Abstract
Intraportal injection is regarded as the current standard procedure of hepatocyte transplantation (HTx). In islet transplantation, which shares many aspects with HTx, recent studies have clarified that instant blood-mediated inflammatory reaction (IBMIR), characterized by strong innate immune responses, can cause poor engraftment, so other transplant sites to avoid such a reaction have been established. Although IBMIR was reported to occur in HTx, few reports have evaluated alternative transplant sites for HTx. In this study, we sought to determine the optimum transplant site for HTx. Rat hepatocytes (1.0 × 107) were transplanted at the 9 transplant sites (intraportal (IPO), intrasplenic (IS), liver parenchyma, subcutaneous, intraperitoneal, renal subcapsular, muscle, inguinal subcutaneous white adipose tissue, and omentum) of analbuminemic rats. The serum albumin levels, immunohistochemical staining (albumin, TUNEL, and BrdU), and in vivo imaging of the grafts were evaluated. The serum albumin levels of the IPO group were significantly higher than those of the other groups (p < .0001). The BrdU-positive hepatocyte ratio of liver in the IS group (0.9% ± 0.2%) was comparable to that of the IPO group (0.9% ± 0.3%) and tended to be higher than that of the spleen in the IS group (0.5% ± 0.1%, p = .16). Considering the in vivo imaging evaluation and the influence of splenectomy, the graft function in the IS group may be almost entirely achieved by hepatocytes that have migrated to the liver. The present study clearly showed that the intraportal injection procedure is more efficient than other procedures for performing HTx
Keywords: hepatocyte transplantation, transplant site, portal, spleen, IVIS
Introduction
Liver transplantation is now established as a standard treatment for end-stage liver disease. However, this approach is suggested to be too invasive for patients suffering from acute liver failure and liver-related metabolic disorders, since merely replacing damaged or enzyme-defective hepatocytes with normal hepatocytes would theoretically be sufficient to cure these diseases1–7 , thereby avoiding the need for invasive whole-organ transplantation. Hepatocyte transplantation (HTx) is thus expected to be a promising alternative therapy for such patients due to its low invasiveness and the potential advantage of using livers that are not suitable for liver transplantation.
HTx has thus far been performed in more than 100 cases worldwide8–10 . In HTx, intraportal (IPO) injection is regarded as the current standard procedure. In pancreatic islet transplantation, which shares many aspects with HTx, IPO injection is also believed to be the most efficient approach. However, several recent studies have clarified that IPO injection procedure is not as effective as expected, and instant blood-mediated inflammatory reaction (IBMIR), characterized by the activation of both coagulation and complement cascades, is a plausible underlying cause for this poor engraftment11–14 .Given these findings, alternative transplant sites for islet transplantation, including the spleen15, liver direct injection (LDI)16, muscle17, renal subcapsular space18, subcutaneous space19, intraperitoneal cavity20, omentum21,22, and inguinal subcutaneous white adipose tissue (ISWAT)23, are eagerly being sought in order to avoid IBMIR.
IBMIR was also reported to occur in HTx24, since hepatocytes as well as pancreatic islets express a substantial amount of tissue factor, which is well recognized as a potent initiator of IBMIR12. However, in HTx, few reports have evaluated other transplant sites. In one previous report, an intrasplenic (IS) approach resulted in extremely efficient hepatocyte engraftment25. Unfortunately, in that report, no comparison was performed between IS and other approaches.
Therefore, in the present study, to determine the optimum transplant site for HTx, we examined the applicability of several different transplant sites for islet transplantation in HTx using an analbuminemic rat transplant model, immunohistochemical analysis, and an in vivo imaging system (IVIS).
Materials and Methods
Animals
For functional evaluation of the HTx, rat livers were obtained from male inbred F344/NSLc rats (age: 9–10 weeks; weight 180–220 g; Japan SLC Inc., Shizuoka, Japan). The analbuminemic rats (age: 10–14 weeks; weight 180–240 g) were provided by Prof. Yuji Nishikawa (Asahikawa Medical College) and were bred at Tohoku University. These analbuminemic rats had a syngeneic background to the donor rats.
In the IVIS evaluation, rat livers were obtained from male luciferase transgenic rats26 (age: 16 weeks; weight 260–300 g) provided by Prof. Eiji Kobayashi (Keio University) and bred at Tohoku University. The recipient rats were male LEW/NSLc rats (age: 12 weeks; weight 260–300 g; Japan SLC Inc., Shizuoka, Japan). All rats were maintained on a 12-h light/dark cycle with ad libitum access to food and water. All animals were handled according to the Guide for the Care and Use of Laboratory Animals27, and the guidelines for animal experiments at Tohoku University. The experimental protocol of the present study (protocol ID: 2016 MdA-138) was approved by the animal experimental committee in the Tohoku University. All surgeries were performed under anesthesia, and every effort was made to minimize suffering.
Hepatocyte Isolation
Rat hepatocytes were isolated by the two-step collagenase perfusion technique as described previously28. First, Ca2+-free Hanks’ balanced salt solution (HBSS) (Sigma-Aldrich, St. Louis, MO, USA) containing ethylene glycol tetraacetic acid was perfused through the portal vein at a rate of 14 ml/min for 5 min. Second, Ca2+-containing HBSS with 0.5 mg/ml of collagenase (Sigma type V; Sigma-Aldrich) was perfused via the same route at the same rate. The isolated cells were suspended in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) containing 10% fetal bovine serum and 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid. The cells were then filtered through a #150 mesh (Ikemoto Scientific Technology, Tokyo, Japan) and purified by gradient centrifugation (50 g, 2 min) at 4°C. Gradient centrifugation (50 g, 20 min) at 4°C was performed again using Percoll density gradient centrifugation media (physical form: Colloidal solution of silica coated with polyvinylpyrrolidone, density max: 1.135 g/ml osmolality: <25 mOsm/kg, viscosity max: 15cP, conductivity: <100 mS/m, pH range: 8.5–9.5) (GE Healthcare Biosciences, Pittsburgh, PA, USA) to obtain a highly purified cell population. The hepatocyte viability was evaluated by a trypan blue exclusion assay. We transplanted 1.0 × 107 hepatocytes with a viability exceeding 90%.
Transplantation Procedures
In the IPO, IS, LDI, and muscle transplant procedures, 1.0 × 107 hepatocytes were spun down, and the pellets were directly injected slowly into the portal vein, splenic pulp, liver parenchyma, and biceps femoris of recipient rats using a 25-G needle with a gastight syringe (Hamilton Company, Reno, NV, USA). The renal subcapsular transplantation was performed, as previously described with slight modifications29. Briefly, small incisions were made in the lower pole of both kidneys of the recipient rats. A specially constructed instrument was inserted to separate the renal capsule from the underlying renal cortex, thereby creating a pocket around the kidney. 5.0 × 106 hepatocytes were spun down, and the pellet was injected into both subcapsular pockets (total 1.0 × 107 cells), with the incision subsequently closed with medical adhesive. In the subcutaneous transplant procedure, small incisions were made in both sides of the recipient rat’s back to create subcutaneous pockets. Then, 5.0 × 106 hepatocytes were spun down, and the pellet was injected into both subcutaneous pockets (total 1.0 × 107 cells), with the incisions sutured using 4-0 nylon. In the ISWAT transplant procedure, skin incisions were made in both inguinal areas of the recipient rats. The inferior epigastric artery and vein were identified on ISWAT, and a small pocket was created superiorly to the vessels. Then, 5.0 × 106 hepatocytes were spun down, and the pellet was injected into both pockets (total 1.0 × 107 cells), with the incisions sutured using 5-0 nylon. In the renal subcapsular, subcutaneous and ISWAT transplant procedures, hepatocytes were transplanted into two pockets (left side and right side) because 1.0 × 107 hepatocytes were too much to place in one pocket. In the intraperitoneal transplant procedure, 1.0 × 107 hepatocytes were spun down, and the pellets were transplanted into the right side of the intraperitoneal space. In the omentum pouch transplant procedure, the greater omentum was spread out onto wet gauze, and 1.0 × 107 hepatocytes were spun down, with pellet placed onto the omentum surface. The omentum margin and gastric serosa were then sutured using 7-0 nylon to create the omental pouch. In the omentum thrombin method, the greater omentum was spread out onto wet gauze, and 1.0 × 107 hepatocytes were centrifuged. The supernatant was discarded, and the hepatocytes were resuspended in analbuminemic rat plasma. After centrifugation a second time, most of the excess plasma was removed, and the slurry of hepatocytes/plasma was collected with a gastight syringe. The hepatocyte/plasma slurry was gently distributed onto the surface of the omentum, and then thrombin (NIHON PHARMACEUTICAL CO., LTD, Tokyo, Japan) was gently dripped onto the graft. The omentum margin and gastric serosa were sutured using 7-0 nylon to create the omental pouch.
Blood samples were taken pre-transplantation and every 2 weeks after transplantation. All groups were evaluated until 10 weeks after transplantation. For comparisons between the IPO and IS groups, the groups were evaluated until 16 weeks after transplantation. In the IPO group, we selected 3 rats that showed relatively high serum albumin levels compared with the other rats and observed them for 72 weeks after transplantation. The serum albumin levels were quantified using a LBIS Rat Albumin ELISA kit (AKRAL-220; FUJIFILM Wako Shibayagi, Gunma, Japan).
Immunohistochemical Staining
In the IPO and IS groups, hepatic tissue and splenic tissue were retrieved at 2 weeks after transplantation (n = 5). On the day 3, 4, 5, 10, 11, 12 after transplantation, 5-bromo-2’-deoxyuridine (BrdU) (Abcam plc, Cambridge, UK) was injected into the intraperitoneal space of the recipients (500 mg/kg). The BrdU and albumin double staining was performed using BrdU immunohistochemistry Kit (Abcam plc) and anti-albumin antibodies (MP Biomedicals, Santa Ana, CA, USA) combined with the SK5100 Vector Red (Vector Laboratories, Inc., Burlingame, CA, USA). The number of albumin-stained, BrdU-stained hepatocytes in each sample was counted by microscopy, and the BrdU and albumin-stained hepatocytes/albumin-stained hepatocytes ratio (BAR) was calculated. In addition, metalloproteinase-2 (MMP-2) and albumin double staining was performed in the IPO and IS groups (n = 3). Hepatic tissue and splenic tissue were retrieved at 8 hours after transplantation. The MMP-2 and albumin double staining was performed using MMP-2 monoclonal antibody (MMP2/8 B4) (Invitrogen, Waltham, MA, USA) combined with the goat anti-mouse IgG1, Alexa Fluor 647 (Thermo Fisher Scientific, Waltham, MA, USA) and rabbit anti-rat albumin, conjugated with FITC (Exalpha Biologicals inc. Shirley, MA, USA). In all groups, tissue samples from the transplant sites were retrieved at 10 weeks after transplantation. Albumin staining was performed using anti-albumin antibodies (MP Biomedicals, Santa Ana, CA, USA) combined with the VECTASTAIN ABC system (Vector Laboratories, Inc., Burlingame, CA, USA), and terminal deoxynucleotidyl transferase-mediated uridine triphosphate nick end labeling (TUNEL) staining was performed using a TACS2 TdT DAB kit (Trevigen Inc., Gaithersburg, MD, USA). In the IPO group, we selected 3 rats that showed relatively high serum albumin levels compared with the other rats, and their hepatic tissue was retrieved at 72 weeks after transplantation. Albumin staining were performed in these tissues.
IVIS
The IVIS Spectrum (PerkinElmer Co., Ltd, Inc., Waltham, MA, USA) was used to detect the luciferase expression. Hepatocytes were obtained from luciferase transgenic rats and transplanted into LEW/NSLc rats using the IPO (n = 8), IS (n = 8), and LDI procedures (n = 3). For in vivo imaging, the 150 mg/kg D-luciferin (Promega Corporation, Madison, WI, USA) was injected into the penile vein. One minute after injection, bioluminescence images were captured for 3 min. Regions of interests (ROIs) were analyzed, and total quantification of bioluminescence was quantified using the Living Image® (PerkinElmer Co., Ltd, Inc.) software program. Imaging was performed at 2 h and 2 and 4 weeks after transplantation. The ROIs at each point were shown as the percentage in comparison to the ROIs at 2 h after transplantation.
The Evaluation of the Influence of Splenectomy on the Serum Albumin Levels in the IS Group
In the IS group, splenectomy was performed at 4 weeks after transplantation (n = 8). Blood samples were taken pre-transplantation and every 2 weeks after transplantation. All groups were evaluated until 10 weeks after transplantation. The influence of splenectomy was evaluated by serum albumin levels among the IS group recipients with (IS splenectomy [+]) and without splenectomy (IS splenectomy [-], n = 8).
Statistical Analyses
All values were expressed as the means and standard deviation. The statistical analyses were performed using the JMP pro 13 software program (SAS institute Inc., NC, USA). The serum albumin levels, the percentage of ROIs and the BAR were analyzed by a two-way analysis of variance, and Tukey-Kramer’s test was used for post-hoc comparisons between the groups. p values of < .05 were considered to indicate statistical significance.
Results
The Comparison of Hepatocyte Engraftment After Transplantation at Various Transplant Sites
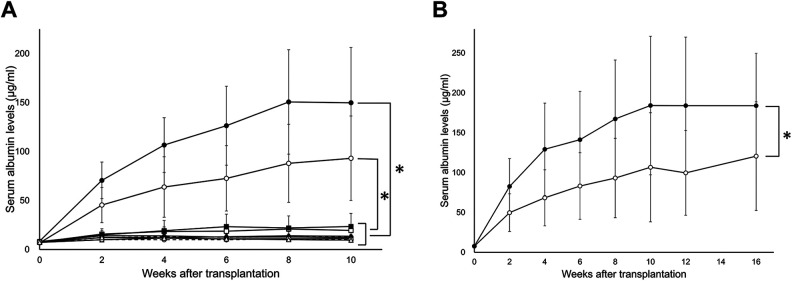
The serum albumin levels of the IPO group and the IS group were significantly higher than those of the other groups investigated in the present study (p < .0001) (Fig. 1A and Table 1).
Figure 1.
A comparison of the serum albumin levels after transplantation at various transplant sites. A total of 1.0×107 hepatocytes were transplanted into the liver via the portal vein (IPO group: •, solid line, n = 8), spleen pulp (IS group: ˆ, solid line, n = 8), liver parenchyma (LDI group: ▪, solid line, n = 8), omentum (pouch method: □, solid line, n = 8; thrombin method: ♦, solid line, n = 8), renal subcapsular (⋄, solid line, n = 8), ISWAT (▴, solid line, n = 8), muscle (△, solid line, n = 8), intraperitoneal cavity (•, dotted line, n = 8), and subcutaneous space (ˆ, dotted line, n = 8). (A) The serum albumin levels of all of the tested groups. The serum albumin levels of the IPO and IS groups were significantly higher than those of the other groups (p < .0001). (B) The serum albumin levels of the IPO and IS groups during the longer observation period. The serum albumin levels of the IPO group were significantly higher than those of the IS group (p < .0001). In both cases, the serum albumin levels appeared to plateau at 10 weeks after transplantation and remained high during the observation period.
Table 1.
Serum albumin levels of IPO, IS, LDI, and Omentum pouch groups.
| Serum albumin levels (μg/ml) | ||||||
|---|---|---|---|---|---|---|
| Pre | 2 weeks | 4 weeks | 6 weeks | 8 weeks | 10 weeks | |
| IPO | 8.5 ± 1.1 | 70.4 ± 18.7 | 106.7 ± 28.0 | 126.4 ± 40.3 | 150.6 ± 53.3 | 149.8 ± 56.3 |
| IS | 6.8 ± 1.4 | 45.4 ± 18.0 | 63.8 ± 30.9 | 72.6 ± 33.4 | 88.0 ± 39.9 | 93.1 ± 43.2 |
| LDI | 8.1 ± 1.3 | 14.9 ± 6.3 | 19.2 ± 10.5 | 23.4 ± 12.7 | 21.9 ± 12.4 | 23.5 ± 13.4 |
| Omentum pouch | 7.6 ± 1.1 | 15.7 ± 3.4 | 18.4 ± 4.6 | 18.7 ± 2.9 | 20.9 ± 2.8 | 19.5 ± 1.6 |
When we focused on transplant sites other than for those in the IPO and IS groups, the serum albumin levels of the LDI group and omentum pouch group were significantly higher than those of the other groups (p < .0001) (Table 1). Concerning the comparison between the IPO and IS groups, the serum albumin levels of the IPO group were significantly higher than those of the IS group (p < .0001), even with a longer observation period (Fig. 1B and Table 2). In both cases, the serum albumin levels appeared to plateau at 10 weeks after transplantation and remained high during the observation period.
Table 2.
Serum Albumin Levels of IPO and IS Groups.
| Serum albumin levels (μg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pre | 2 weeks | 4 weeks | 6 weeks | 8 weeks | 10 weeks | 12 weeks | 16 weeks | |
| IPO | 7.7 ± 1.3 | 82.7 ± 34.9 | 129.3 ± 57.9 | 141.3 ± 60.5 | 167.4 ± 73.8 | 184.2 ± 86.9 | 184.0 ± 85.9 | 183.9 ± 65.7 |
| IS | 7.7 ± 1.3 | 49.8 ± 23.6 | 68.4 ± 35.1 | 83.2 ± 41.8 | 93.2 ± 49.8 | 106.7 ± 68.4 | 99.7 ± 53.2 | 120.6 ± 68.2 |
Immunohistochemical Staining of the Transplanted Hepatocytes
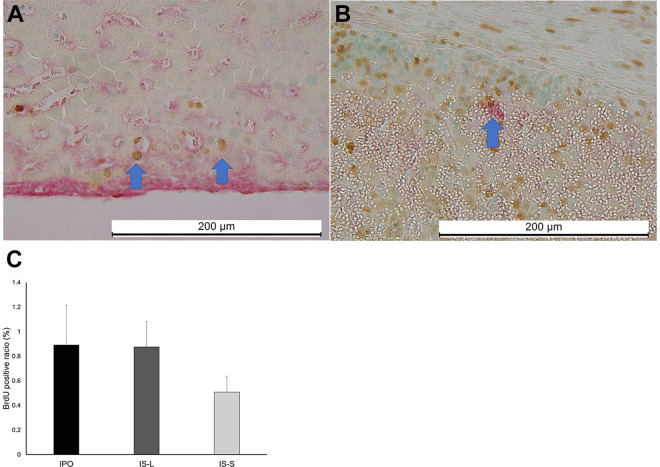
The albumin-positive hepatocyte grafts were easily detected in the livers of the IPO group and in the liver (IS-L) and spleen (IS-S) of the IS group (Fig. 2). In contrast, few albumin-positive hepatocytes were observed in the other groups. No TUNEL-positive hepatocytes were seen in any groups (Fig. 3). BrdU-positive hepatocyte grafts were detected in the IPO, IS-L, and IS-S groups (Fig. 4A, B). The BAR of the IPO group (0.9% ± 0.3%) tended to be higher than that of the IS-S group (0.5% ± 0.1%, p = .16). Notably, the BAR of the IS-L group (0.9% ± 0.2%), which was comparable to that of the IPO group, also tended to be higher than that of the IS-S group (p = .18), suggesting that the hepatocyte engraftment might be dependent on the transplant-site environment rather than the transplant procedures.
Figure 2.
Albumin staining of the transplanted hepatocytes in the IPO and IS groups. (A) The albumin staining of the IPO group. The albumin-positive hepatocytes engrafted in the liver are shown with a black arrow. (B) The albumin staining of the IS-S group. The albumin-positive hepatocytes engrafted in the spleen pulp in the IS group are shown with a black arrow. (C) The albumin staining of the IS-L group. The albumin-positive hepatocytes engrafted in the liver in the IS group are shown with a black arrow.
Figure 3.
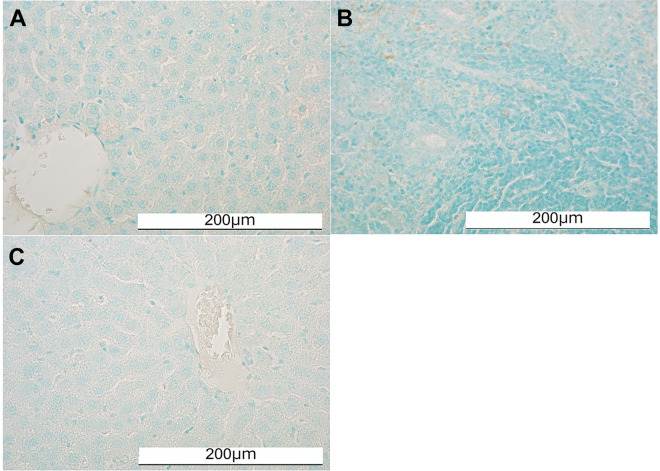
TUNEL staining of the transplanted hepatocytes in the IPO and IS groups. (A) The TUNEL staining of the IPO group. (B) The TUNEL staining of the IS-S group. (C) The TUNEL staining of the IS-L group. No TUNEL-positive hepatocytes were detected in any of the groups.
Figure 4.
BrdU and albumin double staining of the transplanted hepatocytes in the IPO and IS groups. (A) The BrdU and albumin double staining of the IPO group. The BrdU and albumin-positive hepatocytes engrafted in the liver are shown with a blue arrow. (B) The BrdU and albumin double staining of the IS-S group. The BrdU and albumin-positive hepatocytes engrafted in the spleen pulp in the IS group are shown with a blue arrow. (C) The BrdU and albumin-positive hepatocyte/albumin-positive hepatocyte ratio (BAR) of the IPO, IS-L, and IS-S groups. The BAR of the IPO group tended to be higher than that of the IS-S group (p = .16). The BAR of the IS-L group also tended to be higher than that of the IS-S group (p = .18).
In the IPO and IS-L groups, transplanted hepatocytes were widely distributed in the liver sinusoid, and graft shapes were well-maintained. In contrast, the hepatocyte grafts appeared to aggregate together and suffer from high pressure due to the limited transplant space in the IS-S group
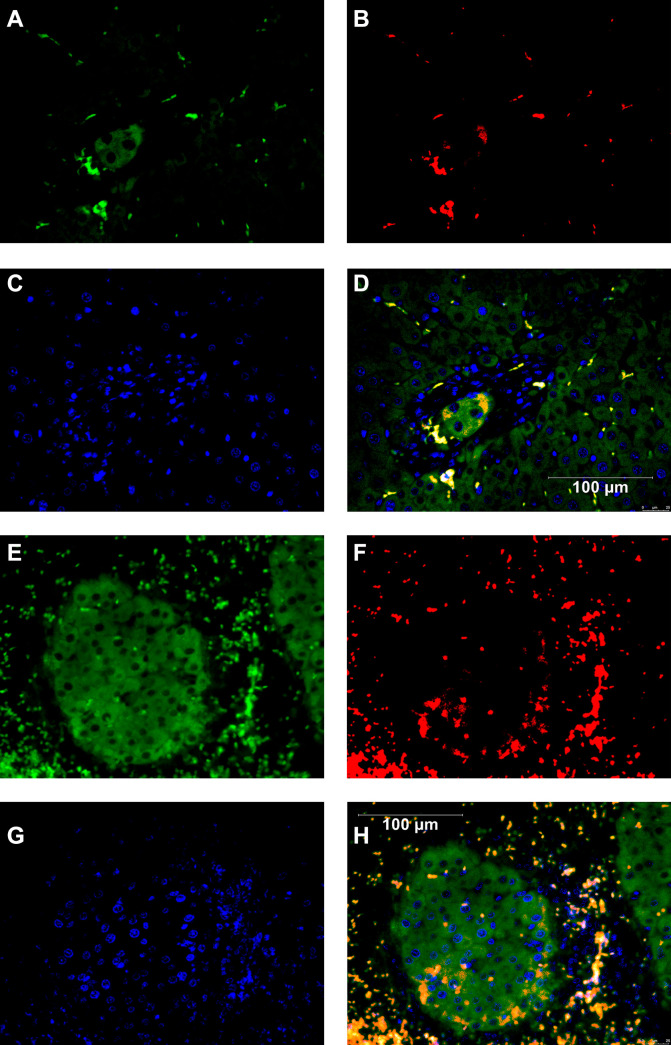
In the IPO and IS-S groups, MMP-2 was detected around transplanted hepatocytes (Fig. 5).
Figure 5.
MMP-2 and albumin double staining of the transplanted hepatocytes in the IPO and IS-S groups. (A) The albumin staining of the IPO group. (B) The MMP-2 staining of the IPO group. (C) The nuclear staining of the IPO group. (D) The figure overlayed albumin, MMP-2, nuclear staining of the IPO group. MMP-2 was detected around transplanted hepatocytes of the IPO group. (E) The albumin staining of the IS-S group. (F) The MMP-2 staining of the IS-S group. (G) The nuclear staining of the IS-S group. (H) The figure overlayed albumin, MMP-2, nuclear staining of the IS-S group. MMP-2 was detected around transplanted hepatocytes of the IS-S group.
The in vivo Imaging Evaluation of Hepatocytes Transplanted into the Portal Vein, Spleen Pulp, and Liver Parenchyma
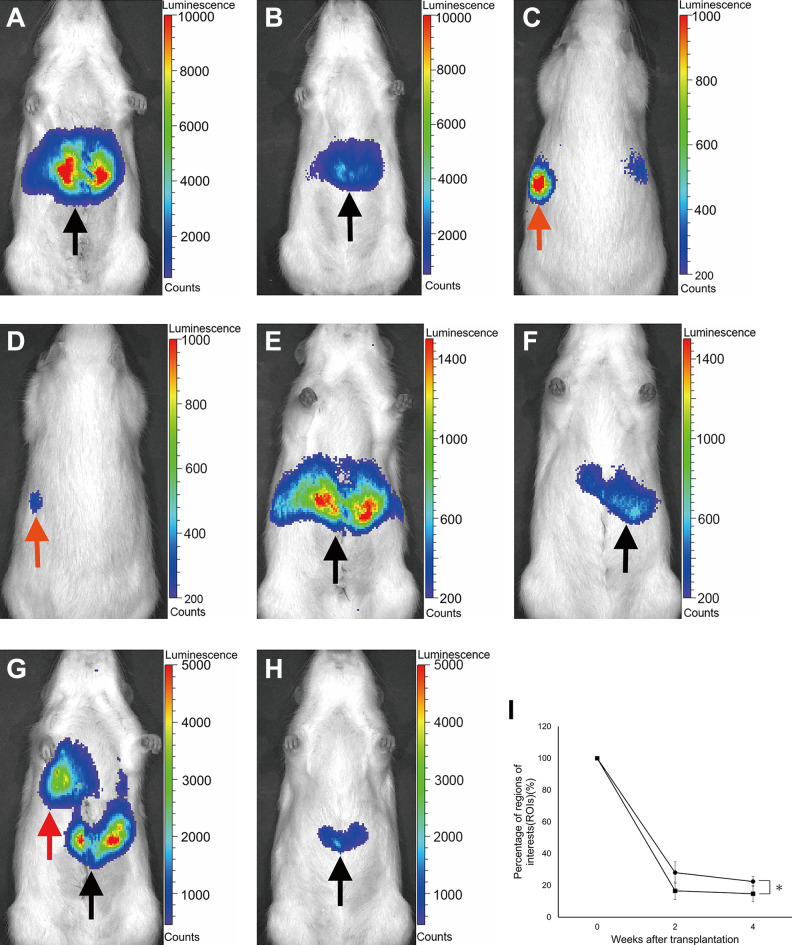
The hepatocytes transplanted into the liver via the portal vein were only distributed in the liver (Fig. 6A, B). The percentage of ROIs of this group were 16.6% ± 5.5% at 2 weeks after transplantation and 14.7% ± 5.0% at 4 weeks after transplantation.
Figure 6.
The in vivo imaging evaluation of hepatocytes transplanted into the portal vein, spleen pulp, and liver parenchyma. (A) The in vivo imaging of the IPO group immediately after transplantation. (B) The in vivo imaging of the IPO group at 4 weeks after transplantation. The grafts were widely distributed in the liver (black arrow). (C) The in vivo imaging of the IS group immediately after transplantation (back side). (D) The in vivo imaging of the IS group at 4 weeks after transplantation (back side). The hepatocyte grafts distributed in the spleen are shown with an orange arrow. (E) The in vivo imaging of the IS group immediately after transplantation (ventral side). (F) The in vivo imaging of the IS group at 4 weeks after transplantation (ventral side). The hepatocyte grafts distributed in the liver are shown with a black arrow. (G) The in vivo imaging of the LDI group immediately after transplantation. (H) The in vivo imaging of the LDI group at 4 weeks after transplantation. The hepatocyte grafts were distributed in the lung (red arrow) and the liver (black arrow) immediately after transplantation. However, the grafts were only detected in the liver at 4 weeks after transplantation. (I) Percentage of ROIs in the IPO and IS-L groups. The black square represents the IPO group (n = 8). The black circle represents the IS-L group (n = 8).
In contrast, the hepatocytes transplanted into the spleen pulp were distributed in both the spleen and liver. In this group, a substantial number of hepatocyte grafts were detected in the liver, even immediately after transplantation (Fig. 6C–F). The percentage of ROIs of the spleen in the IS group were 38.7% ± 22.4% and 22.3% ± 11.9% at 2 and 4 weeks after transplantation, respectively. In contrast, the percentage of ROIs of the liver in this group were 28.1% ± 6.8% and 22.4% ± 3.2% at 2 and 4 weeks after transplantation, respectively. Although the ROIs of the liver in the IS group were approximately 1/2 to 1/4 in comparison to the IPO group, the survival rate of transplanted hepatocytes in the IS-L group was significantly higher than that of the IPO group (p < .0001; Fig. 6I).
The hepatocytes transplanted into the liver parenchyma were distributed in both the liver and lung. Considerable numbers of grafts were distributed in the lung via the hepatic vein to the inferior vena cava, and those grafts had disappeared by 2 weeks after transplantation. In this LDI group, the hepatocyte grafts were only engrafted in the liver (Fig. 6G, H). The percentage of ROIs of this group were 10.0% ± 2.6% at 2 weeks after transplantation and 10.1% ± 2.9% at 4 weeks after transplantation.
The Influence of Splenectomy on the Serum Albumin Levels in the IS Group
The serum albumin levels of the IS-splenectomy [+] group (pre-transplantation: 6.6 ± 0.7 μg/ml, 2 weeks: 50.9 ± 21.0 μg/ml, 4 weeks: 76.5 ± 30.1 μg/ml, 6 weeks: 92.3 ± 48.9 μg/ml, 8 weeks: 97.9 ± 52.9 μg/ml, 10 weeks: 95.2 ± 52.7 μg/ml) were almost the same as those of the IS-splenectomy [-] group (pre-transplantation: 6.9 ± 0.4 μg/ml, 2 weeks: 54.5 ± 22.2 μg/ml, 4 weeks: 73.2 ± 26.0 μg/ml, 6 weeks: 85.2 ± 34.7 μg/ml, 8 weeks: 107.5 ± 41.8 μg/ml, 10 weeks: 96.2 ± 33.9 μg/ml) (Fig. 7).
Figure 7.
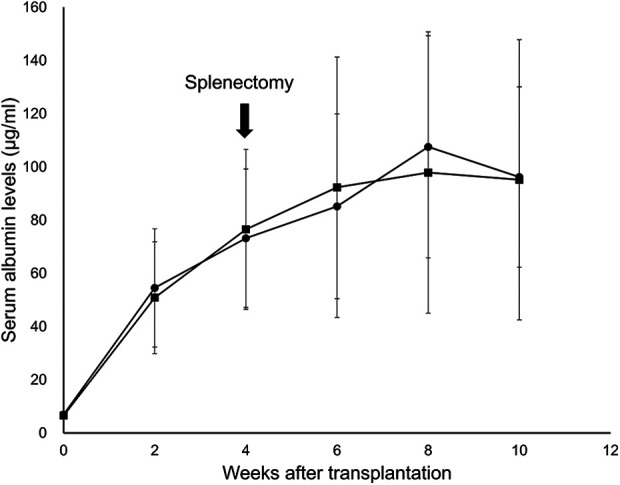
The influence of splenectomy on the serum albumin levels in the intrasplenic (IS) group. The black square represents the IS-splenectomy [+] group (n = 8). The black circle represents the IS-splenectomy [-] group (n = 8). The serum albumin levels of the IS-splenectomy [+] group were almost the same as those of the IS-splenectomy [-] group.
The evaluation of the serum albumin levels and immunohistochemical analyses of intraportally transplanted hepatocytes at 72 weeks after transplantation
The three recipients in the IPO group were observed for a long period (72 weeks) after transplantation. The serum albumin levels continued to increase up to 10-20 weeks after transplantation and then appeared to plateau and remained high during the observation period (Fig. 8A). Albumin-positive hepatocytes were easily detected at both 4 and 72 weeks after transplantation. However, the number of albumin-positive hepatocytes observed in a single view tended to appear higher at 72 weeks after transplantation in comparison to 4 weeks after transplantation (Fig. 8B, C). The transplanted hepatocytes were widely distributed in the liver sinusoid, and the graft shapes were well-maintained (Fig. 8D).
Figure 8.
The evaluation of the serum albumin levels and immunohistochemical analyses of intraportally transplanted hepatocytes 72 weeks after transplantation. (A) The transition of serum albumin levels during 72 weeks after transplantation (n = 3). The serum albumin levels increased up to 10–20 weeks after transplantation and appeared to plateau and remained high during the observation period. (B) The albumin staining of the intraportally transplanted hepatocytes at 4 weeks after transplantation. Magnification: 50×. The albumin-positive hepatocytes are indicated with black arrows. (C) The albumin staining of the intraportally transplanted hepatocytes at 72 weeks after transplantation. Magnification: 50×. The albumin-positive hepatocytes are indicated with black arrows. The number of albumin-positive hepatocytes observed in one view tended to appear higher at 72 weeks after transplantation. (D) The albumin staining of the intraportally transplanted hepatocytes at 72 weeks after transplantation. Magnification: 400×.
Discussion
In the present study, the IPO transplant procedure was clearly more efficient than the other methods for HTx. One possible reason for this finding is the physiological compatibility between the hepatocyte grafts and the transplant site in the IPO procedure. Given that the hepatocytes originate from the liver, this explanation is quite logical. In fact, several crucial factors related to hepatocyte proliferation, including hepatocyte growth factor30,31, transforming growth factor-α32, tumor necrosis factor α33,34, and interleukin 633–35 , are produced in the liver. These factors are particularly strongly activated by transient portal hypertension due to hepatectomy through the up-regulation of urokinase-type plasminogen activator and nitric oxide36,37, subsequently contributing to residual hepatocyte proliferation after hepatectomy. Likewise, transient portal hypertension is frequently induced by the IPO injection of hepatocytes. Given the similarity between hepatectomy and IPO HTx, the beneficial effects of the IPO approach compared with other methods may be explained at least in part by the presence of these preferable growth factors. Indeed, these growth factors may encourage the proliferation and maintenance of the intraportally transplanted hepatocytes for more than 1 year (Fig. 8A, C, D).
Another possible reason for the superiority of the IPO approach to other approaches is a sufficient transplant area. In the IPO group, the transplanted hepatocytes were widely distributed in the liver sinusoid, and the graft shapes were well-maintained. In contrast, the hepatocyte grafts seemed to aggregate together and suffer from high pressure due to the limited transplant space in the IS group. Therefore, substantial number of hepatocyte grafts were distributed in the liver via the splenic vein and engrafted there. Corroborating our findings, Merani et al. reported that the compaction of cells was detrimental to islet transplantation38, so aggregation is likely also deleterious to hepatocytes, since they are much more fragile than pancreatic islets39. Furthermore, it has been reported that MMP-2 and vascular endothelial growth factor contribute to transplanted hepatocyte engraftment and proliferation 40–42 . In fact, MMP-2 was detected in both IPO and IS-S groups (Fig. 5). However, MMP-2 was expressed more extensively in the IPO group than the IS-S group, most likely due to rather small cluster of transplanted hepatocytes in the IPO group. This feature may also suggest that a sufficient transplant area is beneficial for hepatocyte engraftment.
In contrast to the findings of a previous study reported by Ikebukuro et al.25, in the present study, the engraftment efficiency of hepatocytes in the IS group was not as high as we had expected. Ikebukuro et al. reported that the excellent graft function in the IS group completely disappeared after splenectomy, suggesting that the beneficial effects observed in their model might be due in large part to the hepatocytes engrafted in the spleen pulp25. In contrast, no marked influence of splenectomy on the serum albumin levels in the IS group was observed in the present model (Fig. 7). Furthermore, the percentage of BAR in the IS-L group was comparable to that of the IPO group and tended to be higher than that of the IS-S group (Fig. 4C). These findings strongly suggest that hepatocyte engraftment and graft function may be more dependent on the transplant-site environment than the transplant procedure. Taken together, these findings suggest that the liver offers a more suitable environment for hepatocyte engraftment than spleen pulp, and the graft function in the IS group was likely almost entirely achieved by hepatocytes that had engrafted in the liver parenchyma. The IVIS showed a substantial number of hepatocytes transplanted into the spleen pulp were distributed in liver. Of particular note, the survival rate of transplanted hepatocytes in the IS-L group was significantly higher than that of the IPO group. This may suggest that the hepatocytes with a single cell style, such as those migrated to liver parenchyma in the IS group, are suitable for engraftment, since a spleen appeared to have a filtration function. The difference in the hepatocyte graft function between the IPO and IS groups likely depends on the number of hepatocytes engrafted in the liver, as some of the grafts remained in the spleen pulp in the IS group. This may explain why the serum albumin levels of the IPO group demonstrated the best values when the same amount of graft was transplanted. The contradictory outcomes between the previous report25 and the present study may be attributed to differences in the animal models used and hepatocyte-isolation methods, among others, but the full details underlying this discrepancy in findings remain unclear. The clarification on this issue and further analyses of IVIS imaging and BrdU staining on transplanted hepatocytes in the untested transplant sites are topics of interest for our next study.
In the LDI group, hepatocytes were directly injected into the liver parenchyma to minimize IBMIR and locate the grafts near the liver sinusoid. The IVIS clearly demonstrated that a substantial number of hepatocyte grafts were unexpectedly distributed in the lung via the hepatic vein to the inferior vena cava, and those grafts had disappeared by 2 weeks after transplantation (Fig. 6G, H). In the LDI group, only a few hepatocytes were ultimately engrafted in the liver, suggesting that hepatocyte injection through the portal vein may be more efficient for engrafting hepatocytes into the liver sinusoid than the LDI procedure.
In the present study, the transplant efficiency calculated from the serum albumin levels in the recipients (the serum albumin levels of the IPO group/the serum albumin levels of normal rat) and using the IVIS (percentage of ROIs at 4 weeks after transplantation × the graft numbers/whole liver hepatocyte numbers) were almost identical (0.5%), suggesting that these simple systems can be used as reliable tools for evaluating hepatocyte engraftment. In addition, unlike commercially available Nagase analbuminemic rats (SD background), mutant F344 rats with defective albumin production were used as the recipients in this study. Therefore, we can ignore any immunological influences and focus on physiological engraftment by using syngeneic F344 rats as the donors. Furthermore, unlike commercially available Nagase analbuminemic rats, we can perceptively detect the hepatocyte engraftment in this combination, since the basal albumin levels of the recipients are almost zero. These advantages associated with using this animal combination may help increase its reliability as a useful evaluation system39,43.
In this study, IPO injection was proven to be effective for hepatocyte engraftment despite the presence of IBMIR24. In islet transplantation, IBMIR is a strong inhibiting factor for islet engraftment. Furthermore, most islet grafts are unable to reach the liver sinusoid and settle in the portal branches due to the rather large size of islet grafts, resulting in poor engraftment11,44,13. In contrast, hepatocytes are single cells and can therefore easily achieve efficient distribution to the liver sinusoid40. Therefore, in HTx, effective engraftment based on a wide distribution to the liver sinusoid may overwhelm the disadvantages due to IBMIR. However, the IPO procedure is known to carry a risk of not only IBMIR but also portal thrombosis and portal hypertension40,45, causing hepatocyte destruction. In fact, the estimated transplant efficiency in the IPO group in this study was approximately 0.5%, although a transplant efficiency of nearly 5% against whole liver cells is considered necessary in order to cure metabolic liver diseases46–49 . This result implies that a nearly 10-fold greater transplant efficiently may be needed in order to establish HTx as clinical therapy for hepatic metabolic disorders. Therefore, it is necessary to establish a novel strategy for improving the transplant efficiency of the IPO procedure.
In the field of islet transplantation, various methods for suppressing IBMIR, such as low-molecular-weight dextran sulfate (LMW-DS)44, C5a inhibitory peptide50, and islet surface modification51, have been reported. In HTx, LMW-DS52 and hepatocyte surface modification53 have already been reported to be effective for suppressing IBMIR in in vitro models. We must therefore further investigate the above-mentioned methods in order to suppress IBMIR using pre-clinical models.
In conclusion, the present study clearly showed that the IPO injection procedure is more efficient than other procedures for performing HTx. Further investigations on IBMIR inhibition will facilitate the applicability of HTx.
Acknowledgments
The authors thank Kozue Imura (Division of Transplantation and Regenerative Medicine, Tohoku University) and Megumi Goto (Division of Transplantation and Regenerative Medicine, Tohoku University) for their excellent technical assistance, Prof. Yuji Nishikawa (Division of Tumor Pathology, Department of Pathology, Asahikawa Medical University), Norihiko Shimizu (Animal Laboratory for Medical Research, Asahikawa Medical University), Hironobu Chiba (Animal Laboratory for Medical Research, Asahikawa Medical University), Chihiro Hino (Animal Laboratory for Medical Research, Asahikawa Medical University), Tomomi Kibushi (Animal Laboratory, Tohoku University), and Keisuke Nishio (Animal Laboratory, Tohoku University) for breeding and taking care of the analbuminemic rats and Prof. Eiji Kobayashi (Department of Organ Fabrication, Keio University School of Medicine) for providing the luciferase transgenic rats to us. The authors also acknowledge the support of the Biomedical Research Core of Tohoku University, Graduate School of Medicine and TAMRIC (Tohoku Advanced Medical Research and Incubation Center).
Footnotes
Author Contributions: H.O. and M.G. participated in the research design, the performance of the research and the writing of the paper. A.I. participated in the performance of the research and the writing of the paper. I.F. participated in the writing of the paper. T.I., H.Y., Y.S., M.M., K.F., S.M. and Y.N. participated in the performance of the research. K.O. participated in drafting the experimental design. M.U. and T.K. participated in the writing of the paper.
Ethical Approval: The experimental protocol of the present study (protocol ID: 2016 MdA-138) was approved by the animal experimental committee in the Tohoku University.
Statement of Human and Animal Rights: All animals were handled according to the Guide for the Care and Use of Laboratory Animals27, and the guidelines for animal experiments at Tohoku University. All surgeries were performed under anesthesia, and every effort was made to minimize suffering.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by the Japanese Grant-in-Aid for Scientific Research (A) (Grant Number 18H04056), (B) (Grant Number 15H04916) and (C) (Grant Number 16K01355) from the Japan Society for the Promotion of Science (JSPS). The founders played no role in the study design, the collection or analysis of the data, the decision to publish or the preparation of the manuscript.
ORCID iD: Hiroyuki Ogasawara, MD, PhD  https://orcid.org/0000-0003-3213-3783
https://orcid.org/0000-0003-3213-3783
References
- 1.Strom SC, Fisher RA, Rubinstein WS, Barranger JA, Towbin RB, Charron M, Mieles L, Pisarov LA, Dorko K, Thompson MT, Reyes J. Transplantation of human hepatocytes. Transplant Proc. 1997;29(4):2103–2106. [DOI] [PubMed] [Google Scholar]
- 2.Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A, Durham J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6(1):32–40. [DOI] [PubMed] [Google Scholar]
- 3.Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40(6):878–886. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82(4):441–449. [DOI] [PubMed] [Google Scholar]
- 5.Pareja E, Cortes M, Bonora A, Fuset P, Orbis F, Lopez R, Mir J. New alternatives to the treatment of acute liver failure. Transplant Proc. 2010;42(8):2959–2961. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Zhou L, Ma X, Ma W, Wang C, Lu Y, Chen Y, An L, An W, Yang Y. Monitoring of intrasplenic hepatocyte transplantation for acute-on-chronic liver failure: a prospective five-year follow-up study. Transplant Proc. 2014;46(1):192–198. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa S, Miyagawa S. Potentials of regenerative medicine for liver disease. Surg Today. 2009;39(12):1019–1025. [DOI] [PubMed] [Google Scholar]
- 8.Soltys KA, Soto-Gutierrez A, Nagaya M, Baskin KM, Deutsch M, Ito R, Shneider BL, Squires R, Vockley J, Guha C, Roy-Chowdhury J, et al. Barriers to the successful treatment of liver disease by hepatocyte transplantation. J Hepatol. 2010;53(4):769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, Ellis EC, Nowak G, Ericzon BG, Fox IJ, Gomez-Lechon MJ, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012;21(1):1–10. [DOI] [PubMed] [Google Scholar]
- 10.Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272(3):201–223. [DOI] [PubMed] [Google Scholar]
- 11.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48(10):1907–1914. [DOI] [PubMed] [Google Scholar]
- 12.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. [DOI] [PubMed] [Google Scholar]
- 13.Goto M, Groth CG, Nilsson B, Korsgren O. Intraportal pig islet xenotransplantation into athymic mice as an in vivo model for the study of the instant blood-mediated inflammatory reaction. Xenotransplantation. 2004;11(2):195–202. [DOI] [PubMed] [Google Scholar]
- 14.Goto M, Tjernberg J, Dufrane D, Elgue G, Brandhorst D, Ekdahl KN, Brandhorst H, Wennberg L, Kurokawa Y, Satomi S, Lambris JD, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15(4):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson A, Eriksson U, Petersson B, Reibring L, Swenne I. Failure of successful intrasplenic transplantation of islets from lean mice to cure obese-hyperglycaemic mice, despite islet growth. Diabetologia. 1981;20(3):237–241. [DOI] [PubMed] [Google Scholar]
- 16.Eloy R, Kedinger M, Garaud JC, Haffen K, Launay JF, Moody J, Clendinnen G, Grenier AF. Intrahepatic transplantation of pancreatic islets in the rat. Horm Metab Res. 1977;9(1):40–46. [DOI] [PubMed] [Google Scholar]
- 17.Lund T, Korsgren O, Aursnes IA, Scholz H, Foss A. Sustained reversal of diabetes following islet transplantation to striated musculature in the rat. J Surg Res. 2010;160(1):145–154. [DOI] [PubMed] [Google Scholar]
- 18.Toledo-Pereyra LH, Bandlien KO, Gordon DA, MacKenzie GH, Reyman TA. Renal subcapsular islet cell transplantation. Diabetes. 1984;33(9):910–914. [DOI] [PubMed] [Google Scholar]
- 19.Sakata N, Aoki T, Yoshimatsu G, Tsuchiya H, Hata T, Katayose Y, Egawa S, Unno M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab Res Rev. 2014;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 20.Weber CJ, Hardy MA, Lerner RL, Felig P, Reemtsma K. Hyperinsulinemia and hyperglucagonemia following pancreatic islet transplantation in diabetic rats. Diabetes. 1976;25(10):944–948. [DOI] [PubMed] [Google Scholar]
- 21.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3(3):281–285. [DOI] [PubMed] [Google Scholar]
- 22.Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C, Pileggi A.Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes. 2016;65(5):1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasunami Y, Nakafusa Y, Nitta N, Nakamura M, Goto M, Ono J, Taniguchi M. A novel subcutaneous site of islet transplantation superior to the liver. Transplantation. 2018;102(6):945–952. [DOI] [PubMed] [Google Scholar]
- 24.Gustafson EK, Elgue G, Hughes RD, Mitry RR, Sanchez J, Haglund U, Meurling S, Dhawan A, Korsgren O, Nilsson B. The instant blood-mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation. 2011;91(6):632–638. [DOI] [PubMed] [Google Scholar]
- 25.Ikebukuro H, Inagaki M, Mito M, Kasai S, Ogawa K, Nozawa M. Prolonged function of hepatocytes transplanted into the spleens of Nagase analbuminemic rats. Eur Surg Res. 1999;31(1):39–47. [DOI] [PubMed] [Google Scholar]
- 26.Hakamata Y, Murakami T, Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation. 2006;81(8):1179–1184. [DOI] [PubMed] [Google Scholar]
- 27.Bayne K.Revised guide for the care and use of laboratory animals available. American physiological society. Physiologist. 1996;39(4):199, 208-111. [PubMed] [Google Scholar]
- 28.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. [DOI] [PubMed] [Google Scholar]
- 29.Saitoh Y, Inagaki A, Fathi I, Imura T, Nishimaki H, Ogasawara H, Matsumura M, Miyagi S, Yasunami Y, Unno M, Kamei T, et al. Improvement of hepatocyte engraftment by co-transplantation with pancreatic islets in hepatocyte transplantation. J Tissue Eng Regen Med. 2021;15(4):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomiya T, Nishikawa T, Inoue Y, Ohtomo N, Ikeda H, Tejima K, Watanabe N, Tanoue Y, Omata M, Fujiwara K. Leucine stimulates HGF production by hepatic stellate cells through mTOR pathway. Biochem Biophys Res Commun. 2007;358(1):176–180. [DOI] [PubMed] [Google Scholar]
- 31.Maher JJ.Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91(5):2244–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA. 1989;86(5):1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai M, Cui TX, Kitamura H, Saito M, Shimazu T. Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine. 2001;13(1):60–64. [DOI] [PubMed] [Google Scholar]
- 34.Bohm F, Kohler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2(8):294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274(5291):1379–1383. [DOI] [PubMed] [Google Scholar]
- 36.Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143(3):949–958. [PMC free article] [PubMed] [Google Scholar]
- 37.Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci USA. 1998;95(23):13829–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merani S, Schur C, Truong W, Knutzen VK, Lakey JR, Anderson CC, Ricordi C, Shapiro AM. Compaction of islets is detrimental to transplant outcome in mice. Transplantation. 2006;82(11):1472–1476. [DOI] [PubMed] [Google Scholar]
- 39.Fukuoka K, Inagaki A, Nakamura Y, Matsumura M, Yoshida S, Imura T, Igarashi Y, Miyagi S, Ohashi K, Enosawa S, Kamei T, et al. The Optimization of Short-Term Hepatocyte Preservation Before Transplantation. Transplant Direct. 2017;3(7):e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, Novikoff PM. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29(2):509–519. [DOI] [PubMed] [Google Scholar]
- 41.Koenig S, Stoesser C, Krause P, Becker H, Markus PM. Liver repopulation after hepatocellular transplantation: integration and interaction of transplanted hepatocytes in the host. Cell Transplant. 2005;14(1):31–40. [DOI] [PubMed] [Google Scholar]
- 42.Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130(2):507–520; quiz 590. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura M, Imura T, Inagaki A, Ogasawara H, Fukuoka K, Fathi I, Miyagi S, Ohashi K, Unno M, Kamei T, Satomi S, et al. A Simple and useful predictive assay for evaluating the quality of isolated hepatocytes for hepatocyte Transplantation. Sci Rep. 2019;9(1):6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77(5):741–747. [DOI] [PubMed] [Google Scholar]
- 45.Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. J Hepatobiliary Pancreat Surg. 2009;16(2):97–100. [DOI] [PubMed] [Google Scholar]
- 46.Hamman KJ, Winn SR, Harding CO. Hepatocytes from wild-type or heterozygous donors are equally effective in achieving successful therapeutic liver repopulation in murine phenylketonuria (PKU). Mol Genet Metab. 2011;104(3):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338(20):1422–1426. [DOI] [PubMed] [Google Scholar]
- 48.Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND, Rela M, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78(12):1812–1814. [DOI] [PubMed] [Google Scholar]
- 49.Yin Z, Wahlin S, Ellis EC, Harper P, Ericzon BG, Nowak G. Hepatocyte transplantation ameliorates the metabolic abnormality in a mouse model of acute intermittent porphyria. Cell Transplant. 2014;23(9):1153–1162. [DOI] [PubMed] [Google Scholar]
- 50.Tokodai K, Goto M, Inagaki A, Nakanishi W, Ogawa N, Satoh K, Kawagishi N, Sekiguchi S, Nilsson B, Okada N, Okada H, et al. Attenuation of cross-talk between the complement and coagulation cascades by C5a blockade improves early outcomes after intraportal islet transplantation. Transplantation. 2010;90(12):1358–1365. [DOI] [PubMed] [Google Scholar]
- 51.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Larsson R, Korsgren O, et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. [DOI] [PubMed] [Google Scholar]
- 52.Gustafson E, Asif S, Kozarcanin H, Elgue G, Meurling S, Ekdahl KN, Nilsson B. Control of IBMIR induced by fresh and cryopreserved hepatocytes by low molecular weight dextran sulfate versus heparin. Cell Transplant. 2017;26(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asif S, Ekdahl KN, Fromell K, Gustafson E, Barbu A, Le Blanc K, Nilsson B, Teramura Y. Heparinization of cell surfaces with short peptide-conjugated PEG-lipid regulates thromboinflammation in transplantation of human MSCs and hepatocytes. Acta Biomater. 2016;35:194–205. [DOI] [PubMed] [Google Scholar]