Abstract
Background:
The AHA/ACC heart failure (HF) classification has 3 stages. Knowledge regarding the community burden of HF stages is limited, and data on the biomarker profile associated with HF stages are scarce, although higher concentrations of certain biomarkers are associated with preclinical HF.
Objectives:
To describe the prevalence and prognosis of HF stages in the community; to evaluate if preclinical HF stages are characterized by elevation of pro-inflammatory (C-reactive protein), neurohormonal activation (B-type natriuretic peptide renin, and aldosterone), and cardiac stress biomarkers (high-sensitivity troponin I, ST-2, and growth differentiation factor-15).
Methods:
We evaluated 6770 participants (mean age 51 years; 54% women) from the Framingham Study, defining 4 stages: 1) Healthy: no risk factors; 2) Stage A: presence of HF risk factors (hypertension, diabetes, obesity, coronary artery disease), no cardiac structural/functional abnormality; 3) Stage B: presence of prior MI, valvular disease, left ventricular (LV) systolic dysfunction, LV hypertrophy, regional wall motion abnormality, or LV enlargement; 4) Stage C/D: prevalent HF.
Results:
The prevalence of HF stages A, B were 36.5%, 24.2% respectively, rising with age (Odds Ratio [95% CI]: 1.70 [1.64-1.77] per decade increment). In age-, sex-adjusted we observed a gradient of increasing biomarker levels across HF stages (p<0.05; n=3416). Adjusting for age and sex, mortality rose across HF stages (232 deaths, mean follow-up 7 years), with 2-fold and 8-fold mortality risks for Stages B and C/D, respectively compared to healthy.
Conclusions:
Approximately 60% of our sample has preclinical HF, and those in stage B had higher concentrations of HF biomarkers and experienced a substantial mortality risk.
Keywords: heart failure stages, biomarkers, echocardiography, epidemiology
Introduction
Given the high morbidity and mortality, and healthcare cost associated with HF, prevention of HF is a public health priority. In this context, knowledge of the burden of preclinical precursors of HF in the community is a fundamental prerequisite to screen for and prevent the condition. The American Heart Association/American College of Cardiology (AHA/ACC) have categorized HF into three stages (A, B, C/D) (1) with two of these stages (A and B) being preclinical phases, i.e., they are characterized by elevated risk of HF without the overt syndrome (see below). Information regarding the burden of HF stages in the community and the mortality risk associated with these stages is quite limited (2). Additionally, investigators have reported that higher levels of biomarkers mirroring cardiac stress (such as B-type natriuretic peptide [BNP], growth differentiation factor 15 [GDF-15], high sensitivity Troponin I [hsTnI], and ST2) are associated with higher incidence of HF (3). Data on the biomarker profile associated with HF stages is limited, however, although some of the aforementioned biomarkers have been related individually to select preclinical HF phenotypes (such as left ventricular hypertrophy or systolic dysfunction) (4,5). More specifically, the prevalence of HF stages and their association with mortality has not been described in a non-hospital based community sample. Additionally, the associations between HF stages and biomarkers of neurohormonal stress have not been reported comprehensively. Moreover, the relations of the HF stages with cardiovascular disease (CVD) and non-CVD mortality has not been reported. Accordingly, we estimated the prevalence of the various HF stages in the community, assessed the biomarker profile associated with these stages, and evaluated their prognosis using a large community-based sample. We hypothesized that a. the prevalence of preclinical HF stages increases with age and is higher in men versus women; b. there is a gradient of increasing circulating concentrations of BNP, GDF-15 and hsTnI levels across the stages from healthy to preclinical to overt HF; and c. preclinical HF is associated with considerable mortality risk, which is intermediate between the risk observed for ‘healthy individuals and that observed among those with overt HF.
Methods
Study sample
The description of the design, selection criteria, and sampling methods of the Framingham Offspring and Third Generation Studies has been reported (6, 7). For the present investigation, we used two distinct samples. For assessing prevalence of the heart failure (HF) stages and for evaluating the prognostic significance of these stages, 3021 participants of the Framingham Offspring cohort who attended the eighth examination cycle (2005-2008) and 4095 participants of the Framingham Third Generation cohort attending the first examination (2002-2005) were eligible. Participants were excluded for the following reasons: renal insufficiency as indicated by serum creatinine levels ≥ 2 mg/dL (n=21), missing components of HF stage classification (n=48), and missing family classification (n=277), resulting in a total sample of 6770 participants (Sample 1). Participants with renal insufficiency were excluded because the specificity of the Framingham Heart Study HF criteria may be limited in the presence of fluid overload states such as renal failure, and biomarker values could be inflated. For comparing levels of various biomarkers across the HF stages, we evaluated 3532 participants of the Framingham Offspring cohort who attended the sixth examination cycle (1995-1998). Participants in this subsample were excluded for the following reasons: serum creatinine levels ≥ 2 mg/dL (n=15), missing HF stage classification (n=19), missing body mass index (BMI) (n=38), and missing data on biomarkers of interest (n=44), yielding a final sample of 3416 participants (Sample 2). We used 2 different samples for this investigation, driven by the availability of biomarker measurements in Offspring examination cycle 6, and the availability of components defining all heart failure stages in Offspring examination cycle 8 and Third generation examination cycle 1. The Boston University Medical Center Institutional Review Board approved all study protocols. Written informed consent was provided by all participants.
Clinical and Biomarker measurements
Blood was drawn on all Heart Study participants in the morning after an overnight fast (typically between 7:30 and 9:00 AM), and the biosamples were stored at −80° C until assayed. Hypertension was defined as either having a systolic blood pressure of ≥140 or diastolic blood pressure of ≥90 or using anti-hypertension medication. Diabetes mellitus was defined as either having a fasting blood glucose value of ≥126 or using glucose-lowering medication. Obesity was defined as having a BMI ≥30 kg/m2. Coronary artery disease was defined as having a myocardial infarction (8). We measured circulating concentrations of biomarkers representing inflammation (C-reactive protein [CRP]), neurohormonal activation (B-type natriuretic peptide [BNP], renin, and aldosterone), and cardiac stress (high-sensitivity troponin I, ST-2, and growth differentiation factor-15 [GDF-15]). These biomarkers were chosen because they have been associated to incidence of HF in a previous report from our group (3). BNP was measured with the Shionogi assay, ST2 with a high-sensitivity second-generation, enzyme-linked immunosorbent assay (Critical Diagnostics, detection limit 2 ng/mL), high-sensitivity Troponin I was measured with an ultra-sensitive immunoassay using a novel, single-molecule counting technology (Singulex, with a detection limit is 0.2 pg/mL and a range 0.5 to 70 pg/mL), GDF15 levels were measured with a pre-commercial automated electrochemilluminescent immunoassay (Roche Elecsys, with a detection limit of <10 ng/L) (3). High-sensitivity CRP levels were measured with a Dade Behring BN100 nephelometer. Serum aldosterone levels were measured with a radioimmunoassay applied to extracted and fractionated serum (Quest Diagnostics, Cambridge, MA). Plasma renin concentrations were measured with an immunochemiluminometric assay (Nichols assay, Quest Diagnostics, Cambridge, MA). The inter-assay coefficients of variation for each biomarker are as follows: CRP (2.2%); BNP (12.2%); aldosterone (4.0% for high concentrations and 9.8% for low concentrations); renin (2.0% for high concentrations and 10.0% for low concentrations); GDF15 (8-10%); ST2 (<4%); and hsTnI(8%).
Echocardiographic measurements
All study participants underwent standardized two-dimensional transthoracic echocardiography with Doppler color flow imaging. A sonographer or a cardiologist (experienced in echocardiography) read all echocardiograms; all readers were blinded to biomarker results and clinical information. We averaged digital M-mode measurements from 3 or more cardiac cycles and estimated the left ventricular (LV) internal dimensions in end-diastole and systole, and the diastolic thicknesses of the LV posterior wall and the inter-ventricular septum. All measurements were made using the leading edge technique and following the American Society of Echocardiography [ASE] guidelines for echocardiographic measurements (9). Excellent reproducibility of echocardiographic measurements has been previously reported (10). The inter-observer variability ranged from 0.9 to 5% for LV diastolic dimensions (LVDD), 2-2.9% for LV posterior wall thickness in diastole, 3.6-6.5% for the inter-ventricular septum in diastole, and 0.8-4% for LV mass. The intra-observer variability was measured from 0.3 (LVDD) to 4% (inter-ventricular septal thickness). Abnormal left ventricular systolic function was defined as having a fractional shortening value of < 0.29 or qualitative assessment of borderline/mild ejection fraction (mild systolic dysfunction), or having a fractional shortening <0.22 or qualitative assessment of moderate/severe ejection fraction (moderate/severe systolic dysfunction).
Outcome events
The outcome of interest was all-cause mortality during follow-up after the eighth examination cycle. All deaths were verified using information from medical records, local obituaries and national death registries. Heart failure was defined based on the Framingham criteria (11).
Statistical Analysis
We used a categorical variable to classify participants into the healthy category or one of the 3 HF stages, as reported by the AHA/ACC (5): 1) Healthy: healthy participants with no HF risk factors, or symptoms of dyspnea or physical sign of edema; 2) Stage A: participants with at least one of HF risk factors (hypertension; diabetes; obesity; coronary artery disease defined by myocardial infarction, angina pectoris or coronary insufficiency), but with no cardiac structural/functional abnormality on imaging studies; 3) Stage B: participants with any of the following : prior myocardial infarction, valvular heart disease, or echocardiographic evidence of asymptomatic LV systolic dysfunction, hypertrophy by ASE criteria, enlargement or any regional wall motion abnormality; 4) Stage C/D: participants with prevalent HF. In secondary analyses, Stage C/D was further classified based on ejection fraction measured near the time of HF: HF with preserved ejection fraction (HFPEF), HF with reduced ejection fraction (HFREF), and HF with unknown ejection fraction (EF).
We described the prevalence of HF stages by sex using samples 1 and 2 separately. For analysis, we considered the following age groups: <55 years, 55-<65, 65-<75 years, and ≥75 years for an easier comparison with the prevalence of HF stages in Olmsted county as reported by Ammar et al. (2).
Using sample 1, we examined cross-sectional associations of the HF stages (dependent variables) with age and sex (predictor variables) using three separate logistic regression models corresponding to the definition of the variable representing the HF stages, i.e, ≥ stage A vs. healthy, ≥ stage B vs. ≤ stage A, and stage C/D vs. others.
Using sample 2, we examined cross-sectional association of log-transformed biomarkers (dependent variables, separate model for each biomarker) with HF stages using analysis of Covariance, adjusting for age and sex, and further adjusting for BMI in separate models. HF stage was modeled as a continuous variable to test for trend across HF stages and also as categorical variable, treating healthy as the reference group. Primary analyses included circulating B-type natriuretic peptide (BNP), growth differentiation factor-15 (GDF-15), and high-sensitivity cardiac troponin I (hsTnI), whereas ST-2, C-reactive protein (CRP), aldosterone, and renin were included in secondary analyses, given their association with HF in select reports. Additionally, we graphically presented the least square means of the log-transformed standardized biomarkers adjusting for age, sex, and further adjusting for BMI. Furthermore, we plotted the means of biomarkers across the numbers of risk factors, and also created Cox proportional hazards regression models among those in Stage B to evaluate whether participants having Stage B and also highest biomarker levels were at highest risk of developing overt HF (Stage C/D). Finally, we have created tertiles of the biomarkers GDF-15, BNP, and hsTnI, and we have categorized individuals belonging in Stage B (n=976) into 3 groups: a) those having all biomarker values in the lowest tertiles; b) those having at least 2 biomarkers in the highest tertile; and 3) all other participants. We used this grouping variable to create Cox proportional hazards regression models to compare the groups with regards to risk of developing HF (Stage C/D).
Lastly, we evaluated the association between the HF stages and all-cause mortality using age- and sex-adjusted Cox proportional hazards regression models (12), after confirming that the proportionality of hazards assumption was met. We adjusted for familial associations to account for potential correlations between parents, children, and siblings. We also evaluated the interactions of HF stage with age and sex including the corresponding interaction terms in separate models. We also related HF Stages 0-B to the incidence of overt HF by calculating incidence rates.
Statistical significance was assessed based on a two-sided p-value of <0.05. The SAS Software version 9.3 (Cary, NC) was used for all analyses. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
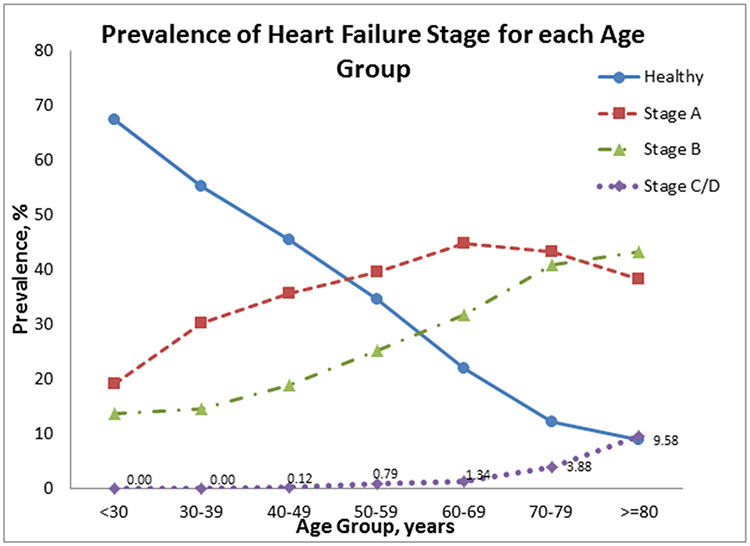
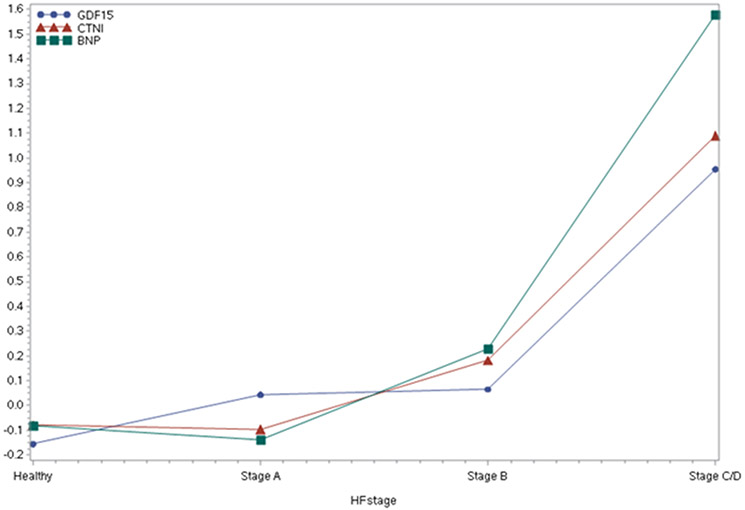
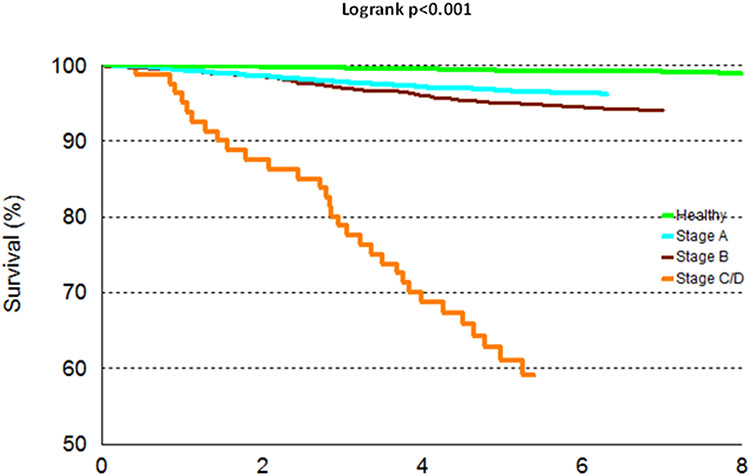
The baseline characteristics of both study samples are shown in Table 1. Participants were middle-aged to older with a mean BMI in the overweight range. About 60% of individuals in our sample had preclinical HF (Stage A or B, Supplementary Table 1), of which nearly 24% had Stage B HF and the remaining 36% had stage A HF. The prevalence of Stages B and C/D and HFREF was substantially higher in men vs. women, whereas HFPEF was more prevalent among women. We also observed an increasing trend in the prevalence of Stages A, B and C/D as age increased (Figure 1); this was confirmed in sex-adjusted models where we observed a statistically significant association between advancing age and the greater odds of having preclinical or clinical HF using 3 different reference categories (Supplementary Table 2). Compared to women, men had 1.6-fold higher odds of having prevalent preclinical or clinical HF (Supplementary Table 2). Notably, age- and sex-adjusted concentrations of circulating biomarkers rose with increasing HF stage (p<0.0001 for BNP, GDF-15, and hsTnI, Figure 2, Supplementary Table 3, Supplementary Figure 1), findings that remain mostly unchanged upon additional adjustment for BMI. Additionally, biomarker levels increased across the number of risk factors (in Stage A), as shown in Supplementary Figure 2. Finally, in age, and sex-adjusted Cox proportional hazard regression models, individuals belonging in Stage B and also having highest levels of biomarkers were at 4 times the hazard of developing overt HF (Stage C/D) as compared to those having lowest values of biomarkers (HR=4.03, p=0.02). Notably, among the individuals in Stage B, those with biomarker values in the highest tertiles were at higher risk of HF compared to those with biomarker values in the lowest tertiles. In prospective analyses, there were 232 deaths (43% women) over a mean follow-up period of approximately 7 years (maximum 10 years). Incidence rates of death rose across the HF stages (Table 3). Higher HF stages were associated with a greater risk of death compared to Healthy, adjusting for age and sex (Hazard Ratio and 95% CI 1.63 [1.37, 1.93, Figure 3). Additionally, 88 deaths (out of the total 232) were observed among those in Stage B, and it is noteworthy that 69% of those deaths were due to non-CVD causes. We did not observe a statistically significant interaction between sex and HF stage on the incidence of death. Finally, we observed increasing rates for incidence of HF across HF stages (Table 4, Supplementary Figure 3).
Table 1.
Clinical characteristics of study samples
| Characteristics | Sample 1† (primary analyses) |
Sample 2 (biomarker sample) |
||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| N = 3657 | N = 3113 | N = 1807 | N = 1609 | |
| Age, years | 51 ± 16 | 51 ± 16 | 59 ± 10 | 59 ± 10 |
| Body mass index, kg/m2 | 26.7 ± 6.1 | 28.3 ± 4.7 | 27.3 ± 5.7 | 28.5 ± 4.4 |
| Obesity (%) | 24.2 | 29.1 | 25.6 | 30.3 |
| Systolic blood pressure, mm Hg | 120 ± 18 | 124 ± 15 | 127 ± 20 | 130 ± 17 |
| Diastolic blood pressure, mm Hg | 72 ± 9 | 77 ± 10 | 74 ± 9 | 77 ± 9 |
| Hypertensive (%) | 29.8 | 38.5 | 38.4 | 44.8 |
| Antihypertensive medication (%) | 23.1 | 27.2 | 25.4 | 30.8 |
| Total cholesterol | 190 ± 35 | 185 ± 37 | 212 ± 39 | 199 ± 41 |
| HDL cholesterol | 62 ± 17 | 48 ± 13 | 58 ± 16 | 43 ± 12 |
| Lipid lowering medication (%) | 18.6 | 25.6 | 0.9 | 1.2 |
| Dyslipidemia (%) | 31.0 | 52.3 | 35.5 | 50.9 |
| Fasting glucose, mg/dL | 97 ± 20 | 103 ± 22 | 100 ± 26.0 | 107 ± 28 |
| Diabetes (%) | 5.5 | 9.1 | 8.0 | 11.9 |
| Diabetes medication (%) | 4.0 | 5.8 | 4.1 | 6.5 |
| Smokers (%) | 13.2 | 14.3 | 15.8 | 14.6 |
| Dyspnea (%) | 2.5 | 1.5 | 21.1 | 17.6 |
| Edema (%) | 20.1 | 6.3 | 10.3 | 4.0 |
| Valvular Heart Disease (%) | 2.2 | 3.2 | 3.7 | 6.4 |
| Left Ventricular mass | 135 ± 29 | 194 ± 41 | 140 ± 31 | 192 ± 43 |
| Left Ventricular Hypertrophy by ASE criteria (%) | 17.0 | 29.3 | 23.8 | 33.2 |
| Regional wall motion abnormality (%) | 1.5 | 5.3 | 2.7 | 11.9 |
| Left Ventricular enlargement (%) | 3.3 | 1.9 | 3.7 | 5.3 |
| Left Ventricular Systolic Dysfunction (%) | 1.3 | 5.0 | 3.6 | 13.0 |
| History of Coronary Artery Disease (%) | 0.1 | 0.3 | 0.0 | 0.0 |
| History of Myocardial Infarction (%) | 1.3 | 4.3 | 1.6 | 7.4 |
| History of Heart Failure (%) | 0.8 | 1.6 | 0.7 | 1.4 |
| HFPEF | 55.2 | 21.6 | 53.8 | 18.2 |
| HFREF | 27.6 | 56.9 | 38.5 | 63.6 |
| Undefined | 17.2 | 21.5 | 7.7 | 18.2 |
| HF Stage frequencies (%) | ||||
| Healthy | 42.1 | 33.6 | 33.2 | 25.5 |
| Stage A | 38.6 | 33.9 | 42.5 | 39.0 |
| Stage B | 18.5 | 30.9 | 23.6 | 34.1 |
| Stage C/D | 0.8 | 1.6 | 0.7 | 1.4 |
| Biomarkers [Median (Q1, Q3)]* | ||||
| Aldosterone, ng/dl | - | - | 11 (7, 15) | 9 (7, 13) |
| B-type natriuretic peptide, pg/ml | - | - | 10.0 (4.1, 20.3) | 6.6 (4.0, 16.7) |
| C-reactive protein, mg/L | - | - | 2.4 (1.0, 5.8) | 1.8 (0.9, 3.8) |
| High-sensitivity cardiac troponin I, pg/ml | - | - | 1.2 (0.8, 1.9) | 1.6 (1.1, 2.7) |
| Growth differentiation factor-15, ng/L | - | - | 1023 (813, 1305) | 1064 (820, 1417) |
| Renin, mU/L | - | - | 11 (6, 19) | 14 (8, 25) |
| ST-2, ng/ml | - | - | 18.8 (15.3, 23.2) | 23.6 (19.2, 29.1) |
Aldosterone, C-reactive protein, renin, and ST-2 were considered only in secondary analyses.
Sample 1 includes participants from both Offspring and Third Generation cohorts (see Supplementary Table 4)
Figure 1.
Prevalence of HF stages by age group
Figure 2.
Age-, Sex-adjusted standardized biomarker means by HF stage
Table 3.
Mortality rates associated with HF stages in the community
| Characteristic | No. events / No. at risk | Person- years at risk |
Crude incidence rate* |
Age- and Sex-adjusted death rate (95% CI)† |
Hazard Ratio (95% CI) |
p-value for Hazard Ratio |
|---|---|---|---|---|---|---|
| HF Stages | ||||||
| Healthy | 23 / 2585 | 17934 | 0.128 | 2.92 (0.12-4.50) | Referent | Referent |
| Stage A | 90 / 2468 | 15506 | 0.580 | 4.11 (2.82-5.35) | 1.97 (1.24-3.13) | 0.0042 |
| Stage B | 88 / 1637 | 9992 | 0.881 | 4.80 (2.97-6.53) | 2.07 (1.29-3.34) | 0.0027 |
| Stage C/D | 31 / 80 | 362 | 8.564 | 12.52 (7.67-16.72) | 7.83 (4.61-13.28) | <0.0001 |
Data shown are number per 100 person-years.
Risk of death over 8 years
Figure 3.
Survival of participants according to Heart Failure stages
Table 4.
Heart Failure Incidence rates associated with HF stages in the community
| Women | Men | Pooled | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. events / No. at risk |
Person- years at risk |
Incidence rate |
No. events / No. at risk |
Person- years at risk |
Incidence rate |
No. events / No. at risk |
Person- years at risk |
Incidence rate |
| HF Stages | |||||||||
| Healthy | 0 / 476 | 2807 | 0.00 | 0 / 234 | 1379 | 0.00 | 0 / 710 | 4186 | 0.00 |
| Stage A | 19 / 772 | 4180 | 0.45 | 14 / 559 | 3063 | 0.46 | 33 / 1331 | 7242 | 0.46 |
| Stage B | 15 / 450 | 2395 | 0.63 | 35 / 575 | 3059 | 1.14 | 50 / 1025 | 5454 | 0.92 |
Data shown are number per 100 person-years.
Discussion
Principal findings
Our findings are three-fold. First, we observed that a very high proportion (~60%) of middle-aged and older adults had preclinical HF defined as Stage A or B according to the AHA/ACC classification schema. A higher proportion of men were classified into more advanced HF stages compared to women. As expected, the prevalence of HF stages increased with age. Of note, nearly 38% of people between ages 65-75 years and 43% of those over age 75 years had evidence of preclinical HF stage B. Additionally, 32% of people <55 years were classified as stage A HF. Second, higher HF stages were associated with greater circulating concentrations of cardiac stress biomarkers, and demonstrated evidence of systemic inflammation and neurohormonal activation. Of note, Stage B HF was associated with a 2-fold mortality hazard and Stage C/D with a 8-fold risk compared to healthy individuals. Interestingly, 69% of deaths occurring among participants with Stage B were due to non-CVD causes, which is likely due to the presence of co-morbidities leading to death before progressing to HF. Finally, we observed increasing incidence rates for HF across the HF stages 0, A and B.
Comparison with the published literature
The comparison of HF stages between our sample and the one used by Ammar et al6 showed significant differences in the prevalence of all stages, notably among those with stages A and B (Table 2). Contrary to the study by Ammar et al,6 we did not observe a statistically significant interaction between sex and HF stage on the incidence of death.
Table 2.
Comparison of prevalence of HF Stages in FHS and Mayo Clinic
| Framingham Heart Study* | Mayo Clinic Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HF Stage |
≤ 54 yr | 55-64 yr | 65-74 yr | ≥ 75 yr | Total | 45-54 yr | 55-64 yr | 65-74 yr | ≥ 75 yr | Total |
| Healthy | 2050 (50.1) | 332 (28.5) | 146 (15.9) | 57 (9.5) | 2585 (38.2) | 281 (46.9) | 225 (36.0) | 107 (20.9) | 27 (9.3) | 640 (31.5) |
| A | 1317 (32.2) | 500 (42.9) | 407 (44.4) | 244 (40.7) | 2468 (36.4) | 167 (27.9) | 157 (25.1) | 94 (18.3) | 36 (12.3) | 454 (22.4) |
| B | 719 (17.6) | 315 (27.1) | 346 (37.7) | 257 (42.9) | 1637 (24.2) | 138 (23.0) | 202 (32.3) | 237 (46.2) | 114 (39.0) | 691 (34.1) |
| C/D | 4 (0.1) | 17 (1.5) | 18 (2.0) | 41 (6.9) | 80 (1.2) | 13 (2.2) | 41 (6.6) | 75 (14.6) | 115 (39.4) | 244 (12.0) |
| Total | 4090 | 1164 | 917 | 599 | 6770 | 599 | 625 | 513 | 292 | 2029 |
Data represent frequencies (%).
Participants from Offspring Exam 8 and Generation 3 Exam 1.
We observed a high prevalence of preclinical HF in our sample, similar to an earlier report (2) from the Olmsted County. Ammar et al also reported increasing circulating BNP concentrations with higher HF stages (2). Our study confirmed these results for blood BNP levels, and expanded the biomarker profile to include 7 biomarkers reflecting inflammation, neurohormonal activation, and cardiac stress. Of note, we observed that participants in stage B had a 2-fold mortality hazard compared to those who were healthy. The Mayo Clinic report (2) demonstrated such an association only among men in their sample (HR = 4.0). To our knowledge, this finding of increased mortality in both sexes with Stage B HF has not been reported previously. The differences between the present investigation and the Mayo clinic report may stem from several important differences between the two studies. Additionally, the participants in the sample from the Olmsted County were older. Moreover, the categorization in the age groupings are very different (notably 60%, 17%, 14%, and 9% in the FHS cohort vs, 30%, 31%, 25%, and 14% in the Olmsted County cohort for the <54, 55-64, 65-74, and >75 years categories, respectively). The mean follow-up in our study was longer (7 years) compared to the Mayo Clinic study (median follow-up 5.5 years). Our sample was larger and included a wider age range compared to the smaller Mayo clinic sample. Additionally there were minor differences in the constituent criteria for the different HF stages in the two reports; for example, the definitions of LVH varied across these studies and the Mayo report used the Goldman SAS questionnaire, which was not used in our study.
Additionally, Wang et al have reported that higher circulating biomarker levels mirroring cardiac stress are associated with the incidence of HF (3). Other investigators have also reported higher circulating concentrations of neurohormones and CRP in individuals with precursors of HF such as LV systolic dysfunction and LVH (4, 5). The present study compares the biomarker profile across the HF stages using a comprehensive panel of biomarkers.
Strengths and limitations
The use of a large community-based sample including the echocardiographic database with comprehensive phenotyping for LVH, and systolic dysfunction, and evaluation of an extensive panel of putative biomarkers implicated in preclinical and overt HF strengthen our investigation. Additionally, we combined cross-sectional prevalence data with longitudinal prognostic information to truly capture the community burden of preclinical and overt HF. A few limitations merit comment. Our sample was predominantly white and middle-aged, which limits the generalizability of our results to other ethnicities and age groups not evaluated. Although our follow-up was longer than that in the Mayo clinic report, we may underestimate the true risk of mortality associated with preclinical HF stages over a longer period of follow-up. Furthermore, individuals with stage A and B HF likely progress over time to overt HF, and the mortality risk in these stages may reflect disease progression itself. We did not specifically evaluate this premise. Finally, separate samples were used for the evaluation of incidence of death and biomarker analyses, based on the availability of data.
Conclusion
In our large community-based sample, a substantial proportion of individuals (nearly 60%) had prevalent preclinical HF (Stage A and B). A staggering 83% of people over the age of 65 years have preclinical HF, and preclinical Stage B HF doubled the mortality hazard relative to healthy people. These observations may serve as forebodings of a substantial rise in the morbidity and mortality due to HF in the community in the future Consistent with our hypotheses, higher HF stages were associated with increased biomarker levels, consistent with activation of different biological pathways across the disease continuum. The likelihood that most individuals with Stage B HF will die of non-CVD causes before they experience overt HF underscores the importance of targeting prevention efforts at comorbidities at the earlier HF stages to avoid death.
Supplementary Material
Perspectives: Core Clinical Competencies and Translational Implications.
Competency in Medical Knowledge:
The AHA/ACC classify heart failure (HF) into 4 stages, 2 of which are preclinical. Nearly 60% of middle-aged to older individuals in the community has preclinical HF, higher circulating concentrations of key HF biomarkers, and experiences a substantially elevated risk of death.
Translational Outlook:
Preclinical HF stages may be characterized by elevation of pro-inflammatory, stress and neuro-hormonal biomarkers as well as with higher risk of death, underscoring the importance of targeting prevention efforts at comorbidities at the earlier HF stages to avoid death.
Acknowledgements and Funding
This work was supported by AHA Clinical Research Program 13CRP14090010 (VX), by N01-HC-25915 (NHLBI, by R00-HL-107642 and from Ellison Foundation (SC). Dr. Januzzi is supported by DeSanctis Clinical Scholar Endowment. Dr. Wollert was supported by the German Ministry of Education and Research, and Dr. Januzzi by the Roman W. DeSanctis Clinical Scholar Endowment. Assays for biomarkers were provided by Critical Diagnostics, Singulex, Inc. and Roche Diagnostics, Inc, but these companies did not have access to study data or analysis and interpretation. Dr. Xanthakis had full access to all study data and takes responsibility for the integrity and accuracy of the data analysis.
Abbreviations
- HF
Heart Failure
- AHA/ACC
American Heart Association/American College of Cardiology
- BNP
B-type natriuretic peptide
- GDF-15
Growth Differentiation Factors-15
- hsTnI
high sensitivity Troponin I
- CVD
Cardiovascular Disease
- BMI
Body Mass Index
- LV
Left Ventricular
- ASE
American Society of Echocardiography
- EF
Ejection Fraction
Footnotes
Disclosures
Dr. Wollert is named as co-inventor on a patent for the use of GDF-15 for cardiovascular applications and has a contract with Roche Diagnostics for the development of a GDF-15 assay.
Dr. Januzzi has reports significant grant funding from Roche Diagnostics, Siemens, Thermo-Fisher, and Singulex, as well as significant consulting income from Roche Diagnostics, Critical Diagnostics, Sphingotec, Amgen, and Novartis.
Reference List
- (1).Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- (2).Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr., Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563–70. [DOI] [PubMed] [Google Scholar]
- (3).Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012;126:1596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr., Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–82. [DOI] [PubMed] [Google Scholar]
- (5).Xanthakis V, Larson MG, Wollert KC, Aragam J, Cheng S, Ho J, Coglianese E, Levy D, Colucci WS, Michael Felker G, Benjamin EJ, Januzzi JL, Wang TJ, Vasan RS. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. J Am Heart Assoc. 2013;2: e000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- (7).Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr., Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–35. [DOI] [PubMed] [Google Scholar]
- (8).Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements: Framingham Heart Study, 30 year follow-up. Section 34 Editors Kannel WB, Wolf PA, Garrison RJ. 1987. Bethesda, MD, Department of Health and Human Services [Google Scholar]
- (9).Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- (10).Sundstrom J, Sullivan L, Selhub J, Benjamin EJ, D'Agostino RB, Jacques PF, Rosenberg IH, Levy D, Wilson PW, Vasan RS. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J 2004;25:523–30. [DOI] [PubMed] [Google Scholar]
- (11).McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–6. [DOI] [PubMed] [Google Scholar]
- (12).Cox DR, Oakes D. Analysis of survival data. London: Chapman & Hall; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.