Abstract
Objective
The objective was to explore the expression and potential functions of long noncoding RNA (lncRNA) and mRNAs in human breast cancer (BC).
Methods
Differentially expressed lncRNAs and mRNAs were identified and annotated in BC tissues by using the Agilent human lncRNA assay (Agilent Technologies, Santa Clara, CA, USA) and RNA sequencing. After identification of lncRNAs and mRNAs through quantitative reverse transcription polymerase chain reaction, we conducted a series of functional experiments to confirm the effects of knockdown of one lncRNA, TCONS_00029809, on the progression of BC.
Results
We discovered 238 lncRNAs and 200 mRNAs that were differentially expressed in BC tissues and para-carcinoma tissue. We showed that differentially expressed mRNAs were related to biological adhesion and biological regulation and mainly enriched in cytokine-cytokine receptor interaction, metabolic pathways, and PI3K-Akt signaling pathway. We created a protein–protein interaction network to analyze the proteins enriched in these pathways. We demonstrated that silencing of TCONS_00029809 remarkably inhibited proliferation, invasion, and migration of BC cells, and accelerated their apoptosis.
Conclusions
We identified a large number of differentially expressed lncRNAs and mRNAs, which provide data useful in understanding BC carcinogenesis. The lncRNA TCONS_00029809 may be involved in the development of BC.
Keywords: Long noncoding RNA, breast cancer, TCONS_00029809, migration and invasion, apoptosis, differential expression
Introduction
Breast cancer (BC) has become one of the most widespread tumors among women in the world, and its incidence is increasing.1,2 An estimated 2.1 million new cases of BC occurred worldwide in 2018.3 BC has the highest mortality rate of tumors in women, and metastasis and recurrence are the leading causes of death.4,5 Although a variety of treatments such as surgery, chemotherapy, radiation therapy, and endocrine therapy can suppress the growth of primary tumors, metastasis remains the greatest challenge in the treatment of BC.6,7 About 90% of all cancer-related deaths are associated with tumor metastasis.8 At present, the early diagnosis of BC is very difficult due to the lack of sensitive diagnostic markers.9 In patients diagnosed with BC, most have already had lymph node metastasis or even distant metastasis in multiple organs, and the clinical treatment and prognosis are unsatisfactory.10,11 Therefore, an in-depth study of BC metastasis-related genes and signaling pathways is of great importance for accurate and early clinical diagnosis, judgment of efficacy, and prediction of progress. This information could help identify new therapeutic targets for BC.
Long noncoding RNAs (lncRNAs) are endogenous single-stranded RNAs that do not encode proteins, with a transcription length from 200 bp to 1000 kb.12,13 LncRNA is transcribed by RNA polymerase II without an open reading frame.14 Studies have shown that lncRNA can regulate DNA methylation, histone modification, and chromosome remodeling via epigenetics, transcriptional regulation, and post-transcriptional regulation, among others.15,16 Moreover, lncRNA participate in various vital biological regulatory processes, including X chromosome silencing, genomic imprinting, transcriptional activation, transcriptional interference, and intracellular transport.17,18 Numerous studies have shown that lncRNAs can affect multiple biological activities, such as cell proliferation, apoptosis, invasion, and migration.19–21 Currently, genome-wide association studies (GWAS) in BC have uncovered a large number of cancer-related lncRNAs.22–24 The tissue-specific expression of lncRNAs and the GWAS results indicate that lncRNAs, as a class of new biomarkers, have numerous potential applications in cancer therapy. However, the exact functions and mechanisms of the many lncRNAs involved in the pathogenesis of BC have not been fully elucidated. Therefore, there is an urgent need to investigate the regulatory mechanism of lncRNAs in BC.
In the current study, we established the expression profiles of lncRNAs and mRNAs in BC tissues and paired adjacent normal tissues using the Agilent human lncRNA assay (Agilent Technologies, Santa Clara, Ca, USA) and RNA sequencing, respectively. We predicted the potential functions and pathways of differentially expressed mRNAs by conducting Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses. We assessed the expression levels of the most promising lncRNAs and mRNAs in BC. Moreover, we demonstrated the effects of knockdown of one such promising lncRNA, TCONS_00029809, on the malignant behavior of BC cells. Our study generated a large amount of data, which might include underlying markers and therapeutic targets for the diagnosis and therapy of BC.
Materials and Methods
Clinical samples
BC tissue and paired para-carcinoma tissue samples (3 cm from the tumor) were collected from patients with BC who underwent surgical treatment in Nanfang Hospital, Southern Medical University. The patients did not receive iodine ablation or other treatment before surgery. The pathologic classification of BC was confirmed by two pathologists using a double-blind method. In the current study, we focused on breast invasive ductal carcinoma (IDC) tissues. Within 15 minutes of collection, samples were stored in liquid nitrogen to prevent secondary freezing-thawing and the samples were transported for analysis on dry ice. All patients provided written informed consent for collection of the samples. This research was approved by the Ethics Committee of the Nanfang Hospital, Southern Medical University (approval number: NFEC-202101-K14-01).
RNA extraction and purification
In accordance with the manufacturer’s instructions, total RNA was extracted using the mirVana miRNA Isolation Kit (cat. no. AM1561, Ambion, Austin, TX, USA). A NanoDrop ND-1000 spectrophotometer (Nanodrop/Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the purity and concentration of total RNAs. The integrity of RNA was confirmed by electrophoresis on a denaturing agarose gel. All RNA samples were checked for purity (optical density 260/280) using a Nanodrop spectrophotometer, and only qualifying RNAs were used. The total RNA was then analyzed using an Agilent Bioanalyzer 2100 (Agilent Technologies).
Agilent human lncRNA assay
Expression profiling of lncRNAs was conducted using the Agilent human lncRNA microarray V.2.0 platform (GPL18109; Agilent Technologies). The microarray analysis was performed by Beijing Genomics Institute/HuaDa-Shenzhen (Shenzhen, China). Briefly, ribosomal RNA was depleted, and depletion was monitored using the bioanalyzer. We used the Low Input Quick Amp Labeling Kit (cat. no. 5190-2305; Agilent Technologies) to amplify and label the total RNA, following by purification using RNeasy mini kit (cat. no. 74106; Qiagen GmBH, Hilden, Germany). Hybridization was conducted using 1.65 μg of Cy3-labeled cRNA in the Gene Expression Hybridization Kit (cat. no. 5188-5242; Agilent Technologies) at 65°C for 17 hours. The slides were then scanned with the Agilent Microarray Scanner (cat. no. G2565CA; Agilent Technologies) and the raw data were then extracted and counted. Quantile normalization and subsequent data processing were performed using Agilent Gene Spring Software 11.5 (Agilent Technologies). Heat maps representing differentially regulated genes were generated using Cluster 3.0 software (Michiel de Hoon, Center for Computational Biology and Bioinformatics, Columbia University, New York, NY, USA). Exogenous RNAs developed by External RNA Controls Consortium were used as controls. The exosomal lncRNA microarray process was performed by KangChen BioTech Co. Ltd. (Shanghai, China).
RNA sequencing
RNA sequencing was conducted by Luen Chuan Biotechnology Co. Ltd. (Hangzhou, China) using an Illumina Novaseq™ 6000. The mRNA library was constructed using purified total RNA, and the Qubit 2.0 Fluorometer (Thermo Fisher Scientific) and Agilent 2100 bioanalyzer were used to determine the concentration and size of libraries. The Illumina HiSeq 2000 System (Illumina Inc., San Diego, CA, USA) was then applied to quantitatively analyze the library. Finally, mRNA expression was analyzed and differentially expressed mRNAs were identified.
Cell culture
Human breast cells (MCF10A) and two BC cell lines (MCF7 and MDA-MB-231) were obtained from ATCC (Rockville, MD, USA). MCF10A cells were grown in DMEM/F12 medium including 5% fetal bovine serum (FBS, Gibco, NY, USA), 20 ng/mL epidermal growth factor, 0.5 μg/mL hydrocortisone, 100 ng/mL cholera toxin, and 10 µg/mL insulin. MCF7 and MDA-MB-231 cells were cultured in DMEM (Gibco) with 10% FBS (Gibco). All cells were incubated at 37°C in a 5% CO2 incubator.
Cell transfection
Negative control (NC)-siRNAs and TCONS_00029809 siRNAs (si-TCONS_00029809) were acquired from GenePharma (Shanghai, China). MCF7 and MDA-MB-231 cells (1 × 105 cells/well) were evenly and gently placed into six-well plates and cultured for 12 hours. The cells were then transfected with NC and si-TCONS_00029809 using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Hierarchical clustering analysis
We conducted hierarchical clustering analysis using the R language package.25 In the cluster diagrams, different colors were used to distinguish different information, and the clustering pattern was adopted to confirm the different experimental conditions.
GO analysis
We defined the GO enrichment of differentially expressed mRNAs between para-carcinoma and BC tissues using the bioinformatics tool DAVID, version 6.8 (https://david.ncifcrf.gov/).26,27
KEGG analysis
We also analyzed the KEGG enrichment pathways of differentially expressed mRNAs between para-carcinoma and BC tissues using KOBAS 2.0 software (http://kobas.cbi.pku.edu.cn/).28
Protein-protein interaction (PPI) network analysis
PPIs of differentially expressed mRNAs between para-carcinoma and BC tissues were predicted and analyzed using STRING (http://string-db.org/) with a comprehensive score >0.9.29
Quantitative reverse transcription polymerase chain reaction assay (RT-qPCR)
We isolated total RNA from BC and para-carcinoma tissues, and from treated MCF10A, MCF7, and MDA-MB-231 cells using TRIzol® reagent (Takara, Dalian, China). The total RNAs were used to synthesize cDNAs using an iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA).30 cDNAs were then used as for quantitative analysis of genes using SYBR Green PCR Master (Takara). The obtained data were calculated using the 2−ΔΔCT method. All primer sequences are listed in Table 1.
Table 1.
Primer sequences used in quantitative reverse transcription polymerase chain reaction assay.
|
ID |
Sequence (5′–3′) |
| GAPDH | Forward: TGTTCGTCATGGGTGTGAAC |
| GAPDH | Reverse: ATGGCATGGACTGTGGTCAT |
| TCONS_00028064 | Forward: GAGAACCAACGAGCTGAGC |
| TCONS_00028064 | Reverse: GTATGGTGATGCTGCTGGTG |
| TCONS_00028800 | Forward: CAAAAGAGGCTCCCCAAACC |
| TCONS_00028800 | Reverse: GCAAAGGAGCTGAGCAGTTT |
| TCONS_00029809 | Forward: CGGAGCTGTGACACCTACTT |
| TCONS_00029809 | Reverse: TTGGGATGGCTTGTTTCTGC |
| TCONS_00007846 | Forward: GGCCGATTTGAGGACCTAGA |
| TCONS_00007846 | Reverse: CTCTCCTCCTCCCCAACTTG |
| TCONS_00010076 | Forward: GGCCTTTGTACCAGCTCTTT |
| TCONS_00010076 | Reverse: ACATGAGGCAGAACGGAAGA |
| TCONS_l2_00028765 | Forward: GTCCATCTGTCTGTCTGCCT |
| TCONS_l2_00028765 | Reverse: GTGACCTCTCTTTGGCTCCT |
| PPAPDC1A | Forward: TGGCTTCACGACGTTCTACT |
| PPAPDC1A | Reverse: CCAGTGATGCTTGTAGTCGC |
| COL10A1 | Forward: AAGGGAGAAAGAGGACCTGC |
| COL10A1 | Reverse: TGGCCCTGTCTCACCTTTAG |
| ADAMDEC1 | Forward: AAGATCCACGACCATGCTCA |
| ADAMDEC1 | Reverse: CATCACACAACTGCCAGAGG |
| SPHKAP | Forward: GAGGGAGACAGAACCAAGCT |
| SPHKAP | Reverse: TGAAGAATCGGGCACTCTGT |
| CLCA4 | Forward: CCAAAGCGAACCCAGAAACA |
| CLCA4 | Reverse: CGCCTGCACCATTATCCAAA |
| ALDH1L1 | Forward: GTTGGGGTTTGTGGCATCAT |
| ALDH1L1 | Reverse: CCGGCCTTTAATGTCAGCTC |
CCK-8 assay
MCF7 and MDA-MB-231 cells (2 × 103 cells/well) in each group were inoculated into 96-well plates and incubated for 48 hours at 37°C, followed by the addition of 10 µL CCK8 solution (Beyotime, Haimen, China) for 3 hours at 37°C. The optical density at 450 nm was then confirmed using a microplate reader (Biotek, VT, USA).
Flow cytometry
MCF7 and MDA-MB-231 cells in each group were harvested by digestion. After centrifugation (1000 ×g for 5 minutes), the cell density was adjusted to 1 × 109/L using phosphate-buffered saline, followed by the addition of 5 μL Annexin V-FITC and 5 μL propidium iodide for 10 minutes in the dark. Apoptotic cells were monitored and analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA).
Transwell assay
For cell migration analysis, treated MCF7 and MDA-MB-231 cells in serum-free medium were inoculated into the upper well of an 8-µm Transwell chamber (Cat. No. 3422; Costar, Cambridge, MA, USA), and DMEM medium supplemented with 10% FBS was added into the lower chamber. After 24 hours at 37°C, migrated cells were fixed and stained using 5% crystal violet, and photographed using an inverted optical microscope. For cell invasion analysis, the upper chambers were pretreated with Matrigel (BD Biosciences) before the addition of cells at 37°C for 30 minutes.
Statistical analysis
All experiments were repeated at least three times and all measurements are presented as means ± standard deviations. Statistical analysis was conducted using SPSS 23.0 statistical software (IBM Corp., Armonk, NY, USA). Numerical data were analyzed with χ2 tests and differences in continuous data between groups were analyzed using Student’s t-tests. P < 0.05 indicates statistical significance.
Results
Expression pattern analysis of differentially expressed lncRNAs in BC
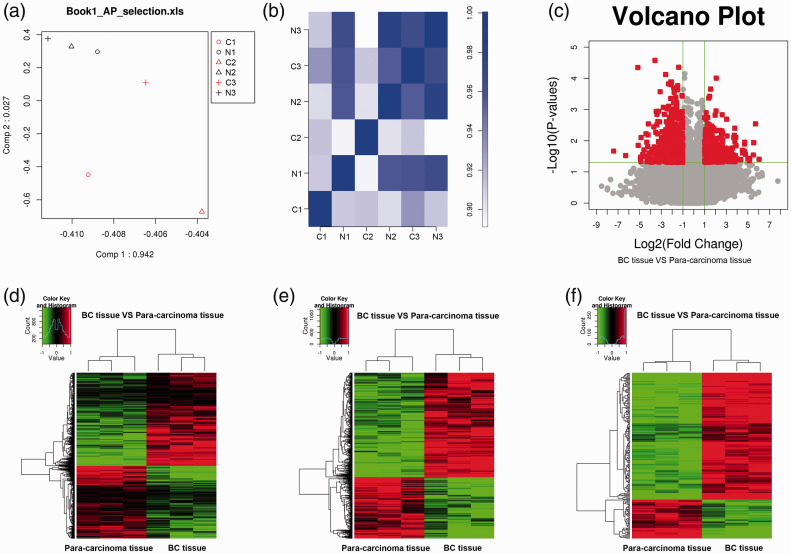
Three BC and three adjacent normal tissues were available for study. The pathological types of BC lesions include IDC, ductal carcinoma in situ (DCIS), invasive lobular carcinoma (IIC), and invasive mucinous carcinoma (IMC). Because IDC is the most common type of pathology in BC, we focused on breast IDC tissues. Differentially expressed lncRNAs were determined using the Agilent human lncRNA assay in breast IDC and para-carcinoma tissues. Based on the data, we first applied principal component analysis (PCA) to conduct dimensionality reduction in different samples (Figure 1A). Pearson correlation coefficient analysis was used to assess biological repeatability and correlation (Figure 1B). Next, in accordance with the results of lncRNA expression, expression profiles were prepared using volcano plots and hierarchical clustering analysis. As shown in Figure 1C and 1D, there were 1855 differentially expressed lncRNAs, including 951 upregulated and 904 downregulated lncRNAs between para-carcinoma and BC tissues, with a criterion of P < 0.05. We also discovered 841 differentially expressed lncRNAs (452 upregulated and 389 downregulated lncRNAs) between para-carcinoma and BC tissues, based on P < 0.05 and fold change ≥2 or ≤0.5 (Figure 1E). Hierarchical clustering revealed 238 differentially expressed lncRNAs (P < 0.01, fold change ≥2 or ≤0.5) from the three patients with BC, and among the lncRNAs, 157 lncRNAs were upregulated and 81 downregulated in BC tissues (Figure 1F). Thus, our results showed numerous lncRNAs potentially involved in BC.
Figure 1.
Expression pattern analysis of long noncoding (lnc)RNAs that were differentially expressed in breast cancer (BC). (a) Principal component analysis of each sample. (b) Pearson correlation coefficient analysis was applied to show the pairwise comparison of lncRNAs in each sample. (c) Volcano plot showing differentially expressed lncRNAs between para-carcinoma and BC tissues (P < 0.05). The red points indicate lncRNAs (P < 0.05) and the gray points indicate nondifferentially expressed lncRNAs. Hierarchical cluster analyses of lncRNAs (P < 0.05). Hierarchical clustering analyses of lncRNAs between para-carcinoma and BC tissues (d) with P < 0.05, (e) with P < 0.05 and fold change ≥2 or ≤0.5, and (f) with P < 0.01 and fold change ≥2 or ≤0.5. g1, para-carcinoma tissues; g2, BC tissues; N, normal tissue (or para-carcinoma tissue); C, BC tissue.
Analysis of differentially expressed mRNAs in BC
We applied mRNA sequencing to screen differentially expressed mRNAs in BC. PCA was also conducted to analyze dimensionality reduction in different samples (Figure 2A). Pearson correlation coefficient analysis was used to assess biological repeatability and correlation. (Figure 2B). Through mRNA sequencing analysis, we discovered 1956 differentially expressed mRNAs in BC tissues compared with para-carcinoma tissues (P < 0.05). The volcano plot and heat map show the expression status of mRNAs (856 upregulated and 1100 downregulated) between para-carcinoma and BC tissues (P < 0.05, Figure 2C and 2D). To further screen differentially expressed mRNAs, 881 mRNAs were screened according to the screening conditions P < 0.05 and fold change ≥2 or ≤0.5, of which 325 were upregulated and 556 were downregulated in BC tissues (Figure 2E). In addition, we discovered 200 differentially expressed mRNAs between para-carcinoma and BC tissues, which contained 47 upregulated and 153 downregulated mRNAs in BC (P < 0.01, fold change ≥2 or ≤0.5). The expression of specific differentially expressed mRNAs in BC is shown using a heat map (Figure 2F).
Figure 2.
Expression pattern analysis of differentially expressed mRNAs in breast cancer (BC). (a) Principal component analysis of each sample. (b) Pairwise comparison of mRNAs in each sample. (c) Volcano plot showing differentially expressed mRNAs between para-carcinoma and BC tissues. The red points indicate differentially expressed mRNAs and the gray points indicate mRNAs that did not change. Hierarchical clustering analyses of mRNAs between para-carcinoma and BC tissues (d) with P < 0.05, (e) with P < 0.05 and fold change ≥2 or ≤0.5, and (f) with P < 0.01 and fold change ≥2 or ≤0.5. g1, para-carcinoma tissues; g2, BC tissues; N, normal tissue (or para-carcinoma tissue); C, BC tissue.
GO analyses of abnormally expressed mRNAs in BC tissues
By GO analysis, we analyzed the related functions of the differentially expressed mRNAs between para-carcinoma and BC tissues. The main enrichment function of the upregulated mRNAs included the biological process (BP) terms biological adhesion (GO:0022610), biological regulation (GO:0065007), cellular component organization or biogenesis (GO:007184); the cellular component (CC) terms cell part (GO:0044464), extracellular matrix (GO:0031012), and extracellular region (GO:0005576); and molecular function (MF) terms antioxidant activity (GO:0016209), binding (GO:0005488), catalytic activity (GO:0003824) (Figure 3A). The downregulated mRNAs were mainly enriched in BP terms biological adhesion (GO:0022621), biological regulation (GO:0065007), and cell killing (GO:0001906); the CC terms cell junction (GO:0030054), cell part (GO:0044464), and extracellular matrix (GO:0031012); and the MF terms antioxidant activity (GO:0016209), binding (GO:0005488), and catalytic activity (GO:0003824), among others (Figure 3B).
Figure 3.
Gene Ontology (GO) analyses of abnormally expressed mRNAs in BC tissues. The GO analysis exhibited biological processes (left), cellular components (middle), and molecular functions (right) of (a) upregulated mRNAs in BC (P < 0.05) and (b) downregulated mRNAs in BC (P < 0.05).
KEGG analyses of abnormally expressed mRNAs between para-carcinoma and BC tissues
By KEGG analysis, we showed that the enriched pathways of the differentially expressed mRNAs between para-carcinoma and BC tissues included cytokine-cytokine receptor interaction, metabolic pathways, pathways in cancer, chemokine signaling pathway, cell adhesion molecules, PI3K-Akt signaling pathway, HTLVI infection, and T cell receptor signaling pathway (Table S1). Analysis suggests that the PI3K-Akt signaling pathway is closely related to BC (Figure 4). Overall, we provided a detailed functional description of differentially expressed mRNAs between para-carcinoma and BC tissues (Table S1).
Figure 4.
Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of abnormally expressed mRNAs in breast cancer (BC) tissues. KEGG analysis was used to analyze the signaling pathways, which were enriched by the differentially expressed mRNAs between para-carcinoma and BC tissues. We have shown the PI3K-Akt signaling pathway. Red represents upregulation, green represents downregulation.
Establishment of PPI network and analysis of genes in BC
To predict the underlying regulatory network of differentially expressed mRNAs between para-carcinoma and BC tissues, we identified feasible co-expressed proteins of these differentially expressed mRNAs and confirmed the connection of these proteins using a PPI. First, we evaluated the entire PPI network to identify possible interrelationships of proteins that might provide the basis for pathway research in BC (Figure 5A). Then, we used STRING protein interaction network analysis to predict protein networks of the PI3K-Akt signaling pathway and positive regulation of cell migration. The PI3K-Akt signaling pathway-related proteins included IL2RB, THBS1, COL6A5, ITGB7, FIGF, IL7R, FN1, COL6A6, FASLG, IL2RG, COL4A1, ITGA8, ANGPT4, LAMB3, ITGA4, JAK3, TNXB, GNGT2, CD19, and GHR (Figure 5B and Table S2). The positive regulation of cell migration-related proteins included MYOC, UTS2, CORO1A, SELL, SOX9, CCR7, RAC2, THBS1, LEF1, CCR2, FIGF, CCL11, FBLN1, FN1, CXCL9, SASH1, F10, CCL7, INSL3, ANGPT4, CEMIP, ITGA4, PTPRC, LRRC15, SEMA4D, DMTN, HIF1A, ADAM8, and CCL5 (Figure 5C and Table S3).
Figure 5.
Protein–protein interaction (PPI) network and PI3K-Akt- or cell migration-related modules analyses of proteins in breast cancer (BC). (a) The entire PPI network in BC. Based on the functional annotation, the proteins related to the PI3K-Akt signaling pathway (b) and positive regulation of cell migration (c) were confirmed by STRING protein interaction network analysis.
Identification of differentially expressed lncRNAs and mRNAs in BC
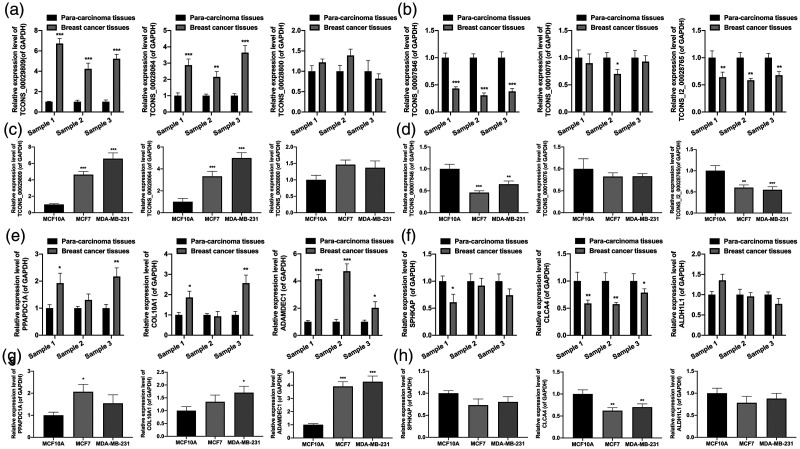
Given the degree of difference in expression, we screened the six most significantly differentially expressed lncRNAs, including three upregulated lncRNAs (TCONS_00028064, TCONS_00028800, TCONS_00029809) and three downregulated lncRNAs (TCONS_00007846, TCONS_00010076, TCONS_l2_00028765) between para-carcinoma and BC tissues; we present detailed information of these lncRNAs in Table 2. Next, through verification by RT-qPCR, we showed that relative to para-carcinoma tissues, TCONS_00028064 and TCONS_00029809 were markedly upregulated (P < 0.01, P < 0.001, Figure 6A) and TCONS_00007846 and TCONS_l2_00028765 were downregulated (P < 0.05, P < 0.01, P < 0.001, Figure 6B) in BC tissues. We also showed that TCONS_00028064 and TCONS_00029809 were upregulated (P < 0.001, Figure 6C) and TCONS_00007846 was downregulated (P < 0.01, P < 0.001, Figure 6D) in BC cells compared with MCF10A cells.
Table 2.
Detailed information on differentially expressed long noncoding RNAs between para-carcinoma and breast cancer tissues.
| Primary accession | Gene symbol | P value | Fold change | Genomic coordinates | Cytoband | Result source |
|---|---|---|---|---|---|---|
| TCONS_00007846 | XLOC_004090 | ≤0.01 | 11.28 | chr4:137734865-137734806 | hs|4q28.3 | Agilent_HUMAN_G3V2 |
| TCONS_00010076 | XLOC_004530 | ≤0.01 | 10.96 | chr5:121176363-121176422 | hs|5q23.1 | Agilent_HUMAN_G3V2 |
| TCONS_l2_00028765 | XLOC_l2_014820 | ≤0.01 | 10.74 | chr9:67282369-67288092 | hs|9q13 | Agilent_HUMAN_G3V2 |
| TCONS_00028064 | XLOC_013794 | ≤0.01 | 0.07 | chr20:50448398-50448339 | hs|20q13.2 | Agilent_HUMAN_G3V2 |
| TCONS_00028800 | XLOC_013872 | ≤0.01 | 0.06 | chr21:20022616-20022675 | hs|21q21.1 | Agilent_HUMAN_G3V2 |
| TCONS_00029809 | XLOC_014244 | ≤0.01 | 0.05 | chr22:39465900-39465959 | hs|22q13.1 | Agilent_HUMAN_G3V2 |
Fold change: LncRNA expression in para-carcinoma/LncRNA expression in BC tissues.
Figure 6.
Expression of long noncoding (lnc)RNAs and mRNAs in breast cancer (BC). (a, b) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis was used to assess the levels of three upregulated lncRNAs (TCONS_00028064, TCONS_00028800, TCONS_00029809) and three downregulated lncRNAs (TCONS_00007846, TCONS_00010076, TCONS_l2_00028765) between para-carcinoma and BC tissues. (c, d) The six differentially expressed lncRNAs were monitored though RT-qPCR analysis in MCF10A, MCF7, and MDA-MB-231 cells. (e, f) RT-qPCR analysis was used to assess the levels of three upregulated mRNAs (PPAPDC1A, COL10A1, and ADAMDEC1) and three downregulated mRNAs (SPHKAP, CLCA4, and ALDH1L1) between para-carcinoma and BC tissues. (g, h) The six differentially expressed mRNAs were assessed by RT-qPCR analysis in MCF10A, MCF7, and MDA-MB-231 cells. *P < 0.05, **P < 0.01, ***P < 0.001.
Similarly, we screened the six most significantly differentially expressed mRNAs, including three upregulated mRNAs (PPAPDC1A, COL10A1, and ADAMDEC1) and 3 downregulated mRNAs (SPHKAP, CLCA4, and ALDH1L1) between para-carcinoma and BC tissues (detailed information of these mRNAs is given in Table 3). The RT-qPCR data showed that ADAMDEC1 was markedly upregulated and CLCA4 was significantly downregulated in BC tissues and cells (P < 0.05, P < 0.001 and P < 0.01, respectively, Figure 6E-6H).
Table 3.
Detailed information of differentially expressed mRNAs between para-carcinoma and breast cancer tissues.
| Gene symbol | Gene name | P value | Fold change | Genomic coordinates | Cytoband | Result source | Sources |
|---|---|---|---|---|---|---|---|
| SPHKAP | SPHK1 interactor, AKAP domain containing | ≤0.01 | 14.94 | chr2:228846461-228846402 | hs|2q36.3 | Agilent_HUMAN_G3V2 | NM_001142644 |
| CLCA4 | chloride channel accessory 4 | ≤0.01 | 22.68 | chr1:87045163-87045222 | hs|1p22.3 | Agilent_HUMAN_G3V2 | NM_012128 |
| ALDH1L1 | aldehyde dehydrogenase 1 family, member L1 | ≤0.01 | 20.26 | chr3:125826021-125824747 | hs|3q21.3 | Agilent_HUMAN_G3V2 | NM_012190 |
| PPAPDC1A | phosphatidic acid phosphatase type 2 domain containing 1A | ≤0.01 | 0.03 | chr10:122349102-122349161 | hs|10q26.12 | Agilent_HUMAN_G3V2 | NM_001030059 |
| COL10A1 | collagen, type X, alpha 1 | ≤0.01 | 0.03 | chr6:116441120-116441061 | hs|6q22.1 | Agilent_HUMAN_G3V2 | NM_000493 |
| ADAMDEC1 | ADAM-like, decysin 1 | ≤0.01 | 0.01 | chr8:24259534-24259593 | hs|8p21.2 | Agilent_HUMAN_G3V2 | NM_001145271 |
Fold change: mRNA expression in para-carcinoma/mRNA expression in BC tissues.
TCONS_00029809 silencing repressed the proliferation, invasion, migration of BC cells and induced apoptosis of BC cells
By identifying lncRNAs in RT-qPCR assay, we discovered that the difference in expression of TCONS_00029809 in BC was the largest, and it was therefore the target for further study. We conducted TCONS_00029809 silencing in MCF7 and MDA-MB-231 cells to further study the roles of TCONS_00029809 on proliferation, apoptosis, invasion, and migration of BC cells. The RT-qPCR analysis showed that TCONS_00029809 was sharply decreased in MCF7 and MDA-MB-231 cells after transfection of siRNAs against TCONS_00029809 compared with the si-NC group (P < 0.01, P < 0.001, Figure 7A). Using the CCK-8 assay, we demonstrated that TCONS_00029809 silencing significantly inhibited cell proliferation in MCF7 and MDA-MB-231 cells (P < 0.05, P < 0.01, Figure 7B). Flow cytometry analysis revealed that knockdown of TCONS_00029809 facilitated apoptosis of MCF7 and MDA-MB-231 cells (P < 0.001, Figure 7C). Additionally, TCONS_00029809 silencing attenuated the invasive and migratory capacities of MCF7 and MDA-MB-231 cells (P < 0.01, P < 0.001, Figure 7D and 7E). Thus, silencing of TCONS_00029809 could suppress malignant behaviors of BC cells.
Figure 7.
Silencing of the long noncoding (lnc)RNA TCONS_00029809 repressed breast cancer (BC) cell proliferation, invasion, and migration, and induced apoptosis. (a) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) assessment of TCONS_00029809 expression in MCF7 and MDA-MB-231 cells transfected with short interfering negative control (si-NC), and short interfering (si)RNAs against TCONS_00029809. (b) Proliferation of MCF7 and MDA-MB-231 cells was estimated by CCK-8 cell counting assay in MCF7 and MDA-MB-231 cells after TCONS_00029809 knockdown. (c) Cell apoptosis was analyzed by flow cytometry in transfected MCF7 and MDA-MB-231 cells. (d, e) Invasive and migratory abilities of MCF7 and MDA-MB-231 cells were evaluated by Transwell assay after TCONS_00029809 knockdown. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC group.
Discussion
BC is a common malignant tumor in clinical practice, and its main clinical manifestations include breast mass, nipple discharge, skin changes, abnormalities of the areola, and axillary lymphadenoma.31,32 At present, the prevention and treatment of BC are crucial areas of research in the study of malignant tumors. However, the cause of BC is not fully understood. BC develops in a complex, multi-stage, multi-step process that can be influenced by various molecules and factors.11,33 LncRNAs are associated with numerous biological functions, including epigenetics, cell differentiation, and cell cycle.34,35 With the development of next-generation sequencing and computer technology, lncRNAs have been widely studied in the pathogenesis, diagnosis, and drug screening of various diseases.36,37 Numerous studies have shown that lncRNAs participate in the development, progression, invasion, and metastasis of BC in a variety of ways. For example, lncRNA SNHG14 induces resistance to trastuzumab in BC by H3K27 acetylation-mediated regulation of PABPC1 expression;38 lncRNA MALAT1 inhibits BC metastasis;39 lncRNA GHSROS has significant promoting effects on the growth and migration of BC;40 and lncRNA NONHSAT101069 induces epirubicin resistance and promotes the migration and invasion of BC cells by targeting the microRNA miR-129-5p to regulate Twist1.41
The expression profile of lncRNA has been analyzed in previous studies.42,43 However, because of tissue specificity, numerous lncRNAs screened through microarray analysis have not yet been identified. In our study, we discovered 238 differently expressed lncRNAs (157 upregulated and 81 downregulated) that might be associated with BC. We showed that TCONS_00028064 and TCONS_00029809 were upregulated and TCONS_00007846 was downregulated in BC tissues and cells. Our functional experiments showed that knockdown of TCONS_00029809 could prevent the proliferation, invasion, and migration of BC cells, and accelerate apoptosis of BC cells. Therefore, inhibition of TCONS_00029809 might have potential value in the treatment of BC.
RNA sequencing has been extensively applied to the identification of differentially expressed genes in cells and tissues of multiple diseases, and has significant applications in genetics and epigenetics.44,45 In our study, we screened 200 differentially expressed mRNAs, including 47 upregulated and 153 downregulated mRNAs in BC tissues relative to that in para-carcinoma tissues. Through GO analysis, we found that the differentially expressed mRNAs might be involved in biological adhesion, biological regulation, cellular component organization or biogenesis, and cell killing. In KEGG pathway analysis, we found that the differentially expressed mRNAs were abundant in cytokine-cytokine receptor interaction, metabolic pathways, pathways in cancer, chemokine signaling pathway, and PI3K-Akt signaling pathway. Several studies have shown that the cytokine-cytokine receptor interaction pathway might be associated with multiple cancers, including renal cell carcinoma,46,47 oral squamous cell carcinoma,48 glioma,49 and colorectal cancer.50 Metabolic pathways have also been shown to contribute to cancer progression.51,52 Research has shown that the chemokine signaling pathway participates in cancer metastasis.53 The PI3K-Akt signaling pathway can be activated by G-protein-coupled receptors and has a significant effect in malignant proliferation, angiogenesis, and drug resistance of tumor cells.54,55 We suggest that differentially expressed mRNAs have an important contribution in the processes of BC development. We further analyzed and predicted the network of proteins related to the PI3K-Akt signaling pathway and positive regulation of cell migration, based on the differentially expressed mRNAs. We verified for the first time that the mRNAs ADAMDEC1 and CLCA4 were differentially expressed in BC. ADAMDEC1 is part of a group of secretory glycoproteins with various functions;56 it has been reported to be closely related to inflammation and tumor progression.57 CLCA4 is a class of calcium-activated chloride channel regulator.58 Research has shown that CLCA1 is connected with the development of colorectal cancer.59 However, the function and mechanism of ADAMDEC1 and CLCA1 in BC are not fully understood. ADAMDEC1 and CLCA1 might be potential targets for the treatment of BC.
This study had several limitations. First, the sample size was small. Furthermore, the mechanism by which TCONS_00029809 silencing prevents the progression of BC, and the regulatory relationship between differentially expressed lncRNAs and genes in BC remain unclear. In addition to TCONS_00029809, the effects and mechanisms of other verified differentially expressed lncRNAs and genes on BC progression also need to be confirmed. Further studies with more samples are therefore needed to verify these potential lncRNAs and mRNAs.
In conclusion, we used high-throughput sequencing to identify 238 candidate lncRNAs and 200 potential mRNAs that might be related to BC. The functions of the differentially expressed mRNAs were annotated through GO and KEGG analyses. The lncRNAs TCONS_00028064, TCONS_00029809, and TCONS_00007846 and mRNAs ADAMDEC1 and CLCA4 were identified as the most-differentially expressed in BC. We showed, for the first time, that TCONS_00029809 knockdown could inhibit malignant processes of BC cells, suggesting that TCONS_00029809 might be a potential disease-causing lncRNA and a diagnostic and therapeutic target for BC. However, further in vitro and in vivo experiments are needed to verify the functions and mechanisms of these potential lncRNAs and mRNAs in different types of BC.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520973137 for Integrated analyses of long noncoding RNAs and mRNAs in the progression of breast cancer by Jingyun Guo, Huining Lian, Minfeng Liu, Jianyu Dong, Zhaoze Guo, Jinlamao Yang and Changsheng Ye in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520973137 for Integrated analyses of long noncoding RNAs and mRNAs in the progression of breast cancer by Jingyun Guo, Huining Lian, Minfeng Liu, Jianyu Dong, Zhaoze Guo, Jinlamao Yang and Changsheng Ye in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520973137 for Integrated analyses of long noncoding RNAs and mRNAs in the progression of breast cancer by Jingyun Guo, Huining Lian, Minfeng Liu, Jianyu Dong, Zhaoze Guo, Jinlamao Yang and Changsheng Ye in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by President Foundation of Nanfang Hospital (2016L007).
ORCID iD: Jingyun Guo https://orcid.org/0000-0003-1049-7156
Supplemental material: Supplemental material for this article is available online.
References
- 1.Harbeck N, Gnant M.Breast cancer. Lancet 2017; 389: 1134–1150. [DOI] [PubMed] [Google Scholar]
- 2.Tung MC, Chang FW, Fan TP, et al. Acidity is one of the main mechanism for hypoxia triggering chemoresistance to mitoxanthrone (MX) in the human breast cancer MCF-7 cell line. Eur J Gynaecol Oncol 2019; 40: 994–999. [Google Scholar]
- 3.Anastasiadi Z, Lianos GD, Ignatiadou E, et al. Breast cancer in young women: an overview. Updates Surg 2017; 69: 313–317. [DOI] [PubMed] [Google Scholar]
- 4.Kimbung S, Loman N, Hedenfalk I.Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 2015; 35: 85–95. [DOI] [PubMed] [Google Scholar]
- 5.Dittmer J.Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol 2017; 44: 72–82. [DOI] [PubMed] [Google Scholar]
- 6.Narod SA, Sopik V.Is invasion a necessary step for metastases in breast cancer? Breast Cancer Res Treat 2018; 169: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medeiros B, Allan AL.Molecular mechanisms of breast cancer metastasis to the lung: clinical and experimental perspectives. Int J Mol Sci 2019; 20: 2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peart O.Metastatic breast cancer. Radiol Technol 2017; 88: 519m–539m. [PubMed] [Google Scholar]
- 9.Jafari SH, Saadatpour Z, Salmaninejad A, et al. Breast cancer diagnosis: imaging techniques and biochemical markers. J Cell Physiol 2018; 233: 5200–5213. [DOI] [PubMed] [Google Scholar]
- 10.Burke EE, Kodumudi K, Ramamoorthi G, et al. Vaccine therapies for breast cancer. Surg Oncol Clin N Am 2019; 28: 353–367. [DOI] [PubMed] [Google Scholar]
- 11.Winters S, Martin C, Murphy D, et al. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 2017; 151: 1–32. [DOI] [PubMed] [Google Scholar]
- 12.Quinn JJ, Chang HY.Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 2016; 17: 47–62. [DOI] [PubMed] [Google Scholar]
- 13.Jarroux J, Morillon A, Pinskaya M.History, discovery, and classification of lncRNAs. Adv Exp Med Biol 2017; 1008: 1–46. [DOI] [PubMed] [Google Scholar]
- 14.Dahariya S, Paddibhatla I, Kumar S, et al. Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol 2019; 112: 82–92. [DOI] [PubMed] [Google Scholar]
- 15.Dykes IM, Emanueli C.Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genomics Proteomics Bioinformatics 2017; 15: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathy NW, Chen XM.Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J Biol Chem 2017; 292: 12375–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo Y, Shinjo K, Katsushima K.Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci 2017; 108: 1927–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ransohoff JD, Wei Y, Khavari PA.The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol 2018; 19: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi Y, Wang D, Wang J, et al. Long non-coding RNA in the pathogenesis of cancers. Cells 2019; 8: 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Y, Fullwood MJ.Roles, functions, and mechanisms of long non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics 2016; 14: 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misawa A, Takayama KI, Inoue S.Long non-coding RNAs and prostate cancer. Cancer Sci 2017; 108: 2107–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol 2019; 12: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian T, Gong Z, Wang M, et al. Identification of long non-coding RNA signatures in triple-negative breast cancer. Cancer Cell Int 2018; 18: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian T, Wang M, Lin S, et al. The impact of lncRNA dysregulation on clinicopathology and survival of breast cancer: a systematic review and meta-analysis. Mol Ther Nucleic Acids 2018; 12: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Zhao LM, Li SL, et al. Differentially expressed lncRNAs and mRNAs identified by NGS analysis in colorectal cancer patients. Cancer Med et al. ; 7: 4650–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen S, Kong J, Qiu Y, et al. Identification of core genes and outcomes in hepatocellular carcinoma by bioinformatics analysis. J Cell Biochem et al. ; 120: 10069–10081. [DOI] [PubMed] [Google Scholar]
- 27.Klopfenstein D, Zhang L, Pedersen BS, et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci Rep et al. ; 8: 10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Shang P, Li Q, et al. : iTRAQ-based proteomic analysis reveals key proteins affecting muscle growth and lipid deposition in pigs. Sci Rep et al. ; 7: 46717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Mering C, Huynen M, Jaeggi D, et al. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res et al. ; 31: 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin S, Zhang R, Xu L, et al. LncRNA Hoxaas3 promotes lung fibroblast activation and fibrosis by targeting miR-450b-5p to regulate Runx1. Cell Death Dis et al. ; 11 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T, Mello-Thoms C, Brennan PC.Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016; 159: 395–406. [DOI] [PubMed] [Google Scholar]
- 32.Tan AR.Cutaneous manifestations of breast cancer. Semin Oncol 2016; 43: 331–334. [DOI] [PubMed] [Google Scholar]
- 33.Nagini S.Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem 2017; 17: 152–163. [DOI] [PubMed] [Google Scholar]
- 34.Charles Richard JL, Eichhorn PJA.Platforms for investigating lncRNA functions. SLAS Technol 2018; 23: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jathar S, Kumar V, Srivastava J, et al. Technological developments in lncRNA biology. Adv Exp Med Biol 2017; 1008: 283–323. [DOI] [PubMed] [Google Scholar]
- 36.Kumar MM, Goyal R.LncRNA as a therapeutic target for angiogenesis. Curr Top Med Chem 2017; 17: 1750–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng WX, Koirala P, Mo YY.LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017; 36: 5661–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong H, Wang W, Mo S, et al. Long non-coding RNA SNHG14 induces trastuzumab resistance of breast cancer via regulating PABPC1 expression through H3K27 acetylation. J Cell Mol Med 2018; 22: 4935–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Piao HL, Kim BJ, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet 2018; 50: 1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas PB, Seim I, Jeffery PL, et al. The long non-coding RNA GHSROS facilitates breast cancer cell migration and orthotopic xenograft tumour growth. Int J Oncol 2019; 55: 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao N, Fu Y, Chen L, et al. Long non-coding RNA NONHSAT101069 promotes epirubicin resistance, migration, and invasion of breast cancer cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene 2019; 38: 7216–7233. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Gao C, Liu C, et al. Four lncRNAs associated with breast cancer prognosis identified by coexpression network analysis. J Cell Physiol 2019; 234: 14019–14030. [DOI] [PubMed] [Google Scholar]
- 43.Deva Magendhra Rao AK, Patel K, Korivi Jyothiraj S, et al. Identification of lncRNAs associated with early-stage breast cancer and their prognostic implications. Mol Oncol 2019; 13: 1342–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stark R, Grzelak M, Hadfield J.RNA sequencing: the teenage years. Nat Rev Genet 2019; 20: 631–656. [DOI] [PubMed] [Google Scholar]
- 45.Hedlund E, Deng Q.Single-cell RNA sequencing: technical advancements and biological applications. Mol Aspects Med 2018; 59: 36–46. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Guo P, Dong K, et al. Identification of key biomarkers and potential molecular mechanisms in renal cell carcinoma by bioinformatics analysis. J Comput Biol 2019; 26: 1278–1295. [DOI] [PubMed] [Google Scholar]
- 47.Wan B, Huang Y, Liu B, et al. AURKB: a promising biomarker in clear cell renal cell carcinoma. PeerJ 2019; 7: e7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Chen X, Liu X, et al. Complex integrated analysis of lncRNAs-miRNAs-mRNAs in oral squamous cell carcinoma. Oral Oncol 2017; 73: 1–9. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Meng Y.Survival analysis of immune-related lncRNA in low-grade glioma. BMC Cancer 2019; 19: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Li H, Sun L, et al. Aberrantly methylated-differentially expressed genes and pathways in colorectal cancer. Cancer Cell Int 2017; 17: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boroughs LK, DeBerardinis RJ.Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 2015; 17: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh SR, Tan M, Rameshwar P.Cancer metabolism: targeting metabolic pathways in cancer therapy. Cancer Lett 2015; 356: 147–148. [DOI] [PubMed] [Google Scholar]
- 53.Lim SY, Yuzhalin AE, Gordon-Weeks AN, et al. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016; 7: 28697–28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murugan AK.Special issue: PI3K/Akt signaling in human cancer. Semin Cancer Biol 2019; 59: 1–2. [DOI] [PubMed] [Google Scholar]
- 55.Carnero A, Blanco-Aparicio C, Renner O, et al. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 2008; 8: 187–198. [DOI] [PubMed] [Google Scholar]
- 56.Jimenez-Pascual A, Hale JS, Kordowski A, et al. ADAMDEC1 maintains a growth factor signaling loop in cancer stem cells. Cancer Discov 2019; 9: 1574–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu T, Deng Z, Xie H, et al. ADAMDEC1 promotes skin inflammation in rosacea via modulating the polarization of M1 macrophages. Biochem Biophys Res Commun 2020; 521: 64–71. [DOI] [PubMed] [Google Scholar]
- 58.Liu CL, Shi GP.Calcium-activated chloride channel regulator 1 (CLCA1): more than a regulator of chloride transport and mucus production. World Allergy Organ J 2019; 12: 100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Hu W, Zhou J, et al. CLCA1 suppresses colorectal cancer aggressiveness via inhibition of the Wnt/beta-catenin signaling pathway. Cell Commun Signal 2017; 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520973137 for Integrated analyses of long noncoding RNAs and mRNAs in the progression of breast cancer by Jingyun Guo, Huining Lian, Minfeng Liu, Jianyu Dong, Zhaoze Guo, Jinlamao Yang and Changsheng Ye in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520973137 for Integrated analyses of long noncoding RNAs and mRNAs in the progression of breast cancer by Jingyun Guo, Huining Lian, Minfeng Liu, Jianyu Dong, Zhaoze Guo, Jinlamao Yang and Changsheng Ye in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520973137 for Integrated analyses of long noncoding RNAs and mRNAs in the progression of breast cancer by Jingyun Guo, Huining Lian, Minfeng Liu, Jianyu Dong, Zhaoze Guo, Jinlamao Yang and Changsheng Ye in Journal of International Medical Research