Summary
The Family Smoking Prevention and Tobacco Control Act of 2009 established the Food and Drug Administration Center for Tobacco Products (FDA-CTP), and gave it regulatory authority over the marketing, manufacture and distribution of tobacco products, including those termed ‘modified risk’. On 8–10 December 2014, IIVS organised a workshop conference, entitled Assessment of In Vitro COPD Models for Tobacco Regulatory Science, to bring together stakeholders representing regulatory agencies, academia, industry and animal protection, to address the research priorities articulated by the FDA-CTP. Specific topics were covered to assess the status of current in vitro technologies as they are applied to understanding the adverse pulmonary events resulting from tobacco product exposure, and in particular, the progression of chronic obstructive pulmonary disease (COPD). The four topics covered were: a) Inflammation and Oxidative Stress; b) Ciliary Dysfunction and Ion Transport; c) Goblet Cell Hyperplasia and Mucus Production; and d) Parenchymal/Bronchial Tissue Destruction and Remodelling. The 2.5 day workshop included 18 expert speakers, plus poster sessions, networking and breakout sessions, which identified key findings and provided recommendations to advance the in vitro technologies and assays used to evaluate tobacco-induced disease etiologies. The workshop summary was reported at the 2015 Society of Toxicology Annual Meeting, and the recommendations led to an IIVS-organised technical workshop in June 2015, entitled Goblet Cell Hyperplasia, Mucus Production, and Ciliary Beating Assays, to assess these assays and to conduct a proof-of-principle multi-laboratory exercise to determine their suitability for standardisation. Here, we report on the proceedings, recommendations and outcomes of the December 2014 workshop, including paths forward to continue the development of non-animal methods to evaluate tissue responses that model the disease processes that may lead to COPD, a major cause of mortality worldwide.
Keywords: COPD, in vitro, in vitro lung, in vitro models, pulmonary models, tobacco regulatory science
Introduction
The Family Smoking Prevention and Tobacco Control Act of 2009 (2009 Act) established the Food and Drug Administration Center for Tobacco Products (FDA-CTP), and gave it regulatory authority over the marketing, manufacture and distribution of tobacco products in the United States. Included among these products are those described as Modified Risk Tobacco Products (MRTPs). The FDA-CTP is required to give guidance on the type of scientific evidence that needs to be submitted to support an application to market a MRTP.
To assist in determining what scientific evidence is needed to support an MRTP application, the FDA requested input from the Institute of Medicine (IoM). The result was a 2012 report, Scientific Standards for Studies on Modified Risk Tobacco Products (1), which advised the FDA to require companies wishing to market an MRTP to include information on the “human health risks of the MRTP, including the risk of tobacco-related diseases”.
Traditionally, much of the information on health risks is interpreted from toxicological experiments conducted on animals. However, the 2007 report, Toxicity Testing in the 21st Century — A Vision and a Strategy, published by the US National Academies Press (2), describes a path forward for toxicology and envisions the use of more human-relevant and predictive in vitro models for estimating human health risks. While many of these methods have been used by both industry and research institutions, their relevance and utility in decision making processes may not yet be well established or publicised within many sectors of the regulatory community. In an effort to help harmonise the in vitro approaches and highlight the potential usefulness of such methods in assessing human health risk within a regulatory framework, the Institute for In Vitro Sciences, Inc. (IIVS) convened a workshop in December 2014. The workshop theme and subject areas to be explored were developed with the input of an independent steering committee, after consideration of the published FDA-CTP research priorities.
The workshop, entitled In Vitro COPD Models for Tobacco Regulatory Science, was conceived and developed by IIVS on the basis of identified needs of the FDA-CTP (as evidenced by the public dissemination of their research priorities; http://www.fda.gov/downloads/tobaccoproducts/newsevents/ucm293998.pdf) and by researchers from different sectors, who are interested in better understanding the adverse health effects of tobacco products. The workshop was meant to address at least parts of the following specific FDA-CTP research priorities:
What in vitro and in vivo assays are capable of comparative toxicity between two different tobacco products, with special attention to cardiotoxicity, respiratory toxicity, carcinogenicity and developmental/reproductive toxicity?
What constituents, compounds, design features and tobacco-use behaviours impact toxicity and carcinogenicity of tobacco products and smoke?
The workshop was held on 8–10 December 2014, in Bethesda, MD, USA, centrally located near both FDA-CTP headquarters and the National Institutes of Health. It was attended by over 60 stakeholders, including regulators, industry, biotechnology providers, research institutions, and the animal protection community. The 2.5-day programme, designed by the steering committee, consisted of four core subject areas covered during presentations by 18 experts in the field (see Table 1), and 21 posters which addressed a wide scope of tobacco-induced chronic obstructive pulmonary disease (COPD) topics. The four core areas were: a) Inflammation and Oxidative Stress; b) Ciliary Dysfunction and Ion Transport; c) Goblet Cell Hyperplasia and Mucus Production; and d) Parenchymal/Bronchial Tissue Destruction and Remodelling. Breakout group sessions were held for three of the four core topics, and were intended to consolidate current views on the assay endpoints, test systems, and related technologies that should be considered for standardisation, and to identify areas that require additional research and/or development. A description of the workshop, and the conclusions from the breakout groups focusing on specific toxicological events expected to lead toward the development of COPD, are presented.
Table 1:
Summary of Contents
| Presenter (where applicable) |
Page | |
|---|---|---|
| Introduction | 129 | |
| Introductory Presentations | 129 | |
| Introduction and Overview of Meeting Plan | Erin Hill | 131 |
| Tobacco Product Regulation and Nonclinical Science | Hans Rosenfeldt | 132 |
| Animals Don’t Smoke: Ending Tobacco Experiments on Animals | Joseph Manuppello | 133 |
| In Vitro Toxicity Testing of Tobacco Products: A Manufacturer’s Perspective | Betsy Bombick | 133 |
| Considerations for Test Method Validation | Rodger Curren | 135 |
| Adverse Outcome Pathways: A Framework for Organizing Mechanistic Information to Improve Chemical Assessment | Kristie Sullivan | 137 |
| Chronic Obstructive Pulmonary Disease: Overview | ||
| Etiology of COPD and In Vitro Models | Holger Behrsing | 138 |
| Overview of the Clinical Aspects of COPD | Sanjay Sethi | 140 |
| Inflammation and Oxidative Stress | ||
| Impact of Tobacco Smoke on Lung Inflammation and Pro-Resolving Pathways in Humans, Mouse Models and In Vitro Models | Richard P. Phipps | 142 |
| Genetic Variants, Inflammation and the Mucous Secretory Phenotypes | Yohannes Tesfaigzi | 143 |
| Overview of Non-Animal Approaches to Address COPD Pathogenesis Associated with Nicotine-Delivering Products | Sherwin Yan | 144 |
| Ciliary Dysfunction and Ion Transport | ||
| Measuring Airway Surface Liquid Volume and Mucus Transport by Fluorescence Microscopy | Robert Tarran | 146 |
| Assessment of Ciliary Dysfunction in COPD Research | Samuel Constant | 150 |
| Understanding the Impact of Tobacco Smoke Exposure on Ciliary Dysfunction and Ion Transport: The Case for In Vitro Testing | Gary Phillips | 151 |
| Goblet Cell Hyperplasia and Mucus Production | ||
| In Vitro Induction of Airway Goblet Cell Hyperplasia in the EpiAirway™ by Th2 Cytokines, Viral Exposure or Cigarette Smoke | Patrick Hayden | 154 |
| Measurement of Mucin Secretion for Potential Evaluation of the Toxicity in Tobacco Products in Human Air–Liquid Interface Airway Models | Xuefei Cao | 155 |
| Combining Systems Biology, Computational Approaches and Human Organotypic Models Exposed to Whole Cigarette Smoke: An Example of 21st Century Toxicology Assessment | Carole Mathis and Julia Hoeng | 156 |
| Parenchymal/Bronchial Tissue Destruction and Remodelling | ||
| Detection of Inflammation and Parenchymal Damage by Using PCLS | Holger Behrsing | 160 |
| The Use of PCLS to Test Physiological and Pathophysiological Lung Responses | Armin Braun | 161 |
| Breakout Discussion Groups | ||
| Overview | 163 | |
| Discussion | 163 | |
| Next steps | 164 | |
| Summary | 164 | |
| Summary of key themes | 164 | |
| Acknowledgements | 164 | |
| References | 165 | |
| Tables | ||
| Table 1: Summary of Contents | 130 | |
| Table 2: The questions used to guide the Breakout Discussion Groups | 150 | |
| Table 3: Outcome of the breakout discussion on Inflammation and Oxidative Stress | 152 | |
| Table 4: Outcome of the breakout discussion on Ciliary Dysfunction and Ion Transport | 158 | |
| Table 5: Outcome of the breakout discussion on Goblet Cell Hyperplasia and Mucus Production | 162 |
Introductory Presentations
Introduction and Overview of Meeting Plan (Erin Hill)
This workshop, which was the first in a series, exemplified what is at the core of IIVS’ not-for-profit mission. That is, to bring together stakeholders from a variety of backgrounds in order to identify promising in vitro and in silico methods, and then to standardise and help validate those methods, to make them ready for regulatory application. Drawing on the list of research priorities set forth by the FDA-CTP, we designed a programme in which invited experts from academia, government and industry would present talks and posters covering key areas in the utilisation of models and assays to investigate the effects of tobacco on human health, such as COPD. The exchange of information was intended to facilitate a better understanding of, and forge a route toward, the standardisation of in vitro methods. On the basis of the workshop discussions, conclusions concerning the readiness of current models for regulatory application and approaches to improving models would be drawn and published, thus advancing the science-based assessment of tobacco products.
Tobacco Product Regulation and Nonclinical Science (Hans Rosenfeldt)
The FDA-CTP was set up in 2009, when the FDA was authorised to regulate the manufacture, marketing and distribution of cigarettes and smokeless tobacco products, by the Family Smoking Prevention and Tobacco Control Act. The FDA has the authority to regulate “tobacco products”, which are defined, in part, as any products “made or derived from tobacco” that are not “drug”, “device”, or combination products under that Act. The FDA currently regulates cigarettes, cigarette tobacco, roll-your-own tobacco and smokeless tobacco. On 25 April 2014, the FDA published a proposed rule, Tobacco Products Deemed to Be Subject to the Food, Drug & Cosmetic Act (Deeming). This would extend the FDA’s tobacco authority to cover additional tobacco products, including electronic cigarettes (e-cigarettes), cigars, pipe tobacco, nicotine gels, water pipe (hookah) tobacco and dissolvables, not already under the Agency’s authority and that meet the definition of a tobacco product. The aim of the Act is to protect public health and make tobacco-related disease part of the US’s past. Specific aims are to prevent young people starting to use tobacco products, to encourage adults to quit use, and to reduce the harm and addictiveness of products for those who continue to use them.
The FDA is using its regulatory authority to gain understanding of tobacco products, restrict changes that might adversely affect public health, prohibit claims of modified risk without sufficient supporting data, ensure compliance with regulation, and educate the public. An important aim is to expand the science base for regulation.
The FDA-CTP is the newest centre at the FDA and it has undergone substantial and rapid growth since its inception. Within the FDA-CTP, the Division of Nonclinical Science employs scientists in areas such as toxicology, pharmacology and environmental science, to review product applications, provide scientific input for guidance and regulation documents, conduct research, and expand knowledge. Unlike other FDA centres, which have regulations that outline the nonclinical studies required of the applicant for clinical studies and product authorisation, the FDA-CTP has no such regulations. Data from in vivo, in vitro, ex vivo and in silico studies are considered and reviewed to inform the agency on scientific decisions.
The FDA-CTP has issued draft guidance that reflects current thinking on nonclinical studies submitted to support modified risk tobacco product applications (MRTPAs). However, both draft and final guidance documents contain non-binding recommendations that represent the Agency’s current thinking. The main difference between a draft and final guidance is that the draft guidance carries an additional disclaimer, indicating that the Agency’s thinking on the subject of the guidance is not final. Draft guidance includes suggestions of items to address when preparing applications, similar to previous ‘points to consider’ documents. The issuing of draft guidance is seen as a good way to share current thinking with stakeholders, and to bridge the gap to finalisation, which can take a substantial period of time. The following example is found in the guidance for testing of MRTPAs:
“FDA recommends that applicants conduct nonclinical studies to address the known clinical toxicities of tobacco products and evaluate a range of potential toxicities of the product as compared to other tobacco products on the market. Applicants should choose appropriate models for nonclinical studies that are sufficiently sensitive for the evaluation of the selected endpoint and be able to provide support for the model used, including an explanation of the sensitivity and probative value of the model chosen. For in vivo animal studies, researchers should administer the test product to animals by a route representative of human exposure, where feasible. Nonclinical toxicology studies should use methods that are sufficiently sensitive to assess the actual differences between use of the product and use of other tobacco products, or between use of the product and non-use of tobacco products.”
Hans Rosenfeldt noted the FDA’s commitment to the Three Rs (Replacement, Reduction and Refinement), and the inclusion of “modernising toxicology” as one of the eight priority areas to address (3, 4). Therefore, the FDA-CTP is very interested in hearing about current assays that could fit into meeting this goal. These should be relevant to humans and address known and potential clinical toxicities of tobacco products. To summarise, the key points are:
The FDA-CTP is the newest centre within the FDA, and is interested in information and knowledge on nonclinical methods that could be relevant to tobacco products.
Draft and final guidance are issued to share the FDA’s current thinking with stakeholders.
Modernisation of toxicology methods, as well as a commitment to the Three Rs, is a priority for the FDA.
The FDA-CTP, as part of its commitment to modernisation of toxicology and the Three Rs, is interested in the investigation into non-animal testing that would be relevant to tobacco products and their toxicity.
[Disclaimer: The information in these materials is not a formal dissemination of information by FDA and does not represent agency position or policy.]
Animals Don’t Smoke: Ending Tobacco Experiments on Animals (Joseph Manuppello)
Joseph Manuppello discussed PETA’s interest in relation to the 2009 Act, and what the related strategy and regulation is likely to mean for animal testing. PETA would like to see a tiered approach to testing introduced, that would minimise the use of animal testing. The FDA guidance to industry lends itself to such an approach, by requesting the submission of clinical data, including endpoints based on validated biomarkers and the use of intermediate clinical endpoints. Crucially, the 2009 Act makes no mention of animal testing. Rather, it stipulates that manufacturers should undertake well-regulated investigations. Even in the case of MRTPs, which if approved are marketed specifically to reduce harm, animal testing is not mentioned. Nevertheless, the IoM noted that, despite the difficulty in making laboratory animals use tobacco products as humans do, and notable inter-species differences that can prevent meaningful extrapolation of data, for MRTPs animal testing might be informative (1). To temper the use of in vivo animal studies, the IoM does recommend that they be preceded by in vitro studies, and that composition and product standards or limits should be set.
As part of the tiered regulatory approach, PETA suggests that, for truly new products, not only should preclinical in vitro studies be done first, but also that the data should be submitted and assessed before any clinical assessments are begun. Preclinical in vitro laboratory tests should also be done, on constituents, rather than just on products. Assays of particular interest are those for cytotoxicity, genotoxicity, cell apoptosis and proliferation, oxidative stress, inflammation, mucus production, and endothelial activation. Only if these assays yield significant findings should animal or human studies be considered. Joseph Manuppello noted that, for existing products, when wishing to make claims of modified risks, epidemiological assessments will play a much more prominent role than laboratory studies, and may be supported by previously reported research on similar products. Likewise, for the investigation of addictive potential, human research (after adequate preclinical screening) seems to be the most externally valid approach, owing in part to difficulties in modelling some kinds of delivery systems, such as snus.
When the 2009 Act was introduced, in vitro methods were not deemed reliable and were limited to a small number of cytotoxicity and genotoxicity tests. Since then, however, many advances have been made, and this is now clearly not the case. Reliable assays are available to also assess air–liquid interface exposure, apoptosis, inflammation, cell transformation, and gene expression. Thus, to support the use of preclinical in vitro testing, PETA suggests that the FDA should exercise its authority under the Act to set standards for the non-animal preclinical testing of tobacco products, including a standard battery of assays.
It has been suggested that there is a risk that e-cigarettes will be deemed to need relevant animal testing, because no products were marketed in 2007, but PETA suggests that there are circumstances that deem this unnecessary. For example, if products have toxicants below certain levels, or use food-grade flavours (or if flavours are prohibited in given products), then animal studies should only be undertaken if the need can be justified by significant in vitro findings and agreed to when opened to public comment.
Some countries, such as Belgium, Estonia, Germany, Slovakia and the UK, have already banned animal testing for tobacco products. Global prohibition, therefore, seems a reasonable goal for manufacturers. In addition, some manufacturers already avoid animal testing wherever possible. To summarise, the key points are:
Since 2009, the availability, breadth and reliability of in vitro assays has grown substantially.
The onus should be on the in vitro testing of products and constituents, where claims of reduced harm are intended.
For existing products, when wishing to make new claims, new epidemiological data and previously published research are more appropriate than new laboratory testing.
The FDA should provide a list of standards, including a battery of standard tests required for regulation.
In Vitro Toxicity Testing of Tobacco Products: A Manufacturer’s Perspective (Betsy Bombick)
Betsy Bombick presented guidance and recommendations on in vitro testing, the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA) experience, and ways to move forward.
A wide range of samples related to tobacco products can be tested in vitro, including cigarette smoke particulate matter, whole smoke, smokeless tobacco, and tobacco product constituents. In vitro tests are faster and less expensive than animal studies and give mechanistic understanding, although they have both strengths and limitations and single assays cannot answer all questions. Therefore, results must be taken in the context of existing data and information. From a regulatory perspective, the use of nonclinical studies (in vitro, in vivo and ex vivo) is encouraged. Health Canada requires annual toxicity testing of cigarettes sold or manufactured in Canada with three required toxicology tests: the Ames bacterial mutagenicity test, the micronucleus assay for genotoxicity, and the Neutral Red Uptake (NRU) assay for cytotoxicity (Figure 1). The FDA is less clear on types of study that should be used for MRTPs and premarket products, recommending, respectively, nonclinical studies and “some combination of in vitro, in vivo, and ex vivo studies” in its Draft Guidance for Industry: Modified Risk Tobacco Product Applications, Applications for Premarket Review of New Tobacco Products (5).
Figure 1: CORESTA Proficiency Trial assays.
a) Ames test: bacterial mutagenicity;
b) micronucleus assay;
c) Neutral Red Uptake assay.
CORESTA, founded in 1956, aims to promote international co-operation in scientific research related to tobacco, in the areas of agronomy and leaf integrity, phytopathology and genetics, smoke science, and product technology. In 2002, the CORESTA In Vitro Toxicity Task Force was formed to establish a rationale and strategy for the in vitro testing of tobacco smoke, and to identify key procedures in line with internationally recognised guidelines, but adapted to take into account the unique properties of tobacco smoke. Recommendations related to these objectives were published in 2004 (6). The suggested battery of tests comprised a bacterial mutation assay (the Ames test), a mammalian cell assay for cytogenetics or mutation (the micronucleus, chromosome aberration or mouse lymphoma assays), and a cytotoxicity assay with appropriate mammalian cells (the NRU assay). The defined test item was total particulate matter from mainstream smoke collected on Cambridge filter pads and extracted in dimethyl sulphoxide. The report also provided useful background information and references.
Proficiency trial assays were performed to improve study conduct and methods with the approved battery of tests, with samples extracted by standardised preparation. Protocols and worksheets were also standardised. The final data were coded, and quality was assessed by a quality assurance expert and a statistician. These studies showed that the Ames test is sufficiently sensitive (excellent concordance between laboratories), that the NRU test results replicated well within a particular cell line, and that the micronucleus assay results differed according to the metabolism component (S9) conditions. Of note is that, in the NRU studies, differences were found between results from the four cell lines used, possibly at least partially driven by differences in metabolic capabilities.
On the basis of these findings, the Task Force concluded the following with regard to proficiency trials: objectives and rationale must be clear before starting; careful selection of samples is important; and statistical analysis might be challenging. These challenges are compounded by variation in laboratory experience and understanding of the strengths and limitations of methods, which might make interpretation and comparison of findings difficult.
In order to effectively test a wide range of different products, the following considerations are necessary before starting testing, namely: products (differences and similarities); sample production and preparation (smoking regimen, sample type and phase); sample storage; exposure systems (equipment and methods); biological test systems (sensitivity, relevance, strengths and limitations); interpretation and context; and utility and application.
Some exciting new tools are available to improve the quality of research and further inform the science, such as Adverse Outcome Pathways (AOPs) and systems biology, in combination with toxicity methods. In vitro models of disease also hold promise. The key points are:
A wide range of samples from tobacco products can be tested in vitro.
CORESTA aims to promote international cooperation in scientific research related to tobacco, and supports in vitro toxicity discussions and proficiency trials.
Proficiency studies with standardised sample preparation and protocols have highlighted the need for planning and understanding of methodological strengths and limitations.
Considerations for Test Method Validation (Rodger Curren)
Validation in some form is generally required by regulatory agencies before data from new test methods are accepted. The classic process of validation is demonstration of the reliability (reproducibility) and relevance (extent to which results can correctly predict outcomes) of a new method for a specific purpose. This definition and the classic sequence of events from conception to validation (presented below) are accepted by many international bodies, such as the Organisation for Economic Co-operation and Development (OECD). However:
Ideas for new test methods come from basic research, but are usually undirected and the suggested methods are often impractical for routine use.
Optimisation of methods increases robustness, and ensures performance across multiple laboratories.
Pre-validation, which is a controlled, small-scale study of reproducibility and performance, is advisable.
A final formal validation stage involves a multi-laboratory blinded assessment of reliability and relevance.
Validation does not need to be done by the agency accepting the test results, but when it is, individual agencies can apply their own standards of rigour to the process. As a result, agencies may vary widely in the number of laboratories required for reproducibility studies, numbers of materials tested, the range of chemistries assessed, and the types of qualitative and quantitative endpoints that must be reported. This highly formal testing is time-consuming and very expensive, and is extended by the slow review of methods by validation authorities. Even if a method is validated, it might take several years to achieve regulatory acceptance. Thus, the process of validation is often severely criticised for the length of time it takes. In this presentation, Rodger Curren explored ways in which the process could be improved.
In 1995, ECVAM proposed a pre-validation stage to assess and refine the validation process, and thereby increase efficiency (7). Three phases of pre-validation were suggested. Phase I: protocol refinement through interaction between two designated laboratories to identify potential adjustments needed to optimise the test method. In addition, the protocol can be developed and put into a format compliant with the regulatory agency’s requirements. Accompanying standard operating procedures may be developed, reproducibility can be confirmed, prediction models can be proposed, and suitability for the next phase of pre-validation can be confirmed. Phase II: protocol transfer, in which a third designated laboratory uses the refined protocol and standard operating procedures to test inter-laboratory transferability; further refinement of the protocol is possible at this stage. If phase II results are acceptable, pre-validation moves into phase III. Phase III: a limited number of materials are coded for blind testing in a small number of laboratories (at least two) to confirm the validity of the prediction model or to suggest its further refinement. If necessary, the method could move on to more rigorous validation involving additional coded materials, or the data obtained during pre-validation could be considered sufficient to establish reliability and relevance.
An efficient validation process can: direct basic research toward gaps in existing testing methodologies and/or knowledge, identified by stakeholders through workshops or directed interactions with regulatory bodies, such as CTP; help move useful methods quickly into development and optimisation; accommodate the use of human biomarkers of effect or disease, rather than — or in addition to — data from animal studies; use a pre-validation stage to refine the protocols in competent laboratories, including those associated with Government agencies, such as the US National Center for Toxicological Research or National Institute for Environmental Health Studies; and include the retrospective evaluation of data (8), including data from robust prior studies (perhaps defined by a regulatory branch, such as the FDA-CTP), so that new studies do not have to be conducted unless truly necessary.
In determining the value of new test methods, more than just the individual predictive capacity (as assessed during the traditional validation process) should be evaluated. Tests should also be considered as part of integrated testing strategies. For example, combining results from several methods of analysis, such as QSAR, read-across, and one or two other complementary in vitro assays, might prove to be highly predictive of subsequent human health effects.
To make any validation process truly useful, a meaningful standard against which to evaluate the new test method needs to be established. For many years the gold standard used in toxicology has been the results obtained from animal tests, yet these often suffer from reproducibility problems, and their ability to predict how humans will respond to the same exposure has frequently been called into question. For example, although the Draize rabbit eye test has been viewed as the gold standard for eye irritation for many years, results for the test vary substantially between laboratories (9, 10) and are often not predictive of human responses. Figure 2, from Bruner et al. (11), illustrates the best correlation relationship that could be expected between two separate laboratories running the Draize eye test, knowing that the coefficient of variation of this test is around 40%. If this is the highest reproducibility for this animal test, clearly an in vitro method could not be expected to perform any better.
Figure 2: Inter-laboratory variation of the Draize rabbit eye test is substantial.
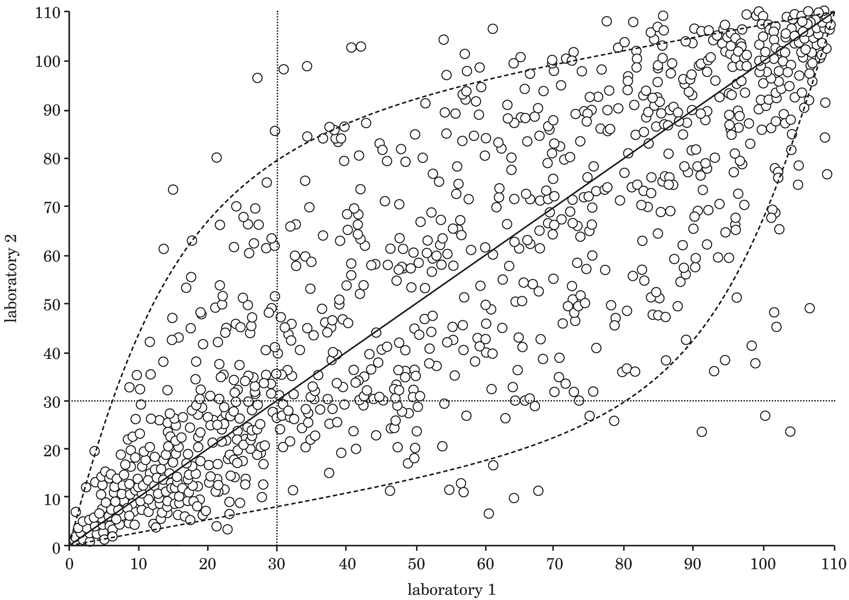
To demonstrate potential inter-laboratory variability of the Draize rabbit eye test, a computer simulation was used to show expected results for two different laboratories testing 1,000 identical substances. The model used assumed the algorithm y = x, which describes the relationship between the results in equally competent laboratories. Values for the full range of the Draize scoring scale (0–110) were utilised. The x and y values plotted on the graph were created from a simulation where a CV of 40% was assumed. (Figure created by Dr Leon Bruner.)
In the specific case of validating in vitro methods for use in estimating the hazard associated with new tobacco products, either combustible or noncombustible, it would clearly be best to validate results against the human response, rather than against animal responses. A potential way forward to this approach would be to validate against human biomarkers that could be obtained in relatively non-invasive ways during clinical studies. Biomarkers of exposure or effect, or both, should be developed and then used as the gold standards during validation trials with new in vitro methods. This approach should be especially useful when used with 3-D human tissue constructs that closely resemble the actual human tissues exposed in situ. If biomarkers from the in vitro systems correlate well with the same biomarkers obtained from individuals in clinical studies after exposure to a given tobacco product, then there would be good evidence that the in vitro method would be predictive of either exposure or disease state for a new tobacco product.
In summary, all test methods to assess tobacco products for regulation need to be validated. The exact steps to be taken for each test cannot always be predicted, but however the validation process is conducted, it must take into account all research agendas to increase the likelihood of regulatory acceptance. Pre-validation performed in competent laboratories and based on guidance documents written by teams with good technical knowledge of the assays, and which address various outcomes, can streamline the process, and may eliminate the need for the formal validation studies currently in place. The key points are:
Stakeholders should collaborate to direct basic research towards gaps in methodologies.
Involvement of the FDA-CTP in designing research and selecting facilities for pre-validation testing would be useful.
The pre-validation stage should be compulsory, with adequate financial support provided for these activities.
All research agendas should be taken into account during the development of assays for regulation.
Adverse Outcome Pathways: A Framework for Organising Mechanistic Information to Improve Chemical Assessment (Kristie Sullivan)
Kristie Sullivan noted that the National Research Council’s Tox 21 report (2) recommended a strategy that included a wide range of methods of testing. The mapping of toxicity pathways (thought to be a finite number) was intended to be a conceptual framework that would help to predict effects of chemicals without animal testing. Ultimately, it led to the concept of Adverse Outcome Pathways (AOPs), which are flexible organisational frameworks that outline linear pathways to adverse outcomes and that can link chemical structures to biological response data. There are a variety of uses of AOPs, dependent on the amount of information available. For instance, they can highlight research needs (e.g. species or genetic differences and effects on toxicity), and can be helpful in the design of testing strategies and frameworks. At the testing level, they can help to identify promising assays, provide mechanistic support for chemical grouping and categorisation and for hazard or risk assessment, and can put assays into biological context, which can remove barriers to regulatory acceptance. However, full mechanistic understanding is not necessary for an AOP to be useful.
The OECD is heavily involved in the development of AOPs. The Extended Advisory Group for Molecular Screening (EAGMST) aims to collect information and enable collaboration, via, for example, tools such as the AOP Wiki (12), and to support regulatory activities.
Concept development can be simple or complex, and can be started without knowing exactly what the key events are (Figure 3). Rather, AOPs can be used to try to establish linkages between key events (i.e. what happens to link one event to the next). A work plan for the AOP can be proposed and submitted for review by expert groups via the AOP Wiki. This can lead to the setting up of workshops and the creation of a main team or laboratory to research the AOP, but with contributions from multiple sources (i.e. ‘crowd-sourcing’ of information). To summarise, the key points are:
Figure 3: Example of AOP concept development.
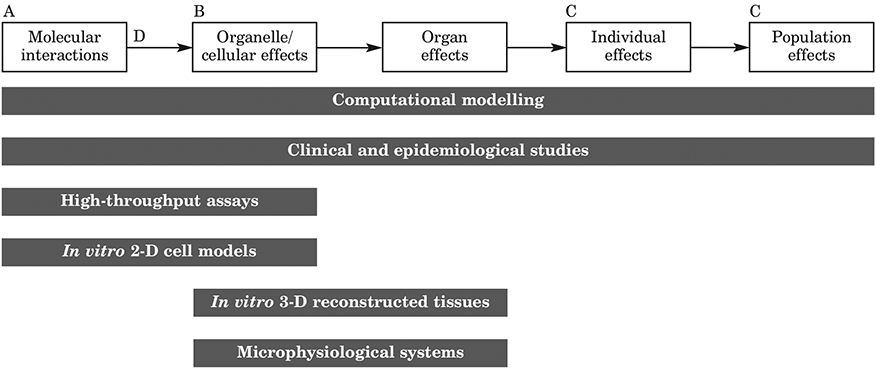
An Adverse Outcome Pathway (AOP) is a linear framework for organising available toxicity information at different levels of biological organisation, and which links mechanistic and apical information to aid in the interpretation of diverse data streams for regulatory application. AOPs can provide context for non-traditional toxicity data and emerging test methods or weight-of-evidence approaches. Key Events (A, B and C) are testable events along a pathway necessary for its continuation; specialised Key Events include a Molecular Initiating Event (A) and one or more Adverse Outcomes (C); Key Event Relationships (D) describe evidence that an upstream Key Event leads to a subsequent downstream Key Event.
AOPs are flexible organisational linear frameworks illustrating pathways to adverse events.
AOPs are useful for highlighting gaps in research and knowledge, for chemical grouping, for providing context for data or methods, and for test-method development.
The OECD AOP Wiki allows information to be shared and aids collaboration.
Chronic Obstructive Pulmonary Disease: Overview
Etiology of COPD and In Vitro Models (Holger Behrsing)
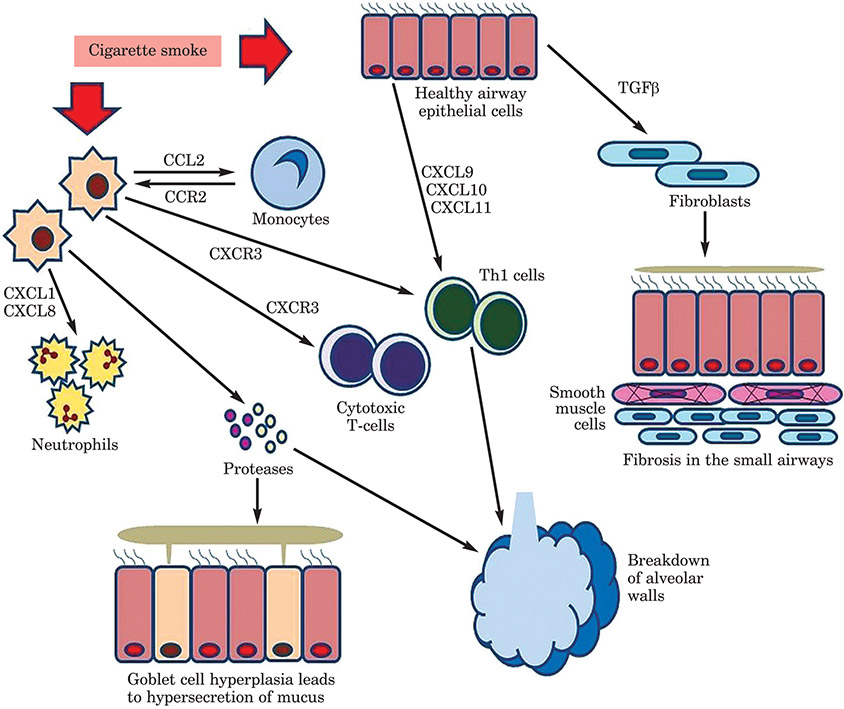
To open this section of the workshop, Holger Behrsing presented an overview of COPD. Patients with COPD can have one or more symptoms of chronic bronchiolitis (excessive mucus production, airway wall thickening, epithelial squamous metaplasia, leukocyte recruitment), emphysema (airspace enlargement, parenchymal destruction), or both. The symptoms vary from individual to individual, but the disease is characterised by (usually) a progressive airflow limitation and chronic inflammatory response to noxious gases and particles. The main risk factor is smoking (Figure 4), but gender, genetics, pre-existing airway disease, and environmental factors can also contribute to the risk. Tobacco smoke exposure changes the lining of the bronchus and leads to oxidative stress. In the early stages, basal cells begin to crowd out columnar cells, which are ciliated. Therefore, cilia become reduced in number and efficiency, and toxic particles are not cleared effectively. Later changes include the total displacement of ciliated columnar cells, which raises the risk of infections and exacerbations of airway limitation, and an increase in abnormal squamous cells, which eventually invade the underlying lung tissue and can become cancerous. Small airways disease, which includes a wide variety of disorders, often includes some form of bronchiolar inflammation and metaplasia of the bronchiolar tissues.
Figure 4:
COPD events after exposure to cigarette smoke
A number of models and assays have been proved fit for purpose for COPD. Various features, such as oxidative stress, inflammatory response, ciliary dysfunction, goblet cell hyperplasia (GCH), and small airway and vascular remodelling, are potential targets for in vitro testing. The selection of models includes cell lines of immortalised cells, primary cells, 3-D airway tissue cultures, and tissue samples for testing ex vivo. These can be employed in areas such as drug development or hazard of exposure assessment — and therefore, the suitability of the models for particular purposes must be considered.
Immortalised cells are economic, straightforward to use, and the results are reproducible. These cell lines can be expanded and are simple to store, although high passage numbers might be associated with genetic drift. However, the cell lines are frequently derived from cancerous tissue and thus do not represent the normal physiological state to the extent that other models do.
Primary cells are often more expensive, because they are not immortalised, are more variable, and their cultivation is more difficult. They are more representative of the population of interest (although subject to donor variability), but may have little capacity to be expanded and stored for future use.
3-D tissue cultures, such as reconstructed airway epithelium, are physiologically relevant, representative of the population of interest, and contain cell types and functions not available in two-dimensional cell lines, which might be amenable to assessment. Each culture, however, can take weeks to complete, which can be expensive, and reproducibility is likely to vary with different donors.
Ex vivo tissue studies can be done by using precision-cut lung slices (PCLS). These are more physiologically relevant, but are representative only of the part of the organ from which they are cut. They might be representative of a population, but frequently are obtained from donors that do not match wider population criteria (e.g. slices are often obtained from lungs discarded from transplant owing to certain disease states). A strength is that they do permit the study of multiple cell types, but their quality and reproducibility are not always high, and are likely to be variable with different donors. Their use is labour intensive. Important considerations in choice of in vitro model are:
Cost.
Reproducibility.
Ease of use and accessibility.
Inter-laboratory transferability.
Endpoints to be modelled.
Tissue origin.
If human, how well can results be translated to the whole body?
If non-human, how well can results be extrapolated to humans?
How amenable is it to high-throughput?
Upcoming technologies that might improve in vitro testing include lung-on-a-chip. The Wyss Institute has created a ‘breathing’ human lung-on-a-chip that recreates expansion and contraction of the airways during breathing, to mimic changes in air and blood flow (13). RTI International, in collaboration with the University of North Carolina, have created a lung-on-a-chip model in which distinct cellular layers are stacked to mimic the structure of airway tissue (14).
To summarise, the key points are:
COPD symptoms can vary from individual to individual and are (usually) characterised by progressive airflow limitation and chronic inflammatory response to noxious gases and particles.
The major etiological factor is smoking.
Various in vitro and ex vivo models are available that permit the relevant testing of exposure to cigarette smoke.
Multiple factors must be taken into account when selecting a model; cheaper options might be less physiologically relevant, whereas more expensive options might be time-consuming to create or are limited in supply.
Variability according to donor is an issue that needs to be addressed, but individuality of tissues may more accurately reflect a population.
Emerging technologies, such as ‘lung-on-a-chip’, could advance the relevance of the models.
Overview of the Clinical Aspects of COPD (Sanjay Sethi)
Sanjay Sethi provided further clinical information about the features and epidemiology of COPD. It is the third most prevalent disease in Canada (15) and the USA, is currently the sixth most prevalent globally, and is predicted to reach the third position by 2020. Despite improvements in treatment and air quality, etc., COPD will remain a major problem because of a high rate of undiagnosed disease. Despite being preventable and treatable, the disease is frequently not diagnosed until progression has occurred — early stage symptoms are generally not serious and frequently go unrecognised by physicians — and some patients are reluctant to seek treatment, as it is a ‘self-inflicted’ disease. High concentrations of autoantibodies are detected, but whether these are causative is unclear, and autoimmune disease elements need to be explored further. COPD incurs high health costs, particularly in relation to exacerbations. The available therapies are mainly symptom driven rather than disease modifying, but most patients do not receive the recommended treatment (~60% are undertreated, and/or ~65% are inappropriately treated). Poorly treated disease is associated with severe extrapulmonary effects and important co-morbid conditions.
Initially, there was a lot of confusion about what constituted COPD. Small-airways disease was overlooked, and the emphasis was on emphysema, but later, it was shown that what happens in the small airways makes a notable contribution (Figure 5a). In a healthy state, the airway is held open by alveolar attachments. In patients with COPD, these attachments are disrupted, which, with mucosal and peribronchial inflammation, fibrosis and hypersecretion of mucus within the lumen, adds to the impairment of airflow. Currently, most in vitro models concentrate on the airway epithelium, but given this feature of COPD, it would be very useful to include alveolar macrophages, especially as attempts to block precursor pathways have so far been unsuccessful.
Figure 5:
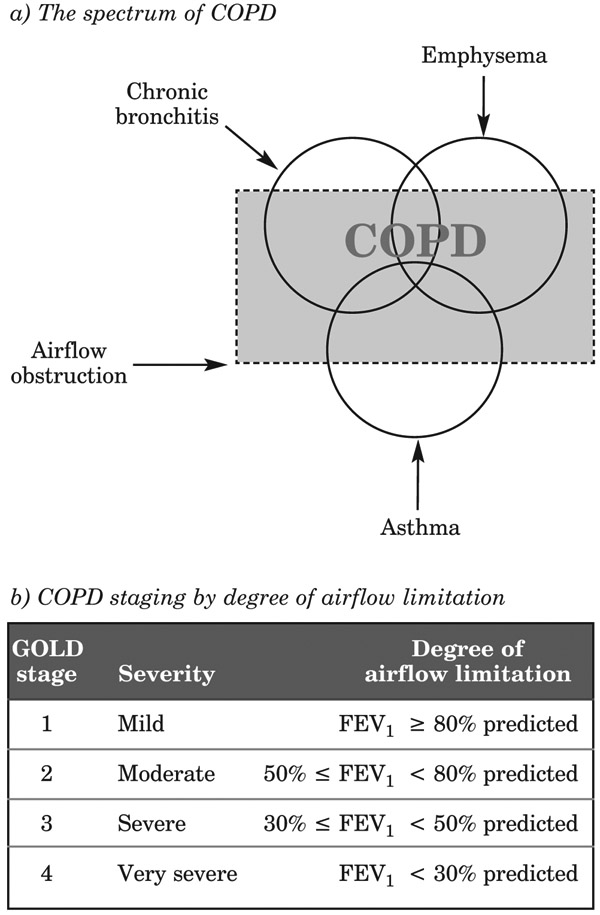
The spectrum of COPD and its staging by degree of airflow limitation
Although tobacco smoke is the main risk factor, individual responses to this and other particle exposures vary substantially (e.g. occupational dusts, and indoor and outdoor air pollution). Lung growth, oxidative stress, female gender, age, respiratory infection, low socioeconomic status, poor nutrition, and comorbidities, are all well-recognised contributing factors and must be taken into account. Genes, however, seem to contribute little to susceptibility.
It appears that diagnosis should be easy: symptoms + risk factors + positive spirometry = COPD, but is not that simple, owing to variability. Physical examination is rarely diagnostic, and spirometry is essential to confirm suspicions and might help to differentiate COPD and asthma. COPD should be considered in patients with any symptoms and a history of exposure to risk factors. Screening might be useful, but as the recommended intervention would require smoking cessation, it is not recommended.
Smoking cessation reduces the risk of COPD, and is the only modality known to change the course of the disease; in some cases, a very low degree of airflow limitation (forced expiratory volume in one second [FEV1]) might lead to self-perpetuating disease. Lung damage starts early, and might be more rapid in these stages than later in the disease course. Even mild disease is associated with exacerbations, airway limitation and physical impairment.
COPD is staged as mild, moderate, severe or very severe, initially based on FEV1 (see Figure 5b), but this classification has a high crossover with asthma. Differentiation used to rely on response to bronchodilators (post-bronchodilator FEV1/ forced expiratory vital capacity [FVC] < 0.70 confirms presence of persistent airflow limitation, and thus COPD), but symptomology should also be taken into account. Asthma and COPD should be viewed and treated as separate diseases.
The current management guidelines are to reduce risk, to relieve symptoms, to improve exercise tolerance and health status, and to prevent disease progression and exacerbations. Where these latter occur, they should be treated adequately to reduce mortality. The use of non-pharmacological management strategies has grown, but some goals have had greater success than others. Smoking cessation is the first priority, and other options are education of patients, rehabilitation (although this is expensive and often not funded), surgery, vaccination, and oxygen therapy. Pharmacological strategy options have also increased, from short-acting bronchodilators and steroids, to long-acting and anti-inflammatory drugs, and the numbers of individual drugs and drug combinations continues to increase. Treatment was originally based on FEV1 staging, but the correlation between FEV1 and quality of life is poor. Therefore, the importance of treating exacerbations has become recognised, and is now included in treatment planning, although improved guidelines are needed. The discovery of disease-modifying treatments would also be helpful (e.g. anti-inflammatory drugs), as bronchodilators are reaching their maximum capacity of usefulness, owing to little difference in outcomes between devices. Treatments to reduce symptoms (such as excess mucus) and risk of infections, and to treat musculoskeletal and gastrointestinal features, would also be useful. To summarise, the key points are:
COPD is a preventable and treatable disease.
Improvements are required in treatments to relieve symptoms and prevent exacerbations.
Increased clinical and basic research offers hope; exacerbations might be a particularly useful target for model development.
Inflammation and Oxidative Stress
Impact of Tobacco Smoke on Lung Inflammation and Pro-Resolving Pathways in Humans, Mouse Models and In Vitro Models (Richard P. Phipps)
Cigarette smoking is a cause of many inflammation-related pulmonary diseases. Tobacco smoke contains more than 4,500 chemicals with various toxic effects, and smoking is associated with around 438,000 deaths per year in the USA. The ideal outcome of inflammation is complete resolution, since, unresolved, inflammation becomes chronic and leads to loss of tissue function. The resolution of inflammation was initially thought to be a passive process occurring after removal of the stimulus. However, the lipid-mediator switch concept describes an active process that involves specialised pro-resolution lipid mediators (SPMs), which provide balance against the pro-inflammatory pathway and help to maintain or regain homeostasis. For example, omega-6 fatty acids (e.g. in peanuts) can lead to inflammatory products, whereas omega-3 fatty acids (e.g. in fish) resolve into SPMs and lead to resolution of inflammation. In smoking-related diseases, two hypotheses are of interest:
The normal resolution pathways are dysregulated, but can be targeted therapeutically.
Tobacco toxic effects can be gauged on the basis of dysregulation of resolution pathways.
Studies of exhaled breath condensate (c. 1ml collected from 10 minutes of breathing), assessed by mass spectrometry, have shown the presence and pattern of SPMs, and reveal dysregulation. Therefore, this non-invasive approach might be a useful way to assess inflammation in COPD patients, where endogenous resolution circuitry has been altered.
Mouse inhalation models of acute lung injury have been used to investigate whether pre-treatment with the SMP resolvin D1 (RvD1) alters the inflammatory process. After twice daily exposure to cigarette smoke for three days, neutrophil infiltration was inhibited and the expression of anti-inflammatory cytokines (e.g. IL-10) and the concentration of macrophage cytokine mRNA were notably increased. Treatment three days after smoke exposure also encouraged the resolution of lung inflammation by a similar mechanism.
A mouse model of emphysema was also assessed. The exposure required is long-term, and symptoms begin after c. 6 months, but is faster in the female A/J mice sensitive strain. Some human features of emphysema are mimicked in mice (e.g. alveolar damage, but not airflow restriction), and these outcomes were at least partially ameliorated by pre-treatment with RvD1.
In vitro studies have been performed to begin addressing the influence of SPMs on human cells. Primary human cells (macrophages, fibroblasts and epithelial cells) are preferable. Macrophages, in particular, are of interest, because COPD patients are highly susceptible to infection, and macrophages play an important role in removing microbes, and are also important in the resolution of inflammation. In human monocyte-derived macrophages, cigarette smoke exposure leads to impaired phagocytosis and the increased production of cytokines, chemokines, COX-2 and PGE2. In cells pre-treated with RvD1, production of the inflammatory cytokine IL-6 is blunted, whereas that of TGFβ, a pro-wound-healing cytokine, is increased (Figure 6). Pre-treatment with RvD1 also reduced cigarette smoke extract-induced inflammatory responses in fibroblasts, and in airway epithelial cells of the small airways, was associated with the reduced production of pro-inflammatory mediators. Human studies show variability in responses, which will be important to take into account when directing responder and nonresponder studies. Prevention with SPMs might be useful, not only for smokers, but also for individuals regularly exposed to toxic smoke (e.g. military personnel, firefighters) or in circumstances of viral-induced cytokine storm. The dietary uptake of omega fatty acids feeds into these resolution pathways. Key points are:
Figure 6: Resolvin D1 blunts cytokine production by monocyte-derived macrophages in cigarette-smoke-induced inflammation.
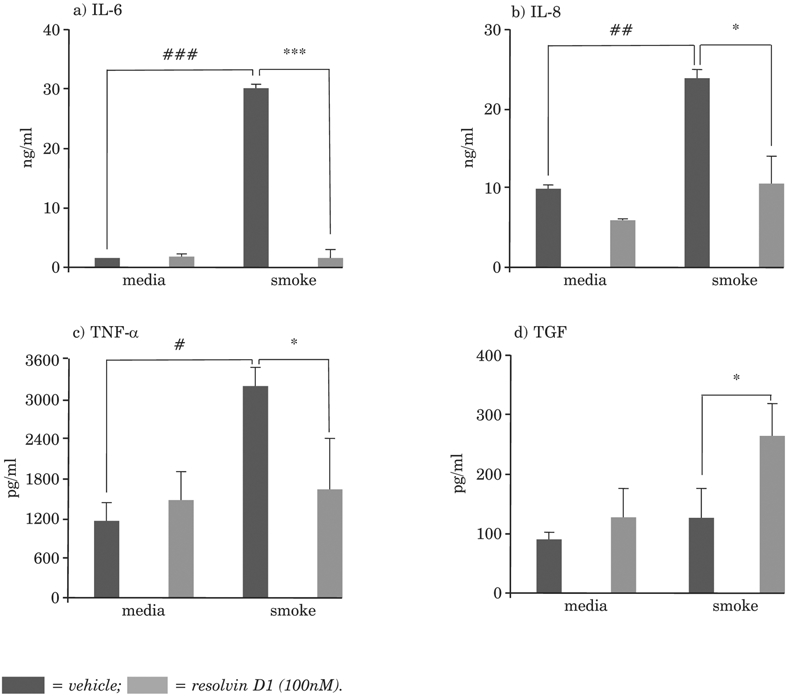
* = compared to no smoke/no resolvin D1 (*p < 0.05, **p < 0.01, ***p < 0.001); # = compared to cigarette smoke/no resolvin D1 (#p < 0.05, ##p < 0.01, ###p < 0.001).
Local tissue inflammation leads to neutrophil infiltration and pro-inflammatory mediators, which in turn lead to apoptosis.
SPMs act via several mechanisms to promote the resolution of acute and chronic inflammation.
The development of SPM therapeutic agents might be a useful route for the management of inflammatory lung disease.
The measurement of changes in SPMs caused by exposure to tobacco products might serve as an indicator of relative toxicity.
Genetic Variants, Inflammation and the Mucous Secretory Phenotypes (Yohannes Tesfaigzi)
After epithelial injury, inflammation results in the proliferation of non-goblet cells that differentiate into mucus-producing cells (mucous-cell hyperplasia [MCH]), and in the differentiation of non-goblet cells into mucin-producing goblet cells (mucous-cell metaplasia [MCM]). These changes lead to thickening of the epithelium. The MCH and MCM decrease after about 10 days, if the inflammation does not continue. The Bcl-2 family of proteins is central in coordinating this resolution process. Bcl-2, which is protective against apoptosis, and Bik, a promoter of apoptosis, are expressed in a timely fashion to reduce the proliferation of hyperplastic cells. The proportion of Bcl-2-positive mucous cells is increased in chronic airways diseases, such as cystic fibrosis and chronic bronchitis. Bik mRNA levels are reduced, but those of other Bcl-2 family genes are not. Thus, the hyperplastic goblet cells do not die and MCM and MCH are not resolved.
The expression of Bcl-2 is suppressed by p53, which destabilises Bcl-2 mRNA. An inverse relation between Bcl-2 and p53 has been shown in p53-deficient mice, where Bcl-2 levels remain high and resolution of MCM and MCH is obstructed.
The single-nucleotide polymorphism in TP53 modifies Arg72Pro. This change in amino acid is within the second of five PXXP motifs of the proline-rich domain, and encodes two p53 variants, 72Arg and 72Pro. Airway epithelial cells with the 72Pro variant have reduced expression of Bcl-2 and mucus production, thereby attenuating loss in lung function. In individuals with TP53 Arg72Pro, airway epithelial cells are also more susceptible to death caused by DNA-damaging agents, because of reduced Bcl-2 levels. The overexpression of p53Arg increases, and of p53Pro decreases, mucus production via the differential regulation of SPDEF, a transcription factor that drives the mucous differentiation pathway. Humans with COPD and the p53Arg variant are at increased risk of chronic disease, and pollutants other than cigarette smoke are likely to have an additive effect.
Therefore, it is important to consider DNA polymorphisms in human disease. This will be important for the development of relevant in vitro models. Key points are:
The Bcl-2 family of proteins are important in inflammatory lung diseases.
They have a variety of actions: guardians, effectors and sensors.
p53 destabilises Bcl-2 mRNA, and is crucial for the resolution of MCM.
In individuals with the TP53 Arg72Pro single-nucleotide polymorphism, the expression of Bcl-2 and mucus production are reduced.
DNA polymorphisms should be considered in the development of in vitro models.
Overview of Non-Animal Approaches to Address COPD Pathogenesis Associated with Inhaled Nicotine-Delivering Products (Sherwin Yan)
Smoking, the main etiological factor in COPD, is an important exogenous source of reactive oxygen species (ROS), and upregulates release of endogenous ROS. In addition, activated epithelial cells produce inflammatory mediators, such as TNF, GMCSF, IL-1 and IL-8. Exposure to smoke, however, impairs the innate and adaptive immune responses of the airway epithelium, and the likelihood of infections is increased.
COPD is multifaceted, so no single model can produce all the relevant results. The most frequently used cell types are human sinonasal, lung fibroblast, and small airway and bronchial epithelial cells. Some ready-to-use models are also available, such as fully and pseudo-differentiated epithelial models (basal, goblet and ciliated cells), and human primary epithelial cells co-cultured with fibroblasts (to show interactions of different cells types).
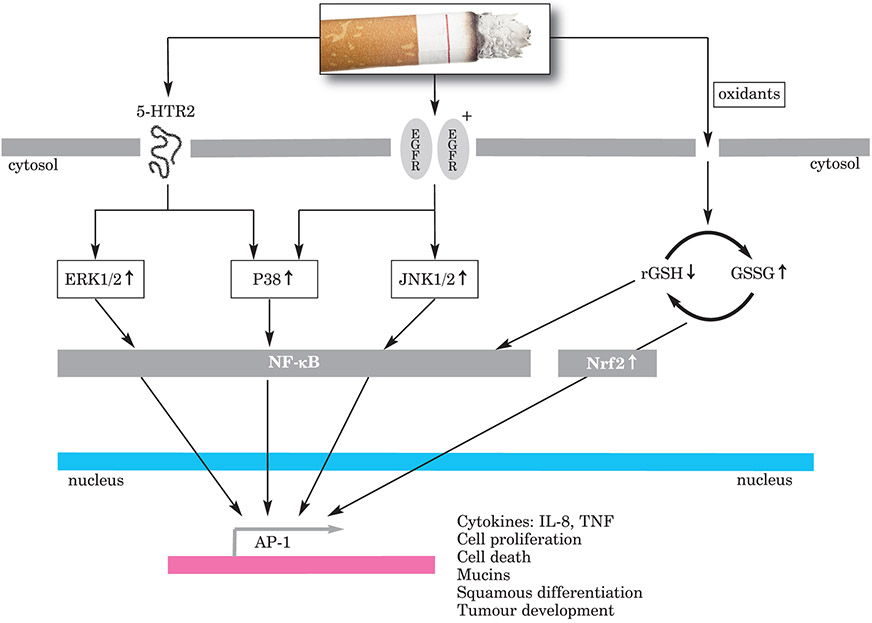
T-lymphocytes, especially CD8+ cells and macrophages, are prevalent inflammatory cells in healthy smokers and in smokers with mild COPD. In smokers with severe COPD, CD4 and NF-κB are also overexpressed. Macrophages secrete proteases MMP-2, MMP-9, MMP-12, etc., which are potent activators of inflammation. NF-κB is a pro-inflammatory transcription factor involved in the control of genes for many inflammatory mediators expressed in COPD, and is upregulated in the macrophages and airway epithelial cells of people with this disease. The up-regulation of NF-κB in epithelial and endothelial cells may contribute to the differential prevalence of cell infiltration (Figure 7).
Figure 7:
Oxidative stress and inflammation induced by cigarette smoke
The serine-threonine mitogen-activated protein kinase (MAPK) pathway, which regulates inflammation, is heavily discussed in relation to COPD. Within this pathway are three important kinase pathways that can be targeted separately: ERK, JNK and p38. In vitro models have been used to investigate MAPK pathways and cascades. Cigarette smoke activates 5-HTR2, which induces disrupted MAPK cascades through many different types of cellular events in all three pathways. However, the different components of the MAPK pathway have different roles. For example, the targeted inhibition of p38 or the ERK kinases MEK 1/2 suppresses IL-8 release after exposure to cigarette smoke, but the inhbition of JNK does not. The knockdown of MEK 1 specifically blocks cigarette-smoke-induced release of IL-8.
The lung is one of the most vulnerable targets to oxidative damage in the body, due to its location, anatomy and function. Oxidant–anti-oxidant imbalance in favour of oxidants increases oxidative stress in COPD. Increased numbers of neutrophils and macrophages in the alveolar space in smokers increase the oxidant burden. The imbalance is further increased by increased free Fe2+ concentrations (Haber–Weiss reaction) and lipid peroxidation. Antioxidant defence is normally mounted by antioxidants in the respiratory tract lining fluid, such as glutathione, mucins, uric acid, ascorbic acid and albumin. Cigarette smoke reduces antioxidant capacity and actions.
In the acute phase of COPD, reduced intracellular glutathione or increased concentrations of ROS can result in translocation to the nucleus of the transcription factor Nrf2. The activation of this transcription factor induces the up-regulation of detoxifying genes and pro-inflammatory cytokines.
Overall, the evidence from human studies supports the hypotheses generated by in vitro model studies for COPD.
In e-cigarettes, the aerosol is much less complex than that in cigarette smoke, including greatly reduced CO levels because of non-combustible heating. Studies suggest that there is a substantially lower inflammation response (IL-8 release) to aerosol than to cigarette smoke. Indeed, there are substantially fewer harmful and potentially harmful consituents in aerosol, and either no or minimal amounts of nitrosamines. However, reliable, validated methods are needed to assess exposure to nicotine. The key points are:
NF-κB is an important factor in the ongoing inflammatory process in COPD.
The MAPK pathways are disrupted by exposure to cigarette smoke, which leads to the release of pro-inflammatory cytokines, such as IL-8.
Human studies support the findings of in vitro model studies.
Ciliary Dysfunction and Ion Transport
Measuring Airway Surface Liquid Volume and Mucus Transport by Fluorescence Microscopy (Robert Tarran)
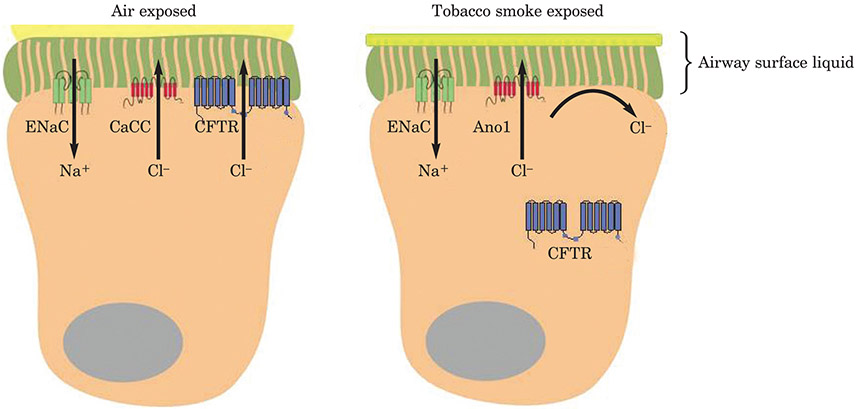
Epithelial cells are the first point of contact for cigarette smoke. Research in the past 10 years has shown they regulate immune responses, mainly by mucus and mucin production. Airway surface liquid (ASL) comprises mucus to trap inhaled particles and a peri-ciliary liquid layer that keeps mucus at an optimum distance from the underlying epithelia for clearance. Ion channels in endothelial cells are damaged by exposure to cigarette smoke which, over the long term, can lead to the dehydration of ASL in patients with COPD, as is seen in cystic fibrosis soon after birth, and possibly, to asthma. Increased Na+ absorption increases mucus dehydration, which has been shown to cause lung disease in mice.
The measurement of ASL height is straight-forward, and ASL hydration assays have proved extremely predictive of the human in vivo effects of cystic fibrosis physiology (Figure 8). In addition, they can be coupled to other assays, such as viability and inflammation assays, to investigate the effects of chronic smoke exposure. Bronchial epithelial cultures differentiate into basal, goblet and ciliary cells, and form the peri-ciliary liquid and mucus layers, but keep growing in height. The cultures can sense changes and alter hydration, as in in vivo airways. Thus, ASL height can be tracked over time in cultures, by using an inverted confocal microscope. 3-D rendering software enables the comparison of differences between cultures. As well as ASL volume depletion, cigarette smoke exposure correlates with changes in ciliary beating, which affects the speed of clearance.
Figure 8: Representation of ASL height.
Tobacco-induced inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR) leads to reduced fluid secretion and a decrease in airway surface liquid height.
ENaC = epithelial sodium channel; CaCC = calcium-activated chloride channel; Ano1 = Anoctamin 1.
Cell cultures of primary airway or alveolar lung cells are best; cell lines can be used, if they polarise. The choice of substrate is key, as this has a marked effect on how the cells grow. Fluorescent dextran is mainly used for labelling, because there is a wide choice of products for different budgets. Mucus can be labelled with fluorescent latex beads. The size of beads is important, as they will yield different results: 100nm beads are used to aid visualisation of the ultrastructure, and 1μm beads are used to track speed of movement (e.g. slowing, as mucus becomes dehydrated). Effects can be assessed with an inexpensive microscope. Fluorescent bacteria can also be used, or viruses expressing green fluorescent protein (GFP). However, these are living organisms, so whether adding them to the culture alters outcomes and/or whether the culture has potential effects on the bacteria, must be considered.
The use of an inverted confocal microscope is best for the assessment of cultures, but upright microscopes with dry or dipping lenses can be used with spinning disc and XZ stacks. XZ line scanning is ideal, if the galvometric stage is used to permit high-speed scanning. The type of lens greatly influences the quality of the images. The optimum is a bright (high numerical aperture) lens with a long working distance, particularly for an inverted microscope, but such lenses can be expensive. Cheaper dry lenses with a good working distance but a lower numerical aperture, are good alternatives. More dye in the ASL might be helpful, if brightness is an issue.
Cultures in media are sterile. If they are taken out of media, sterility might be lost, so they should only be opened under a tissue culture hood. ASL time-courses can run for two days, and smoke exposure protocols for two weeks. Under the hood, the culture can be transferred to a second plate with Ringer’s glucose solution in media. Experiments should be performed in the second plate, and the culture transferred to the original plate after the chosen time points are reached (this works > 95% of the time).
ASL height can vary substantially from culture to culture, so, for analysis, five areas per culture with different numbers of points should be assessed manually, for calculating an average height. ImageJ freeware can be used to process microscopy images. For 7μm ASL, five or six cultures are needed per group, to show a 50% decrease in ASL height. Another option to increase output is an automated microscope that can collect 20 images per culture in around 20 seconds and can be coupled to the automated analysis software.
To ensure good results, cultures with good ion transport, grown for 3–5 weeks should be used; those grown for longer periods will yield poor images. Primary cultures are best. During their growth, cultures might need to be washed, to ensure that too much mucus does not develop, as this will reduce the quality of ASL height images. When assessing ASL responses, multiple features, such as ion transport and tight junctions, aquaporin channel proteins (AQPs), G protein-coupled receptors (GPCRs), etc. should be considered. The key points are:
The measurement of ASL height is a straightforward approach to determine airway hydration, and there is a choice of products to meet different budget needs. Ciliary beating and ion transport can be measured on the same cultures.
Mucus can be labelled with fluorescent latex beads of various sizes, to allow visualisation of different features, such as ultrastructure and speed of movement.
The use of an inverted confocal microscope is best for the assessment of cultures, but upright microscopes with dry or dipping lenses can be used.
To ensure good results, cultures with good ion transport grown for 3–5 weeks should be used; those grown for longer periods will yield poor images.
Assessment of Ciliary Dysfunction in COPD Research (Samuel Constant)
Sam Constant presented Epithelix’s MucilAir™ airway models and how they can be used in a toxicology setting. Mucus is secreted to trap pollutants, but the cilia comprise a sychronised beating ‘motor’ of the clearance system. Each cilium consists of > 600 proteins, organised into complexes that work as nanomachines. In healthy individuals the cilia have inner and outer axonemal dynein arms, which ensure that cilia move in the same direction. In damaged cilia, the inner arms, or both sets, might be missing, leading to abnormal function. When arms are missing, movement becomes asynchronous. In patients with airways diseases characterised by primary ciliary dyskinesia, the different frequencies and directions of beating can be visualised with light microscopy. Various mechanisms have been suggested for the sychronisation, but the generally accepted theory is that hydrodynamic coupling forces exist between adjacent beating cilia.
Effective mucociliary clearance (MCC) is essential for clear respiratory health. It involves the beating cilia, but also relies on the properties of the mucus. Mucins are secreted as long strands that interact with globular proteins to create the viscoelastic properties that enable particles to be trapped and transported. The thickness of the layer can be affected by over-hydration and under-hydration, because, when the mucus becomes, respectively, not sticky enough or too sticky (and sticks to cells), clearance can be impaired. Thus, MCC may be affected at the structural (modification of cilia), functional (desynchronisation of cilia) and physiological (mucus) levels, and might be exacerbated by external pollutants and infections. For instance, repeated exposure to pollutants can lead to reduced numbers of ciliary cells and increased numbers of mucus-secreting cells, and influenza viruses can attach to cilia and lead to disorientation or addition and/or decreases in central microtubules. In COPD, the cilia become shortened, and are eventually lost due to ROS interacting with ciliary proteins after exposure to cigarette smoke, leading to the autophagy of ciliary proteins. Ciliary beating is also depressed in COPD patients.
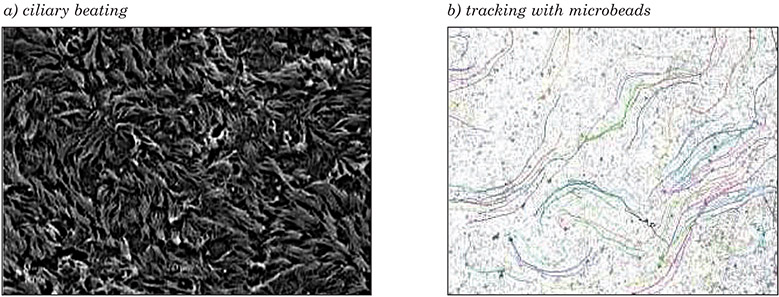
3-D models of the airway epithelium (MucilAir™) have been used to assess ciliary dysfunction in vitro, and have a shelf life of about 1 year due to slow cell turnover in the epithelium. Nasal, tracheal and bronchial primary human cells can undergo air–liquid differentiation to basal, ciliary and goblet cells that produce mucus, and closely resemble host epithelium. Ciliogenesis is seen by 21 days, and increases up to 45 days, after which cell numbers remain stable. To ensure that correct mucus thickness is maintained, the model should be washed at least twice per month. Cilia beating frequency can be measured with an inverted microscope, high-speed camera, and high-throughput measurement software (Figure 9a). Microbeads seeded onto an apical surface can be tracked to calculate the velocity and direction of movement (Figure 9b). When mucus is dehydrated and viscous, the cilia still beat, but particles are not moved.
Figure 9: Ciliary beating and tracking with microbeads on a 3-D human airway epithelium.
Images courtesy of Charles River Laboratories.
The analysis of ciliary dysfunction is relevant for COPD research, as MCC and ciliary beating frequency are informative endpoints of dysfunction. 3-D air–liquid interface cultures can also be used to measure the chronic effects of airborne xenobiotics on ciliary function and ciliogenesis. Non-destructive methods to measure cilia lengths are still needed. Novel models that better recapitulate the physiology of the human airway for in vitro testing (e.g. 3-D printing) might also be useful. Another possibility with the 3-D model is the use of genomic, proteomic, metabolomic, lipidomic and glycanomic methods to identify relevant biomarkers. To summarise, the key points are:
Effective MCC is essential for clear respiratory health, and requires good ciliary beating frequency and correctly hydrated mucus.
In COPD, the cilia become shortened and are eventually lost.
3-D models of the airway epithelium can be used to assess particle clearance under normal and altered conditions.
The assessment of chronic effects of exposure to pollutants is possible.
Understanding the Impact of Tobacco Smoke Exposure on Ciliary Dysfunction and Ion Transport: The Case for In Vitro Testing (Gary Phillips)
Gary Phillips continued the discussion on the use of in vitro models to elucidate mechanisms that contribute to the development of COPD. Several publications now advocate the use of in vitro methods for toxicity testing based on an AOP approach. In this way, the use of in vitro models helps to reduce costs, improve human relevance, and provide a degree of predictability, because the tests are based on a mechanistic understanding of the toxicity pathway. To date, such models have been developed and are used in various industrial settings, either as a consequence of the ban on animal testing, as is seen in the cosmetic industry, or as a part of the Three Rs approach in the pharmaceutical and chemical industries. In addition, in vitro methods that are robust and acceptable for regulatory purposes are being used in the tobacco industry for the assessment of the next generation of nicotine delivery and tobacco-related products.
Studies on MCC have been conducted for more than 40 years and have demonstrated that, as in many disease states, including COPD, the inability of the lung to clear excessive mucus can lead to airflow impairment, inflammation, and a clinically important decline in lung function. Impaired clearance can be a consequence of many different processes, including overproduction of mucus, increased numbers of mucus-secreting cells, decreased number and function of cilia, and inability of the airway epithelium to maintain adequate hydration of this matrix. Although in vitro models of MCC are being used more frequently, in many instances the use of cell lines might not be not suitable. They often lack the structural and biochemical features associated with known in vivo functions of MCC. Primary cells and tissue models have helped overcome this problem, as they are able to maintain their in vivo metabolic competence in vitro, and are structurally similar to the cells found in the conducting airways. Tissue samples derived from most areas of the respiratory system (nasal to small airways) can easily be obtained from healthy individuals and those with various disease states, which allows more-relevant studies to be conducted on the effects of exposure to smoke toxicants. More-complex in vitro models, such as PCLS and the lung-on-chip system, are also gaining prevalence. These models more closely mimic the complex structural and functional aspects of the lung than do culture models and they have a good shelf-life, which allows studies to be conducted that involve chronic and repeated exposures to smoke and aerosols.
When reproducing real-life exposure, how aerosols and particles are collected and presented to in vitro models needs careful consideration. The testing of cigarette smoke has in the past concentrated on the particulate phase of the aerosol. However, toxicants present in the vapour phase are known drivers of COPD, so appropriate smoke generation and delivery systems have been developed that allow for physiologically more accurate and more appropriate aerosol exposures.
In vitro models offer notable benefits in terms of facilitating repeat exposure studies with improved relevance and ethics. The harmonisation of approaches is now needed, especially with regard to the way in which aerosols from all tobacco products and nicotine delivery devices are generated, characterised and delivered to in vitro models. Key points are:
In vitro assays are gaining momentum to address concerns related to the ethics and relevance of testing and to meet regulatory requirements.
The development of primary cell and tissue models has improved the human relevance of the tests and the ability to address the effects of chronic exposure to tobacco smoke.
Many different disease-relevant and toxicologically specific endpoints are being measured, and combination of these endpoints supports understanding of the effects of smoke exposure on the lung.
Harmonisation is required between all industrial and academic laboratories, to agree on the approach to generate smoke aerosols and the in vitro models required to address specific disease concerns.
Goblet Cell Hyperplasia and Mucus Production
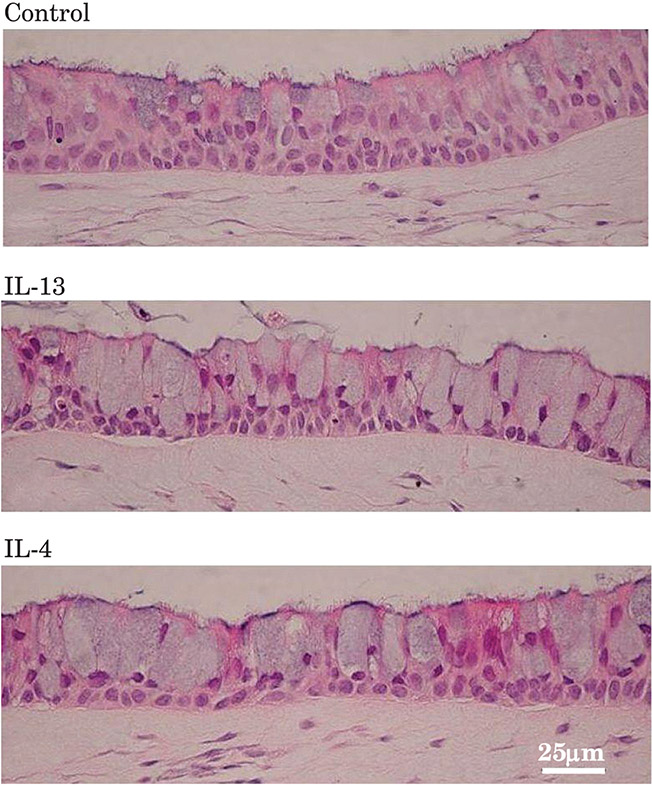
In Vitro Induction of Airway Goblet Cell Hyperplasia in the EpiAirway™ Model by Th2 Cytokines, Viral Exposure or Cigarette Smoke (Patrick Hayden)
Patrick Hayden discussed MatTek’s 3-D human tracheal/bronchial epithelial model (EpiAirway™) and a recently developed alveolar model (EpiAlveolar™). The EpiAlveolar model is a co-cultured model of alveolar epithelial cells and pulmonary endothelial cells of human origin. Both models are suitable for the testing of tobacco products, pharmaceuticals, airborne chemicals, and pathogens. Cultures for both models are grown on microporous membranes, so that the apical surface is exposed at the air–liquid interface to mimic in vivo exposure conditions. A full-thickness version of EpiAirway incorporates pulmonary fibroblasts in a sub-epithelial stromal matrix. The available formats include individual tissues (6, 12 and 24 wells) and high-throughput (24 and 96 wells).
The advantages of the EpiAirway model are a long functional lifespan (> 3 months), a pseudostratified morphology (basal, ciliated, goblet and club cells), functional tight junctions, beating cilia, mucus secretion, and the expression of drug-metabolising enzymes and transporters. The use of primary human cells also avoids potential problems that are often encountered with immortalised cells lines, which might have functional defects or show changes in function over time. Furthermore, the models avoid the problematic inter-species differences that are seen with in vivo animal studies or animal cells in vitro. EpiAirway cultures are typically created from single donors, and a wide selection of donors are available to vary age, sex, ethnicity, smoking history and disease status. Large cell inventories are available for each donor, to support long-term projects. These commercial tissue cultures are cost effective and are supported by a large database of use, by numerous peer-reviewed papers and abstracts in the literature, and by technical support.
The EpiAirway and EpiAlveolar models can be used to mimic systemic exposure by additions to the medium, or can mimic inhalation exposure of the apical surface at the air–liquid interface. Thus, the models are relevant for general respiratory toxicology, nanoparticle toxicology, tobacco toxicology, basic airway research, and lung permeation, and to assess the effects of aerosol exposure and inhaled drug delivery. The availability of numerous donors, including those with reported smoking history and disease conditions, such as asthma and COPD, permits the evaluation of individual variability and research on airway diseases.
A specific application that was presented in detail at the workshop, involved the reproduction of GCH in the EpiAirway model by exposure to Th2 cytokines (e.g. IL-13 and IL-4), human rhinovirus and tobacco smoke. The effect on GCH of azithromycin, an antibiotic that also has anti-inflammatory properties, was also investigated. Th2-induced GCH was reproduced by exposure to IL-4 or IL-13 for six days (Figure 10). Pronounced changes could be seen in the number and size of goblet cells, accompanied by notable changes in gene expression. Azithromycin notably reduced the induction of Th2-induced GCH and gene-expression changes. To simulate viral exposure, the cultures were treated with poly(I:C), which induced substantial and unique changes in the full-thickness EpiAirway model, including the extensive formation of goblet cells in the middle and at the surface of the epithelium. Infection with rhinovirus in non-diseased non-smokers, non-diseased smokers and asthmatic smokers, led to the sustained up-regulation of MUC5AC at 48 hours. The response to rhinovirus exposures was stronger in asthmatic smoker donors than in nonasthmatic donors. The exposure of EpiAirway cultures to cigarette smoke showed small, but statistically significant, differences in GCH induction between exposed tissues in smokers and controls. Some changes in mucin gene expression were induced by the smoke exposure (e.g. MUC5AC). Azithromycin had a smaller effect on reducing smoke-induced GCH in the full-thickness model than in the partial-thickness model. Taken together, the results demonstrate that the in vitro airway models provide translational data that are highly relevant to the assessment of human clinical airways disease. In summary, the key points are:
Figure 10: Th2-mediated GCH in a 3-D epithelial airway model.
Figure from BéruBé et al. (24).
The use of 3-D in vitro human airway tissues, including models representing the tracheal/bronchial epithelium and alveolar spaces, can be effectively used for airway toxicological, disease and basic research, and to assess the effects of inhaled drug delivery and respiratory infection.
GCH can be induced in vitro by Th2 cytokines, viral exposure or tobacco smoke.
GCH can be influenced by co-culture with fibroblasts, individual variability, pre-existing disease (e.g. asthma), and exposure conditions (e.g. smoking history).
Measurement of Mucin Secretion for Potential Evaluation of the Toxicity in Tobacco Products in Human Air–Liquid Interface Airway Models (Xuefei Cao)
Batteries of in vitro tests are recommended by the FDA and CORESTA for assessing the cytotoxicity and genotoxicity of cigarette smoke. However, tests for evaluating specific disease outcomes, which could be useful for assessing and predicting the health risks of exposure to cigarette smoke, are not included. In patients with COPD, disturbances of the normal redox state, mucus secretion, extracellular-matrix remodelling, and levels of inflammatory signalling lead to chronic inflammation and airway obstruction. Potentially, the pathophysiology of COPD could guide the development of in vitro tests for assessing disease outcomes of exposure to cigarette smoke.
The human airway expresses around ten mucin genes, with MUC5AC and MUC5B being the major mucins secreted by the airway epithelium. Well-differentiated human in vitro air–liquid interface models of airway tissue possess many of the structural and physiological characteristics of the human bronchial epithelium, including mucin production, and thus may be suitable for investigating the pathological roles of the mucin proteins in the progression of respiratory diseases. The quantitative measurement of mucin secretion could conceivably provide valuable information for risk assessment on the toxic effects of inhaled toxicants, such as cigarette smoke. A pilot study was performed to compare the mucin-inducing effects of two sets of whole-smoke solutions prepared according to the International Organisation for Standardisation (ISO), or Health Canada intense, machine smoking regimens. Each set contained two whole-smoke solutions generated by machine smoking 60 sticks of either Marlboro Red (R60) or Marlboro Silver (S60) cigarettes. The apical surface of the air–liquid interface model was exposed to whole-smoke solutions for four hours each day for five days. Mucus was collected before the start of each exposure. After the last treatment, the cultures were allowed to recover for two weeks, during which time mucus was collected at the end of each week (the apical surface was washed daily). The secretion of mucin proteins was measured with mucin ELISA assays.
With whole-smoke solutions prepared under the ISO smoking regimen, MUC5AC expression was increased significantly by the R60 and S60 exposures, with the amount of mucin generally dependent upon doses of whole-smoke solution and the number of treatments. The kinetics of MUC5B secretion differed from those of MUC5AC, in that its induction was delayed until the third treatment, at which point the induction of MUC5B was significantly higher than that in the vehicle-treated control. In general, mucin induction by R60 was stronger than that with S60, on the basis of the lowest effective dose. With whole-smoke solutions prepared with the samples obtained under the Health Canada intense regime, the induction patterns of MUC5AC and MUC5B were similar to those seen with the ISO whole-smoke solutions, but the differences between the R60 and S60 effects were diminished. Furthermore, compared with ISO whole-smoke solutions, the Health Canada intense samples appeared to be stronger inducers of MUC5B secretion, since changes in MUC5B induction were seen after one treatment. During the recovery period, the secretion of the two mucin proteins decreased after one week and returned to baseline levels at two weeks.
Changes in goblet cell density and morphology were also examined after exposure to Health Canada intense whole-smoke solutions. Five daily treatments with the highest doses resulted in a significant increase in GCH, compared with the vehicle-treated control. Treatment with the Health Canada intense whole-smoke solutions also led to morphological changes, such as decreased size and mis-shapen cells (hypotrophy). These observations correlated well with those made on mucin induction. Recovery following the five daily treatments with whole-smoke solutions, resulted in the gradual loss of GCH, hypotrophy and size alterations.
Concurrent exposure with N-acetylcysteine significantly decreased MUC5AC hypersecretion induced by ISO whole-smoke solutions, which suggests that mucin induction is a downstream effect of oxidative stress.
In summary, mucin measurement is a sensitive and quantifiable endpoint for human in vitro air–liquid interface cultures exposed to whole-smoke solutions. The findings are relevant to the progression of smoking-related diseases, specifically COPD, which makes this endpoint potentially useful for evaluating the health impact of exposure to cigarette smoke. A major challenge for this measurement is to develop smoke exposure regimens, including the appropriate machine smoking protocols, exposure doses and exposure schedules, to better mimic in vivo cigarette exposure in humans. To use such an assay for regulatory testing, standard operating procedures will need to be established for culture exposure conditions, methods and schedules for mucin collection, and ELISA assay procedures. Furthermore, donor variation in primary airway epithelial cells should be taken into account, and a sample size should be established to ensure a desired detection sensitivity with minimum donor effects. The key points are:
Methods to assess disease outcomes would be useful for evaluating the health impact of inhaled toxicants, such as cigarette smoke.
Exposure of human airway air–liquid interface cultures to whole-smoke solutions resulted in increased levels of mucin secretion.
The levels of mucin protein secretion were consistent with morphological and density changes in goblet cells.
Oxidative stress is involved in cigarette-smoke-induced mucin protein hypersecretion.
Procedures need to be established for optimising the sensitivity of mucin measurements, while mitigating donor effects.
Combining Systems Biology, Computational Approaches and Human Organotypic In Vitro Models Exposed to Whole Cigarette Smoke: An Example of 21st Century Toxicology Assessment (Carole Mathis and Julia Hoeng)
Systems toxicology is an approach intended to decode the toxicological blueprint of active substances that interact with living systems (16). It is an integrative approach that harnesses multidisciplinary expertise to gain a detailed understanding of toxicants. The systems aspect involves transcriptomics, genomics, proteomics and lipidomics. An important aim is to develop dynamic AOPs that include detailed mechanistic information, predict adverse outcomes, and provide a new paradigm for risk assessment, and direct research toward safer products and environmental protection. Methods used are computational models, molecular measurements and application of ‘omics’ technologies (17).
Smoking cigarettes strongly contributes to the development and progression of a number of serious diseases. Although quitting is preferable to reduce smoking-related health risks, MRTPs are being developed to provide alternatives for smokers who do not quit. The biological impact of novel products and related disease risks are being compared with those of conventional cigarettes to assess the differences in effects from conventional cigarettes and smoking cessation. The approach allows for the systematic generation of experimental data, by measurement of responses in tissues that are contextualised by molecular measurements in pathway models (mechanisms), and outcomes are quantified to compute biological impact factors. Many of the protocols and data are being made publicly available (18).
Owing to bans in various countries on animal testing for tobacco products, manufacturers have moved toward in vitro assays for genotoxicity and cytotoxicity testing. PETA expressed a wish that the boundaries of non-animal testing should be pushed further to test whole-product and whole-system effects. To try to establish reliable in vitro systems to support the principles of the Three Rs, work is being conducted to translate findings between other species and human experimental systems. Studies so far have included data from rats and human cellular models, and the scientific community has been asked to help identify which systems are translatable.
As part of its in vitro programme, Philip Morris International is looking for organotypic models. Sampling the bronchial epithelium to identify potential biomarkers of exposure response and disease has yielded significant insights, and has shown that many of the smoking-related changes in the bronchial epithelia are also present in nasal and buccal epithelia. With a climatic chamber exposure model, whole-smoke and aerosol experiments have been performed in nasal and bronchial tissue cultures (Figure 11). Linear correlations were found for nicotine and eight different carbonyls, which is of note, owing to changes in aerosol (19). A difficulty with this method is understanding how to achieve reliable exposure.
Figure 11: In vitro whole smoke exposure experiments.
Figure modified from Majeed et al. (19).
Of clinical importance is what actually reaches the tissue from smoke and aerosol. Computational fluid dynamics has been used in an attempt to model transport and evolution of aerosols and compute deposition at the air–liquid interface. Features to take into account are droplet size, number and intensity, the required residence time for the aerosol to reach a uniform particle number concentration in the exposure system, the stability and physical characteristics of the aerosol in the dilution system, and the influence of operating systems and conditions. Many different substances are involved, but the degree of exposure can be measured with the above system and characterised with gas chromatography time-of-flight analysis. The exposure of organotypic human primary bronchial epithelial cell cultures to cigarette whole-smoke clearly shows a dose–response relationship with 3R4F in terms of inflammatory cytokine release, but also for other endpoints, such as gene expression, microRNA, MMP-1 release, and cell counts and survival (20).
The translation of in vivo smoking exposure, based on a specific gene signature, was assessed in thousands of inserts, based on four publicly available bronchial data sets for smokers and nonsmokers. Similar results were obtained with the in vitro organotypic bronchial epithelial model. The results for microRNA were also assessed, but only one data set was available for comparison. However, differentially expressed microRNAs were found after exposure to cigarette smoke, that were common to the in vivo and in vitro data sets and were associated with functions, such as inflammation and cell cycle processes.
As interest has been expressed in global biological responses, the similarities of nasal and bronchial tissue gene expression changes have been assessed. There is some overlap, but nasal tissue cannot necessarily be used to predict COPD. However, this tissue can be used to identify smokers. Culture studies with bought cultures show increases in changes with increasing exposure time and dose. Likewise, changes have been shown in cellular composition.
Transcriptomic studies have also shown striking similarities in responses to cigarette smoke in the bronchial and nasal tissues. The observation suggests that the xenobiotic responses in the bronchial and nasal epithelial cells of smokers were similar to those observed in their respective organotypic models exposed to cigarette smoke. Furthermore, the results suggest that nasal tissue is a reliable surrogate to measure xenobiotic responses in bronchial tissue (21). The effects of exposure to 8% whole cigarette smoke on nasal organotypic cultures resemble those in bronchial organotypic cultures at the gene expression level. Thus, these are promising experimental systems to reduce the need for animal research.
The international scientific crowdsourcing initiative, sbv IMPROVER, which stands for Systems Biology Verification combined with Industrial Methodology for Process Verification in Research (Figure 12), aims to provide a measure of quality control of industrial research and development by verifying the methods used (https://sbvimprover.com; 22). The scientific community is able to look at entire data sets to complement the classic peer-review system. As there is little or no standardisation in operating procedures, assays, etc. so far, this approach helps to provide a measure of R&D quality control and to highlight gaps in knowledge or the research pipeline. It has potential applications in risk assessment to enhance insight into mechanistic understanding and improve product development. To summarise, the key points are:
Figure 12:
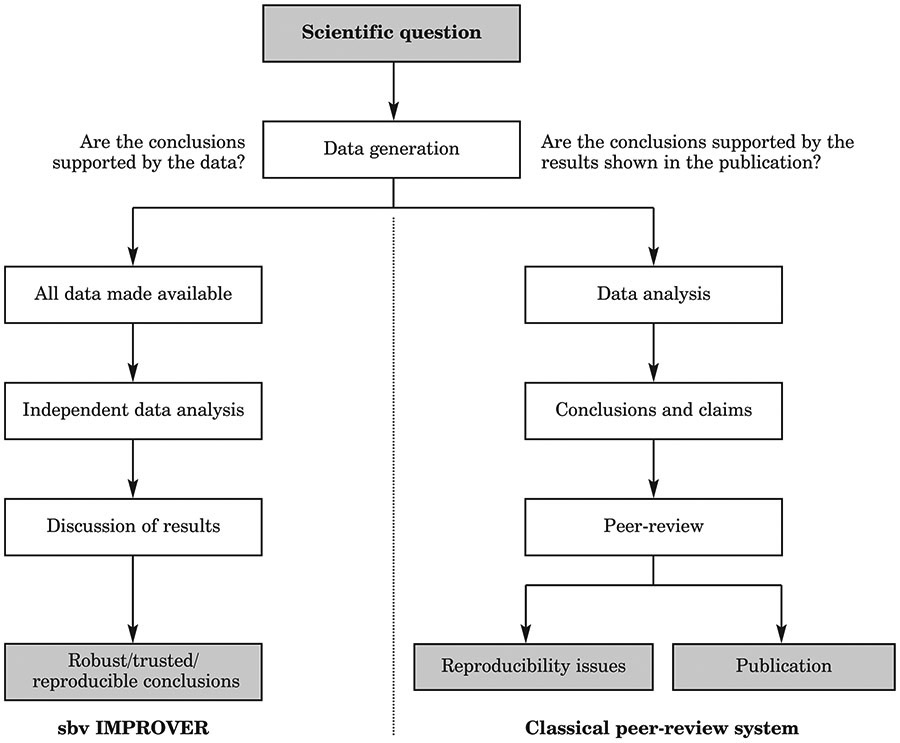
Comparison between the classical peer-review system and the Systems Biology Verification combined with Industrial Methodology for Process Verification in Research (sbv IMPROVER)
Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air–liquid interface resemble bronchial epithelium from human smokers.
Many biological functions known to be directly affected by exposure to cigarette smoke were identified by transcriptomic analysis in vivo and in vitro.
Gene expression profiling has demonstrated a remarkably similar transcriptomic response to cigarette smoke in nasal and bronchial tissues from current smokers and never smokers.
Modelling the transport and evolution of aerosol droplets is important for understanding aerosol deposition in the in vitro exposure system.
The sbv IMPROVER program aims to provide a measure of quality control in research pipelines.
Parenchymal/Bronchial Tissue Destruction and Remodelling
Detection of Inflammation and Parenchymal Damage by Using PCLS (Holger Behrsing)
Organ slice culture has been around for nearly 100 years, but precision cutting only became available in the past 30–40 years. The advantages of using PCLS are that they can be cultured for days to weeks, and that endogenous cell types are retained, which helps with the assessment of complex tissue responses.
PCLS are taken from whole lungs that are inflated with agarose solution that gels. The use of whole lungs eases proper inflation, and means that samples can be obtained from multiple regions. However, many of the lungs used are transplant discards, meaning that disease could make the region of interest unusable. Thus, acceptance criteria (e.g. age, pretreatment, etc.) should be set and agreed with the vendor. Slices are obtained from tissue cores. Methods of cutting slices are similar across laboratories, but how slices are processed and cultured differ (e.g. roller systems, shaking flasks, rocker platforms, and well inserts).
At eight days and also much later, at 28 days, when using the roller culture method, endogenous cellularity is fairly well preserved and a range of cell types are present. Key is the retention of activated macrophages, which are important in COPD research as they mediate inflammation and illustrate inflammatory responses through the secretion of several pro-inflammatory cytokines (e.g. TNF, IL-1β and IL-6). Exposure to carmustine is associated with increased numbers of macrophages that infiltrate the alveolar walls by day seven. Exposure to bleomycin can result in numerous diffuse activated macrophages, plus clusters that fill the alveolar spaces. Collagen deposition is also seen in sloughs and ribbon-like patterns that may vary from slice to slice (Figure 13). Thus, these features and biomarker content (e.g. ALP and LDH) can be used to demonstrate toxic effects to the lungs. Human PCLS may also be used to assess parenchymal damage. For instance, aminoflavone exposure leads to cytokine increase and severe tissue damage by around day seven, although meaningful results could be obtained on earlier days.
Figure 13:
Collagen deposition on lung slices
Of note is that the process of creating PCLS leads to increased cytokine (and chemokine) production, owing to mechanical disruption of the tissue, but values resolve to baseline levels after around days 2–3. Thus, exposure should not be started until after day 2, if confounding results of cytokine expression are to be avoided.
Establishing whether there is a no-effect level with the exposure compound is important. For example, exposure to Phortress, a pro-drug in the class of CYP-activated DNA-interactive anticancer agents, was associated with acute cytokine/chemokine increases. After low-dose exposure (25μM) was stopped, these effects were reversible, whereas, with higher doses, the levels continued to increase after exposure removal. A dose of 10μM was shown to have no toxicological effect.
PCLS are an attractive model for COPD, because the natural architecture is retained and long-term culture is possible. The important features, such as cytokine/chemokine induction, inflammation, collagen deposition and parenchymal destruction, can be measured in PCLS. Other features, such as ciliary function, increased mucus secretion, reduced airflow (diameter of airways) and chronic inflammation, should be measureable in a manner reflecting tobacco-induced changes that may lead to COPD. PCLS might also be suitable for a much wider range of biomarker and other assessments. Disadvantages are that the hardware rate of slice production, experimental capacity and methods for exposure of PCLS to smoke, could all be improved, but few significant changes have been made for many years. Obtaining slices from human lungs would be ideal to avoid the use of animals, and to avoid the need for cross-species extrapolation, but the supply of good-quality human lungs is limited. How utilisation and storage could be improved to extend the use of this model, need to be explored (e.g. freezing of slices so more can be taken from individual donor lungs for use in a later study). The key points are:
PCLS have been used in research for many years, and retain natural lung architecture and endogenous cell types that permit the study of complex cell interactions that may be required for the COPD disease state.
Current culture methods permit PCLS culture for weeks, and the assessment of biomarkers of acute and chronic exposure, such as early cytokine release, activation of macrophages and collagen deposition — i.e. markers associated with tobacco exposure.
The PCLS model could benefit from modernising of the equipment used to create and culture slices, a greater pool of human donor tissue available for research, and the ability to cryopreserve samples.
The Use of PCLS to Test Physiological and Pathophysiological Lung Responses (Armin Braun)
Armin Braun discussed what PCLS can offer compared with simple cell cultures, and whether inflammation is the main driver of disease. In PCLS, the tissue is kept alive, is in 3-D, and contains many of the types of cells expected to be present in the lungs, including smooth-muscle cells, fibroblasts and mast cells, as well as mucous glands, microvessels and other structures. Thus, these ex vivo samples bridge the gap between cell lines and the in vivo situation.
Airways and lungs are affected by many exposures, including cold, heat, biologicals, and endogenous stress, which might be physiological or pathophysiological. Neurogenic inflammation is stimulated by endogenous and exogenous activators. With PCLS, these can be mimicked ex vivo.
PCLS can be tested as part of an integrated strategy. Ideally, this would include a test system with standard operating procedures, clear endpoints, a predetermined data interpretation procedure, and a procedure for comparison of results with those for reference substances. The results should be used to develop prediction models to provide a point of reference for validation studies. For example, exposure of human lung to lipopolysaccharide followed by segmental lavage showed a strong correlation between in vivo and ex vivo responses, which provided a point of reference. Bronchoconstriction after the addition of agents to PCLS can be seen microscopically. Therefore, these tissue samples provide a useful way to test and rank the prevention of airway constriction with anti-COPD drugs. Of note, human PCLS are not consistently available, but there are substantial variations in PCLS reactions between species, so careful selection of the samples and the questions to be addressed are important issues.
The standardisation of testing methods is also important. To assess standardisation between laboratories, a joint study was conducted by the Fraunhofer Institute for Toxicology and Experimental Medicine, BASF and the University of Aachen. In the three laboratories, 20 chemicals were applied to PCLS, followed by 24 hours of no exposure, yielding 927 fitted curves. EC50 values obtained for the WST-1, LDH and BCA assays were very similar in all the participating laboratories. Differences between toxic substances and those not yielding dose–response curves could be clearly seen, and showed excellent correlations with in vivo data. After intensive training for about two years, the technique was fully reproducible and transferable between laboratories (23).
A limitation of the above method is that it only measured acute responses. Longer-term experiments were performed, and showed that lung function decreased over time. The changes were related to dose. Live/dead staining in a Triton repeat-dose study showed clear dose–responses that did not differ very much over time.
A new exposure system for gases and aerosols enables the customisation of exposure within a single air–liquid interface plate, and has a compact design, although it has some difficulty with longer-term exposures (high cell death after three hours). Good results have been seen for cytotoxic and inflammatory responses. In addition, the establishment of viral infection, such as with rhinovirus, could be assessed in human PCLS, as performed in the framework of the Innovative Medicines Initiative/EU project, U-BIOPRED. Human PCLS infected with rhinovirus showed differences between donors that were likely to reflect individual susceptibility, while the response within each donor was robust. The transcriptomics of human rhinovirus can be used to produce heat maps to indicate the antiviral response fingerprint.
In summary, PCLS can be used to test different types of toxic effects in various disease models, after exposures to various chemical or biological agents, including cigarette smoke and infections. Key points are:
PCLS are suitable for acute and repeat-dose testing, but inter-species differences must be considered at the research planning stage (Figure 13).
PCLS have been used to assess bronchoconstriction and the effects of anti-COPD drugs that might affect tissues.
Exposure systems are available to test multiple exposures simultaneously in PCLS.
Breakout Discussion Groups
Overview
Moderated breakout groups were held in three subject areas: inflammation and oxidative stress, ciliary function and ion transport, and GCH and mucus production. All three groups had the same goals:
To confirm that the biological effects addressed by the group are indeed relevant to tobacco-induced COPD.
To characterise any proposed in vitro models and test methods for their relevance to predicting the key events.
To identify the limitations of the test method and propose activities to address those limitations or gaps in mechanistic understanding.
Each group was provided with a set of questions to guide the discussions, as listed in Table 2. The outcomes of the discussions in the three breakout groups are detailed in Tables 3, 4 and 5.
Table 2:
The questions used to guide the Breakout Discussion Groups
| A: | What is the similarity of the model to any part of the human respiratory tract? |
|---|---|
| 1. | Is it of human origin? |
| 2. | Is it 3-D? |
| 3. | Does it have similar morphology? |
| 4. | Are all appropriate cell types present? |
| 5. | What area of the human respiratory tract is modelled? |
| 6. | Is it proprietary? |
| 7. | Is it suitable for long-term studies? |
| 8. | Does it respond appropriately to known stimulants or inhibitors? |
| 9. | Does the model have any known shortcomings? |
| B: | What is the relationship of the assay endpoint(s) to the clinical manifestations of COPD? |
| 1. | Is the endpoint measurable in humans? Quantitatively? |
| 2. | Is it a precursor to the disease state or a constituent? |
| 3. | Does it measure progression to the disease? |
| 4. | Is it useful for screening out either active or inactive materials? |
| 5. | Does the assay have any known shortcomings? |
| C: | How well characterised is the assay? |
| 1. | Ease of use and throughput? |
| 2. | Intra-laboratory reproducibility established? |
| 3. | Number and relevance of materials tested? |
| 4. | Used by (available to) other laboratories? |
| 5. | Portability to other laboratories? |
| 6. | Inter-laboratory reproducibility established? |
| 7. | Used with tobacco-related materials (chemicals)? |
| D: | What research activities can be proposed to address any gaps and limitations? |
| 1. | For models? |
| 2. | For assays? |
Table 3:
Outcome of the breakout discussion on Inflammation and Oxidative Stress
| Model characteristics | |
|---|---|
| Cell lines | Suitable for screening for exposure effects as part of a larger package of tests (potentially human and non-human cell lines). Everyone can use the same cell lines, provided no genetic drift has occurred between sources of cells or between laboratories. They are easy to grow and can be sensitive (possible advantage and disadvantage). Metabolic activity is often low, which limits applicability. Characteristics of some cells change when brought to the air–liquid interface (e.g. formation of tight junctions at confluence; possible advantage and disadvantage). Signal transduction pathways are abnormal. Cells might be altered or abnormal in different, unknown ways. Cell lines can only demonstrate potential for a process rather than the process itself. For ROS measurement, it might even be possible to utilise a reporter system, such as Nrf. |
| Primary cells (2-D monolayers) | Cells are closer (more ‘normal’) than cell lines to those in the in vivo population of interest and, therefore, have greater predictive power. The potential of stem cell-derived primary cells exists. Signal transduction pathways are typically retained more than in cell lines. Some heterogeneity exists between donors, which could be an advantage or disadvantage. There is some control over the donor population (e.g. diseased versus healthy or by sex). The lifespan is limited and accessibility to human donors is finite. Primary cells are more expensive and harder to grow and maintain than cell lines. |
| 3-D models (typically constructed of primary cells) | Availability of healthy or diseased donor tissue sources allows assessment of disease states in COPD research. Relevant structures can be maintained, although not necessarily the full structure and, therefore, models potentially need to increase in complexity to improve physiological relevance. However, complexity can lead to cell–cell cross-talk. Cell types (e.g. monocytes, macrophages, neutrophils, and ciliary and mucous cells) should be included to enable investigation of downstream effects; some key cell types (e.g. dendritic cells and macrophages) are missing; gene expression and metabolic pathways are retained. The cultures remain reasonably stable, which makes them suitable for chronic or repeated dose experiments. The possibility that dosimetry will not be consistent throughout the model must be taken into account. The model can be technically challenging and costly to create/utilise in research. Inter-donor variability of model characteristics and response to challenge requires consideration. |
| Ex vivo (i.e. PCLS) | Advantages are similar to those for 3-D cultures, plus organelles, biomarkers, etc. are easy to label and disease characteristics, such as broncho-obstruction/constriction, collagen deposition, ciliary beating, alveolar scarring, MMP secretion, differentiation between irritation and sensitisation, can be viewed as they were in vivo. The lung region of interest can be selected from the whole lungs. Disadvantages are similar to those for 3-D models, plus availability is one human donor per experiment and PCLS have only short-term to medium-term stability. The question was raised of whether human lung tissue would be made available for tobacco research. Few historical data are available compared with those available for some of the other models. Long-term stability needs to be studied further. |
| All models are capable of showing changes related to inflammation and oxidative stress. Whatever model is used, quantitative sensitivity is crucial and inter-species translation might need to be considered if human cells or cultures are not available. For the simple detection of oxidative stress, non-cellular (chemical-reaction-based) detection was suggested but this system would not capture cell-generated ROS that might play into more complex cellular mechanisms. | |
| Assay characteristics | |
| Lists of markers of inflammation and oxidative stress related to COPD were identified: | |
| Inflammation markers | NF-κB Reporter for NF-κB Interleukins Pro- and anti-PPARG (blocks NF-κB) Chemokines Cytokines γ-interferon (not regulated by NF-κB) Toll-like receptors — driven by NF-κB and dysregulation of Toll-like receptors MMP-2 and MMP-9 Aryl hydrocarbon receptor (AHR) Lipid and lipid mediators (prostaglandins, resolvins) — PGE2 Reporter assay for MAPK and ERK |
| Oxidative stress markers | Glutathione (reduced versus oxidised) expression level (HIP pathway) γ-GCS (related to glutathione enzyme) Nrf2 Hif-1α Mitochondrial ROS (quantitative by flow cytometry or dyes) F2 isoprostanes Lipid peroxidation Haemoxygenase 1 |
| Whether all would need to be tested or whether it is possible to select a specific representative panel (e.g. clinically relevant markers) was highlighted as an issue. All the markers listed are related to early biological events, but, even if they are strongly associated, none is unique to COPD and, therefore, the predictive value needs to be investigated. The choice of markers and changes thereof need to impart a reasonable expectation of disease risk. Certain markers might need to be considered in specific sequences (e.g. if NF-κB is not induced, a host of other markers will not be present). Although different approaches in testing for oxidative stress and inflammation were described (small sets of markers versus large sets by different participants), specific positive controls were identified: | |
| Oxidative stress positive controls | H2O2, potassium bromate and l-sulphoraphane |
| Inflammation positive controls | LPS, IL-1β, TNF |
| Recommendations and conclusions | |
| Research recommendations | As regulatory bodies want tests to show safety, but because product development requires more screening of effects, it was suggested that a consensus should be sought about which markers might be most useful to include in testing panels. As not enough information is available to easily eliminate possible cell models, the following paths to reduce the marker panel size were proposed: — Focus on those that predict the clinical situation. — Base the panel on the assays that are already available to measure them. — Review the literature to investigate whether biomarkers in the clinic have been reported as suitable for laboratory assessment. — Consider whether they contribute to prediction models to aid interpretation of data. |
| General recommendations | — Creation of collaborative partnership to evaluate endpoints and to provide recommendations on which might be most useful. — Standardisation of methods used for models, exposure protocols (including dosimetry) and reference materials. — Positive or negative cutoffs should be determined for assay sensitivity, through development of prediction and data interpretation models. — Correlation of in vitro results to the in vivo situation is extremely important. |
| Conclusions | — Many in vitro models and assays can be used to detect adverse events, such as oxidative stress and inflammation. These events, however, can be resolved and when assessed in an acute manner may not reflect downstream events associated with progression of COPD pathology. — More correlation between acute, initiating events and downstream key COPD events is needed, and/or focus should be assigned to long-term in vitro models capable of modelling chronic states of oxidative stress and inflammation. |
Table 4:
Outcome of the breakout discussion on Ciliary Dysfunction and Ion Transport
| Model characteristics | |
|---|---|
| 3-D bronchial models | Bronchial epithelial models cultured from human cells retain morphology similar to that found in vivo. Any reconstructed model should include basal, goblet and ciliary cells as standard; co-culture with fibroblasts might be useful as long as these cells do not change the phenotype from what is found in vivo; other desirable cells to add might be macrophages and club cells. Having a profile of phase I and phase II metabolising enzymes similar to that found in the human respiratory tract is important for the testing of xenobiotics. Some 3-D models of the upper respiratory tract can be used for long-term studies (e.g. ≥ 6 weeks) and respond to known toxicants and stimulators of hyperplasia and increased mucus production. Some commercial, proprietary models are available, but they are expensive and information about all medium constituents might not be available. Lack of definitive information about the medium could make the interpretation of certain experiments very difficult. Shortcomings of the current models are that they lack glands and are not capable of mimicking bronchial constriction. |
| Nasal tissue models | Nasal epithelial cells can be obtained directly from individuals by swab and tissue inserts can be created from the samples. Most of the properties are similar to those for 3-D bronchial models. There is a need to distinguish nasal respiratory and nasal olfactory epithelia. Presently there do not seem to be any good models for the latter. A shortcoming is that if excised tissues are required for studies, they are better taken from bronchial epithelium, as samples are better and larger. |
| Small airways models | Although of human origin, the current small airways models consist of cells grown in a monolayer rather than 3-D more complex models. Some commercial, proprietary cultures are available. Despite probably being the tissue most relevant to COPD, since the small airways of the lung is where the disease starts to develop, few models are available because samples are difficult to obtain, even from lung transplant discards, and because micro-dissection is required. |
| Alveolar models | Human type 2 epithelial cells can be isolated and de-differentiated into type 1 cells, but this method of obtaining type 1 cells is not ideal. Cell types present in most of the models are type 1 epithelial cells, other endothelial cells and macrophages. Some commercial, proprietary models are available. Responses to known toxicants are unclear, although some data are available on ion channel effects. Of note, airway surface liquid (ASL) is generally thicker than in vivo, which might alter the results obtained from in vitro models. |
| PCLS | PCLS that can be used ex vivo can be derived from humans and other species; the latter might be helpful to correlate ex vivo and existing animal in vivo data after similar exposures. Animal tissues will likely be easier. PCLS are useful in terms of the mixture of cell types (basal, interstitial, goblet, ciliary, fibroblasts, macrophages, etc. [although not neural cells]) they contain and the fact that they retain the endogenous structure. They can also be obtained from different lung regions of interest. Once prepared, PCLS can be used to assess inflammatory responses over several weeks, but there is a short initial timeframe for collection and culture. Some functions decline quite rapidly if they are being used to assess MCC or bronchoconstriction, etc. Scarcity of human tissue donors hinders the possibility for collection, culture and experimentation. The tissues show reasonable responses to known toxicants. Because it is difficult to orient the axis of the cutting, PCLS are often in the wrong plane to be able to study ciliary movement. However, the assessment of ciliary beating frequency has been reported in some studies. Additional shortcomings are: — Most tissues are obtained from lungs that are already unhealthy (e.g. the lungs might contain tumorigenic tissue); — Achieving a consistent air–liquid interface for the duration of the experiment can be difficult; — Sensitivity and specificity, for instance in tracking inflammatory responses, might be hindered by the rich variety of cell types. |
| Assay characteristics | |
| Important endpoints are MCC, ciliary beating frequency, ion transport and ASL, which cover progressive changes that appear before full disease development. All endpoints can be used for screening. | |
| Endpoints | MCC is easy to use and has good intra-laboratory reproducibility (inter-laboratory not established), although throughput is low. Has been tested quite extensively with multiple drugs and some individual compounds (e.g. nitrosamines). Suitable for use with cigarette smoke and smoke condensate, but whether it can distinguish between similar products is unclear. PCLS have been shown to distinguish between the effects of MRTPs and reference cigarettes. Ion transport assays have good intra- and inter-laboratory reproducibility and are suitable for use with many materials, including tobacco-related products (although few data are published for the latter). ASL is simple to use but only with the correct equipment. Thus, although reproducibility is good and assays can be used with tobacco product samples and constituents, use might not be widespread. |
| Recommendations and conclusions | |
| Research recommendations | — For 3-D models, investigate new supports and flexible substrates to enable assessment of mechanical properties of airway constriction; — Models to assess MCC require improved hardware and software for assessment and standardisation of test conditions and controls; — Comparisons of in vitro and in vivo data need to be made to provide background information for mechanisms of disease development and resolution, speed of transfer of particles, etc.; — Expansion of ion channel models to enable assessment of individual smoke constituents and validation of the assays for in vitro testing would be useful; — Finding ways to overcome the difficulties with creating small airway models would be an important advance; — More bridging is needed between the in vitro and in vivo responses for these parameters. This could be accomplished with non-invasive studies (nose, lung?) to compare clinical versus in vitro findings. Such data should be compiled so that they are easily accessible by all researchers. |
| General recommendations | — Standardisation of in vitro assays for regulatory purposes is necessary. This includes protocols, standard operating procedures, acceptance criteria, performance standards, and a discussion of donor variability for PCLS; — A process should be developed to make human pulmonary tissue more available to researchers; — Efforts should be made to understand how induced pluripotent stem cell technology can contribute to human tissue models; — Close interactions between regulators and researchers would dramatically shorten the development of useful in vitro models; — Development of pulmonary toxicology AOPs for tobacco products would be beneficial and should include molecular and cellular network information; — Funding for practical, regulatory-focused research should be made available; — Interactions with investigators in the field of air pollution and aerosolised pharmaceuticals should be encouraged. |
| Conclusions | — MCC is a key event in a COPD AOP and can be measured in vitro. Hence, the development of standardised and qualified in vitro assays to measure proxies of MCC has clear potential to predict and inform on tobacco-product hazards; — There are practical considerations that govern the use of individual in vitro assays. Therefore, depending on the specific purpose of the study, various combinations of assays will likely be used. |
Table 5:
Outcome of the breakout discussion on Goblet Cell Hyperplasia and Mucus Production
| Model characteristics | |
|---|---|
| 3-D models | Are commercially available (not proprietary) and can be developed in-house from human primary cells. Show organised and stratified epithelium. Morphology is analogous to that in the human airway epithelium in vivo. Dependent on the specific model, the requisite functional airway epithelial cells responsible for mucus production and clearance are present (i.e. basal epithelial cells, ciliated epithelial cells and goblet cells). Co-culture with fibroblasts may be helpful to enhance responses, which might modulate the magnitude of mucus production. Other cells types, such as club cells, might also be desirable, but their roles in chemically-induced GCH and changes in mucus production need to be better understood. In general, the complexity of the model should not exceed the experimental requirements. Theoretically, the models address GCH and mucus production throughout the respiratory tract, but the specific modelling may be that of the upper large airway epithelium, as the cells are typically derived from the tracheal or bronchial epithelium. The breakout group members recognised that there are notable differences between the upper and lower respiratory tracts (e.g. the reduction or absence of cilia in the small airways). However, to meet the objectives of establishing a model for evaluating chemically-induced GCH and changes in mucus production, these differences were noteworthy but not known to be limiting to the applicability. Models have been demonstrated to be sufficiently stable to allow for repeat exposure or dosing over several weeks, and have demonstrated GCH and changes in mucus production to known stimulants and inhibitors, including cigarette smoke, in as little as a few days. |
| PCLS | The breakout group members recognised that PCLS have the potential to be used to evaluate chemically induced GCH and changes in mucus production, similar to assessments done in 3-D reconstructed human respiratory epithelial models, but considered this model to be early in the conceptual process. Further understanding of GCH and mucus production will help in the evaluation and understanding of the potential strengths and limitations of this model. |
| Assay and endpoint characteristics | |
| Assay | ELISA technology may be used to quantify specific mucins (such as MUC5AC and MUC5B profiles), but availability/quality of commercial ELISA kits may be limited; it might be possible to develop assays in-house. Other useful detection methods could include ‘-omics’ technologies and chromatography. The production of airway cultures and the subsequent repeat exposures of the test systems is expected to be time and labour intensive and, therefore, adequate time must be budgeted for these activities. |
| Endpoints | The two main overall endpoints for clinical manifestations and disease progression are GCH/metaplasia (increases in numbers of goblet cells and changes in morphology) and changes in mucus production. These endpoints can be determined in vivo by collecting airway biopsy samples, or sputum or bronchial lavage samples. PAS staining of histology sections and specific immunofluorescence staining of mucins were deemed useful for qualitative assessment of changes in mucus production. Histology sections would enable comparative goblet cell counts and evaluation of changes in goblet cell morphology. |
| Recommendations and conclusions | |
| Research recommendations | — A better fundamental understanding of the mechanisms of action of normal and chemically-induced GCH and mucin production; — Population of a database with high-quality in vivo data of GCH and mucin production parameters in humans, optimised to improve in vitro model and endpoint development; — Improved understanding of how or whether any of the in vivo endpoints are predictive of the progression toward COPD and whether the relationships, if any, will need to be investigated; — Overall performance characteristics of models and quality-control need to be evaluated, and test system (i.e. tissue model) and assay endpoint quality and intra- and inter-laboratory reproducibility need to be characterised; — Optimisation and standardisation of assay endpoints; — Development of performance standards for the tissues; — Assessment of suitability of models to assess combustible tobacco smoke and aerosol exposures; it is envisioned that the tissue model platforms and the smoke and aerosol or vapor exposure systems will need to be optimised to enhance compatibility; — Extend availability of specific ELISA assays for detecting and quantifying mucin profiles; — Establish sensitivity and dynamic range in GCH and mucus production, cell counts and other metrics; — Establish reference substances and positive and negative controls (e.g. poly(I:C), air pollutant material [supply to NIST for analysis, storage and distribution] standard whole-smoke extract, total particulate matter, stable extract tobacco from standard smokeless tobacco products, etc.) for use in evaluating the performance of the test systems and assay endpoints. |
| Conclusions | — The breakout group accepted the premise that one can model goblet cell hyperplasia and changes in mucus production in vitro by using relevant 3-D reconstructed human airway models, and that currently available technologies can be utilised for qualitative and quantitative endpoint measurements; — To meet the widespread acceptance of these prototypic test methods, it was recommended that considerable development activities should be conducted to define the requisite characteristics of the systems/tissue models, develop and optimise the test system exposures, and optimise the various endpoint methodologies; — Establish reference materials to aid in the evaluation of the proposed test models. |
Discussion
The workshop programme addressed the FDA-CTP priorities and was met with a general enthusiasm by the attendees and speakers. The speakers discussed the scientific and medical aspects associated with tobacco and other related product toxicities. The poster presentations and ample networking opportunities fostered new relationships among researchers and strengthened existing ones. Informal discussions, as well as speaker panel Q&A sessions, focused on existing technologies, approaches to solving problems, and reiteration that collaborative efforts would be the best mechanism to advance the science of applying in vitro models to better understand tobacco exposure-driven adverse events in COPD aetiology.
The breakout group conclusions yielded priority areas to pursue, a summary of which was given during the 2015 annual Society of Toxicology conference, held in San Diego, CA. Statements by the workshop attendees affirmed their substantial interest in the application of in vitro methods for assessing adverse human health effects by respiratory toxicants. Prevalent throughout the breakout group sessions and informal workshop discussions was widespread enthusiasm for the further development of standardised methods that employ robust biomarkers of key events in lung disease progression. However, it was recognised by the attendees that progress will be slow in the absence of relevant funding mechanisms to assist the development of in vitro systems that could potentially provide meaningful data to support decisionmaking processes. In addition, collaborative, inter-laboratory efforts that incorporate a transparent process (ideally involving regulatory scientists) will be needed to accelerate the acceptance of such technologies.
Next steps
In response to recommendations from the workshop, IIVS hosted a follow-on Technical Workshop, In Vitro Models for Goblet Cell Hyperplasia, Mucus Production, and Ciliary Beating Assays, in June 2015, which resulted in suggestions for in vitro assay protocols to assess specific tobacco-related hazards. Currently, a multi-laboratory proof of principle effort is under way to further assess and standardise these protocols.
The attendees also identified additional subject areas that require exploration in a workshop format. For example, many of the attendees recognised that the current methods for tobacco product relevant exposures to respiratory models are not standardised, thus making difficult the evaluation of the lung disease modelling endpoints in the in vitro models. Accordingly, IIVS convened a workshop on In Vitro Exposure Systems and Dosimetry Assessment Tools for Inhaled Tobacco Products in April 2016. The programme aimed to address the inherent difficulty in making dose–response correlations of adverse/toxic effects from tobacco and e-cigarette constituents and aerosols, and to explore approaches to optimising and standardising the in vitro exposure and dosimetry methods to support potential regulatory decision processes.
Summary
The In Vitro COPD Models for Tobacco Regulatory Science workshop described here was considered a success in bringing together experts and stakeholders who contributed to creating a vibrant programme that addressed topics in alignment with the FDA-CTP’s mission. It yielded a path forward that identified three in vitro assays with the potential to be useful tools in assessing adverse pulmonary effects associated with COPD. These assays will undergo a technical proof of principle phase, which includes evaluation in multiple laboratories. These activities all support the identification, validation and dissemination of robust in vitro methods for the evaluation of tobacco products and their constituents, a process necessary for modernising and advancing regulatory decisionmaking to protect human health.
Summary of key themes
Several plausible in vitro test systems, models and assay endpoints are currently available, with the potential to be used for assessing the adverse impacts of tobacco product exposure in respiratory tissues. These models and assay endpoints should be further evaluated for their use as hazard assessment tools in a regulatory arena.
Of particular interest are 3-D reconstructed, human, organotypic tissue models of the respiratory airways; these should reduce the need to extrapolate results across species, thus strengthening the relevance of the results obtained.
Standardisation of promising test methods should be a high priority.
Relevant reference standards should be developed and made easily available to the research community.
Access to information from non-invasive or minimally invasive clinical studies is needed, to bridge the gap between in vitro and in vivo results.
The development of test methods for regulatory purposes is unlikely to be funded through the traditional grant process; more-direct funding programmes should be developed.
Close communication should be maintained between regulators and the research community, in support of the most efficient development of useful in vitro methods to assess relative risk.
Acknowledgments
Conference Director: Erin Hill; Principal Investigator: Holger Behrsing; Workshop Chairperson: Martha Moore. Steering Committee Members: Holger Behrsing, Marianna Gaça, Robert Heflich, Martha Moore, Richard Phipps, Hans Raabe, Kristie Sullivan and Rob Tarran. Funding for this conference was made possible, in part, by the Food and Drug Administration through Grant 1 R13 FD 005299-01. The views expressed in written conference materials or publications, and by speakers and moderators, do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organisations imply endorsement by the United States Government. Figures 1-14, as published in this Workshop Report, are illustrative of the points made in the workshop presentations; for more in-depth detail of their content, please contact the corresponding author (hbehrsing@iivs.org).
Figure 14: Results in PCLS vary substantially between species.
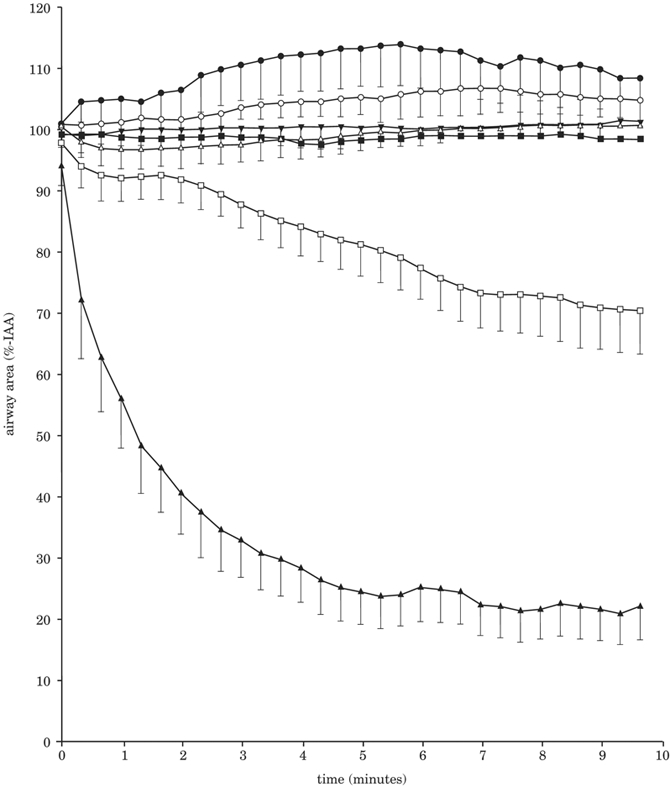
● = mouse;▾ = rat;▴ = guinea-pig;▵ = sheep;∎ = marmoset;◻= human responder; ○= human non-responder. Results show PCLS treatment with 10μM capsaicin.
Figure reproduced from Schlepütz et al. (25), published under Creative Commons Attribution CC BY.
References
- 1.Anon. (2012). Scientific Standards for Studies on Modified Risk Tobacco Products, 370pp. Report by the Committee on Scientific Standards for Studies on Modified Risk Tobacco Products, Board on Population Health and Public Health Practice, Institute of Medicine of the National Academies of Sciences. Washington, DC, USA: National Academies Press. [Google Scholar]
- 2.Anon. (2007). Toxicity Testing in the 21st Century — A Vision and a Strategy, 216pp. Washington, DC, USA: National Academies Press. [Google Scholar]
- 3.Anon. (2011). FDA Modernizing Regulatory Science. Silver Spring, MD, USA: US Food and Drug Administration. Available at: http://www.fda.gov/forconsumers/consumerupdates/ucm268201.htm (Accessed 16.03.16). [Google Scholar]
- 4.Goodman JL (2011). Advancing Regulatory Science at FDA: Strategic Plan. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/ScienceBoardtotheFoodandDrugAdministration/UCM268756.pdf (Accessed 16.03.16). [Google Scholar]
- 5.Anon. (2012). Draft Guidance for Industry: Modified Risk Tobacco Products Applications, 50pp. Silver Spring, MD, USA: US Food and Drug Administration. Available at: http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297751.pdfAccessed 30.04.16). [Google Scholar]
- 6.Anon. (2004). The Rationale and Strategy for Conducting In Vitro Toxicology Testing of Tobacco. Report of the CORESTA In Vitro Toxicology Task Force, 20pp. Paris, France: CORESTA. Available at: http://www.coresta.org/Reports/IVT_TF_Rationale-IVT-Testing-Tob.-Smoke_Report_Jun04.pdf (Accessed 16.03.16). [Google Scholar]
- 7.Curren RD, Southee JA, Spielmann H, Liebsch M, Fentem JH & Balls M (1995). The role of prevalidation in the development, validation and acceptance of alternative methods. ATLA 23, 211–217. [Google Scholar]
- 8.Hartung T, Bremer S, Casati S, Coecke S, Corvi R, Fortane S, Gribaldo L, Halder M, Hoffmann S, Roi AJ, Prieto P, Sabbioni E, Scott L, Worth A & Zuang V (2004). A modular approach to the ECVAM principles on test validity. ATLA 32, 467–472. [DOI] [PubMed] [Google Scholar]
- 9.Weil CS & Scala RA (1971). Study of intra- and interlaboratory variability in the results of rabbit eye and skin irritation tests. Toxicology & Applied Pharmacology 19, 276–360. [DOI] [PubMed] [Google Scholar]
- 10.Cormier EM, Parker RD, Henson C, Cruse LW, Merritt AK, Bruce RD & Osborne R (1996). Determination of the intra- and interlaboratory reproducibility of the low volume eye test and its statistical relationship to the Draize eye test. Regulatory Toxicology & Pharmacology 23, 156–161. [DOI] [PubMed] [Google Scholar]
- 11.Bruner LH, Carr GJ, Harbell JW & Curren RD (2002). An investigation of new toxicity test method performance in validation studies: 2. Comparison of three measures of toxicity test performance. Human & Experimental Toxicology 21, 313–323. [DOI] [PubMed] [Google Scholar]
- 12.Anon. (2016). Adverse Outcome Pathway Wiki. Available at: https://aopwiki.org/wiki/index.php/Main_Page (Accessed 16.03.16).
- 13.Anon. (2012). Researchers mimic pulmonary edema in lung-on-a-chip. Cambridge, MA, USA: Wyss Institute for Biologically Inspired Engineering. Available at: http://wyss.harvard.edu/viewpage/404/ (Accessed 16.03.16). [Google Scholar]
- 14.Anon. (2014). RTI International develops novel lung-on-a-chip. Research Triangle Park, NC, USA: RTI International. Available at: http://www.rti.org/newsroom/news.cfm?obj=503D8210-E344-7120-553A0AD769F494B4 (Accessed 16.03.16). [Google Scholar]
- 15.Gershon AS, Warner L, Cascagnette P, Victor JC & To T (2011). Lifetime risk of developing chronic obstructive pulmonary disease: A longitudinal population study. Lancet 378, 991–996. [DOI] [PubMed] [Google Scholar]
- 16.Sturla SJ, Boobis AR, Fitzgerald RE, Hoeng J, Kavlock RJ, Schirmer K, Whelan M, Wilks MF & Peitsch MC (2014). Systems toxicology: From basic research to risk assessment. Chemical Research in Toxicology 27, 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeng J, Kenney RD, Pratt D, Martin F, Sewer A, Thomson TM, Drubin DA, Waters CA, de Graaf D & Peitsch MC (2012). A network-based approach to quantify the impact of biologically active substances. Drug Discovery Today 17, 413–418. [DOI] [PubMed] [Google Scholar]
- 18.Kuehn D, Majeed S, Guedj E, Dulize R, Baumer K, Iskandar A, Boue S, Martin F, Kostadinova R, Mathis C, Ivanov NV, Frentzel S, Hoeng J & Peitsch MC (2015). Impact assessment of repeated exposure of organotypic 3D bronchial and nasal tissue culture models to whole cigarette smoke. Journal of Visualized Experiments (96), e52325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majeed S, Frentzel S, Wagner S, Kuehn D, Leroy P, Guy PA, Knorr A, Hoeng J & Peitsch MC (2014). Characterization of the Vitrocell® 24/48 in vitro aerosol exposure system using mainstream cigarette smoke. Chemistry Central Journal 8, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathis C, Poussin C, Weisensee D, Gebel S, Hengstermann A, Sewer A, Belcastro V, Xiang Y, Ansari S, Wagner S, Hoeng J & Peitsch MC (2013). Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air–liquid interface resemble bronchial epithelium from human smokers. American Journal of Physiology — Lung Cellular & Molecular Physiology 304, L489–L503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iskandar AR, Martin F, Talikka M, Schlage WK, Kostadinova R, Mathis C, Hoeng J & Peitsch MC (2013). Systems approaches evaluating the perturbation of xenobiotic metabolism in response to cigarette smoke exposure in nasal and bronchial tissues. Biomedical Research International 2013, 512086. [doi: 10.1155/2013/512086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer P, Alexopoulos LG, Bonk T, Califano A, Cho CR, de la Fuente A, de Graaf D, Hartemink AJ, Hoeng J, Ivanov NV, Koeppl H, Linding R, Marbach D, Norel R, Peitsch MC, Rice JJ, Royyuru A, Schacherer F, Sprengel J, Stolle K, Vitkup D & Stolovitzky G (2011). Verification of systems biology research in the age of collaborative competition. Nature Biotechnology 29, 811–815. [DOI] [PubMed] [Google Scholar]
- 23.Hess A, Wang-Lauenstein L, Braun A, Kolle SN, Landsiedel R, Liebsch M, Ma-Hock L, Pirow R, Schneider X, Steinfath M, Vogel S, Martin C & Sewald K (2016). Prevalidation of the ex-vivo model PCLS for prediction of respiratory toxicity. Toxicology in Vitro 32, 347–361. [DOI] [PubMed] [Google Scholar]
- 24.Bérubé K, Pitt A, Hayden P, Prytherch Z & Job C (2010). Filter-well technology for advanced three-dimensional cell culture: Perspectives for respiratory research. ATLA 38, Suppl. 1, 49–65. [DOI] [PubMed] [Google Scholar]
- 25.Schlepütz M, Rieg AD, Seehase S, Spillner J, Perez-Bouza A, Braunschweig T, Schroeder T, Bernau M, Lambermont V, Schlumbohm C, Sewald K, Autschbach R, Braun A, Kramer BW, Uhlig S & Martin C (2012). Neurally mediated airway constriction in human and other species: A comparative study using precision-cut lung slices (PCLS). PLoS One 7, 347344. [DOI] [PMC free article] [PubMed] [Google Scholar]