stuck L, Ali A, Yukich J, et al. Operational Coverage and Timeliness of Reactive Case Detection for Malaria Elimination in Zanzibar, Tanzania. Am J Trop Med Hyg 2019;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk AM, Ramanathapuram L, Sutton PL, Kanagaraj D, Sri Lakshmi Priya G, Ravishankaran S, et al. What is the value of reactive case detection in malaria control? A case-study in India and a systematic review. Malar J 2016;15 (1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, van den Hoogen LL, Slater H, Walker PG, Ghani AC, Drakeley CJ, et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015;528(7580):S86–93. [DOI] [PubMed] [Google Scholar]
- Yukich JO, Taylor C, Eisele TP, Reithinger R, Nauhassenay H, Berhane Y, et al. Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J 2013;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.