Abstract
Globally, there are now over 160 million confirmed cases of COVID-19 and more than 3 million deaths. While the majority of infected individuals recover, a significant proportion continue to experience symptoms and complications after their acute illness. Patients with ‘long COVID’ experience a wide range of physical and mental/psychological symptoms. Pooled prevalence data showed the 10 most prevalent reported symptoms were fatigue, shortness of breath, muscle pain, joint pain, headache, cough, chest pain, altered smell, altered taste and diarrhoea. Other common symptoms were cognitive impairment, memory loss, anxiety and sleep disorders. Beyond symptoms and complications, people with long COVID often reported impaired quality of life, mental health and employment issues. These individuals may require multidisciplinary care involving the long-term monitoring of symptoms, to identify potential complications, physical rehabilitation, mental health and social services support. Resilient healthcare systems are needed to ensure efficient and effective responses to future health challenges.
Keywords: epidemiology, health service research, infectious diseases, public health, respiratory medicine, COVID-19, long COVID, post-COVID-19 syndrome, persistent COVID-19 symptoms
Introduction
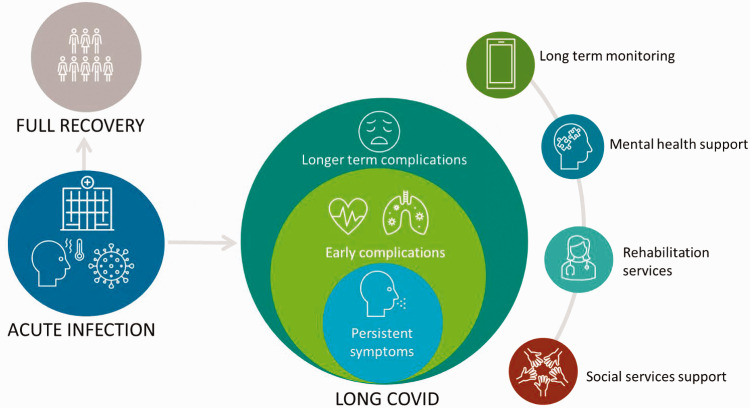
Globally, there are now over 160 million confirmed cases of COVID-19 and more than 3 million deaths.1 The majority of people with COVID-19 experience mild-to-moderate illness, while approximately 10%–15% develop severe illness and 5% become critically ill.2 The average recovery time from COVID-19 is 2–3 weeks depending on symptom severity.3–5 However, 1 in 5 people, regardless of the severity of their acute infection, may exhibit symptoms for 5 weeks or more, while 1 in 10 may have symptoms lasting 12 weeks or more.6 There is yet to be a consensus on the appropriate definitions for situations where COVID-19 symptoms persist beyond the acute phase of infection. The patient-coined term ‘long COVID’ was proposed7,8 and there were calls for its full adoption in scientific literature.9,10 The UK’s current National Institute for Health and Care Excellence guideline states that long COVID encompasses ongoing symptomatic COVID-19 (where symptoms last for 4 to 12 weeks) and post-COVID-19 syndrome (when they persist beyond 12 weeks in the absence of an alternative diagnosis).11 Figure 1 depicts the potential clinical course of COVID-19. This review summarises the current evidence on symptom prevalence, complications and management of long COVID and highlights priority areas for research.
Figure 1.
Depiction of the clinical course of long COVID.
Methods
We searched the Living Systematic Review database12 on 8 February 2021 for articles that described persistent COVID-19 symptoms. This database retrieves scientific publications related to COVID-19 from PubMed, EMBASE, MedRxiv and BioRxiv. We also conducted hand searching of reference lists of selected articles. The authors who performed the screening discussed and agreed on the included articles. Critical appraisal of the studies was conducted using the modified Newcastle Ottawa Scale.
Eligibility criteria
Inclusion
Quantitative and qualitative studies of adults with ongoing symptomatic COVID-19 or post-COVID syndrome.
Published since 1 January 2020.
English language.
Exclude
Studies that focus only on acute COVID-19 (<4 weeks).
Narrative reviews, commentaries, opinion pieces and letters that do not report primary findings.
Symptomatology and lived experiences of long COVID
Search results
We retrieved 1809 entries from our database search and two independent researchers screened 1574 after removing duplicates. Full texts for 94 articles were examined and 24 were selected. Three more articles were identified through hand searching, bringing the total number of included articles to 27.
Long COVID symptoms
Patients with long COVID experience the persistence of a wide range of physical and mental/psychological symptoms. Table 1 shows the symptoms of long COVID described by 27 primary research articles we included in this review.3,4,13–36 According to National Institute for Health and Care Excellence classification, 19 articles described ongoing symptomatic COVID-19 (symptoms lasting 4–12 weeks)3,4,13–19,21,22,24,25,27,28,31–33,36,37 and 8 reported post-COVID-19 syndrome (symptoms persisting beyond 12 weeks).18,20,23,26,27,30,34,36 Three studies provided data for both categories.18,27,36 Box 1 summarises the critical appraisal of these studies.
Table 1.
Signs and symptoms of long COVID.
| Symptoms | |
|---|---|
| Cardiopulmonary | Fatigue3,4,13,14,16,18,20–25,27,31,34–36,a |
| Shortness of breath (dyspnoea)3,4,13–15,17–22,24,25,27,29,33,35,b | |
| – Shortness of breath at rest16 | |
| – Shortness of breath with exertion16,17,30,34,36 | |
| Chest pain3,4,13–15,18–21,23,25,30,35,36 | |
| Palpitations13,15,16,18,21,23,36 | |
| Chest tightness16,21 | |
| Wheezing16,29 | |
| Naso-oropharyngeal | Loss of smell (anosmia)3,4,13–18,20,21,23,24,26,27,29,30,32,35,37,c |
| Dysgeusia (altered taste)3,13,14,16–18,20,21,23,24,26,29,32,37,c | |
| Sore throat3,4,14,16,21–23,29 | |
| Cough3,4,13,14,16–21,24,27,29,30,34,35 | |
| Tinnitus13,18,25,36 | |
| Sputum production14,24 | |
| Hoarse voice4/voice change22 | |
| Aphonia13 | |
| Rhinitis14,21/rhinorrhea3,29 | |
| Sneezing21 | |
| Chronic sinusitis/congestion13,16 | |
| Ear pain21,29 | |
| Hearing loss26 | |
| Diarrhoea3,4,14–16,18,21–24,27,29,35,d | |
| Nausea3,16,18,21 | |
| Loss of appetite4,14,22,23 | |
| Abdominal pain3,4,16,29,35 | |
| Weight loss15,21/anorexia16,19 | |
| Vomiting21,29 | |
| Gastritis19 | |
| Musculoskeletal | Joint pain (arthralgia)13–16,18,19,21,23–25,27,29,30,35,e |
| Muscle pains (myalgia)3,4,13–15,19,21,23,24,26,29,30,35,f | |
| Neuro-psychological | Memory loss (amnesia)13,16,18,20,22,26,27,36 |
| Difficulty thinking/inability to concentrate18,20,22/brain fog/cognitive impairment3,36/ disorientation13,16,18,24,29/delirium4 | |
| Sleep disorders such as insomnia13,16,18–20,23,28,30,35,36 | |
| Visual disturbances13,21,25,27,29 | |
| Anxiety and depression22/anxiety16,19,25,28,g | |
| Depression25,28 | |
| Mood change26 | |
| Thoughts of self‐harm22/suicide28 | |
| Neuralgia/neuropathy13,26/needle pains in arms and legs (paraesthesia)/tingling18,36 | |
| Tremors26 | |
| Seizures29 | |
| Miscellaneous | Fever/chills3,4,13,15,16,18,21,23–25,29,35,36 |
| Headache3,4,13–16,18,21,23–27,29,34–36,f | |
| Dizziness18,36/vertigo14,16,21,23 | |
| Skin rash18,23,29/pruritus13,16/cutaneous signs15,27/red spots on feet21 | |
| Significant hair loss20,23 | |
| Red eyes14/eye irritation24,29 | |
| Asthenia30/weakness16,18 | |
| Unspecified pain13/body ache16 | |
| Bladder incontinence22 | |
| Hot flushes21 | |
| Sweats16 | |
| Sicca syndrome14 | |
| Ulcer24 |
Banda combined malaise and fatigue (International Classification of Diseases (ICD) 10 Code).
Chopra combined shortness of breath/chest tightness/wheezing.
Carvalho & Chopra & Moreno combined anosmia and ageusia.
Huang recorded as diarrhoea or vomiting.
Moreno combined muscle and joint pain.
Carvalho combined myalgia, headache and/or asthenia.
Anxiety/depression measured using EQ-5D-5 L.
Box 1.
Critical appraisal of studies that reported the prevalence of long COVID symptoms.
| The modified Newcastle Ottawa Scale was used to evaluate the quality of the included studies. The design and methodological issues identified are presented here. However, these issues have to be considered with caution given the fast and constantly evolving nature of the pandemic, which might make them unavoidable for researchers. |
| • Virtually all the primary articles considered for this review excluded individuals with issues such as delirium. This might have led to missing some of the neuropsychiatric complications of long COVID. |
| • While the studies reviewed reported the prevalence of ongoing symptoms, there was a general lack of detail on their severity. A few studies provided detail on symptom severity.28,36 |
| • The lack of matched controls for most of the studies means that it was difficult to ascertain which symptoms were actually linked to long COVID and which might be related to ageing or co-morbidities. |
| • Majority of the studies recruited previously hospitalised patients therefore there is a lack of data on long COVID in patients who had mild-to moderate acute infections self-managed at home. |
| • Some studies involved patients with suspected COVID-19, in the absence of confirmatory testing, which raises the possibility that some of the symptoms reported might actually be related to other infections.35,36 |
| • A few studies obtained their data from social media and internet sources, which may not be entirely reliable or verifiable.13,21 |
| • Some studies relied on self-selection, and patient requests for follow-up which might have led to selection bias.16,18 |
| • A number of studies included in this review are still undergoing peer review and so care needs to be taken when interpreting or using their findings.4,13,18,19 |
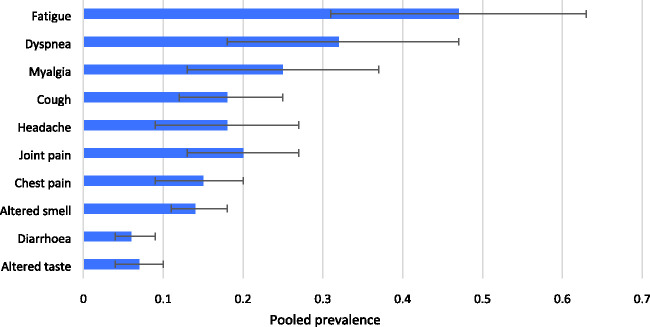
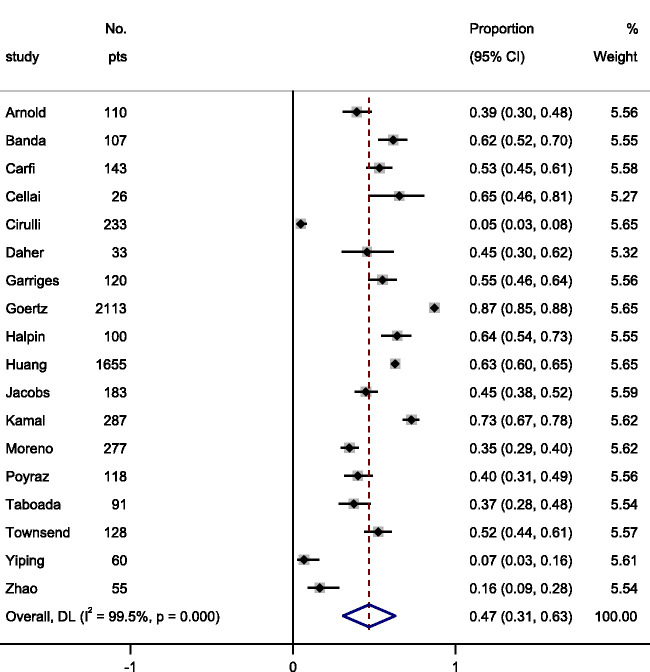
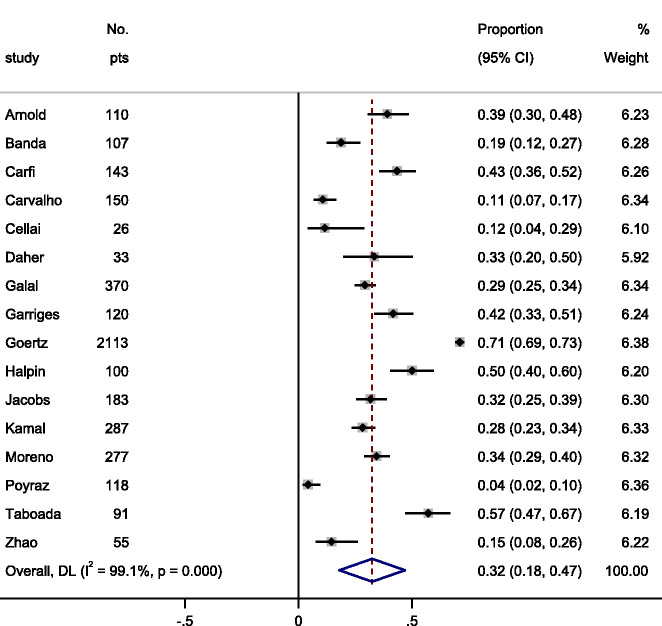
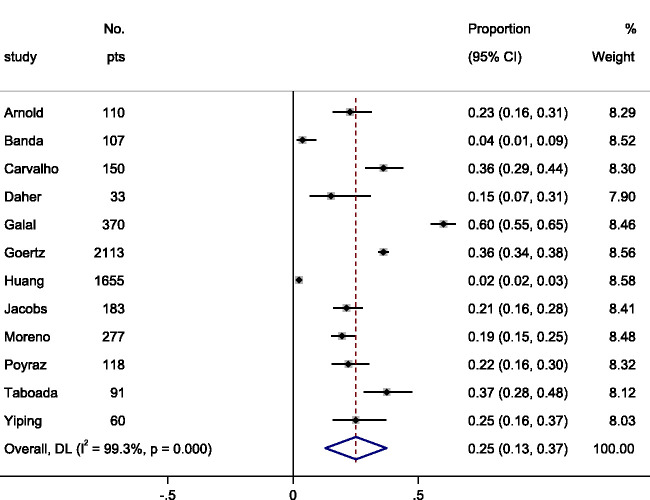
We pooled prevalence data from the included articles (Figure 2) and the 10 most prevalent reported symptoms were: (i) fatigue 47% (95% CI 31–63); (ii) dyspnoea (shortness of breath) 32% (95% CI 18–47); (iii) myalgia (muscle pain) 25% (95% CI 13–37); (iv) joint pain 20% (95% CI 13–27); (v) headache 18% (95% CI 9–27); (vi) cough 18% (95% CI 12–25); (vii) chest pain 15% (95% CI 9–20); (viii) altered smell 14% (95% CI 11–18); (ix) altered taste 7% (95% CI 4–10); and (x) diarrhoea 6% (95% CI 4–9). Figures 3 to 5 show individual forest plots for fatigue, dyspnoea (shortness of breath) and myalgia (muscle pain) (Figures S1–S7 in Supplement for the others).
Figure 2.
Pooled estimates for the 10 most common symptoms in patients with long COVID-19.
Figure 3.
Pooled estimate of the prevalence of fatigue in patients with long COVID-19.
Other common symptoms were cognitive impairment ‘brain fog’, amnesia (memory loss), sleep disorder, palpitations (awareness of heartbeat) and sore throat. Less common symptoms such as runny nose, sneezing, hoarseness, ear pain and rare, but important outcomes, including thoughts of self‐harm and suicide, seizures, and bladder incontinence, were only reported by the ongoing symptomatic COVID-19 studies. Conversely, hair loss, hearing loss and tremors were only reported by articles focused on post-COVID-19 syndrome.20,23,26 Sicca syndrome, also known as Sjögren syndrome, a condition characterised by dry eyes and dry mouth, was reported by only one study.13 Two main symptom clusters of long COVID were identified namely: (i) those comprising exclusively of fatigue, headache and upper respiratory complaints; and (ii) those with multi-system complaints including ongoing fever and gastroenterological symptoms.4
Long COVID symptoms in relation to severity of acute COVID-19
The presence of more than five symptoms in the first week of acute infection was significantly associated with the development of long COVID irrespective of age or gender.4
Although the number of symptoms have been shown to decline from acute COVID-19 to follow-up,29 several studies demonstrated that a significant number of patients continue to experience persistent symptoms regardless of the severity of the initial illness.14–16,18,35 For instance, a study that grouped patients as having mild, moderate or severe acute COVID-19 based on their need for oxygen supplementation and/or intensive care showed 59% (16/27) of patients with mild disease continued to experience COVID-19 symptoms at 8–12 weeks of follow-up from symptom onset.35 In comparison, 75% (49/65) and 89% (16/18) of patients in the moderate and severe groups had ongoing symptoms within the same period.35 Breathlessness and fatigue were the most common symptoms regardless of the severity of the acute illness.35
In a single-centre study of 143 previously hospitalised patients, 87.4% had at least one symptom (predominantly fatigue and dyspnoea) and 55% reported three or more symptoms at the time of assessment, approximately eight weeks from the onset of the first symptom.14
A study of 26 patients with mild COVID-19 reported that 96.2% experienced at least one symptom and 69.2% had four or more symptoms six weeks after symptom onset.16 Similarly, another study found that in a sample of 150 patients with mainly mild-to-moderate acute COVID-19 (77.3%, 116/150), at least one persistent symptom was reported at eight weeks in 66% of patients.15 Notably, anosmia/ageusia (loss of sense of smell/taste), myalgia (muscle pain) or headache were the symptoms that persisted in patients with mild-to-moderate COVID-19 in this study.15 In a sample of patients with mostly mild confirmed infection (96.6%, 225/233), a study found that nearly a quarter still had at least one symptom after 12 weeks.18 Finally, a study with a six-month follow-up after symptom onset found that 76% (1265/1733) of previously hospitalised patients reported at least one symptom, and the proportion was higher in women.23 Women were also found to have higher odds for fatigue or weakness than men.23
Determinants of the occurrence of persistent symptoms
Older age,4,15,24 female gender,4,24 hospital admission at symptom onset,4,15 initial dyspnoea,15,18 chest pain,18 abnormal auscultation findings (sounds from the heart, lungs or other organs),15 symptom load during the acute phase4,29 and co-morbidities29 particularly asthma4 were found to be significantly associated with an increased risk of developing persistent symptoms. The need for oxygen therapy, pre-existing hypertension and chronic lung conditions were highlighted as the main determinants of long-term symptoms.19
Older age, self-reported health status before the onset of symptoms, pre-existing co-morbidities and the number of symptoms during acute infection were found to significantly predict the number of symptoms patients with long COVID may experience at follow-up.21 Although Moreno-Pérez et al. found a significant association between anosmia-dysgeusia and younger age (<65 years) at 10–14 weeks,27 this was not statistically significant in the analysis done by another study at 8 weeks.32
Lived experience
The mid- and long-term effects and impact of illness due to COVID-19 is yet to be fully understood. However, there is evidence that the impact of acute COVID-19 on patients, regardless of severity, extends beyond hospitalisation in severe cases, to ongoing impaired quality of life, mental health and employment issues.14,15,17,24 People living with long COVID have indicated that they are suffering with a range of symptoms, feel ‘abandoned’ and ‘dismissed’ by healthcare providers and receive limited or conflicting advice.38 More than one-third (48/130) of the patients in a study reported they still felt ill or in a worse clinical condition at eight weeks than at the onset of COVID-19.15
Quality of life
The generic EuroQol Five Dimension (EQ-5D) index score, EuroQol Visual Analog Scale (EQ-VAS),3,14,23,27,30 RAND Short Form-36 questionnaire (SF-36)35 and the PROMIS® Global Health instrument33 were used to assess the quality of life of patients with long COVID. Existing evidence suggests that people with long COVID experience significant reductions in quality of life.
Quality of life at 4–12 weeks
In a study of previously hospitalised patients with COVID-19 in the United States, scores on the PROMIS® Global Health-10 instrument indicated worse general health after their acute illness compared to baseline.33 The patients’ summary t scores in both the physical health and mental health domains were slightly above the United States mean at baseline. However, both scores were significantly lower and patients reported a reduced ability to carry out social activities 4–6 weeks after hospitalisation.33
Overall EQ-5D index score was 0.86 (standard deviation 0.20) and EQ-VAS was 70.3% (standard deviation 21.5) at approximately eight weeks in a cohort of patients who had severe acute COVID-19.3 Another study stated that previously hospitalised patients had an average EQ-VAS score of 63%, and based on their data, 44.1% of the patients experienced a reduction in quality of life (defined as a 10-point difference in health status) before COVID-19 versus eight weeks of follow-up.14 However, these findings are difficult to interpret without data from age-/gender-matched controls without COVID-19.
Scores obtained from the administration of the SF-36 at 8–12 weeks to patients with mild, moderate and severe acute COVID-19 showed an impairment in reported health status across all domains compared with age-matched population norms.35 Physical scores were significantly lower in the severe group in comparison to patients in the mild-to-moderate groups.35
Quality of life at 12 weeks or more
A comparison of EQ-VAS scores showed a significant difference in overall quality of life for patients with ongoing symptoms and those who reported no symptoms after their acute infection at 10–14 weeks (43.2% vs. 66.9%, p = 0.0001).27 In a study with a six-month follow-up of previously hospitalised patients, an overall EQ-VAS score of 80% was reported indicating persistent reductions in quality of life.23
At six months of follow-up, in a study of previously hospitalised patients with COVID-19–related acute respiratory distress syndrome (ARDS), 67% (61/91) had a decrease in their quality of life.30 Comparison of EQ-5D index scores before acute infection and six months after showed a significant difference in quality of life (before = 0.965, after = 0.705, p < 0.001). Their EQ-VAS scores were also significantly different (before = 87.6%, after = 66.4%, p < 0.001).30 Similarly, there was a significant impairment in functional status among the patients with only 30.8% reporting no limitations in their everyday life (based on the Post-COVID-19 Functional Status scale).30 In a study that included individuals who regularly engaged in sports before hospitalisation for COVID-19 (details of the sporting activities were not given), 72% (28/39) were able to resume physical activity after three months, and nearly half of those were only able to do so at lower intensity.20
Impact on mental health
At eight weeks after acute infection, nearly half of all patients (238/488) surveyed in a study were emotionally affected by their experiences of long COVID with 28 requiring further mental health care.17 At six months of follow-up after the onset of symptoms, another study found that 23% of previously hospitalised patients suffered from anxiety or depression with women having higher odds than men.23
However, a study that utilised the Warwick-Edinburgh Mental Wellbeing Scales reported its scores were comparable with published population norms, and there were no significant differences between patients with mild, moderate or severe infection.35 According to data from Patient Health Questionnaire 9 (PHQ-9) and Generalized Anxiety Disorder 7 (GAD-7) questionnaires administered to a group of patients with severe acute COVID-19 approximately eight weeks after hospital discharge, patients mostly suffered from mild depression and anxiety.3
A study conducted in Turkey focused on the mental health of patients previously treated at a tertiary hospital at eight weeks of follow-up. Data collected using the Impact of Events Scale-Revised (IES-R) showed a quarter of the patients (72/284) had moderate-to-severe post-traumatic stress disorder (PTSD) symptoms while 18.3% (52/284) had mild PTSD symptoms.28 Over 40% reported co-morbid depression. Based on responses for the Mini-International Neuropsychiatric Interview suicidality scale, 7.4% (21/284) patients had a positive response to one or more items. Of these, six had a ‘moderate’ current risk of suicide, based on their Mini-International Neuropsychiatric Interview combined score.28 In addition, the study found that the occurrence of PTSD was significantly higher and more severe in women. Patients with severe acute COVID-19 had a significantly higher occurrence of PTSD symptoms and those with a higher mean acute symptom burden where more likely to exhibit PTSD symptoms.28 However, a significant proportion of patients with moderate-to-severe PTSD symptoms had a past psychiatric diagnosis.
Inadequate social support was linked to the occurrence and severity of PSTD symptoms.28 Social stigmatisation and discrimination appeared to influence the severity of PTSD symptoms as the study found that patients who felt stigmatised were more likely to experience moderate-to-severe PTSD symptoms.28 COVID-19–related stigmatisation has also been linked to a sense of hopelessness in patients.38,39
Impact on employment
A study found that among 195 patients who were employed before hospitalisation, 40% were unable to return to work within eight weeks of discharge due to ongoing health problems or job loss.17 Of those who returned to work, a quarter needed to reduce their working hours or alter their duties for health reasons.17 Another study reported nearly 70% (38/56) of previously hospitalised patients were unable to return to work at three months after hospitalisation.20
Risk of readmission
Ongoing fever and skipped meals were reported to be strong predictors of a subsequent hospital visit.4 A few studies have reported incidences of readmission of previously hospitalised patients with long COVID ranging from 1.4% to 15%.17,23,33 The study by Chopra et al. found that 15% (189) of previously hospitalised patients were symptomatic enough to require readmission within eight weeks of discharge.17
Complications associated with long COVID-19
COVID-19 is a multi-systemic disease, which may occur with complications at presentation or developing during the acute phase of illness. These complications may be respiratory,40 cardiovascular,41–45 renal,46–48 gastrohepatic,49–53 thromboembolic,54–58 neurological,59–61 cerebrovascular62–64 and autoimmune13,65 among others.
Beyond persistent symptoms, patients with long COVID may have clinical complications related to the disease.39 The epidemiology and pathophysiology of complications in long COVID are presently not well understood. While some studies have described some initial findings which are presented here, there is an urgent need for research into the underlying mechanisms (Box 2).66,67
Box 2.
Priority areas and considerations for future research.
| • Treatment options are currently limited as there is insufficient understanding of the mechanisms that underpin long COVID. Longer-term longitudinal observational studies are needed to fully understand the pathophysiology of the symptoms and complications associated with long COVID-19, its clinical course, symptom clusters and syndromes. This evidence will be crucial to understand the natural history of long COVID and the types of interventions that may be required. Qualitative research into the lived experiences of patients could provide the insight required for the planning of effective care pathways and lead to improved clinical outcomes. Clinical trials are urgently needed to evaluate interventions for long COVID that address the wide range of symptoms and complications identified in this review. |
| • Racial differences in the incidence of acute COVID-19 infections have been well documented. However, such differences have not been well researched in patients with long COVID and need further exploration. |
| • Most studies have focused on hospitalised patients and there is an urgent need for studies to investigate long COVID in non-hospitalised COVID-19 patients who have been underrepresented in the current research literature. |
Cardiovascular abnormalities
Evidence of myocarditis or prior myocardial injury by cardiac magnetic resonance imaging was found in 12/26 (46%) college athletes 12–53 days after their acute COVID-19 infection despite the fact that none were hospitalised; less than half had mild symptoms and the rest asymptomatic.68 A study of 100 patients showed that 78% had abnormal findings based on cardiac magnetic resonance imaging results 2–3 months after the onset of COVID-19 and 60% had evidence of myocardial inflammation independent of the severity and overall course of their acute illness.69 As with most of the literature available on long COVID, the sample selection was not random and may be biased. However, the possibility of cardiovascular abnormalities occurring in patients with long COVID was supported by another study which reported that up to 40% of COVID-19 patients presented with pericarditis or myocarditis > 70 days after infection.70
Pulmonary abnormalities
Lung function tests
A study conducted lung function tests in a sample of 57 patients 30 days after discharge for acute COVID-19 and reported a decrease in lung diffusion capacity for carbon monoxide (DLCO) in 53% and diminished respiratory muscle strength in 49% of patients.71 In another study, lung function abnormalities were detected in approximately a quarter of patients (14/55) at three months after hospital discharge.34 The commonest lung function abnormality (16.36%) was DLCO. A higher level of D-dimer at admission was significantly associated with DLCO% < 80% suggesting that D-dimer might be a potential biomarker for the prediction of DLCO decline patients with COVID-19.34 Of the patients with lung function abnormalities, 12 also had radiological changes, including evidence of lung fibrosis.34 At six months of follow-up, Huang et al. also found lung diffusion impairment among 34% (114/334) of patients previously hospitalised for acute COVID-19.23
Chest computed tomography scans
Varying degrees of radiological abnormalities in the chest computed tomography scans of 71% (39/55) of patients who were previously admitted for COVID-19 were discovered by another study at approximately three months after discharge.34 One to three lung segments were involved in about half of the patients with radiological abnormalities. Thirteen patients (23.64%) showed bilateral involvement and 15 (27.27%) had evidence of fibrosis (interstitial thickening).34 Persistent symptoms were also reported by 64% of the patients.34 Ground glass opacity was the most common high-resolution computed tomography pattern observed in the study by Huang et al. at six months after discharge.23
Neurological abnormalities
The occurrence of encephalitis, seizures and other conditions such as major mood swings and cognitive impairment (brain fog) have been reported in patients up to two to three months after the onset of acute illness.72 Magnetic resonance imaging scanning (diffusion tensor imaging and three-dimensional T1-weighted imaging) of previously hospitalised patients with COVID-19 suggested possible disruption to micro-structural and functional brain integrity at three months of follow-up,26 thus signifying the neuro-invasive capabilities of the SARS-CoV-2 virus and the potential for long-term consequences of the infection.
Renal complications
In one study, approximately one-third of previously hospitalised patients, who had acute kidney injury during the acute phase of COVID-19, did not fully regain renal function at discharge or post-hospitalisation follow-up.73
Endocrine disorders
Two studies reported newly diagnosed diabetes mellitus in patients after hospitalisation.25,74 However, more research is required to fully understand the aetiology.
Comparison to other coronaviruses
Although the clinical manifestations of COVID-19 are distinct, the persistence of dyspnoea and fatigue were similarly reported for the severe acute respiratory syndrome (SARS) and the Middle East Respiratory Syndrome (MERS) coronavirus infections.22,24 The findings on pulmonary abnormalities in patients with long COVID are similar to those from a study of patients, who recovered from SARS, but still had abnormal computed tomography findings and DLCO anomalies after a year.75 A meta-analysis of 28 follow-up studies found that six months after hospital discharge, approximately 25% of patients hospitalised with SARS and MERS had reduced lung function and exercise capacity.22,76
In the longer term, PTSD, depression and anxiety, and reduced quality of life were observed at one year after infection with SARS and MERS.76 In addition, a study found that up to 40% of patients who had SARS continued to experience fatigue and psychiatric illnesses for nearly 3.5 years after the acute infection.77 These findings are similar to those from a six-month follow-up study of previously hospitalised patients with COVID-19, which showed that patients mainly struggled with fatigue or muscle weakness, sleep difficulties, and anxiety or depression.23 This suggests that in the longer term, patients with long COVID may also experience a similar disease trajectory to that of patients who had SARS or MERS.22
Management of long COVID
Treatment options are currently limited as there is insufficient understanding of the mechanisms that underpin long COVID (Box 2). While there are still uncertainties about the optimal management of patients with long COVID, a number of countries have produced clinical guidelines to assist clinicians.11,78 Patients may require multidisciplinary care involving the long-term monitoring of ongoing symptoms, to identify potential complications for clinical intervention and the need for physical rehabilitation, mental health and social services support (Figure 1).
Aspects of management
Physical rehabilitation
Patients with severe acute COVID-19 who are managed in intensive care units may develop muscle weakness, deconditioning, myopathies (muscle disease) and neuropathies (nerves damage or dysfunction), which are the physical domains of post-intensive care syndrome.79 It is recommended that appropriate rehabilitation to prevent this syndrome should start in intensive care units as soon as sedation and clinical stability permit.79 Pulmonary rehabilitation may help improve patients’ breathing, exercise capacity, muscle strength, quality of life and functional outcome.80 Early mobilisation would help to improve functional, cognitive and respiratory conditions in these patients and may shorten hospital stay.79
Non-hospitalised patients with long COVID may also require physical rehabilitation, especially those with cardiopulmonary problems who may need significant rehabilitation, in order to improve their ability to engage in activities of daily living. However, identifying this group of patients may be challenging due to under-recognition and under-investigation of symptoms. There is also a risk that non-hospitalised patients with long COVID with mild-to-moderate symptoms, who are likely to represent a significant proportion of long COVID sufferers, may not be prioritised for follow-up care.38
Management of pre-existing co-morbidities
A significant proportion of patients who experience severe acute COVID-19 have underlying co-morbidities. It is therefore essential that these are adequately managed in order to avoid clinical deterioration and the need for readmission in these patients.81
Mental health support
Psychological and mental health issues such as anxiety, depression, PTSD and suicidal ideation have been discussed earlier as some of the long-term consequences of long COVID.16,19,22,25,26,28 There is a need to ensure that appropriate mental health support is available and accessible to those patients who require such services. Patients may be screened as part of their follow-up care and those identified as requiring extra support referred for specialist management. However, care should be taken not to pathologise patients as physical manifestations of COVID-19 may distort responses to assessment tools.81
Social services support
Due to persistent symptoms, a significant number of patients with long COVID are unable to return to work and may require long-term governmental financial support.17,20 Some patients may be unable to cope with day-to-day living especially if they also suffer significant social isolation and or stigmatisation.38,39 These groups of patients would benefit from social services support.
Strategies to facilitate the management of patients
A role for patient-reported outcomes
Patient-reported outcomes may be used for long-term follow-up care of patients with long COVID to monitor their symptoms and assess the impact on quality of life. The collection and use of patient-reported outcomes can help identify patients with ongoing symptoms, especially those who were not previously hospitalised and so not receiving formal follow-up. There is evidence that patient-reported outcomes are capable of detecting adverse events in patients even before clinical parameters.82 Patient-reported outcome data may alert clinicians to the development of potentially life-threatening complications in patients with long COVID.83 As shown by a number of studies discussed in this review, patient-reported outcome data may also indicate which patients are struggling to cope physically and mentally with their condition.14,23,30,35 In research settings, they can also provide valuable information on the effectiveness, safety and tolerability of drug interventions.84 The International Consortium for Health Outcomes Measurement initiative has developed a core outcome set for COVID-19 studies.85 Adoption of such standards would enable the collection of globally comparative data.
Harnessing the capabilities of digital technologies
Digital technologies are currently being used for the public health response to the COVID-19 pandemic through population surveillance, case identification, contact tracing and evaluation of interventions.86 A study found up to 30.5% (382) of previously hospitalised patients required follow-up with a primary care physician. Of these, 42% was via videoconferencing.17 Where possible, videoconferencing could be used for follow-up of patients with long COVID. This would also reduce the need for in-person contact and the risk of reinfection while the pandemic continues.
Moving forward, a digital therapeutics approach could be implemented where non-pharmacological interventions such as rehabilitative breathing exercises can be delivered via a digital platform according to patients’ presentations where feasible. This may ensure that a greater number of patients are cared for than would be possible with in-person care alone. Dedicated in-person COVID-19 rehabilitation services would require a substantial amount of resources87 and tele-rehabilitation may be potentially cost-effective in the long-term.
Advances in digital technology have facilitated the collection of electronic patient-reported outcome data. Electronic patient-reported outcomes and other measures such as temperature, oxygen saturation and blood pressure (measurable by wearable devices) may be collected remotely for sharing with clinical teams. Such data can also be analysed using machine learning and artificial intelligence to monitor and identify at-risk patients for early clinical intervention and rehabilitation.88
Conclusion
The wide range of potential symptoms and complications patients with long COVID may experience highlights the need for a deeper understanding of the clinical course of the condition. There is an urgent need for better, more integrated care models to support and manage patients with long COVID-19 in order to improve clinical outcomes. Resilient healthcare systems are required to ensure efficient and effective responses to future health challenges.
Supplemental Material
Supplemental material, sj-pdf-1-jrs-10.1177_01410768211032850 for Symptoms, complications and management of long COVID: a review by Olalekan Lee Aiyegbusi, Sarah E Hughes, Grace Turner, Samantha Cruz Rivera, Christel McMullan, Joht Singh Chandan, Shamil Haroon, Gary Price, Elin Haf Davies, Krishnarajah Nirantharakumar, Elizabeth Sapey, Melanie J Calvert and on behalf of the TLC Study Group in Journal of the Royal Society of Medicine
Acknowledgements
None.
Declarations:
Competing Interests: OLA receives funding from the NIHR Birmingham Biomedical Research Centre (BRC), NIHR Applied Research Centre (ARC), West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation, Innovate UK (part of UK Research and Innovation), Gilead Sciences Ltd, and Janssen Pharmaceuticals, Inc. OLA declares personal fees from Gilead Sciences Ltd, GlaxoSmithKline (GSK) and Merck outside the submitted work. SEH is funded by the NIHR ARC, West Midlands. SEH is company director of Narra Consulting Ltd. and declares personal fees from Cochlear Ltd. outside the submitted work. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. ES reports grant funding from Health Data Research UK, Wellcome Trust, Medical Research Council (MRC), British Lung Foundation, the NIHR, Engineering and Physical Sciences Research Council (EPSRC) and Alpha 1 Foundation. MC is Director of the Birmingham Health Partners Centre for Regulatory Science and Innovation, Director of the Centre for Patient Reported Outcomes Research and is a National Institute for Health Research (NIHR) Senior Investigator. She receives funding from the NIHR Birmingham Biomedical Research Centre, the NIHR Surgical Reconstruction and Microbiology Research Centre and NIHR ARC West Midlands at the at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UK Research and Innovation), Macmillan Cancer Support, UCB and GSK Pharma. MC has received personal fees from Astellas, Takeda, Merck, Daiichi Sankyo, Glaukos, GSK and the Patient-Centered Outcomes Research Institute (PCORI) outside the submitted work. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. Other authors declare no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was jointly supported by the National Institute for Health Research (NIHR) and UK Research and Innovation (UKRI) (grant number COV-LT-0013).
Ethics approval: Not applicable.
Guarantor: OLA.
Provenance: Not commissioned; peer-reviewed by Dr Eleftheria Vasileiou.
Supplemental material: Supplemental material for this article is available online.
Contributorship
All authors contributed to the conceptualisation of the work. OLA, SEH, SCR, CM and JSC screened the articles. OLA and GT extracted the data. SEH designed Figure 1. GT conducted the data analysis and produced Figures 2–5. OLA drafted the initial manuscript. All the authors reviewed, revised and approved the final manuscript.
Figure 4.
Pooled estimate of the prevalence of dyspnoea in patients with long COVID-19.
Figure 5.
Pooled estimate of the prevalence of muscle pain in patients with long COVID-19.
ORCID iDs
Olalekan Lee Aiyegbusi https://orcid.org/0000-0001-9122-8251
Sarah E Hughes https://orcid.org/0000-0001-5656-1198
Melanie J Calvert https://orcid.org/0000-0002-1856-837X
References
- 1.WHO. WHO Coronavirus Disease (COVID-19) Dashboard. See https://covid19.who.int/ (last checked 20 May 2021).
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 3.Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Resp Med 2020; 174: 106197–106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv2020: 2020.2010.2019.20214494. DOI: 10.1101/2020.10.19.20214494.
- 5.Burn E, Tebé C, Fernandez-Bertolin S, et al. The natural history of symptomatic COVID-19 during the first wave in Catalonia. Nature Commun 2021; 12: 777–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ONS. The Prevalence of Long COVID Symptoms and COVID-19 Complications. See https://www.ons.gov.uk/news/statementsandletters/theprevalenceoflongcovidsymptomsandcovid19complications (last checked 26 January 2021).
- 7.Perego E, Callard F, Stras L, et al. Why we need to keep using the patient made term “Long Covid”. BMJ 2020. https://blogs.bmj.com/bmj/2020/10/01/why-we-need-to-keep-using-the-patient-made-term-long-covid/ (accessed 2 July, 2020).
- 8.Callard F, Perego E. How and why patients made long covid. Soc Sci Med 2021; 268: 113426–113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long COVID: let patients help define long-lasting COVID symptoms. Nature 2020; 586: 170. [DOI] [PubMed]
- 10.Sivan M, Taylor S. NICE guideline on long covid. BMJ (Clinical Research ed) 2020; 371: m4938–m4938. [DOI] [PubMed] [Google Scholar]
- 11.NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. NICE Guideline [NG188]. See https://www.nice.org.uk/guidance/ng188 (last checked 26 January 2021).
- 12.Project C-OA. Living Evidence on COVID-19. See https://ispmbern.github.io/covid-19/living-review/ (last checked 30 June 2021).
- 13.Banda JM, Singh GV, Alser OH, et al. Long-term patient-reported symptoms of COVID-19: an analysis of social media data. medRxiv 2020: 2020.2007.2029.20164418. DOI: 10.1101/2020.07.29.20164418.
- 14.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. DOI: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed]
- 16.Cellai M and O’Keefe JB. Characterization of prolonged COVID-19 symptoms in an outpatient Telemedicine clinic. Open Forum Infect Dis 2020; 7(10): ofaa420. [DOI] [PMC free article] [PubMed]
- 17.Chopra V, Flanders SA, O'Malley M, et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med 2020. DOI: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed]
- 18.Cirulli ET, Schiabor Barrett KM, Riffle S, et al. Long-term COVID-19 symptoms in a large unselected population. medRxiv 2020: 2020.2010.2007.20208702. DOI: 10.1101/2020.10.07.20208702.
- 19.Galal I, Hussein AARM, Amin MT, et al. Determinants of persistent post COVID-19 symptoms: value of a novel COVID-19 symptoms score. medRxiv 2020: 2020.2011.2011.20230052. DOI: 10.1101/2020.11.11.20230052.
- 20.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020; 6: 00542–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PloS One 2020; 15: e0243882–e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamal M, Abo Omirah M, Hussein A, et al. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract; 75(3): e13746. [DOI] [PMC free article] [PubMed]
- 26.Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. EClinicalMedicine 2020; 25: 100484. [DOI] [PMC free article] [PubMed]
- 27.Moreno-Pérez O, Merino E, Leon-Ramirez J-M, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. DOI: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed]
- 28.Poyraz BÇ, Poyraz CA, Olgun Y, et al. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res 2021; 295: 113604–113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stavem K, Ghanima W, Olsen MK, et al. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2020: thoraxjnl-2020-216377. DOI: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed]
- 30.Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Brit J Anaesth 2020. DOI: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed]
- 31.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One 2020; 15: e0240784–e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaira LA, Hopkins C, Petrocelli M, et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol 2020; 134: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weerahandi H, Hochman KA, Simon E, et al. Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med 2021. DOI: 10.1007/s11606-020-06338-4. [DOI] [PMC free article] [PubMed]
- 34.Zhao Y-m, Shang Y-m, Song W-b, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. [DOI] [PMC free article] [PubMed]
- 35.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2020: thoraxjnl-2020-216086. DOI: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed]
- 36.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. medRxiv 2020: 2020.2012.2024.20248802. DOI: 10.1101/2020.12.24.20248802. [DOI] [PMC free article] [PubMed]
- 37.Otte MS, Eckel HNC, Poluschkin L, et al. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol 2020; 140: 1032–1035. [DOI] [PubMed] [Google Scholar]
- 38.Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 2020; 20: 1144–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020; 324: 1723–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020; 5: 831–840. [DOI] [PubMed] [Google Scholar]
- 42.Hendren NS, Drazner MH, Bozkurt B, et al. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 2020; 141: 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasitlumkum N, Chokesuwattanaskul R, Thongprayoon C, et al. Incidence of myocardial injury in COVID-19-infected patients: a systematic review and meta-analysis. Diseases 2020; 8(4): 40. [DOI] [PMC free article] [PubMed]
- 44.Creel-Bulos C, Hockstein M, Amin N, et al. Acute cor pulmonale in critically ill patients with Covid-19. New Engl J Med 2020; 382: e70–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayek SS, Brenner SK, Azam TU, et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ (Clinical Research Ed) 2020; 371: m3513–m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L, Wang X, Ren J, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open 2020; 10: e042573–e042573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart DJ, Hartley JC, Johnson M, et al. Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc Health 2020; 4: e28–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross O, Moerer O, Weber M, et al. COVID-19-associated nephritis: early warning for disease severity and complications? Lancet (London, England) 2020; 395: e87–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunutsor SK, Laukkanen JA. Hepatic manifestations and complications of COVID-19: a systematic review and meta-analysis. J Infect 2020; 81: e72–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. United Eur Gastroenterol J 2020; 8: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijarnpreecha K, Ungprasert P, Panjawatanan P, et al. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol DOI: 10.1097/meg.0000000000001817. [DOI] [PMC free article] [PubMed]
- 52.El Moheb M, Naar L, Christensen MA, et al. Gastrointestinal complications in critically ill patients with and without COVID-19. JAMA 2020; 324: 1899–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao R, Qiu Y, He J-S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The Lancet Gastroenterol Hepatol 2020; 5: 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest 2020. DOI: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed]
- 55.Boonyawat K, Chantrathammachart P, Numthavaj P, et al. Incidence of thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Thromb J 2020; 18: 34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA 2020; 324: 799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to D-Dimer levels. Radiology 2020; 296: E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020; 7: e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Favas TT, Dev P, Chaurasia RN, et al. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol Sci 2020; 41: 3437–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chua TH, Xu Z, King NKK. Neurological manifestations in COVID-19: a systematic review and meta-analysis. Brain Inj 2020; 34: 1549–1568. [DOI] [PubMed] [Google Scholar]
- 61.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol 2020; 19: 767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamakawa M, Kuno T, Mikami T, et al. Clinical characteristics of stroke with COVID-19: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2020; 29: 105288–105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nannoni S, de Groot R, Bell S, et al. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 2021; 16: 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ntaios G, Michel P, Georgiopoulos G, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the Global COVID-19 Stroke registry. Stroke 2020; 51: e254–e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 2020; 16: 413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altmann DM, Boyton RJ. Decoding the unknowns in long covid. BMJ (Clinical Research Ed) 2021; 372: n132–n132. [DOI] [PubMed] [Google Scholar]
- 67.Fraser E. Long term respiratory complications of covid-19. BMJ (Clinical research ed) 2020; 370: m3001–m3001. [DOI] [PubMed] [Google Scholar]
- 68.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021; 6: 116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eiros R, Barreiro-Perez M, Martin-Garcia A, et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: a cross-sectional descriptive study in health-care workers. medRxiv , 2020. doi: 10.1101/2020.07.12.20151316. [DOI]
- 71.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Resp Res 2020; 21: 163–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 2020; 77: 1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2021; 32: 151–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akter F, Mannan A, Mehedi HMH, et al. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabet Metab Syndr 2020; 14: 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005; 128: 2247–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020; 52: jrm00063–jrm00063. [DOI] [PubMed] [Google Scholar]
- 77.Lam MH-B, Wing Y-K, Yu MW-M, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169: 2142–2147. [DOI] [PubMed] [Google Scholar]
- 78.Burgers J. “Long covid”: the Dutch response. BMJ (Clinical Research Ed) 2020; 370: m3202–m3202. [DOI] [PubMed] [Google Scholar]
- 79.Candan SA, Elibol N, Abdullahi A. Consideration of prevention and management of long-term consequences of post-acute respiratory distress syndrome in patients with COVID-19. Physiother Theory Pract 2020; 36: 663–668. [DOI] [PubMed] [Google Scholar]
- 80.Grigoletto I, Cavalheri V, Lima FFd, et al. Recovery after COVID-19: the potential role of pulmonary rehabilitation. Braz J Phys Ther 2020; 24: 463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ (Clinical Research Ed) 2020; 370: m3026–m3026. [DOI] [PubMed] [Google Scholar]
- 82.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aiyegbusi OL, Calvert MJ. Patient-reported outcomes: central to the management of COVID-19. The Lancet 2020; 396: 531–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calvert MJ, O'Connor DJ, Basch EM. Harnessing the patient voice in real-world evidence: the essential role of patient-reported outcomes. Nat Rev Drug Discov 2019; 18: 731–732. [DOI] [PubMed] [Google Scholar]
- 85.International Consortium for Health Outcomes Measurement. The Standard Set – COVID-19, https://www.ichom.org/portfolio/covid-19/ (last checked 5 February 2021).
- 86.Budd J, Miller BS, Manning EM, et al. Digital technologies in the public-health response to COVID-19. Nature Med 2020; 26: 1183–1192. [DOI] [PubMed] [Google Scholar]
- 87.Iannaccone S, Alemanno F, Houdayer E, et al. COVID-19 rehabilitation units are twice as expensive as regular rehabilitation units. J Rehabil Med 2020; 52(6): jrm00073. [DOI] [PubMed]
- 88.Lassau N, Ammari S, Chouzenoux E, et al. Integrating deep learning CT-scan model, biological and clinical variables to predict severity of COVID-19 patients. Nature Commun 2021; 12: 634–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jrs-10.1177_01410768211032850 for Symptoms, complications and management of long COVID: a review by Olalekan Lee Aiyegbusi, Sarah E Hughes, Grace Turner, Samantha Cruz Rivera, Christel McMullan, Joht Singh Chandan, Shamil Haroon, Gary Price, Elin Haf Davies, Krishnarajah Nirantharakumar, Elizabeth Sapey, Melanie J Calvert and on behalf of the TLC Study Group in Journal of the Royal Society of Medicine