Abstract
Clinical Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly spread globally from late 2019, reaching pandemic proportions.
Epidemiology
The related disease, COVID-19, exacerbates and progresses due to patients' abnormal inflammatory/immune responses, widespread endothelial damage, and complement-induced blood clotting with microangiopathy. COVID-19 manifests mainly as a respiratory illness. In cases of severe viral pneumonia, it may lead to acute respiratory distress syndrome, respiratory failure, and death.
Challenges
Many extrapulmonary manifestations commonly occur, and a substantial proportion of patients with severe COVID-19 exhibit signs of kidney damage. Clinically, kidney involvement ranges from mild/moderate proteinuria and hematuria to acute kidney injury (AKI) requiring renal replacement therapy (RRT). The pathophysiologic mechanisms of kidney damage and AKI in patients with COVID-19 remain unclear but are known to be multifactorial. Current knowledge implies direct SARS-CoV-2-dependent effects on kidney cells (tubular epithelial cells and podocytes) and indirect mechanisms through the systemic effect of viral infection secondary to the critical pulmonary illness and its management.
Prevention and Treatment
Standard-of-care strategies apply, as there is no specific evidence to suggest that COVID-19 AKI should be managed differently from other types in severely ill patients. If conservative management fails, RRT should be considered. The choice of RRT approaches and sequential extracorporeal therapies depends on local availability, resources, and expertise. The focus should now be on the long-term follow-up of COVID-19 patients, especially those who developed kidney injury and dysfunction. This represents an opportunity for integrated multidisciplinary research to clarify the natural history of COVID-19 renal sequelae and the best therapeutic interventions to mitigate them.
Introduction
Severe acute respiratory syndrome caused by coronaviruses has been recognized as being of particular epidemiological concern because it can be fatal [1, 2]. This has been observed with the two coronaviruses that induced the human epidemic diseases Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) in 2003 and 2012, respectively [3]. In December 2019, a cluster of patients with pneumonia of unknown etiology, linked initially to a seafood market in Wuhan, China, was reported [4]. Since then, the novel coronavirus that causes this interstitial pneumonia has been isolated and identified through high-throughput sequencing of the viral RNA genome and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the World Health Organization (WHO). It belongs to the β-coronavirus cluster, which also comprises the viruses that cause SARS and MERS [5]. The related disease was then named coronavirus disease 2019 (COVID-19). The infection spread rapidly within and outside of China, and on 31 January 2020 the WHO declared a global emergency.
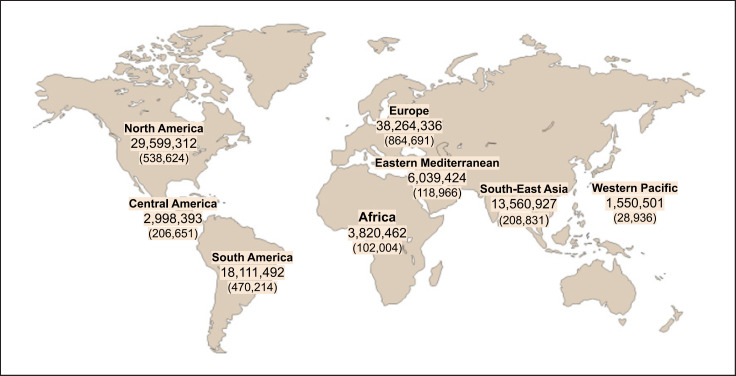
However, international responses to this emergency were neither immediate nor decisive, probably due to differing perceptions across countries. The situation was therefore underestimated, resulting in responses with different levels of urgency. Meanwhile, the initial evidence of progressively increasing admissions to intensive care units warned of the need to be prepared for extracorporeal organ support in a large number of patients [6]. Thus, SARS-CoV-2 infection continued to spread worldwide, and on 11 March 2020, the WHO declared the COVID-19 outbreak a global pandemic, as there were already over 118,000 reported cases, of which 40,000 had been diagnosed in 114 countries outside of China, with a resulting 4,291 deaths [7]. As of 3 March 2021, there have been over 114 million cases of confirmed COVID-19 worldwide (though the true number of cases will be much higher), and the death toll attributed to this viral infection has passed 2.55 million [7] (Fig. 1). As a result, communities and healthcare resources around the world have been pushed to their limits [8].
Fig. 1.
Map of the confirmed COVID-19 cases and deaths reported in different regions of the world up to 3 March 2021. Number of deaths is given in brackets. (https://www.coronavirus.jhu.edu).
The clinical spectrum of SARS-CoV-2 infection is wide, encompassing asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death [9]. According to retrospective data from China regarding 1,099 patients with laboratory-confirmed COVID-19 [10], at the time of admission to hospital, the most common symptoms were cough, fever and fatigue, and, less frequently, myalgia/arthralgia and a sore throat and headache, while nausea, vomiting and diarrhea were uncommon. The clinical characteristics encountered in European and US COVID-19 patients in the early phase of the infection are similar.
The scientific community has made an enormous effort, unparalleled in modern history, to better understand the nature and pathophysiology of this disease, with the hope that these advances will expedite the creation of safe and effective therapeutic interventions. Clinical experience and the emerging literature, however, have shown that, in addition to the lung, COVID-19 often also affects other organs and tissues, including the kidneys, which accounts for the many extrapulmonary manifestations of the disease. In some instances, this multiorgan dysfunction also contributed through superimposed bacterial sepsis, necessitating therapeutic strategies to control both viral infection and sepsis, as well as extracorporeal organ support.
Here, we review the current understanding of the pathogenic mechanisms involved in SARS-CoV-2 infection and the progression of COVID-19; discuss how an uncontrolled immune response, widespread endothelial damage, and systemic microangiopathy contribute to the development of acute and chronic kidney complications; and report the impact of COVID-19 in patients with preexisting chronic kidney disease (CKD) or kidney transplantation.
Pathogenic Mechanisms of SARS-CoV-2 Infection
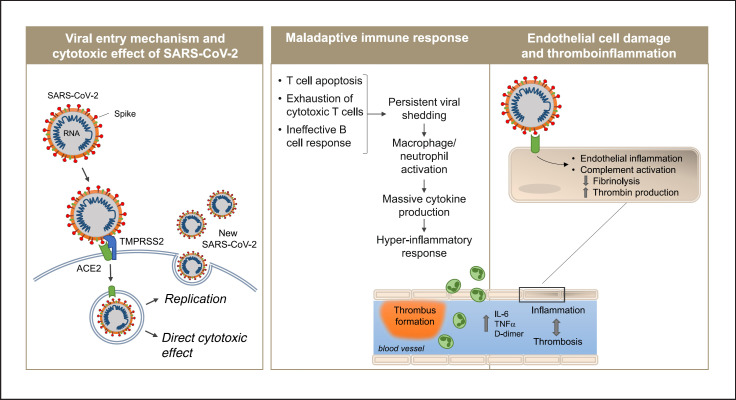
SARS-CoV-2 employs mechanisms for receptor recognition similar to those used by virulent coronaviruses that emerged earlier, such as SARS-CoV [11, 12]. The coronavirus spike protein facilitates the entry of the virus into target cells. The spike subunit of SARS-CoV-2 engages the host protease angiotensin-converting enzyme 2 (ACE2) as an entry receptor (Fig. 2) [13]. In addition, cell entry requires priming of the spike protein by the cellular transmembrane serine protease 2 (TMPRSS2) or other proteases [13, 14]. Coexpression on the cell surface of ACE2 and TMPRSS2 is required for the completion of this entry process, which may occur through endocytosis or via direct fusion of the viral envelope with the host cell membrane. Moreover, the efficiency with which the virus binds to ACE2 is a key determinant of transmissibility [15]. Recent studies have demonstrated that SARS-CoV-2 has a higher binding affinity for ACE2 than SARS-CoV does, which may partially explain the increased transmissibility of SARS-CoV-2 [16]. Once inside the target cell, the viral particles are uncoated, and the released viral genome is translated in the endoplasmic reticulum; the viral structural proteins move into the secretory pathway and the assembled progeny are then released from the host cell by exocytosis [17].
Fig. 2.
Proposed pathogenetic mechanisms of COVID-19. Following the infection of the lung, SARS-CoV-2 enters epithelial cells interacting with the ACE2 receptor through its spike protein in the presence of TMPRSS2 required to complete the entry process. The virus induces cell damage through direct cytotoxic effect and after newly formed virions are released by exocytosis into the extracellular compartment (left panel). A dysregulated immune response eventually leads to hyperinflammation (central panel). In parallel, activation of the complement system and massive production of cytokines activate endothelial cells and disrupt vascular integrity leading to vessel coagulation and thrombosis (right panel). ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2.
The human-to-human transmission of SARS-CoV-2 occurs primarily through direct or indirect respiratory tract exposure to droplets or aerosols generated during sneezing and coughing [18]. The virus has tropism for the respiratory tract, given the high expression of ACE2 in multiple epithelial cell types of the airway, including alveolar epithelial type II cells in the lung parenchyma [18, 19]. Indeed, reverse transcriptase-polymerase chain reaction has been able to detect live SARS-CoV-2 virus and viral subgenomic mRNA isolated from the upper airway. Later in the course of the illness, viral replication may occur in the lower respiratory tract [20], which manifests in severe cases as pneumonia and acute respiratory distress syndrome (ARDS). As part of the viral replication cycle, the infected epithelial cells of the lower respiratory tract undergo apoptotic death. This direct toxic response is associated with vascular leakage within alveoli that induces initial local inflammation and the recruitment of immune cells to eliminate extracellular virus and destroy virus-infected cells [21]. Proinflammatory cytokines further recruit leukocytes within the lung, contributing to the propagation of the inflammatory response that underlies the pathology of the atypical interstitial bilateral pneumonia observed in patients with COVID-19. The lung disease can rapidly progress to the severe illness ARDS, a hyperinflammatory state involving multiorgan dysfunction [22], with a mortality rate of approximately 10% in the most severe cases [23, 24].
Dysregulation of the host immune response and cytokine release syndrome due to overactivation of innate immunity in the setting of T cell lymphodepletion characterizes the presentation of severe COVID-19 [25, 26]. Growing evidence suggests that a suboptimal or unrestrained immune response during SARS-CoV-2 infection drives clinical patterns, disease severity, and the progression of COVID-19. Indeed, patients with unfavorable COVID-19 progression have been observed to have a dysregulated cytokine and chemokine profile, characterized by an increased level of IL-2, IL-6, G-CSF, TNF, and with the highest predictive values for poor outcomes for the combination of IFNγ-inducible protein 10 and monocyte chemoattractant protein 3 [27, 28]. However, the concept of considering the immune response to SARS-CoV-2 a sort of “cytokine storm,” as observed in other viral infections, sepsis, or autoimmune diseases [29], has recently been questioned [30]. Nonetheless, the evidence suggests that proinflammatory CD68+ macrophages bearing ACE2 on their surface can be directly infected by SARS-CoV-2 [31], and their virus-induced activation seems relevant to the initiation and spread of the hyperinflammatory response [32, 33]. Notably, single-cell RNA sequencing of cells from bronchoalveolar lavage fluid from patients with severe COVID-19 has demonstrated the expansion of proinflammatory monocytes/macrophages and a decrease in tissue-resident reparative alveolar macrophages [34]. The dysregulation of the adaptive immune response, such as a marked reduction in the absolute number of T cells, especially of the CD8+ subset, and of natural killer (NK) cells, is a common feature of severe COVID-19 and also associated with disease-related mortality [35, 36]. The increase in inflammatory cytokine levels caused by SARS-CoV-2 and/or direct apoptotic cell death induced by the virus might account for the depletion of T and NK cells, with a subsequent possible impairment of the host immune response to the foreign viral proteins. Nonetheless, there is evidence of circulating SARS-CoV-2-specific CD4+ (and CD8+) T cells in a large proportion of patients who have recovered from severe COVID-19, which could have contributed to destroying infected cells [37]. Moreover, a few studies also support the hypothesis of a dysregulated B cell response to SARS-CoV-2, as shown by the expansion in the peripheral blood of oligoclonal plasmablasts and reduced memory B cell frequencies in patients with severe disease, compared with patients with mild COVID-19 or healthy subjects [38].
Other complementary pathophysiologic mechanisms of COVID-19 suggest that the loss of vascular barrier integrity and the development of a procoagulant endothelium may contribute to the initiation and progression of ARDS in this disease [39]. SARS-CoV-2 enters endothelial cells through ACE2, whose expression has been documented in the arterial and venous endothelium of several organs [39]. Histopathological studies have provided evidence of SARS-CoV-2 viral particles in lung endothelial cells [39]. As in the epithelial cells, the virus induces cell injury and subsequent inflammation, eventually promoting the generation of a prothrombotic milieu [39]. In patients with COVID-19, virus-mediated endothelial injury and endotheliitis trigger excessive thrombin production, inhibit fibrinolysis, and activate complement, ultimately leading to microthrombi deposition and microvascular dysfunction [40, 41, 42]. In addition, hypoxia-induced hyperviscosity following acute lung injury may also contribute to the prothrombotic state of COVID-19 [43].
Together, the available evidence highlights the critical role of the dysregulation of the innate and adaptive immune response, in association with widespread endothelial damage, complement-induced blood clotting, and systemic microangiopathy in the initiation and exacerbation of COVID-19 lung disease.
Renal Involvement in COVID-19
Sustained viral shedding and hyperactivation of the immune response greatly increase the risk of systemic multiorgan failure. However, few could have anticipated the profound effect that the COVID-19 pandemic would have on the field of kidney health care. Indeed, the kidney is one of the main sites of COVID-19 complications, with abnormal renal function a major risk factor for death in severely ill patients [44]. Acute kidney injury (AKI) is infrequent in the context of mild to moderate SARS-CoV-2 infection [45]. In these patients, the most common kidney abnormalities are subclinical. Notably, in a prospective cohort study of 701 patients with COVID-19 hospitalized in Wuhan, China, on admission 43.9% had proteinuria, 26.9% hematuria, while 5.1% of patients experienced AKI during hospitalization [46]. The reported rates of AKI are extremely variable, probably reflecting the different definitions or staging of AKI adopted [44]. Although initial reports indicated that rates of AKI were negligible [10, 47], more recent evidence suggests that AKI likely affects over 20% of hospitalized COVID-19 patients and >50% of patients in the intensive care unit [48, 49, 50, 51, 52, 53]. Clinical reports have suggested a high prevalence of proteinuria during the course of COVID-19, with 34% and 63% of patients developing proteinuria at the time of admission or during hospitalization [54], respectively. In most COVID-19 patients, abnormal kidney function in the presence of low-level proteinuria may reflect tubular injury. In other cases, proteinuria is abundant or contains albumin, suggesting glomerular impairment [55]. Moreover, in these patients, elevated levels of baseline serum creatinine or blood urea nitrogen, AKI stage, proteinuria or hematuria has been shown to be independent risk factors for in-hospital mortality [46]. Other studies have shown that in COVID-19 patients, severe AKI is a negative prognostic factor associated with high mortality [56, 57].
Histopathological data, although limited, confirm kidney involvement in COVID-19. In autopsy specimens, the spectrum of renal pathological abnormalities includes loss of brush border and vacuolar degeneration in tubular epithelial cells, with debris composed of necrotic epithelium in the tubular lumen [58]. As seen in the lung [59], postmortem examinations of kidney specimens also revealed that the deposition of C5b-9 on tubule cells and marked interstitial infiltration of macrophages are hallmarks of kidney complications in severely ill patients [60]. The importance of microvascular thrombosis in the kidney is still a matter of investigation. One autopsy study identified limited fibrin thrombi in glomerular capillaries of kidney specimens from patients with severe COVID-19 disease [58]. However, renal vasculopathy has not been found consistently in the kidney and no significant glomerular disease has been described, except for collapsing focal segmental glomerulosclerosis [61]. This glomerulopathy has been reported in a few patients and appears to be associated with the presence of genetic “risk” variants of APOL1 [62, 63]. Thus, in individuals with high-risk APOL1 genotypes, SARS-CoV-2 infection and the resulting hyperinflammation may act as a “second hit” that leads to podocyte dysregulation, injury, and collapsing glomerulopathy [64]. Other glomerular diseases, such as antineutrophil cytoplasmic antibody-associated vasculitis [65, 66, 67], anti-glomerular basement membrane disease [68], and immunoglobulin A vasculitis without nephropathy [69] have been reported in a small number of patients with COVID-19. Similarly, in kidney biopsy findings in COVID-19 patients, membranous nephropathy, minimal change disease and IgA nephritis have also been observed [70, 71]. Whether these patients are predisposed to a specific glomerular pathology and SARS-CoV-2 is the ultimate trigger, or these represent incidental histopathological findings unrelated to SARS-CoV-2, remains ill defined. Together, this evidence indicates that the kidney is severely affected in some patients with COVID-19.
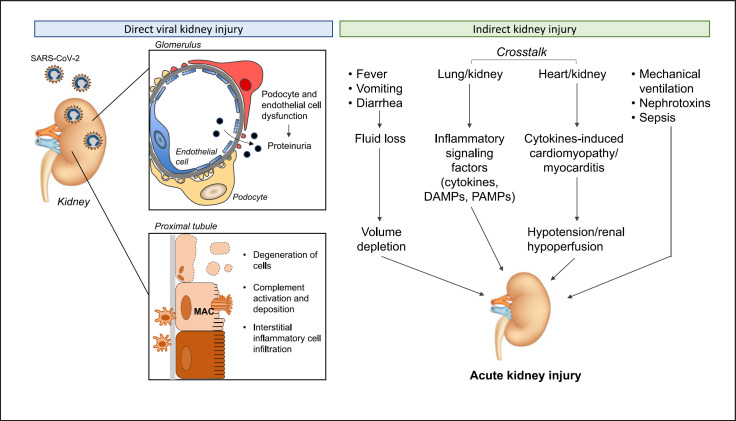
Our current understanding of the pathophysiology of COVID-19-associated AKI implies (i) direct and (ii) indirect pathogenetic mechanisms (Fig. 3). Indeed, emerging evidence suggests that the human kidney is a specific target of SARS-CoV-2 that may have a direct effect on kidney cells [72, 73, 74, 75]. Tubular epithelial cells and podocytes exhibit high levels of ACE2 and TMPRSS2, indicating that they are possible SARS-CoV-2 targets [76, 77]. ACE2 internalization following SARS-CoV-2 infection could influence an imbalance in the renin-angiotensin-aldosterone system, with increased angiotensin II (Ang II) signaling leading to proinflammatory and fibrotic processes in the kidney [78, 79]. The critical role of ACE2 in SARS-CoV-2 kidney infection is emphasized by findings from in vitro studies showing that SARS-CoV-2 can infect human kidney organoids and that this infection can be limited by the expression of human recombinant soluble ACE2, a decoy receptor [80]. The hypothesis that SARS-COV-2 exerts tropism in the kidney is supported by the finding of clusters of viral particles in the tubular epithelium and podocytes, at electron microscopy examination of autopsy samples from 26 patients who died with COVID-19 [58]. Moreover, the microdissection of autoptic tissue demonstrated a detectable SARS-CoV-2 viral load in 3 of 6 deceased patients; within the three SARS-CoV-2 positive samples, the virus was detected in all kidney compartments examined, with preferential targeting of glomerular cells [81]. However, electron microscopy, light microscopy and/or immunohistochemistry analyses cannot conclusively ascertain whether the identified particles are actually SARS-CoV-2 or merely virus-like structures. In particular, the identification of viral particles in kidney tissue through electron microscopy has been questioned, given the resemblance of these particles to other cellular structures, such as clathrin-coated vesicles [82]. Moreover, several studies have been unable to confirm the presence of virus in the kidney [65, 70, 83, 84]. Studies of SARS-CoV-2 in biological fluids have also rarely shown viral shedding in the urine [55], raising the possibility that there may not be a large source of SARS-CoV-2 in the kidney. Despite these uncertainties, investigators have documented enrichment of SARS-CoV-2 in glomerular epithelial cells through in situ hybridization [81]. Together, the available evidence, though still inconclusive, suggests that SARS-CoV-2-dependent kidney injury can be the result of a direct cytopathic effect on renal tubular cells, tubular deposition of complement C5b-9 and/or a cytotoxic action of infiltrating interstitial macrophages. These tubulointerstitial injuries are complemented by direct SARS-CoV-2-induced podocyte and glomerular endothelial cell dysfunction that may eventually contribute to the development of AKI in the setting of COVID-19.
Fig. 3.
Pathophysiology of COVID-19-associated AKI. Kidney injury in patients with COVID-19 is multifactorial resulting from SARS-CoV-2 direct effects on podocytes, glomerular endothelial cells, and proximal tubular cells, and/or systemic consequences of viral infection, organ cross talk through inflammatory signals and hemodynamic alterations, as well as result of management of the severe illness. DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns; AKI, acute kidney injury.
In addition to direct pathophysiological mechanisms, renal dysfunction in the context of COVID-19 might also arise through the systemic effects of SARS-CoV-2 infection and critical illness [22, 44]. COVID-19 patients may experience hyperpyrexia and gastrointestinal symptoms, such as vomiting or diarrhea, which can cause considerable insensible fluid loss, resulting in volume depletion, an important potential contributor to AKI in other settings. Similarly, the contribution of organ cross talk, the complex and reciprocal biological communication between distant organs mediated by signaling factors, including cytokines and damage-associated molecular patterns (DAMPs) [53] may also be relevant. For example, in the lung-kidney axis, a bidirectional relationship between alveolar and tubular damage as a consequence of the overproduction and release of cytokines, chemokines, DAMPs and vasoactive substances in SARS-CoV-2 has been proposed that may sustain the development of AKI [53]. In addition, in this setting, tissue injury can also be mediated by other factors, such as pathogen-associated molecular patterns (PAMPs) that could explain clinical features similar to sepsis [85]. Notably, it has been suggested that this cross talk may mediate AKI in the setting of ARDS [86, 87, 88], which may also cause renal medullary hypoxia, an additional insult to tubular cells [53]. Tissues other than lung tissue might also serve as sources of toxic tubular substances. This may be the case in rhabdomyolysis, reported in 19.3% of COVID-19 patients [54], which would result in the release of myoglobin from injured skeletal muscle [89]. In addition, cardiorenal syndrome, or heart-kidney cross talk, particularly right ventricular failure secondary to COVID-19 pneumonia, might lead to kidney congestion and subsequent AKI [22]. Similarly, cytokine release syndrome-induced cardiomyopathy and acute viral myocarditis may lead to low cardiac output, arterial underfilling, hypotension, and renal hypoperfusion, contributing to a reduction in glomerular filtration rate and AKI in patients with COVID-19 [53]. Another potential mechanism is nosocomial sepsis, since individuals who develop secondary infections, as occurs in COVID-19 patients, are at increased risk of secondary sepsis-associated AKI [90]. Additionally, patients with severe COVID-19-associated pneumonia may require high levels of mechanical ventilation, which can potentially induce AKI by increasing sympathetic tone and inducing secondary activation of the renin-angiotensin-system [91, 92]. The neurohumoral activation results in intrarenal blood flow alterations and other hemodynamic changes that impair renal perfusion.
Mechanical ventilation also induces lung injury which, through the release of inflammatory mediators, may further increase the risk of AKI in patients with COVID-19 [93]. Other contributors to AKI might include the development of microemboli and microthrombi in the context of COVID-19-associated hypercoagulopathy and endotheliitis [34, 94]. Critically ill COVID-19 patients might be exposed to nephrotoxic drugs, in particular antibiotics, as part of their clinical management, which can cause tubular injury or acute interstitial nephritis, eventually promoting AKI [95, 96].
COVID-19 AKI should be managed similarly to other causes of AKI in critically ill patients, following measures recommended by KDIGO guidelines [97], which are largely supportive. As indicated by the Consensus Report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup [44], individualized fluid and hemodynamic management after adequate assessment of cardiovascular status is critical for severely ill COVID-19 patients to reduce the risk of AKI, as a similar strategy has been shown to be effective in patients with septic shock [98]. For the expansion of intravascular volume in patients at risk of or with AKI, initial management could involve balanced crystalloids, unless there is a specific indication for the use of other fluids. Special attention should be paid to limiting, where possible, exposure to nephrotoxic drugs and to carefully monitoring patients when nephrotoxins are required. If conservative management fails, the initiation of renal replacement therapy (RRT) should be considered, especially for fluid management in patients with severe hypoxemia, with the decision being personalized [97]. Since no clear benefit with any specific RRT modality has been reported, the selection of the approach depends on local availability, resources, and expertise. Nonetheless, continuous RRT (CRRT) is the preferred modality, since it is better tolerated in hemodynamically unstable patients, such as those with COVID-19 [97]. A dramatic increase in the need for RRT in critically ill patients would, however, require judicious resource planning, including the use of shared CRRT protocols, and the use of acute peritoneal dialysis in selected patients [22, 99]. This approach to treating COVID-19 patients with AKI has been adopted in Latin America [100], for example. In the absence of contraindications, patients with COVID-19 may require systemic anticoagulation during RRT [22].
COVID-19 in Preexisting Chronic Kidney Disease and Kidney Transplantation
Diabetes, hypertension, cardiovascular diseases, and cancer have been listed as risk factors for severe COVID-19. CKD has also recently been reported as one of the most common risk factors for severe COVID-19 [101, 102, 103]. It has been clearly shown that the risk for severe illness increases as the estimated glomerular filtration rate (eGFR) decreases. However, it is still unknown whether patients with preserved eGFR but increased albuminuria (i.e., earlier CKD stages) are also at increased risk of severe COVID-19. Indeed, the Open SAFELY study, a health analytic platform that covers 40% of all patients in England, analyzes factors associated with COVID-19 death in 17 million adult patients [101]. After adjusting the analysis for confounding factors and age, the authors found that CKD stage 4 and 5 (aHR 2.52), transplant recipients (aHR 3.53), and dialysis patients (aHR 3.69) are three of the top four risk categories for COVID-19 death [101]. Notably, the risk for COVID-19 death in these subgroups was higher than in diabetic patients and in patients with chronic heart disease. These findings have been confirmed by data from across Europe from the ERA-EDTA Registry [104], showing that 28-day mortality was 20.0% in patients who were receiving dialysis and 19.9% in kidney transplant recipients. Although the mortality risk was higher in transplant patients than age- and sex-matched dialysis patients, in both groups it was mainly related to age and, for dialysis, to frailty conditions [105]. The observed differences between kidney transplant recipients and dialysis patients in relation to death associated with COVID-19 may be attributed to multiple comorbidities, and especially to these vulnerable patients' suppressed immune systems [106]. There is also evidence that the need for dialysis may contribute to enhancing the risk of SARS-CoV-2 infection in patients with end-stage kidney disease [107].
In addition, a recent Global Burden of Disease (GBD) study estimated the number of subjects at increased risk of severe COVID-19 by age and sex for 188 countries [102]. CKD was the most prevalent risk factor for severe COVID-19 worldwide, as frequent as cardiovascular disease at any age. The GBD analysis estimated that CKD explained the increased risk of severe COVID-19 for approximately 1 in 4 high-risk individuals worldwide, namely in over 86 million individuals globally.
Emerging SARS-CoV-2 Variants
Like other RNA viruses, SARS-CoV-2 is susceptible to genetic evolution resulting in multiple variants with different characteristics compared to its inherited strains. Initially, the genetic evolution of SARS-CoV-2 was minimal with the appearance of the globally dominant D614G variant, characterized by increased transmissibility [108]. At the same time, in the middle of 2020, another variant was found in humans as possible result of transmission from infected farmed minks in Denmark [109]. However, this variant was not associated with increased transmissibility. Since then, several variants of SARS-CoV-2 have been identified and classified by the Centers for Disease Control and Prevention (CDC) in United States and by the WHO into variants of concern (VOCs) and variants of interest. Definition of VOC was based on their potentials to cause increased transmissibility or virulence, their ability to evade detection, and lower neutralization by antibodies obtained through natural infection [110]. The B.1.1.7 variant was unveiled in the middle of December 2020 as the first VOC described in the UK, being 43–82% more transmissible than the original SARS-CoV-2 [111, 112]. The rapid transmission of B.1.1.7 variant may explain why it is becoming the dominant lineage responsible for the upcoming infections in Europe and USA. Notably, studies have also shown that this variant was associated with increased mortality compared to other non-B.1.1.7 SARS-CoV-2 [113, 114]. The second VOC that has raised global concern is the B.1.351, a lineage first reported in South Africa in October 2020. It soon replaced the circulating viruses in that region becoming the dominant variant [115], which suggests higher transmission rate, although no evidence of greater disease severity has been reported [110]. Another new relevant variant of SARS-CoV-2, known as P.1 or B.1.1.28.1 variant, was identified in early January 2021 in Brazil, where it has become the dominant circulating virus and the main responsible for the rapid increase in the number of hospital admission by COVID-19 [115]. More recently, the B.1.617 VOC has become common in India, but yet not proven to be one potential factor for the COVID-19 crisis the country is still facing. This Indian coronavirus variant is now spreading in the UK as easily as the more highly transmissible B.1.1.7 variant that dominates infections in the country. Currently, the research effort aims to establish whether and to which extent the mutations introduced into the SARS-CoV-2 genome exert resistance to the action of the immune response induced by the available vaccines.
Take-Home Messages
Today we are facing one of the biggest pandemic events of the last few centuries of human history, caused by SARS-CoV-2. The related pathogenic condition has been called COVID-19 and is associated with a broad clinical spectrum of presentations, from subclinical presentation to severe lung disease with respiratory distress and often multiorgan failure. Almost 10% of all critically ill patients subsequently die. The inflammatory/immune response, widespread endothelial damage, complement-induced blood clotting, and systemic microangiopathy play a critical role in COVID-19 exacerbation and progression.
Kidney involvement, through direct SARS-CoV-2 infection or, more importantly, as a consequence of multiorgan dysfunction, is a major risk factor for poor COVID-19 outcomes in severely ill patients. These can range from proteinuria and hematuria to AKI, requiring RRT. Little is known about the prevention and management of AKI, and therefore the currently available AKI strategies are adopted empirically to treat patients with COVID-19. Moreover, several reports, albeit not from controlled randomized studies, suggest that extracorporeal blood purification techniques could be useful for selected individual COVID-19 patients to remove circulating inflammatory molecules, DAMPS and PAMPs, as well as SARS-CoV-2 particles [44]. Similarly, some critically ill patients with COVID-19 could benefit from CRRT with special membranes that can remove target molecules by adsorption, diffusion, or convection [44].
While we wait for the widespread use of vaccines globally and the development of more effective specific therapies for SARS-CoV-2, our focus should be on the long-term follow-up of COVID-19 patients who have recovered, including those who have developed kidney injury and dysfunction [116]. Monitoring and treating these COVID-19 patients will create an opportunity to conduct integrated multidisciplinary research that could help to improve our understanding of the natural history of COVID-19 sequelae and to assess the efficacy of therapeutic interventions to mitigate them. Our ultimate goal for the future is, however, prevention: to avoid the transmission of viruses from one animal host to another and then to humans, eventually causing new outbreaks. This would require improvements in the management and monitoring of urban sanitation [117] and, more importantly, ensuring adequate hygiene in food markets. Medical doctors and scientists may need to work more closely with veterinary doctors to gain greater insights into the characteristics of viruses found in animals and how they sometimes jump species, including to humans.
Disclosure Statement
Norberto Perico: No conflicts of interest to declare. Luca Perico: No conflicts of interest to declare. Claudio Ronco: He has been consulting or part of advisory boards for ASAHI, Astute, Baxter; Biomerieux, B. Braun, Cytosorbents, ESTOR, FMC, GE, Jafron, Medtronic, Toray. Giuseppe Remuzzi: Speaker honorarium/Travel reimbursements: Alnylam, Boehringer Ingelheim, Inception Sciences Canada. Advisory Board Consultancy: Alexion Pharmaceuticals Inc, Janssen Pharmaceutical, Akebia Therapeutics, Biocryst Pharmaceuticals, Catalyst Biosciences, Menarini Ricerche SpA. Consultancy fees for Advisory Board. No personal remuneration is accepted, compensations are paid to his institution for research and educational activities.
References
- 1.Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014 Jun 26;370((26)):2499–505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 2.Drosten C, Günther S, Preiser W, van der Werf S, Brodt H-R, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003 May 15;348((20)):1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Zhao S, Teng T, Abdalla AE, Zhu W, Xie L, et al. Systematic comparison of two animal-to-human transmitted human coronaviruses SARS-CoV-2 and SARS-CoV. Viruses. 2020 Feb 22;12((2)):244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan of novel coronavirus-infected pneumonia. N Engl J Med. 2020 Mar 26;382((13)):1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020 Jun;92((6)):548–51. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020 Mar;8((3)):240–1. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns Hopkins CSSE COVID-19 Map − Johns Hopkins Coronavirus Resource Center [accessed 2020 Feb 15] Available at www.coronavirua.jhu.edu.
- 8.Perico N, Fagiuoli S, Di Marco F, Laghi A, Cosentini R, Rizzi M, et al. Bergamo and Covid-19. how the dark can turn to light. Front Med. 2021;8:609440. doi: 10.3389/fmed.2021.609440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China a retrospective cohort study. Lancet. 2020 Mar 28;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 May;581((7807)):215–20. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 Nov 27;426((6965)):450–4. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr 16;181((2)):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020 May 26;117((21)):11727–34. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005 Sep 16;309((5742)):1864–8. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 May 14;181((4)):894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen K-Y. Coronaviruses − drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15((5)):327–47. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020 May;26((5)):681–7. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W, Li T. COVID-19 towards understanding of pathogenesis. Cell Res. 2020 May;30((5)):367–9. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581((7809)):465–9. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 21.Cao X. COVID-19 immunopathology and its implications for therapy. Nat Rev Immunol. 2020 May;20((5)):269–70. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020 Jul;8((7)):738–42. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8((4)):420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021 Jan;17((1)):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007 Oct;13((10)):1248–52. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020 Aug;584((7821)):463–9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19 consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395((10229)):1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020 Jul;146((1)):119–127.e4. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 Jun;19((6)):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha P, Matthay MA, Calfee CS. Is a ‘cytokine storm’ relevant to COVID-19? JAMA Intern Med. 2020 Sep 1;180((9)):1152–4. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBio Medicine. 2020 Jul;57:102833. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen SF, Ho Y-C. SARS-CoV-2 a storm is raging. J Clin Invest. 2020 May 1;130((5)):2202–5. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bost P, Giladi A, Liu Y, Bendjelal Y, Xu G, David E, et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020 Jun 25;181((7)):1475–1488.e12. doi: 10.1016/j.cell.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020 Jun;26((6)):842–4. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 May 1;130((5)):2620–9. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 May;17((5)):533–5. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020 Jun 25;181((7)):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020 Jul;26((7)):1070–6. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis and angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383((2)):120–8. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395((10234)):1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020 Aug;98((2)):314–22. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song W-C, FitzGerald GA. COVID-19 hemostatic activation and complement. J Clin Invest. 2020 Aug 3;130((8)):3950–3. doi: 10.1172/JCI140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta N, Zhao Y-Y, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019 Sep;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Nadim MK, Forni LG, Mehta RL, Connor MJ, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020 Dec;16((12)):747–64. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020 Jun 1;318((6)):F1454–F62. doi: 10.1152/ajprenal.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97((5)):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China. JAMA. 2020 Mar 17;323((11)):1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020 Nov 1;180((11)):1436–47. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020 Jul;98((1)):209–18. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 Apr 28;323((16)):1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8((5)):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020 Jun;31((6)):1157–65. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020 Jun;16((6)):308–10. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Izzedine H, Jhaveri KD. Acute kidney injury in patients with COVID-19 an update on the pathophysiology. Nephrol Dial Transplant. 2021 Jan 25;36((2)):224–6. doi: 10.1093/ndt/gfaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021 Jan;32((1)):151–60. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali H, Daoud A, Mohamed MM, Salim SA, Yessayan L, Baharani J, et al. Survival rate in acute kidney injury superimposed COVID-19 patients a systematic review and meta-analysis. Ren Fail. 2020 Nov;42((1)):393–7. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim J-H, Park S-H, Jeon Y, Cho J-H, Jung H-Y, Choi J-Y, et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020 Jun 3;9((6)):1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 Jul;98((1)):219–27. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection a report of five cases. Transl Res. 2020 Jun;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021 May 4;12((1)):2506. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020 Sep;27((5)):365–76. doi: 10.1053/j.ackd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020 Jun;5((6)):935–9. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, et al. COVID-19-related glomerulopathy a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020 Aug;2((4)):488–92. doi: 10.1016/j.xkme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman DJ, Pollak MR. Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab. 2016 Apr;27((4)):204–15. doi: 10.1016/j.tem.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19-associated kidney injury a case series of kidney biopsy findings. J Am Soc Nephrol. 2020 Sep;31((9)):1948–58. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uppal NN, Kello N, Shah HH, Khanin Y, De Oleo IR, Epstein E, et al. De Novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020 Nov;5((11)):2079–83. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moeinzadeh F, Dezfouli M, Naimi A, Shahidi S, Moradi H. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy a case report. Iran J Kidney Dis. 2020 May;14((3)):239–42. [PubMed] [Google Scholar]
- 68.Prendecki M, Clarke C, Cairns T, Cook T, Roufosse C, Thomas D, et al. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020 Sep;98((3)):780–1. doi: 10.1016/j.kint.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allez M, Denis B, Bouaziz J-D, Battistella M, Zagdanski A-M, Bayart J, et al. COVID-19-related IgA vasculitis. Arthritis Rheumatol. 2020 Nov;72((11)):1952–3. doi: 10.1002/art.41428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020 Sep;31((9)):1959–68. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suso AS, Mon C, Oñate Alonso I, Galindo Romo K, Juarez RC, Ramírez CL, et al. IgA vasculitis with nephritis (Henoch-Schönlein Purpura) in a COVID-19 patient. Kidney Int Rep. 2020 Nov;5((11)):2074–8. doi: 10.1016/j.ekir.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durvasula R, Wellington T, McNamara E, Watnick S. COVID-19 and kidney failure in the acute care setting our experience from Seattle. Am J Kidney Dis. 2020 Jul;76((1)):4–6. doi: 10.1053/j.ajkd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naicker S, Yang C-W, Hwang S-J, Liu B-C, Chen J-H, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020 May;97((5)):824–8. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020 Aug;31((8)):1683–7. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abbate M, Rottoli D, Gianatti A. COVID-19 attacks the kidney ultrastructural evidence for the presence of virus in the glomerular epithelium. Nephron. 2020;144((7)):341–2. doi: 10.1159/000508430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu D, Xue G, Peng B, Feng Z, Lu H, Gong L. High-throughput docking and molecular dynamics simulations towards the identification of potential inhibitors against human coagulation Factor XIIa. Comput Math Methods Med. 2020;2020:2852051. doi: 10.1155/2020/2852051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme implications for albuminuria in diabetes. J Am Soc Nephrol. 2006 Nov;17((11)):3067–75. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 78.Perico L, Benigni A, Remuzzi G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;144((5)):213–21. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samavati L, Uhal BD. ACE2, much more than just a receptor for SARS-COV-2. Front Cell Infect Microbiol. 2020;10:317. doi: 10.3389/fcimb.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 May 14;181((4)):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020 Aug 6;383((6)):590–2. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller SE, Goldsmith CS. Caution in identifying coronaviruses by electron microscopy. J Am Soc Nephrol. 2020 Sep;31((9)):2223–4. doi: 10.1681/ASN.2020050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol. 2020 Aug;31((8)):1688–95. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020 Sep;31((9)):2158–67. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aboudounya MM, Heads RJ. COVID-19 and Toll-like receptor 4 (TLR4) SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm. 2021;2021:8874339. doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joannidis M, Forni LG, Klein SJ, Honore PM, Kashani K, Ostermann M, et al. Lung-kidney interactions in critically ill patients consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020 Apr;46((4)):654–72. doi: 10.1007/s00134-019-05869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armutcu F. Organ crosstalk the potent roles of inflammation and fibrotic changes in the course of organ interactions. Inflamm Res. 2019 Oct;68((10)):825–39. doi: 10.1007/s00011-019-01271-7. [DOI] [PubMed] [Google Scholar]
- 88.Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H, et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019 Jul 1;9((1)):74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020 Jul;26((7)):1618–20. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, Bodro M, Blasco M, Poch E, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020 May 16;395((10236)):e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan China. Eur J Clin Nutr. 2020 Jun;74((6)):871–5. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dudoignon E, Moreno N, Deniau B, Coutrot M, Longer R, Amiot Q, et al. Activation of the renin-angiotensin-aldosterone system is associated with acute kidney injury in COVID-19. Anaesth Crit Care Pain Med. 2020 Aug;39((4)):453–5. doi: 10.1016/j.accpm.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hepokoski ML, Malhotra A, Singh P, Crotty Alexander LE. Ventilator-induced kidney injury are novel biomarkers the key to prevention? Nephron. 2018;140((2)):90–3. doi: 10.1159/000491557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020 Apr 23;382((17)):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Welch HK, Kellum JA, Kane-Gill SL. Drug-associated acute kidney injury identified in the United States Food and Drug Administration Adverse Event Reporting System Database. Pharmacotherapy. 2018 Aug;38((8)):785–93. doi: 10.1002/phar.2152. [DOI] [PubMed] [Google Scholar]
- 96.Izzedine H, Jhaveri KD, Perazella MA. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 2020 Jun;97((6)):1297–8. doi: 10.1016/j.kint.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kidney Disease. Improving Global Outcomes. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2((Suppl 1)):1–138. [Google Scholar]
- 98.Douglas IS, Alapat PM, Corl KA, Exline MC, Forni LG, Holder AL, et al. Fluid response evaluation in sepsis hypotension and shock a randomized clinical trial. Chest. 2020 Oct;158((4)):1431–45. doi: 10.1016/j.chest.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.American Society of Nephrology Recommendations on the care of hospitalized patients with COVID-19 and kidney failure requiring renal replacement therapy. 2020. https://www.asn-online.org/g/blast/files/AKI_COVID-19_Recommendations_Document_03.21.2020.pdf.
- 100.Rizo-Topete LM, Claure-Del Granado R, Ponce D, Lombardi R. Acute kidney injury requiring renal replacement therapy during the COVID-19 pandemic what are our options for treating it in Latin America? Kidney Int. 2021 Mar;99((3)):524–7. doi: 10.1016/j.kint.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using Open SAFELY. Nature. 2020 Aug;584((7821)):430–6. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020 a modelling study. Lancet Glob Health. 2020 Aug;8((8)):e1003–e17. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.ERA-EDTA Council ERACODA Working Group Chronic kidney disease is a key risk factor for severe COVID-19 a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021 Jan;36((1)):87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020 Dec;98((6)):1540–8. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, et al. COVID-19-related mortality in kidney transplant and dialysis patients results of the ERACODA collaboration. Nephrol Dial Transplant. 2020 Nov 1;35((11)):1973–83. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vinson AJ, Chong AS, Clegg D, Falk C, Foster BJ, Halpin A, et al. Sex matters COVID-19 in kidney transplantation. Kidney Int. 2021 Mar;99((3)):555–8. doi: 10.1016/j.kint.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rincón A, Moreso F, López-Herradón A, Fernández-Robres MA, Cidraque I, Nin J, et al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J. 2020 Aug;13((4)):542–9. doi: 10.1093/ckj/sfaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking Changes in SARS-CoV-2 Spike Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020 Aug 20;182((4)):812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, et al. SARS-CoV-2 infection in farmed minks the Netherlands April and May 2020. Euro Surveill. 2020 Jun;25((23)):2001005. doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gómez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines (Basel) 2021 Mar 11;9((3)):243. doi: 10.3390/vaccines9030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021 May;593((7858)):266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 112.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 Apr 9;372((6538)):eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1 matched cohort study. BMJ. 2021 Mar 9;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davies NG, Jarvis CI, CMMID COVID-19 Working Group, Edmunds WJ, Jewell NP, Diaz-Ordaz K, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021 May;593((7858)):270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buss LF, Prete CA, Abrahim CMM, Mendrone A, Salomon T, de Almeida-Neto C, et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021 Jan;371((6526)):288–92. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021 Jan;397((10270)):173–5. doi: 10.1016/S0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pael A, Evans B, Ahilan S, Ban R, Blackett I, Hawkins P, et al. Estimating safely managed sanitation in urban areas; lessons learned from a global implementation of excreta-flow diagrams. Front Environ. 2020 doi. 10.3389/fenvs.2020.00001. [Google Scholar]