Abstract
Objective:
To describe the physiologic swallowing impairments (MBSImP™©) associated with safety/efficiency impairments (DIGESTsafety/DIGESTefficiency grades) at 3–6 months after transoral robotic surgery (TORS) or radiation therapy (RT).
Study Design:
Secondary analysis of registry data.
Setting:
Single, academic institution.
Methods:
Two hundred and fifty-seven patients with HPV+ oropharynx cancer were stratified by primary treatment (75 TORS, 182 RT). Modified barium swallow studies were analyzed at baseline and 3–6 months using MBSImP scores and DIGESTsafety/DIGESTefficiency grades. DIGESTsafety/DIGESTefficiency grades and MBSImP were compared groupwise and associations between DIGESTsafety/DIGESTefficiency grades and MBSImP were explored by ordinal logistic regression. Exploratory analyses were stratified by multimodality treatment.
Results:
Neither DIGESTsafety/DIGESTefficiency differed significantly between groups at baseline or 3–6 months. Laryngeal vestibule closure was impaired more frequently in the RT group (RT: 41% vs. TORS: 27%; p = 0.02) while the TORS group had significantly more pharyngeal contraction impairment (63%; p < 0.001) compared to RT at 3–6 months.
Conclusion:
The results suggest a focal injury associated with DIGESTsafety/DIGESTefficiency post-TORS in contrast to a low-level diffuse physiologic impairment associated with post-RT dysphagia.
Keywords: dysphagia, head and neck cancer, radiation, surgery
1 ∣. INTRODUCTION
The recent increase in the incidence of oropharynx cancers (OPCs) is largely driven by human papilloma virus (HPV) associated tumors, which are rising in epidemic proportions.1 Much of the cohort of OPC is composed of patients with low-to-intermediate risk stage of disease, based on tumor, nodal volume, HPV status, and smoking.1 Patients with low to intermediate risk OPC present with favorable survival probability, thus likely to progress into lifelong survivorship.
Standard of care treatment for OPC over the past two decades has relied on radiotherapy (RT), with iterative advancements in conformal methods such as intensity modulated radiation therapy designed to sculpt dose to minimize normal tissue injury by sparing radiation dose to critical structures. Transoral robotic surgery (TORS) has been rapidly adopted as another primary treatment option for low-to-intermediate risk OPC.2,3 The increase in TORS is driven by the potential to de-escalate or avoid postoperative, multimodality treatments altogether.3
There are conflicting findings regarding which primary treatment strategy results in the best swallowing outcomes in low-to-intermediate risk disease.4,5 Neither TORS or RT is, however, dysphagia-free despite the function-preserving focus of both primary treatment methods.4-6 Regardless of which modality is best to preserve swallow function, acute dysphagia is expected for either approach.5,6 A current knowledge gap exists in understanding pathophysiology of functional impairment in early survivorship with modern treatment options as clinical observations, in fact, suggest that the mechanism of swallowing injury differs.
Pharyngeal swallowing function is two-fold, often broken into safety and efficiency. Swallow safety is commonly measured using the penetration-aspiration scale,7 which captures depth and corresponding sensation or reaction to airway entry on an ordinal scale. Pathophysiology that drives any underlying swallowing safety impairment is commonly measured using parameters describing the completeness, timing, and duration of laryngeal vestibule closure (LVC).8-10 Swallow efficiency is commonly measured using visuo-perceptual rater estimates11,12 and more objective quantitative spatial measures of postswallow residue. Pathophysiology of swallow inefficiency is theoretically driven by factors such as poor pharyngeal constriction, pharyngeal cavity area, esophageal opening, and impaired lingual and tongue-base propulsion.13,14 Knowledge of the underlying pathophysiology of impaired swallow safety and efficiency is needed to plan personalized treatment, select appropriate diagnostics, and inform patients regarding their post-treatment expectations.
The Dynamic Imaging Grade of Swallowing Toxicity (DIGEST™) and the Modified Barium Swallow Impairment Profile (MBSImP ™©) are two methods commonly used to grade dysphagia severity, per safety and efficiency of bolus clearance, and to characterize the physiological impairments of the swallow in patients with head and neck cancer. DIGEST is a validated method used to grade the severity of pharyngeal dysphagia from MBS and is based on the interaction of both swallow safety (per the penetration-aspiration scale7) and efficiency (per estimated amount of pharyngeal residue).12 MBSImP is a standardized approach for visuo-perceptual ordinal grading of 17 oral and pharyngeal swallowing physiology components from the MBS.15
The authors have previously characterized the prevalence and severity of subacute dysphagia after primary TORS or RT for OPCs using the DIGEST grading method.6 Approximately 7% of TORS and 16% of RT patients presented with DIGEST grades of 2 or higher, indicating moderate-to-severe dysphagia 3–6 months persisting after treatment. DIGEST analysis enabled the characterization of dysphagia severity among treatment subgroups and by tumor location. Alternatively, prospective, cross-sectional studies have applied an MBSImP component analysis to compare swallow physiology among OPC patients treated with surgery or RT to age-matched controls.16 Specific MBSImP components, such as initiation of pharyngeal swallow (100%, 19/19), decreased hyolaryngeal excursion (53%, 10/19), and decreased tongue base retraction (74%, 14/19), were highly prevalent approximately 12 months post-treatment. These published data confirm the presence and pattern of dysphagia in OPCs after surgery or RT, yet we lack data that detail the underlying pathophysiological components that contribute to the swallowing safety and efficiency impairment, or differences in these relationships by primary treatment strategy (i.e., TORS or RT).
Therefore, the overall objective of the present study was to compare MBSImP profiles of swallowing physiology at subacute recovery (3–6 months) after primary treatment to determine which physiologic impairments are associated with safety (per DIGESTsafety) and efficiency (per DIGESTefficiency) grades after two primary oncologic treatment strategies: TORS versus RT. Specific aims included a comparison between primary TORS and RT groups:
Patterns of functional impairment (DIGESTsafety and DIGESTefficiency grades),
Physiologic impairment profiles (per MBSImP), and
Associations between MBSImP physiologic impairments with functional impairments (DIGESTsafety and DIGESTefficiency).
Finally, as most studies aggregate swallowing outcomes of all cases after TORS regardless of adjuvant therapies, we know very little regarding the cumulative effects of treatment as it relates to impairment profiles after single versus multimodality regimens.6 Therefore, exploratory subgroup analyses were conducted for patients who received single (TORS alone, RT alone) versus multimodality treatments (TORS with adjuvant RT/chemoRT, RT with chemotherapy).
2 ∣. METHODS
2.1 ∣. Setting and study design
This is a secondary analysis of prospective registry data from the M. D. Anderson Oropharynx Cancer Registry (PA14-0947) Patient-Reported Outcomes and Function (PROF) Core. The registry enrolls all consenting patients with oropharyngeal malignancies of the head and neck at the University of Texas M. D. Anderson Cancer Center (MDACC) beginning in March 2015. The sample for this analysis comprised those enrolled on PA14-0914 from March 2015 to September 2019. Eligibility criteria were (1) cancer of the oropharynx, and (2) TORS or RT as primary treatment approach at MDACC. Primary treatment was determined by Multidisciplinary Tumor Board presentation of all cases in accordance with standard institutional practices.17-19 Data analysis occurred under approval of the Institutional Review Board (protocol PA11-0809). Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at MDACC.20,21 REDCap is a secure, web-based software platform designed to support data capture for research studies.
2.2 ∣. Patient inclusion and eligibility
Two hundred and fifty-seven patients with HPV-associated low to intermediate risk OPC were sampled from a prospective registry and stratified by primary treatment of either TORS (n = 75) or RT (n = 182; Table 1). The full database sampling consort has been published elsewhere.6
TABLE 1.
Participant demographic and clinical details
| Variable | Value | All patients n = 257 No. of patients |
Percentage | Primary TORS n = 75 No. of patients |
Percentage | Primary RT n = 182 No. of patients |
Percentage |
p value |
|---|---|---|---|---|---|---|---|---|
| Age at primary treatment start | Mean (SD) | 59 | 9 | 58 | 10 | 59 | 9 | 0.32 |
| Sex | Female | 35 | 14% | 10 | 13% | 25 | 14% | 0.55 |
| Male | 222 | 86% | 65 | 87% | 157 | 86% | ||
| Primary tumor site | Tonsil | 135 | 53% | 38 | 51% | 97 | 53% | 0.51 |
| BOT | 116 | 45% | 34 | 45% | 82 | 45% | ||
| GPS | 6 | 2% | 3 | 4% | 3 | 2% | ||
| Clinical T classification of primary, 7th edition | 1 | 124 | 48% | 40 | 53% | 84 | 46% | 0.50 |
| 2 | 126 | 49% | 34 | 45% | 92 | 51% | ||
| 3 | 7 | 3% | 1 | 1% | 6 | 3% | ||
| Baseline primary tumor volume, median (range) (cm3) | 6.50 (0.30–29.30) | 5.15 (0.30–21.90) | 6.95 (0.50–29.30) | 0.18 (0.04–0.29)a | ||||
| Clinical N classification of primary, 7th edition | N0 | 50 | 19% | 31 | 41% | 19 | 10% | <0.001 |
| N1 | 35 | 14% | 15 | 20% | 20 | 11% | ||
| N2a | 19 | 7% | 4 | 5% | 15 | 8% | ||
| N2b | 153 | 60% | 25 | 33% | 128 | 70% | ||
| Induction chemotherapy | Yes | 21 | 8% | 4 | 5% | 17 | 9% | 0.21 |
| Radiation therapy | Yes | 219 | 85% | 37 | 49% | 182 | 100% | <0.001 |
| No | 38 | 15% | 38 | 51% | 0 | — | ||
| Concurrent chemotherapy and XRT | Yes | 162 | 63% | 15 | 20% | 147 | 81% | <0.001 |
| RT dose (Gy) | Mean (minimum, maximum) | 6786 | (5000, 7000) | 6000 | (5000, 6996) | 6996 | (6000, 7000) | <0.001 |
| RT laterality | Unilateral | 54 | 21% | 17 | 23% | 37 | 20% | <0.001 |
| Bilateral | 164 | 64% | 19 | 25% | 145 | 80% | ||
| Neck dissection | Yes | 93 | 36% | 75 | 100% | 18 | 10% | <0.001 |
| Baseline MDADI composite score | Mean (SD) | 91.7 | 9.6 | 90.4 | 11.2 | 92.3 | 8.9 | 0.285 |
| Subacute MDADI composite score | 81.8 | 14.8 | 83.0 | 15.2 | 81.4 | 14.6 | 0.474 | |
| Subacute feeding tube | Yes | 16 | 6% | 2 | 1% | 14 | 5% | 0.009 |
| No | 234 | 91% | 71 | 28% | 163 | 63% | ||
| Missing | 7 | 3% | 2 | 1% | 5 | 2% |
Note: The italic values indicate statistical significance (p < 0.05).
Abbreviations: BOT, base of tongue; CI, confidence interval; GPS, glossopharyngeal sulcus; MDADI, MD Anderson Dysphagia Inventory; RT, radiation therapy; TORS, transoral robotic surgery; XRT, radiation therapy.
Effect size (95% CI).
2.3 ∣. Swallowing outcome assessment
Patients enrolled in the prospective registry (PA14-0914) undergo standardized modified barium swallow (MBS) studies at regular timepoints including baseline and 3–6 months after locoregional treatment. Dysphagia was graded using DIGEST and MBSImP criterion.11,15 MBS acquisition followed a standard protocol by a speech pathologist (SLP).11 The MBS were recorded using 30 frames/s and archived via the TIMS DICOM System©.
The MBS bolus protocol included six trials thin liquid (5 cc, 10 cc, cup sip), and two trials each of pudding and cracker with barium pudding (Varibar® oropharyngeal contrast, Bracco Diagnostics, Inc.). Independent review of MBS DICOM files for DIGEST and MBSImP analyses were conducted by registered MBSImP clinicians who met >80 exact agreement reliability standard.11,15 Rating SLPs were blinded to all clinical details.
The DIGEST method follows a standard decision tree to derive safety and efficiency grades that converge on an overall grade of 1 (mild), 2 (moderate), 3 (severe), and 4 (life threatening/profound) pharyngeal dysphagia aligned to the NCI's CTCAE toxicity grading framework.11 MBSImP provides 17 measures of swallowing physiology parsed out by swallow stage: oral (items 1–6), pharyngeal (items 7–16), and esophageal (item 17). MBSImP components are ordinal, with select components varying from 0 (normal function) to 3 or 4 (Table 2). MBS were graded for DIGESTsafety and DIGESTefficiency grades in addition to all MBSImP pharyngeal items. Oral component 6, initiation of the pharyngeal swallow, was included as it has been identified as a potential impairment in the head and neck cancer (HNC) dysphagia profile.16
TABLE 2.
MBSImP component scores, DIGEST grade, DIGESTsafety, and DIGESTefficiency at baseline and 3–6 months by primary treatment
| Baseline | 3–6 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TORS (75) | RT (182) | TORS (75) | RT (182) | |||||||
| Variable, Value | No. of patients |
Percentage | No. of patients |
Percentage |
p value |
No. of patients |
Percentage | No. of patients |
Percentage |
p value |
| 6_Initiation of Pharyngeal Swallow | ||||||||||
| 0 | 3 | 4% | 6 | 3% | 0.69 | 2 | 3% | 3 | 2% | 0.73 |
| 1 | 5 | 7% | 18 | 10% | 5 | 7% | 10 | 5% | ||
| 2 | 36 | 48% | 86 | 47% | 27 | 36% | 78 | 43% | ||
| 3 | 28 | 37% | 65 | 36% | 25 | 33% | 54 | 30% | ||
| 4 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 4% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| 7_Soft Palate Elevation | 0.45 | 0.28 | ||||||||
| 0 | 67 | 89% | 167 | 92% | 57 | 76% | 134 | 74% | ||
| 1 | 5 | 7% | 8 | 4% | 1 | 1% | 11 | 6% | ||
| 2 | 0 | — | 0 | — | 1 | 1% | 0 | — | ||
| 3 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| 4 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 5% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| 8_Laryngeal Elevation | 0.01 | 0.25 | ||||||||
| 0 | 36 | 48% | 58 | 15% | 19 | 25% | 37 | 20% | ||
| 1 | 35 | 47% | 116 | 64% | 40 | 53% | 105 | 58% | ||
| 2 | 0 | — | 0 | — | 0 | — | 3 | 2% | ||
| 3 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 4 | 5% | 8 | 4% | 16 | 21% | 37 | 20% | ||
| 9_Anterior Hyoid Excursion | 0.64 | 0.86 | ||||||||
| 0 | 11 | 15% | 31 | 17% | 6 | 8% | 16 | 9% | ||
| 1 | 61 | 81% | 144 | 79% | 53 | 71% | 129 | 71% | ||
| 2 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 4% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| 10_Epiglottic Movement | 0.77 | 0.78 | ||||||||
| 0 | 61 | 81% | 151 | 83% | 48 | 64% | 116 | 64% | ||
| 1 | 10 | 17% | 20 | 11% | 9 | 12% | 21 | 12% | ||
| 2 | 1 | 1% | 4 | 2% | 2 | 3% | 8 | 4% | ||
| Missing | 3 | 5% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| 11_LVC | 0.48 | 0.02 | ||||||||
| 0 | 57 | 76% | 147 | 81% | 39 | 52% | 70 | 38% | ||
| 1 | 14 | 19% | 28 | 15% | 20 | 27% | 72 | 40% | ||
| 2 | 0 | — | 0 | — | 0 | — | 2 | 1% | ||
| Missing | 4 | 5% | 7 | 4% | 16 | 21% | 38 | 21% | ||
| 12_Pharyngeal Stripping Wave | 0.69 | 0.73 | ||||||||
| 0 | 32 | 43% | 73 | 40% | 16 | 21% | 36 | 20% | ||
| 1 | 40 | 53% | 102 | 56% | 43 | 57% | 109 | 60% | ||
| 2 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 4% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| 13_Pharyngeal Contraction (A/P) | 0.17 | <0.001 | ||||||||
| 0 | 39 | 52% | 109 | 60% | 11 | 15% | 45 | 25% | ||
| 1 | 28 | 37% | 64 | 35% | 34 | 45% | 91 | 50% | ||
| 2 | 4 | 5% | 1 | 1% | 13 | 17% | 3 | 2% | ||
| 3 | 0 | — | 0 | — | 0 | — | 2 | 1% | ||
| Missing | 4 | 5% | 8 | 4% | 17 | 23% | 41 | 23% | ||
| 14_Pharyngoesophageal Segment Opening | 0.23 | 0.6 | ||||||||
| 0 | 18 | 24% | 32 | 18% | 13 | 17% | 26 | 14% | ||
| 1 | 54 | 72% | 143 | 79% | 45 | 60% | 118 | 65% | ||
| 2 | 0 | — | 0 | — | 1 | 1% | 1 | 1% | ||
| 3 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 4% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| 15_Tongue Base Retraction | 0.09 | 0.67 | ||||||||
| 0 | 0 | — | 4 | 2% | 2 | 3% | 2 | 1% | ||
| 1 | 46 | 61% | 123 | 68% | 19 | 25% | 55 | 30% | ||
| 2 | 22 | 29% | 45 | 25% | 33 | 44% | 62 | 34% | ||
| 3 | 4 | 5% | 3 | 2% | 5 | 7% | 26 | 14% | ||
| 4 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 4% | 7 | 4% | 16 | 21% | 37 | 20% | ||
| DIGEST grade | 0.06 | 0.93 | ||||||||
| 0 | 53 | 71% | 147 | 81% | 25 | 33% | 72 | 40% | ||
| 1 | 16 | 21% | 28 | 15% | 29 | 39% | 47 | 26% | ||
| 2 | 3 | 4% | 1 | 1% | 4 | 5% | 23 | 13% | ||
| 3 | 0 | — | 0 | — | 1 | 1% | 6 | 3% | ||
| 4 | 0 | — | 0 | — | 0 | — | 0 | — | ||
| Missing | 3 | 4% | 6 | 3% | 16 | 21% | 34 | 19% | ||
| DIGESTsafety | 0.51 | 0.53 | ||||||||
| 0 | 64 | 85% | 161 | 88% | 44 | 59% | 106 | 58% | ||
| 1 | 7 | 9% | 15 | 8% | 13 | 17% | 29 | 16% | ||
| 2 | 1 | 1% | 0 | — | 2 | 3% | 8 | 4% | ||
| 3 | 0 | — | 0 | — | 0 | — | 4 | 2% | ||
| 4 | 0 | — | 0 | — | 0 | — | 1 | 1% | ||
| DIGESTefficiency | 0.10 | 0.72 | ||||||||
| 0 | 58 | 77% | 155 | 85% | 30 | 40% | 79 | 43% | ||
| 1 | 10 | 13% | 19 | 10% | 24 | 32% | 41 | 23% | ||
| 2 | 2 | 3% | 1 | 1% | 3 | 4% | 16 | 9% | ||
| 3 | 2 | 3% | 1 | 1% | 2 | 3% | 12 | 7% | ||
| 4 | 0 | — | 0 | — | 0 | — | 0 | — | ||
Note: The italic values indicate statistical significance (p < 0.05).
Abbreviations: RT, radiation therapy; TORS, transoral robotic surgery.
Patients completed the MD Anderson Dysphagia Inventory (MDADI) prior to primary treatment and at their 3–6 months MBS. The MDADI is a patient-administered questionnaire that indicates the swallowing related quality of life.22 The questions include 19 items pertaining to various aspects of swallowing (emotional, functional, physical) in addition to one item related to overall function. Results from all three subscales are pooled and averaged to yield a composite score, which was used in this analysis. Data were unavailable for one participant in the surgical group who was deceased at subacute follow-up.
2.4 ∣. Statistical analysis
DIGESTsafety and DIGESTefficiency grades and MBSImP profiles were compared between groups (primary TORS and primary RT) using chi-square (Fisher's exact, twosided) and Kruskall-Wallis with post hoc Dunn's test and Šidák correction, respectively (Aims 1 and 2). Associations of MDADI scores MBSImP components with feeding tube placement at 3–6 months (yes/no) between groups were explored using the Wilcoxon signed-rank test and chi-square (Fisher's exact). Correlations between MDADI composite scores and dichotomized MBSImP components 6–15 were explored using point biserial correlations with multiple comparison correction. MBSImP components for each group (primary TORS and primary RT) associated with DIGESTsafety (TORS vs. RT) and DIGESTefficiency (TORS vs. RT) were then explored further by ordinal logistic regression with Bonferroni correction (Aim 3) controlled for baseline DIGEST. Furthermore, exploratory analyses by treatment modality (single vs. multiple) involved descriptive summaries with stratification by four groups: TORS alone and RT alone (single modality), versus TORS with adjuvant treatment (TORS+), and RT with induction chemotherapy/concurrent chemotherapy (RT+; multimodality).
3 ∣. RESULTS
A total of 257 patients (mean age, 59) were included in this study (29%, 75/257 underwent primary TORS and 71%, 182/257 received primary RT) as briefly summarized in Table 1; populations details are previously published.6 The primary TORS group had significantly lower N classification and RT dose. All TORS patients received upfront neck dissection, in contrast with only 10% of those with primary RT receiving neck dissection. Multimodality treatment was common: 49% (37/75) TORS patients required adjuvant treatment and 84% (152/182) primary RT patients received chemo/systemic therapy.
3.1 ∣. Differences in DIGESTsafety and DIGESTefficiency grades (Aim 1)
Neither DIGESTsafety [X2 (1) = 0.4, p = 0.51], nor DIGESTefficiency[X2 (1) = 2.7, p = 0.10] differed significantly between the two groups at baseline or at the subacute, 3–6 month timepoint post-treatment (DIGESTsafety: X2 (1) = 0.4, p = 0.53; and DIGESTefficiency: X2 (1) = 0.1; p = 0.72). Dysphagia prevalence between groups at 3–6 months did not differ between the two primary treatment methods (DIGEST >0; TORS: 45%, 34/75; RT: 42%, 76/182; p = 0.44; Table 2).
3.2 ∣. Differences in MBSImP physiologic impairment profiles (Aim 2)
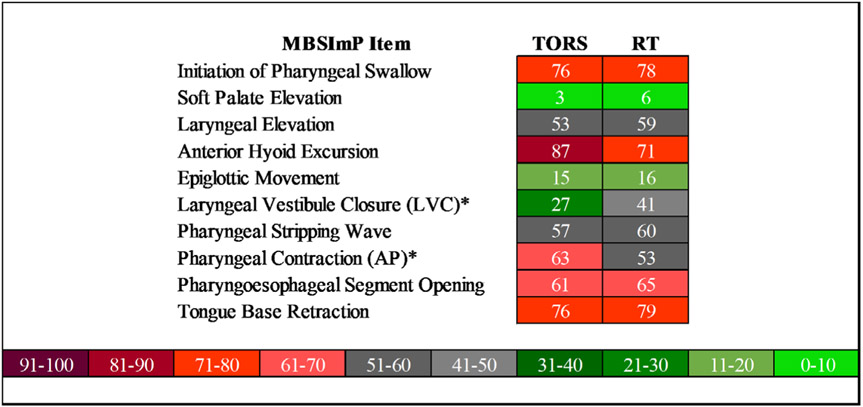
MBSImP components at baseline significantly differed only for the laryngeal elevation component score with higher frequency of impairment in the RT group [X2 (1) = 6.4, p = 0.01] but the laryngeal elevation component scores did not significantly differ at 3–6 months between the groups (p = 0.25). When comparing the two treatment groups at 3–6 months, only severity of laryngeal vestibular closure [X2 (1) = 5.4, p = 0.02] and pharyngeal contraction [X2 (1) = 10.5, p = 0.001] differed between the two groups. When dichotomized, MBSImP scores reveal greater LVC impairment in the RT group and an increased impairment in pharyngeal contraction in the TORS group (Figure 1).
FIGURE 1.
Proportion of patients with MBSImP component scores ≥1. “*” denotes significant MBSImP components between the groups
3.3 ∣. Associations between DIGESTsafety/DIGESTefficiency and physiologic MBSImP components (Aim 3)
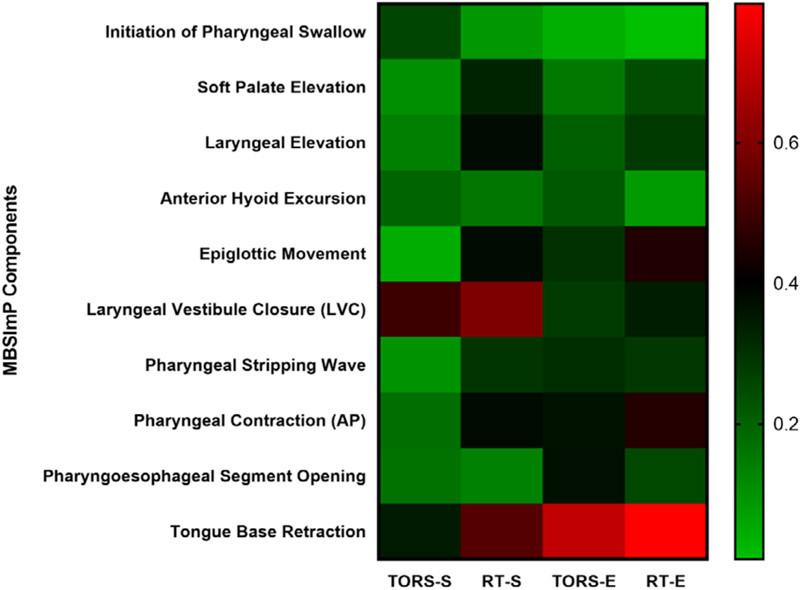
Controlling for baseline DIGEST, we found significant correlates of post-treatment DIGESTsafety in the TORS group including laryngeal vestibule closure (odds ratio [OR] 16.1, p < 0.001); while significant associations with DIGESTsafety grades for the RT group included all MBSImP components with the exception of initiation of pharyngeal swallow, anterior hyoid excursion, and PE opening (p = 0.23, respectively; Figure 2). Significant correlates of DIGESTefficiency for the TORS group included epiglottic movement (OR 5.4, p = 0.003), PE opening (OR 11.2, p = 0.004), and tongue base retraction (OR 32.6, p < 0.001), while all MBSImP components were significant in the RT group, with the exception of initiation of pharyngeal swallow (OR 1, p = 0.84) and anterior hyoid movement (OR 1.7, p = 0.29).
FIGURE 2.
Correlation between DIGESTsafety and DIGESTefficiency with MBSImP pharyngeal components for TORS vs RT in low-to-intermediate risk OPC (n = 257). Red boxes depict stronger correlations
3.4 ∣. Single versus multiple modality exploratory comparisons
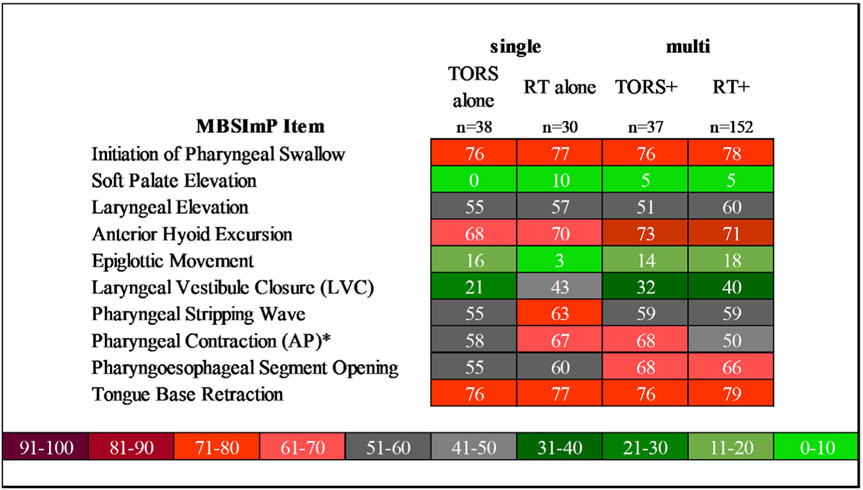
Exploratory descriptive analysis found relatively similar prevalence in LVC impairment between all treatment subgroups except single modality TORS (single modality TORS: 21%, all other groups: 32%–43%). While prevalence of impaired pharyngeal contraction was similar between the four subgroups, inspection of ordinal severity of the pharyngeal contraction component revealed a noteworthy increase in the proportion of TORS+ patients who presented with lateralized pharyngeal constriction impairment (unilateral bulging on MBSImP; 27%, 10/37) versus those patients who underwent TORS alone (8%, 3/38). Proportion of patients with MBSImP component scores ≥1 by treatment combination can be found in Figure 3.
FIGURE 3.
Proportion of patients with MBSImP components ≥1 among patients treated with TORS or RT (with and without adjuvant) in low-to-intermediate risk OPC (n = 257). “*” denotes significant MBSImP components between the groups
3.5 ∣. Functional and patient reported outcome by MBSImP component analysis
There were no significant differences between MDADI composite scores between TORS or RT groups at either baseline of 3–6 months; p = 0.28, 0.47. MDADI composite scores were not significantly correlated with MBSImP components for either group or timepoint (Table 3). At 3–6 months, the RT group had significantly higher feeding tube rates (5%, 14/257) than the TORS group, p = 0.009. One MBSImP component, pharyngeal contraction was significantly associated with feeding tube at 3–6 months, p = 0.05.
TABLE 3.
Correlations between MDADI with MBSImP components for patients treated with primary TORS or RT for low-to-intermediate risk OPC (n=257).
| Baseline | 3–6 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| TORS (75) | RT (182) | TORS (75) | RT (182) | |||||
| r pb | p value | r pb | p value | r pb | p value | r pb | p value | |
| 6_Initiation of Pharyngeal Swallow | −0.080 | 0.531 | −0.122 | 0.119 | −0.105 | 0.451 | −0.041 | 0.638 |
| 7_Soft Palate Elevation | 0.198 | 0.116 | 0.026 | 0.743 | 0.126 | 0.365 | −0.040 | 0.650 |
| 8_Laryngeal Elevation | −0.150 | 0.241 | −0.116 | 0.139 | 0.205 | 0.137 | −0.016 | 0.859 |
| 9_Anterior Hyoid Excursion | −0.167 | 0.189 | 0.064 | 0.417 | −0.137 | 0.325 | −0.104 | 0.234 |
| 10_Epiglottic Movement | −0.163 | 0.197 | −0.051 | 0.514 | 0.027 | 0.848 | −0.200 | 0.022 |
| 11_LVC | −0.219 | 0.085 | −0.100 | 0.201 | −0.214 | 0.120 | −0.168 | 0.055 |
| 12_Pharyngeal Stripping Wave | −0.008 | 0.953 | −0.103 | 0.190 | −0.178 | 0.198 | −0.167 | 0.056 |
| 13_Pharyngeal Contraction (A/P) | 0.019 | 0.882 | −0.232 | 0.003 | −0.075 | 0.595 | −0.213 | 0.016 |
| 14_Pharyngoesophageal Segment Opening | 0.077 | 0.547 | −0.137 | 0.079 | 0.195 | 0.157 | −0.058 | 0.510 |
| 15_Tongue Base Retraction | — | — | −0.006 | 0.935 | −0.069 | 0.623 | −0.060 | 0.496 |
Note: rpb = point biserial correlations. The italic values indicate statistical significance (adjusted p value = 0.005).
Abbreviations: MDADI, MD Anderson Dysphagia Inventory; RT, radiation therapy; TORS, transoral robotic surgery.
4 ∣. DISCUSSION
Given favorable response to current therapies, as the incidence of low-to-intermediate risk OPCs rises, so will the increase in survivors with dysphagia. While previous studies confirm the persistence of dysphagia after primary TORS or RT for low-to-intermediate risk OPC,6 the patterns of functional and physiologic deficits are largely unknown. Clinicians should be mindful of the pathophysiological factors related to different primary treatment modalities for OPC. Differences in the pathophysiology and dysphagia profiles after primary TORS and RT represent key targets in rehabilitation planning and clinical decision making. Our results demonstrate differences in the pathophysiology of dysphagia between primary TORS and primary RT. Specifically, there are distinctions in LVC and pharyngeal contraction as well as different patterns of pathophysiology associated with swallowing safety and efficiency. While DIGEST grade of safety and efficiency impairments did not significantly differ between primary TORS or RT groups, physiological correlates of DIGESTsafety and DIGESTefficiency impairments did differ. Our findings support the necessity for individualized approach to dysphagia management because of the distinct underlying pathophysiology of dysphagia depending upon the primary treatment method.
Despite dysphagia prevalence in almost half of survivors at 3–6 months per gold-standard MBS, it is encouraging to report a low severity of subacute dysphagia after primary RT for low-intermediate risk OPC. While diffuse, low-grade physiologic impairments appeared to contribute to radiation-associated dysphagia severity (per DIGEST), impaired LVC stood out as more prevalent in the RT group and most strongly correlated with safety impairment.
This result is supported by previous studies attempting to characterize swallow physiology in OPC patients treated with RT.8,10,23 Based on the available data, laryngeal deficits are highly prevalent among individuals treated with radiation therapy. Specifically, Starmer et al. and Barbon et al. found measures related to degree of laryngeal closure (LVC mechanisms, laryngeal elevation) or timing of the laryngeal vestibule to be impaired in irradiated OPC patients.8,10 It is postulated that edema and neuromuscular damage to the laryngeal framework in the radiation field manifest sensory and motor impairments within the laryngeal complex that are recognized as impaired LVC in these patients. These results have the potential to influence RT planning priorities that may mitigate post-RT dysphagia in the subacute stage post-treatment.
Despite studies demonstrating similarly favorable early post-TORS swallowing outcomes,5,6,24-26 little is published regarding the physiologic impairments that contribute to post-TORS dysphagia.27 The authors previously reported 23% prevalence of radiographically confirmed moderate to severe dysphagia in the acute postoperative period.6 The current results further detail the prevalence of (any grade) dysphagia at the subacute period (3–6 months) and newly identified impaired pharyngeal contraction/base of tongue retraction as significant efficiency-based pathophysiologic features and likely drivers of subacute post-TORS dysphagia. This result aligns with aspects of swallowing decompensation reported post-TORS in a prospective case series of 10 patients,24 wherein MBS conducted approximately 1-month post-TORS revealed unilateral pharyngeal weakness and impaired base of tongue motion in patients whose DIGESTefficiency scores were >0. The current data suggest that impaired pharyngeal constriction remains prevalent during the subacute period post-TORS, as pharyngeal contraction proved significant prior to Bonferroni correction and retained a large odds ratio (OR 3.4, p = 0.10). These results are aligned with the between-group analysis (Aim 2), which identified pharyngeal constriction impairment more prevalent post-TORS compared with primary RT. Oncologic resection of the pharyngeal constrictors and/or associated pharyngeal musculature along with resection of, or injury to the glossopharyngeal nerve in TORS are likely explanations for the impaired pharyngeal constriction identified in these data. Interestingly, our results also indicate increased pharyngeal impairment related to the presence of feeding tube at 3–6 months, although feeding tube dependence was extremely low at this time in both groups.
Despite similar proportions of dysphagia at 3–6 months evidenced by DIGEST grade and MDADI scores, underlying mechanistic differences exist between modalities. The results correlating physiologic impairments with swallow safety and efficiency suggest a more focal injury associated with DIGESTsafety and DIGESTefficiency post-TORS in contrast to a low-level diffuse physiologic impairment associated with post-RT dysphagia (Figure 2). The diffuse physiologic impairment post-RT in OPC by Starmer et al. is aligned with our findings.28 Impaired base of tongue retraction (35%), poor velopharyngeal closure (27%), and reduction in epiglottic tilt (70%) along with a reduction in hyoid excursion (42%) and velopharyngeal closure (27%) were all highly prevalent at 3–6 months post-RT. Wall et al. also reported nine pharyngeal phase deficits prevalent in a review of HNC patients treated with chemoRT; eight of which align with our findings.29 The novelty of the present analysis is the demonstration of the independent significant associations between these previously reported physiologic impairments with impaired swallow safety and efficiency, and comparison between primary treatment strategies. Nevertheless, measurement variation in these publications limits direct comparison of findings.
There are trade-offs between surgical pharyngeal injury and RT dose that coexist in patients receiving multimodality treatment. The addition of postoperative RT or chemoRT augments the likelihood of a complicated toxicity profile that has yet to be examined with this degree of specificity on physiologic profiles. This is critical to our understanding as approximately half of TORS patients treated with primary surgery receive adjuvant radiation or chemoradiation.3 Although we are unable to detect differences in swallow pathophysiology in the subgroup analysis stratifying on single versus multimodality treatments, trends emerged regarding significant differences in pharyngeal contraction among the groups (Figure 3). Observation of the ordinal pharyngeal contraction component revealed a large proportion of TORS+ patients with unilateral bulging, while patients with TORS alone were less impaired regarding pharyngeal contraction. There appears to be a difference in the severity pharyngeal contraction impairment between primary single modality TORS versus TORS plus adjuvant or RT that we posit reflects a loss of compensation for ipsilateral post-TORS pharyngeal injury after RT.
Our work demonstrates distinct pathophysiological underpinnings of functional impairment for patients with OPC who have been treated with primary TORS or RT. Strengths include the use of validated swallowing outcome measures and a large prospectively derived cohort. Limitations of this work include the lack of randomization between the groups with inherent imbalance of clinical factors relating to oncologic treatment selection and the low-to-intermediate risk sampling in our study, which provides an overall bias toward less severe dysphagia. Also, there were missing data that equated to approximately 20% at the subacute timepoint. These missing data are also attributed to the nature of the prospective registry. Additionally, measures of tumor location (e.g., proximity to midline or swallow critical structures) were not controlled for in our analyses representing a source of potential uncontrolled bias. Our data confirm the presence of subacute, mild dysphagia which has previously been reported in the literature. Targeting contributing pathophysiologic features early after treatment may be important in the mitigation of long-term dysphagia. Moving forward, future analysis aiming to characterize pathophysiology and swallow impairment should be stratified further for single versus multimodality treatment in larger cohorts.
5 ∣. CONCLUSION
Although the dysphagia severity did not differ between primary TORS or RT for OPC at 3–6 months posttreatment, the pathophysiology of the dysphagia is distinct for each primary treatment strategy. These results may have particular implication on dysphagia-sparing adjuvant RT planning considerations. We have identified pharyngeal constriction impairment to be common among the post-TORS group, whereas impaired LVC was more common in the post-RT group. The results correlating physiologic impairments with swallow safety and efficiency suggest a more focal injury associated with DIGESTsafety and DIGESTefficiency post-TORS in contrast to a low-level diffuse physiologic impairment associated with post-RT dysphagia. These nuances in swallowing profiles should be considered for targeted rehabilitative planning.
Footnotes
CONFLICT OF INTEREST
Dr Hutcheson reports receiving research grants and contracts from the National Institutes of Health and the Patient Centered Outcomes Research Institute and personal fees from MedBridge, ATOS Medical, and the American Speech-Language-Hearing Association (ASHA) outside of this work. Dr Lai reported receiving grants from the National Institutes of Health and the Cancer Prevention and Research Institute of Texas and personal fees from Cardinal Health outside the submitted work. Dr Fuller reported receiving grants and personal fees from the National Institutes of Health, grants and personal fees from Elekta AB, and personal fees from Greater Baltimore Medical Center, Tianjin Memorial Hospital, and the American Association of Physicists in Medicine outside the submitted work. Dr Gross reported receiving personal fees from Intuitive Surgical and non-financial support from MedRobotics outside the submitted work.
This work was presented at the 2020 Dysphagia Research Society annual meeting on March 17, 2020.
DATA AVAILABILITY STATEMENT
Data are available on request due to privacy/ethical restrictions.
REFERENCES
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liederbach E, Lewis CM, Yao K, et al. A contemporary analysis of surgical trends in the treatment of squamous cell carcinoma of the oropharynx from 1998 to 2012: a report from the national cancer database. Ann Surg Oncol. 2015;22(13):4422–4431. [DOI] [PubMed] [Google Scholar]
- 3.Cracchiolo JR, Baxi SS, Morris LG, et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer. 2016;122(10):1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. 2012;87(3):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols AC, Theurer J, Prisman E, et al. A phase II randomized trial for early-stage squamous cell carcinoma of the oropharynx: radiotherapy versus trans-oral robotic surgery (ORATOR). Paper presented at: American Society for Clinical Oncology; May31, 2019; Chicago, IL. [Google Scholar]
- 6.Hutcheson KA, Warneke CL, Yao C, et al. Dysphagia after primary transoral robotic surgery with neck dissection vs nonsurgical therapy in patients with low- to intermediate-risk oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2019; 145(11):1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbek JC. A penetration-aspiration scale. Dysphagia. 1996; 11:93–98. [DOI] [PubMed] [Google Scholar]
- 8.Barbon CEA, Chepeha DB, Hope AJ, Peladeau-Pigeon M, Waito AA, Steele CM. Mechanisms of impaired swallowing on thin liquids following radiation treatment for oropharyngeal cancer. J Speech Lang Hear Res. 2020;63(9):2870–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele CM, Peladeau-Pigeon M, Barbon CAE, et al. Reference values for healthy swallowing across the range from thin to extremely thick liquids. J Speech Lang Hear Res. 2019;62(5):1338–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starmer HM, Tippett D, Webster K, et al. Swallowing outcomes in patients with oropharyngeal cancer undergoing organ-preservation treatment. Head Neck. 2014;36(10):1392–1397. [DOI] [PubMed] [Google Scholar]
- 11.Hutcheson KA, Barrow MP, Barringer DA, et al. Dynamic imaging grade of swallowing toxicity (DIGEST): scale development and validation. Cancer. 2016;123(1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989;70(10):767–771. [PubMed] [Google Scholar]
- 13.Stokely SL, Peladeau-Pigeon M, Leigh C, Molfenter SM, Steele CM. The relationship between pharyngeal constriction and post-swallow residue. Dysphagia. 2015;30(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard R, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: the pharyngeal constriction ratio. Ann Otol Rhinol Laryngol. 2006;115(12):897–901. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment-MBSImp: establishing a standard. Dysphagia. 2008;23(4):392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky MB, McFarland DH, Dozier TS, et al. Respiratory-swallow phase patterns and their relationship to swallowing impairment in patients treated for oropharyngeal cancer. Head Neck. 2010;32(4):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chronowski GM, Garden AS, Morrison WH, et al. Unilateral radiotherapy for the treatment of tonsil cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):204–209. [DOI] [PubMed] [Google Scholar]
- 18.Garden AS, Dong L, Morrison WH, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(4):941–947. [DOI] [PubMed] [Google Scholar]
- 19.Kamal M, Mohamed ASR, Volpe S, et al. Radiotherapy dose-volume parameters predict videofluoroscopy-detected dysphagia per DIGEST after IMRT for oropharyngeal cancer: results of a prospective registry. Radiother Oncol. 2018;128(3): 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M.D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–876. [PubMed] [Google Scholar]
- 23.Kotz T, Costello R, Li Y, Posner MR. Swallowing dysfunction after chemoradiation for advanced squamous cell carcinoma of the head and neck. Head Neck. 2004;26(4):365–372. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus CL, Ganz C, Ru M, Miles BA, Kotz T, Chai RL. Prospective instrumental evaluation of swallowing, tongue function, and QOL measures following transoral robotic surgery alone without adjuvant therapy. Head Neck. 2019;41(2):322–328. [DOI] [PubMed] [Google Scholar]
- 25.Heah H, Goepfert RP, Hutcheson KA, et al. Decreased gastrostomy tube incidence and weight loss after transoral robotic surgery for low- to intermediate-risk oropharyngeal squamous cell carcinoma. Head Neck. 2018;40(11):2507–2513. [DOI] [PubMed] [Google Scholar]
- 26.Amit M, Hutcheson K, Zaveri J, et al. Patient-reported outcomes of symptom burden in patients receiving surgical or nonsurgical treatment for low-intermediate risk oropharyngeal squamous cell carcinoma: a comparative analysis of a prospective registry. Oral Oncol. 2019;91:13–20. [DOI] [PubMed] [Google Scholar]
- 27.Hutcheson KA, Holsinger FC, Kupferman ME, Lewin JS. Functional outcomes after TORS for oropharyngeal cancer: a systematic review. Eur Arch Otorhinolaryngol. 2014;272(2):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starmer HM, Yang W, Raval R, et al. Effect of gabapentin on swallowing during and after chemoradiation for oropharyngeal squamous cell cancer. Dysphagia. 2014;29(3):396–402. [DOI] [PubMed] [Google Scholar]
- 29.Wall LR, Ward EC, Cartmill B, Hill AJ. Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: a systematic review. Dysphagia. 2013;28(4):481–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request due to privacy/ethical restrictions.