Abstract
The brain undergoes substantial maturation during adolescence, and repeated exposure to ethanol at this time has been shown to result in long-lasting behavioral and neural consequences. During the broad period of adolescence, different neuronal populations and circuits are refined between early and late adolescence, suggesting the possibility that ethanol exposure at these differing times may lead to differential outcomes. The goal of the current study was to evaluate the impact of adolescent intermittent ethanol (AIE) during early and late adolescence on the formation of goal-directed and habitual behavior in adulthood. Male and female Sprague-Dawley rats were exposed to ethanol via intragastric gavage (4.0 g/kg, 25% v/v) every other day from postnatal day (P) 25–45 or P45–65, considered early and late adolescence, respectively. In adulthood (~P70 early or ~P90 late), rats were gradually food-restricted and began operant training on a fixed ratio 1 schedule. Rats were then transitioned onto random interval schedules and eventually underwent a sensory-specific satiation procedure as a model of reward devaluation. Few differences as a result of adolescent ethanol exposure were found during instrumental training. Following reward devaluation, rats exposed to water and ethanol during early adolescence exhibited reductions in lever pressing, suggestive of a goal-directed response pattern. In contrast, late AIE males and females demonstrated persistent responding following both devalued and non-devalued trials, findings representative of a habitual behavior pattern. The shifts from goal-directed to habitual behavior noted only following late AIE contribute to the growing literature identifying specific behavioral consequences as a result of ethanol exposure during distinct developmental periods within adolescence. More work is needed to determine whether the greater habit formation following late AIE is also associated with elevated habitual ethanol consumption.
Keywords: adolescence, goal-directed, habit formation, intermittent ethanol exposure, sex differences
Introduction
In the United States, approximately 55% of the population that is 12 years or older consumes alcohol at least monthly, with roughly 15 million people meeting the criteria for dependence and an alcohol use disorder (AUD) in 2018 (Substance Abuse and Mental Health Services Administration, 2019). Alcohol use is commonly initiated during early adolescence (Morean, Corbin, & Fromme, 2012; Morean, L’Insalata, Butler, McKee, & Krishnan-Sarin, 2018; Ohannessian, Finan, Schulz, & Hesselbrock, 2015), and an earlier age of initiation is a strong predictor of the development of an AUD (Patrick & Schulenberg, 2013). Adolescents also exhibit high rates of heavy drinking, with approximately 1.2 million adolescents aged 12 to 17 years old engaging in binge drinking during the previous month, defined as 4 or more drinks per occasion for females and 5 for males (Substance Abuse and Mental Health Services Administration, 2019). Consumption can reach even higher levels, particularly later in adolescence. As recently reported by Patrick and Terry-McElrath (2019), up to 11% of late adolescents/emerging adults in the United States consume 10–15 drinks or more per occasion, a drinking pattern termed “high-intensity drinking”. With a significant portion of the adolescent population in the United States binge drinking and consuming alcohol in large quantities, it is important to understand the long-term impacts of these drinking patterns.
The consequences of early initiation of drinking and binge/high intensity consumption in adolescence have been thoroughly studied in terms of relatively immediate effects in humans (for review see, White & Hingson, 2013). However, study of the long-lasting impact of adolescent drinking is difficult in humans due to practical and ethical limitations. In studies examining consumption of alcohol (i.e., ethanol) using rodent models, consummatory behavior of adolescents is often greater than that of adults (Doremus, Brunell, Rajendran, & Spear, 2005; Vetter-O’Hagen, Varlinskaya, & Spear, 2009), suggesting some potential conservation of underlying biological contributors to these age differences across species.
In recent years, research using animal models and different routes of ethanol administration has revealed a variety of long-term behavioral, neural, and epigenetic changes that persist into adulthood following adolescent intermittent ethanol (AIE) exposure (for review, see Crews et al., 2019). Consistent behavioral effects of AIE include increased general (Pandey, Sakharkar, Tang, & Zhang, 2015; Sakharkar et al., 2019; Varlinskaya, Hosová, Towner, Werner, & Spear, 2020) and social anxiety-like behaviors (Dannenhoffer et al., 2018; Varlinskaya, Truxell, & Spear, 2014), reduced sensitivity to the aversive properties of ethanol (Saalfield & Spear, 2015; Varlinskaya et al., 2014; Williams, Nickel, & Bielak, 2018), impaired fear conditioning (Bergstrom, McDonald, & Smith, 2006; Broadwater & Spear, 2013), as well as elevated ethanol intake in adulthood under some circumstances (see Towner & Varlinskaya, 2020 for references).
Intermittent ethanol exposure during adolescence has also been reported to reduce behavioral flexibility in adulthood when measured by behaviors such as set shifting (Fernandez & Savage, 2017; Gass et al., 2014; Varlinskaya et al., 2020) and reversal learning (Coleman, He, Lee, Styner, & Crews, 2011; Coleman, Liu, Oguz, Styner, & Crews, 2014; Galaj, Kipp, Floresco, & Savage, 2019; Vetreno et al., 2020). The inability to update behavior based on previous outcomes may contribute to elevations in ethanol consumption due to a failure to reduce drinking after experiencing a negative outcome that, under normal circumstances, would curb subsequent intake. The observed behavioral inflexibility following AIE could also be associated with a greater sensitivity to form habit-like behaviors. Initial drug use, including that of ethanol, is commonly associated with goal-directed behavior that is susceptible to devaluation, whereas repeated exposure tends to produce a shift toward habitual use that becomes resistant to devaluation (Barker & Taylor, 2014; Corbit, Nie, & Janak, 2012; Leong, Berini, Ghee, & Reichel, 2016; Zapata, Minney, & Shippenberg, 2010). Thus, repeated ethanol exposure during adolescence may induce elevated ethanol consumption not only due to changes in behavioral flexibility but also via promoting habit formation, potentially resulting from alterations in brain regions underlying these behaviors. In fact, Barker and colleagues (Barker, Bryant, Osborne, & Chandler, 2017) found that AIE exposure led to a shift from goal-directed to habitual behavior in adulthood, a finding specific to females.
Goal-directed and habitual behaviors are influenced by distinct regions within the striatum (see Burton, Nakamura, & Roesch, 2015; Lipton, Gonzales, & Citri, 2019, for reviews). The dorsolateral striatum contributes to habitual responding, whereas the dorsomedial striatum is implicated in regulating goal-directed responding. Both systems are heavily influenced by the prefrontal cortex (PFC), and this top-down control has been shown to modify the development/expression of both goal-directed and habitual behavior (Smith & Laiks, 2018).
The striatum, frontostriatal connectivity, as well as dopaminergic innervation into the PFC undergo significant age-related remodeling throughout the broad adolescent period – maturation that is important for the formation of goal-directed behavior (DePasque & Galván, 2017; Hoops, Reynolds, Restrepo-Lozano, & Flores, 2018; Insel, Kastman, Glenn, & Somerville, 2017; Larsen & Luna, 2015; 2018; Somerville & Casey, 2010). Brain maturation during early adolescence is associated with reductions of volume within the striatum (Goddings et al., 2014; Lenroot et al., 2007), and increases in dopaminergic innervation (Hoops et al., 2018; Willing, Cortes, Brodsky, Kim, & Juraska, 2017) and synaptic pruning in the PFC (Drzewiecki, Willing, & Juraska, 2016; Mallya, Wang, Lee, & Deutch, 2019). In contrast, maturational changes during late adolescence and emerging adulthood are characterized by further refinement and strengthening of circuitry involving the PFC (Baker et al., 2015; Uematsu et al., 2017; van Duijvenvoorde, Westhoff, de Vos, Wierenga, & Crone, 2019). Thus, ethanol exposure during adolescence may influence different aspects of brain maturation due to the timing of that exposure. This possibility, however, has received limited attention to date (although see Alaux-Cantin et al., 2013; Desikan, Wills, & Ehlers, 2014; Saalfield & Spear, 2015; Sanchez-Roige, Peña-Oliver, Ripley, & Stephens, 2014; Varlinskaya et al., 2014; Varlinskaya et al., 2020).
The goal of the current study was to determine the impact of intermittent ethanol exposure during different adolescent developmental sub-periods on goal-directed behavior in adulthood. In order to test goal-directed behavior, we used a sensory-specific satiation procedure. With the maturation of striatal circuitry undergoing more expansive changes during early rather than late adolescence, we hypothesized that early AIE would promote habit-like responding following reward devaluation, whereas late AIE would have less of an effect. The findings from the current study suggest the opposite, with late AIE leading to significantly increased habit-like responding in adult males (along with a similar trend in females), whereas early water and ethanol exposures were associated with goal-directed responding.
Method
Subjects
Male and female Sprague-Dawley rats bred/reared in our colony at Binghamton University were used in the current study. All animals were maintained in a temperature- (21–23 °C) and humidity-controlled vivarium, with the lights on a 12-hour on/off schedule (lights on at 7:00 AM). Food (Purina Rat Chow; Lowell, Massachusetts, United States) and water were available ad libitum until the beginning of experimental testing. On the day after birth, postnatal day (P) 1, litters were culled to 10 rats with a sex ratio of six males to four females when possible. In order to limit the influence of litter effects on experimental outcomes (Zorrilla, 1997), a maximum of one rat per sex per litter was assigned to each condition. Sample sizes at the beginning of operant testing were between 14 and 16 animals, based on our previous work using reward devaluation procedures (Towner, Fager, & Spear, 2020). All rats were weaned and pair-housed with a same-sex littermate on P21. Animals were left undisturbed other than for routine cage cleaning until ethanol administration began. Experimental testing took place during the light cycle between 12:00 noon and 4:00 PM. Treatment, experimental manipulations, and maintenance of rats were conducted in agreement with the National Institutes of Health guidelines for animal care using protocols approved by Binghamton University Institutional Animal Care and Use Committee.
Adolescent intermittent ethanol (AIE) exposure
Rats were exposed to either tap water or ethanol during early (P25–45) or late (P45–65) adolescence/emerging adulthood. Ethanol (4 g/kg, 25% in tap water) was delivered via intragastric gavage every other day for a total of 11 exposures throughout the 20-day exposure period. This intragastric gavage procedure was performed by a trained experimenter. We have previously found blood ethanol concentrations (BECs) from this ethanol exposure model to be between 125 and 200 mg/dL, with minimal differences between sexes (Kim, Varlinskaya, Dannenhoffer, & Spear, 2019). Control rats were given an isovolumetric equivalent of tap water and were otherwise handled identically. Following the final ethanol exposure, all rats were given a period of approximately 25 days to allow for aging into adulthood prior to behavioral testing.
Behavioral procedure
Instrumental Training.
In adulthood (~P70 for early and ~P90 for late adolescent-exposure groups), rats were individually housed and gradually food-restricted to 85–90% of free-feeding weights across five days, using our prior data of free-feeding weight trajectories (see Vetter, Doremus-Fitzwater, & Spear, 2007). During operant testing, all animals gained weight, and thus the food restriction had little seeming impact on normal growth patterns. Three days prior to the onset of operant training, all animals were exposed to approximately 40 sucrose pellets, including both flavors to be used during the training and devaluation test sessions. Conditioning and extinction testing were conducted in operant chambers (ENV-007 Med Associates Inc.; St. Albans, Vermont, United States) fitted with two retractable levers on opposing sides of a food trough (magazine) and a house-light centered at the top of the wall opposite of the magazine. Upon each correct lever press, a pellet dispenser delivered a single 45-mg sucrose pellet that was either banana- or berry-flavored (Bio-Serv; Frenchtown, New Jersey, United States), with half of the animals per group pseudo-randomly assigned to the banana-flavored reinforcer and the remaining animals to the berry-flavored reinforcer.
On the first day of testing, animals were placed into sex-specific operant chambers and trained to retrieve sucrose pellets from the magazine provided on a variable interval (VI) 30-second schedule. The following day, animals were pseudo-randomly assigned to either the left or the right lever and trained on a fixed ratio (FR) 1 schedule. FR1 training was conducted over four consecutive days with an opportunity to attain a maximum of 50 sucrose pellets across the 60-minute trial. The criterion to move onto the next training phase was receipt of 50 pellets during at least one of the prior FR1 training sessions. Rats were then trained on a random interval (RI) 30-second schedule for two sessions and then progressed to a RI60 second schedule for the next four sessions. Both RI30 and RI60 training sessions had a maximum sucrose pellet delivery of 30 and a trial length of 60 minutes. Exclusion criterion during RI training was a failure to complete at least one training session, although no animals met this criterion.
Reward devaluation.
To test reward devaluation, we implemented a sensory-specific satiation protocol comparable to that used in our previous study (Towner et al., 2020). Prior to devaluation testing, rats were given 1-hour ad libitum access to either banana- or berry-flavored sucrose pellets. In the devalued condition, each animal received access to the flavor sucrose pellet that matched the reinforcer received during operant testing. In contrast, animals in the non-devalued condition were allowed access to the flavor of sucrose pellet that the animal had not previously experienced as a reinforcement during operant testing. Following the 1-hour access period, rats were immediately placed into the operant chambers for a 10-minute extinction trial, during which no rewards were delivered upon lever press. Over the next two days, animals were retrained on a RI60 schedule to re-establish lever pressing. The day after the second reinstatement RI60 session, animals went through the same sensory-specific satiation protocol; however, the opposite-flavored sucrose pellet was used. Animals then underwent extinction testing as before. Using this method, all animals received exposure to both the devalued and non-devalued conditions, counter-balanced within each group. Immediately following the second extinction session, rats were given simultaneous access to both flavored sucrose pellets for 15 minutes to determine efficacy of the satiation procedure.
Data analysis
Instrumental training of males and females, both FR and RI data, was analyzed using separate repeated-measures three-way ANOVAs with factors of exposure condition (water; ethanol), age of exposure (early; late), and training day included. Since assessment of sex as a biological variable recommends that data should be disaggregated by sex (Clayton, 2018), and given previously reported sex-specific behavioral alterations induced by AIE (Barker et al., 2017; Hauser et al., 2019; Kasten et al., 2020; Varlinskaya et al., 2020), analyses were performed separately for each sex. Lever presses during extinction trials that followed sensory-specific satiation were analyzed separately for each sex using repeated-measures three-way ANOVAs including factors of exposure condition, age of exposure, and reward devaluation conditions. To determine the size of the devaluation effect (see Renteria, Baltz, & Gremel, 2018), a devaluation index was calculated using the following equation: [(non-devalued lever presses – devalued lever presses)/(non-devalued lever presses + devalued lever presses)]. The devaluation index scores for each condition were then compared using a one-sample t test against a hypothetical mean of 0 (indicative of no difference in lever pressing between devaluation conditions). Sucrose pellet consumption during the satiation procedure and during post-consumption testing was also tested using similar three-way ANOVAs within each sex, with factors of exposure condition, age of exposure, and devaluation condition. When indicated, significant main effects and/or interactions were further analyzed using multiple comparisons with Sidak corrections.
Results
Instrumental training.
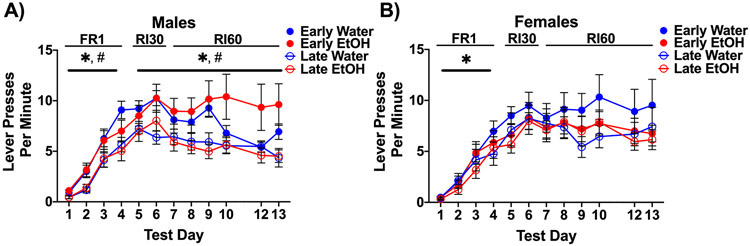
During FR1 training, 0–3 animals per group failed to meet the criterion of successful completion of one session. As mentioned previously, no animals were removed following FR training and therefore the final N per group was between 12 and 16 rats. When comparing FR1 training among males, there was an increase in responding across days. In addition, early-exposed males lever pressed more compared to late adolescent-exposed rats (see test days 1–4 in Figure 1A). A repeated-measures three-way ANOVA revealed a significant main effect of training day, F(3,153) = 128.50, p < 0.0001, a main effect of age of exposure, F(1,51) = 9.55, p < 0.01, and a training day by age of exposure interaction, F(3,153) = 3.52, p < 0.05. Follow-up analyses of the interaction revealed greater lever pressing of early-exposed males compared to late-exposed males, specifically on test days 2–4 (all p values < 0.05). Female rats also increased lever responses across FR1 training, as indicated by a significant main effect of training day, F(3,150) = 77.08, p < 0.0001. However, no other main effects or interactions were evident in female subjects. As can be seen in Figure 1A-B, all groups increased lever pressing across FR1 training sessions (test days 1–4), regardless of exposure age or adolescent exposure condition (ethanol or water).
Figure 1.
Lever presses per minute made during operant training. All groups increased lever pressing across FR1 training. For males, a decrease in lever pressing across RI training was evident, regardless of age of exposure and exposure condition (A). In addition, early-exposed males tended to make more lever presses per minute than late-exposed males. In contrast, no differences were seen for females (B). * indicates main effect of test day and # indicates main effect of age of exposure.
For RI training, males decreased lever responding across testing sessions, regardless of age of exposure, as noted by a significant main effect of training day, F(7,357) = 6.02, p < 0.0001 (see Figure 1A, test days 5–12). Similar to FR1 training, early-exposed males continued to lever press significantly more than late-exposed males, indicated by a main effect of age, F(1,51) = 9.01, p < 0.01. A trend for a three-way interaction of age of exposure, exposure condition, and training day was evident, F(7,357) = 2.02, p = 0.052, whereas no other main effects or interactions were found. For females, no significant main effects (p > 0.05) of age of exposure, exposure solution, training day, or their interactions were found (see test days 5–12 in Figure 1B). Males, regardless of exposure condition and age of exposure, generally had a slight reduction in lever pressing during RI training, whereas the responding of females remained relatively stable across trials.
Reward devaluation.
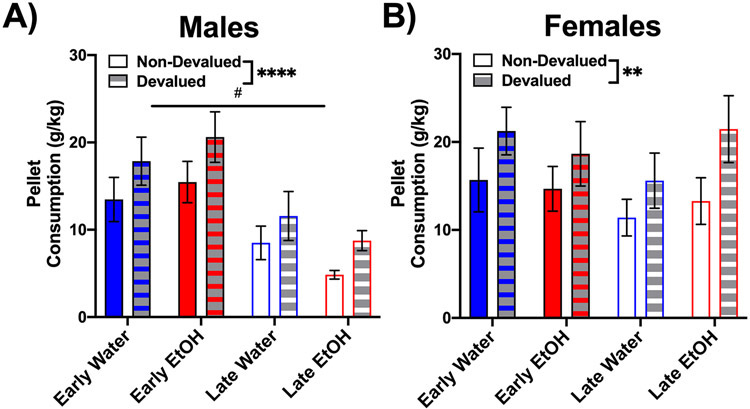
In order to test whether AIE altered goal-directed behavior, we used a sensory-specific satiation protocol, during which animals had free access to either the reinforcement used during operant training (devalued) or a never-reinforced sucrose pellet (non-devalued). Repeated-measures three-way ANOVAs of sucrose pellet consumption during the 1-hour free access period within each sex revealed a significant main effect of devaluation type (all F values ≥ 11.45, p < 0.05), with all groups consuming more sucrose pellets during the devaluation condition compared to non-devalued (Figure 2A-B). In males, a main effect of age was also evident, F(1,53) = 18.23, p < 0.0001, with early adolescent-exposed males consuming more sucrose pellets than late adolescent-exposed males. No other significant differences were evident (p > 0.05). During the ad libitum access to the different-flavored sucrose pellets, all groups had greater intake of the more familiar sucrose pellet (devalued condition) as can be seen in Figures 2A-B, a likely effect of some degree of initial neophobia to the less familiar tastant.
Figure 2.
The amount of sucrose pellets consumed (g/kg) during the 1-hour free access periods prior to extinction testing. All groups (A–B) had increased consumption of pellets in the devalued condition in comparison to the non-devalued condition. Early-exposed males also had greater sucrose pellet consumption compared with late adolescent-exposed males. ** (p < 0.01) and **** (p < 0.0001) indicate main effect of devaluation and # indicates main effect of age of exposure, p < 0.05.
To evaluate whether responding during the devaluation period was a result of intake during the pre-testing consumption phase, we assessed correlations between the amount of sucrose pellets consumed and lever pressing during the extinction trial. For early adolescent-exposed males and females, negative correlations between g/kg consumed and lever pressing were found when animals were given access to the non-devalued condition sucrose pellets (females r = −0.48, p = 0.01; males r = −0.59, p = 0.002). In contrast, responding during the devalued condition was not dependent on the amount of sucrose pellets consumed for early-exposed animals (p > 0.05). Similar correlations conducted in late adolescent-exposed males and females did not reveal any significant relationships (p > 0.05) between pre-test consumption of either the devalued or non-devalued sucrose pellets and lever pressing during extinction (see Supplemental Figure 1A-D). With a lack of significant relationships for responding following access to the devalued condition in all groups, we are confident that lever pressing in the extinction trial following reward devaluation was not driven solely by how much was consumed in the previous access period.
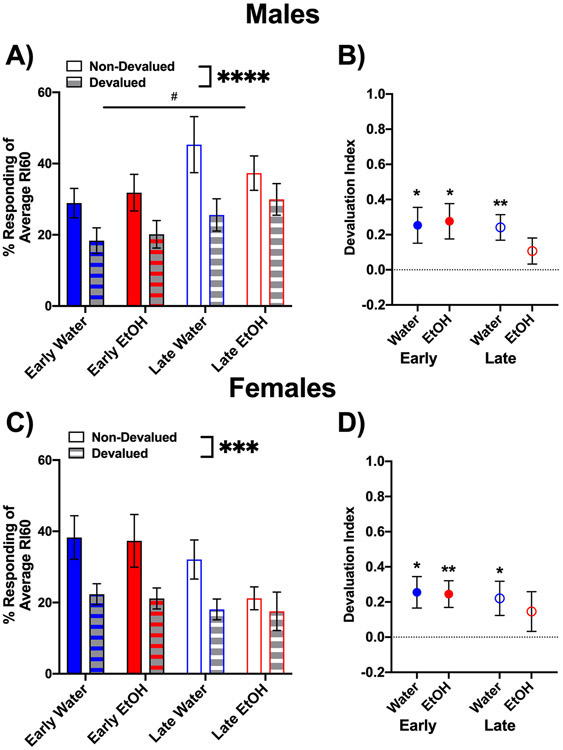
To account for differences in lever pressing during RI training, we normalized the lever pressing following devaluation with respect to the lever pressing of each animal over the RI60 training sessions. To create a percent baseline, we divided the lever presses per minute made during extinction by the averaged lever presses per minute across RI60, with this value then multiplied by 100. Using repeated-measures three-way ANOVAs to assess the normalized lever pressing following reward devaluation within each sex, a main effect of devaluation type was found in both males, F(1,48) = 20.60, p < 0.0001, and females, F(1, 47) = 16.42, p < 0.001. As seen in Figures 3A and C, all groups had decreased lever pressing after reward devaluation when compared with responding following the non-devalued condition. In males, there was also a significant main effect of age, F(1,48) = 5.37, p < 0.05, with early-exposed males lever pressing less in extinction compared with late-exposed males.
Figure 3.
Normalized responding made during the 10-minute extinction trial following the sensory-specific satiation procedure. For both sexes, early and late adolescent water- and ethanol-exposed animals (A and C) had reduced lever press responses following devaluation. When assessing the devaluation index (B and D), late AIE males and females did not display different response patterns between devalued and non-devalued conditions. *** (p < 0.001) and **** (p < 0.0001) indicate main effect of devaluation and # indicates main effect of age of exposure, p < 0.05 (A and C) and devaluation index significantly different from 0 indicated by * (p < 0.05) and ** (p < 0.01, B and D).
When assessing the degree of devaluation with the devaluation index, this index differed significantly from 0 in early-exposed animals regardless of sex and exposure condition (all t values ≥ 2.48, p < 0.05). In contrast, in late-exposed animals, significant differences from 0 were only evident for water-exposed males and females (t values ≥ 2.27, p < 0.05), whereas these indices did not differ significantly from 0 in late adolescent ethanol-exposed animals of both sexes. Assessments of the devaluation index confirmed that early-exposed males and females as well as late water-exposed animals were sensitive to reward devaluation displaying goal-directed behavior. However, these assessments also revealed that the devaluation effect was minimal in late ethanol-exposed males and females, suggestive of a more habitual pattern of responding (Figures 3B and D).
To control for order effects of devaluation testing, we conducted two-way ANOVAs within each exposure group comparing the first and second extinction test sessions. No main effects of test day were evident for any exposure group (all F values ≤ 1.64, p > 0.05).
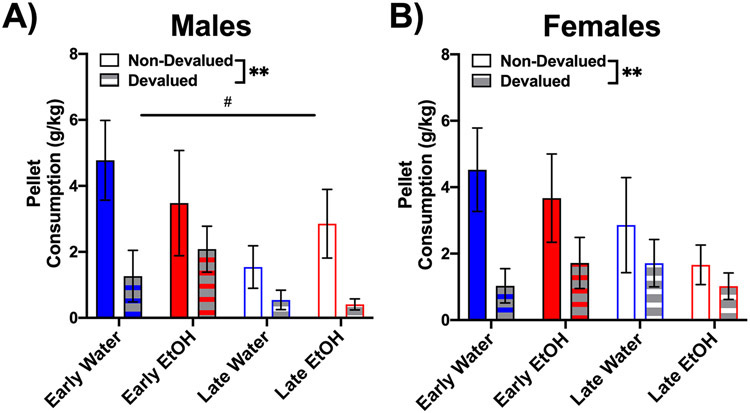
To ensure that the sensory-specific satiation procedure was effective, we gave animals simultaneous access to both flavored sucrose pellets for 15 minutes following the extinction trial, referred to as a post-consumption test. As seen in Figures 4A and B, all groups tended to consume more of the pellets that were not devalued prior to testing that day in comparison to the sucrose pellets that were devalued, confirmed by a main effect of devaluation type for both males and females (F values ≥ 7.97, p < 0.01). In males, early adolescent-exposed rats had greater consumption than late adolescent-exposed animals, as evidenced by a main effect of age, F(1,43) = 8.32, p < 0.01. These findings support that the implemented sensory-specific satiation procedure was effective.
Figure 4.
Post-consumption test to ensure the effectiveness of the sensory-specific satiation procedure. All conditions had greater intake of the sucrose pellets that were not devalued prior to testing (A and B). Early adolescent-exposed males also consumed more than late adolescent-exposed males. ** (p < 0.01) indicates main effect of devaluation and # indicates main effect of age of exposure, p < 0.05.
Discussion
Many studies have found that adolescent ethanol exposure leads to persistent deficits in behaviors of adult rats (see Crews et al., 2019, for review), including the inability to update behavior using tasks of behavioral flexibility (Coleman et al., 2011; 2014; Fernandez & Savage, 2017; Gass et al., 2014; Varlinskaya et al., 2020). The behavioral inflexibility observed following AIE may be associated with the development of habitual behavior, although shifts from goal-directed behavior to habit formation have not been thoroughly studied after AIE. In the current study, we tested the hypothesis that AIE during early, but not late, adolescence would lead to increased habit formation following a reward devaluation task. Contrary to our hypothesis, both male and female early AIE rats exhibited reductions in responding following reward devaluation similar to that of water-exposed rats, a finding suggesting a pattern of goal-directed behavior in these animals. In contrast, late AIE rats were less sensitive to reward devaluation, with similar rates of responding following devalued and non-devalued test sessions. The persistence of elevated lever pressing following devaluation in late AIE rats is representative of a more habitual response pattern.
During the FR1 training phase, all groups increased lever pressing across days, regardless of previous age of exposure, exposure solution, and sex. The observed increase in lever pressing during FR1 training was consistent with animals learning the response/outcome contingency. After completion of FR1 training, when rats progressed to RI training, both early- and late-exposed male rats exhibited modest reductions in lever pressing across days. The slight reduction seen in early- and late-exposed males could be associated with reductions in effort due to learning that the reward contingency is no longer closely associated with effort (number of lever presses). Females exposed to water and ethanol during both adolescent developmental periods maintained similar rates of lever pressing across RI training. Taken together, the results of the present study demonstrated that AIE had limited effects on FR1 and RI training. The minimal effects under these circumstances are similar to other studies that have shown adolescent ethanol exposure to have little impact on instrumental conditioning (Jury et al., 2017; Risher et al., 2013; Varlinskaya et al., 2020). Importantly, the lack of differences in instrumental training observed after AIE suggests that differences during the reward devaluation task are associated with alterations to the development of goal-directedness and habitual behavior and not to alterations in conditioning per se.
Using a sensory-specific satiation procedure, we evaluated the impact of AIE on goal-directed behavior and found that early AIE (P25–45) had no effect. More specifically, rats of both sexes exposed to ethanol and water during early adolescence reduced lever pressing behavior following reward devaluation. The lack of early AIE-induced changes in habitual behavior was particularly surprising, as our previous findings using a behavioral flexibility task revealed moderate impacts of ethanol exposure during the early adolescent period (Varlinskaya et al., 2020). Likewise, AIE-induced changes in behavioral flexibility as a result of ethanol exposure during early-mid adolescence have also been found (Coleman et al., 2014; Gass et al., 2014; Sey, Gómez-A, Madayag, Boettiger, & Robinson, 2019). Furthermore, Barker et al. (2017) found that AIE from P28–44 resulted in habitual responding in adulthood, an effect evident only in females. However, the lack of habitual responding in males exposed to AIE is similar to the observed findings in the present study (Barker et al., 2017). The different findings between the current study and previous work (Barker et al., 2017; Coleman et al., 2014; Gass et al., 2014; Sey et al., 2019; Varlinskaya et al., 2020) could be due to a number of factors, including route and dose of ethanol exposure, rat strain, and the behavioral task used to measure behavior.
The circuitry involved in the development of goal-directed and habitual behavior undergoes substantial remodeling during early adolescence (DePasque & Galván, 2017; Hoops et al., 2018; Insel et al., 2017; Larsen & Luna, 2015; 2018; Somerville & Casey, 2010). Previous studies have reported adolescent ethanol-induced alterations to parts of the striatum involved in habit formation and goal-directed behavior (Broadwater et al., 2018; Cuzon Carlson, Grant, & Lovinger, 2018; Evrard et al., 2006; Jones, Cservenka, & Nagel, 2016). However, none of these studies evaluated changes in goal-directed behavior per se. These previous reports of striatal dysfunction following AIE support the potential for impairment in behaviors such as habit formation. However, the age during which the ethanol exposure occurred in these studies typically spanned from early to late adolescence, a factor that might have contributed to the lack of effect in the present study of rats exposed to ethanol only during early adolescence.
Traditional analyses using normalized responding during extinction following devaluation revealed an omnibus effect of devaluation type for all late-adolescent exposure groups, suggesting that late exposure to either ethanol or water did not alter goal-directed behavior in these animals. However, using a devaluation index to assess the strength of this devaluation effect, it was found that male and female rats exposed to late AIE (P45–65) had consistent lever pressing between the devalued and non-devalued conditions, revealing a more habit-like response pattern in these animals. These findings are similar to studies that exposed animals to ethanol across early and late adolescence and found alterations to behaviors such as set-shifting (Fernandez & Savage, 2017) and reversal learning (Contreras et al., 2019; Fernandez, Lew, Vedder, & Savage, 2017; Galaj et al., 2019). However, Fisher and colleagues (Fisher, Bright, Gallo, Pajser, & Picken, 2017) reported that ethanol consumption spanning early and late adolescence did not influence subsequent reversal learning or habit formation, a finding possibly due to the low blood ethanol concentrations achieved as a result of the voluntary consumption. The greater propensity of late AIE animals to form habits is also consistent with findings from studies of chronic intermittent ethanol (CIE) exposure in adulthood (Barker et al., 2017; Renteria et al., 2018). For example, Renteria, Baltz, and Gremel (2018) found increased habit formation among CIE-exposed mice using a reward devaluation procedure similar to that used in the current study. Renteria et al. (2018) also reported a reduction in the excitability of the orbitofrontal cortex to dorsal medial striatum circuitry in animals exposed to CIE, and suggested that this alteration could contribute to the greater habit formation observed in that study. Indeed, a restoration of goal-directed behavior was observed following chemogenetic excitation of this circuit in CIE rats in that study. Although these findings were observed in animals exposed to ethanol in adulthood, similar alterations to the circuitry involved with goal-directedness and habit formation have also been found following adolescent ethanol exposure (Broadwater et al., 2018; Cuzon Carlson et al., 2018; Evrard et al., 2006; Jones et al., 2016). For example, Johnson, Liput, Homanics, and Lovinger (2020) found that intermittent ethanol during adolescence led to disruptions in glutamatergic signaling in the dorsolateral striatum. In the current study, it is possible that our late AIE exposure resulted in a long-term impact on the frontostriatal circuit leading to shifts from goal-directedness to habitual behavior. Follow-up studies to test this hypothesis are needed.
The importance of AIE timing has received little attention, although previous findings suggest that behavioral consequences resulting from AIE can be specific to age at exposure (for review see Spear, 2015). The results from the current study showing that late, but not early, AIE led to greater susceptibility for habit formation in adulthood further contribute to findings of timing-specific behavioral alterations. The differential maturation occurring between early and late adolescence (DePasque & Galván, 2017; Hoops et al., 2018; Insel et al., 2017; Larsen & Luna, 2015; 2018; Somerville & Casey, 2010) may underlie the observed ethanol-induced changes to habit formation. For example, Galvan et al. (2006) found that the orbitofrontal cortex develops substantially later in adolescence than the nucleus accumbens. It is possible that late AIE exposure influenced such orbitofrontal maturation in the present study, subsequently increasing habit formation in these animals. In contrast, previous work from our laboratory has shown that early AIE affects brain regions important for regulating behaviors such as social anxiety-like behavior (Dannenhoffer et al., 2018; Kim et al., 2019), with these effects not evident after late AIE. Thus, it is likely that early and late AIE exposures affect distinct brain regions that mediate specific behaviors as a result of the brain maturational processes occurring at the time the ethanol was administered.
The differential impact of AIE timing in the current study could also be due to the age at which the ethanol exposure was terminated. Adolescence is thought of as a critical/sensitive period (Aoki, Romeo, & Smith, 2017; Larsen & Luna, 2018), and the end of an insult prior to the closure of this period may allow for recovery or compensation to occur. Previous studies have found that a substantial amount of neural development occurs post-puberty (see Juraska & Willing, 2017, for review). In the present study, the early adolescent-exposure period occurred predominantly prior to puberty in both males and females. We may have failed to see effects in early-exposed animals due to greater neural development occurring post-pubertally versus pre-pubertally, and thus having a limited impact on the developing system at this time. Moreover, the early AIE exposure period ends well within the adolescent sensitive period, and hence animals in this group may exhibit greater recovery/compensation from deficits resulting from that exposure. Thus, plasticity occurring during the latter portion of adolescence may allow for a restoration of normal behavior after early AIE. In contrast, our late adolescent exposure occurs post-pubertally and ends in early adulthood. The ending of the ethanol exposure outside of the adolescent sensitive period in the late AIE rats likely provides less opportunity for compensatory processes to emerge, resulting in persistent behavioral deficits as noted by the increased habit formation in these animals. Future work is needed to determine whether termination of AIE within the adolescent sensitive period allows for the development of compensatory mechanisms and a recovery of goal-directed behavior.
A number of behaviors appear to be affected by AIE in a sex-specific manner (Carzoli et al., 2019; Dannenhoffer et al., 2018; Hauser et al., 2019; Kasten et al., 2020; Logrip et al., 2013; Kim et al., 2019; Madayag, Stringfield, Reissner, Boettiger, & Robinson, 2017; Ruby et al., 2018; Varlinskaya, Kim, & Spear, 2017; Varlinskaya et al., 2020). However, in the current study, males and females displayed generally similar patterns of behavior when tested on the reward devaluation procedure, regardless of ethanol exposure and age of exposure. A lack of sex differences following AIE on a Pavlovian conditioned approach task have been found elsewhere (Madayag et al., 2017). Similarly, after adult CIE exposure, no sex differences were reported in the development of habitual behavior (Renteria et al., 2018). Together, these findings suggest that the effect of adolescent ethanol exposure on goal-directedness and habit formation in adulthood may be independent of sex.
Previous studies have found that stress during early life (Grissom et al., 2012; Patterson, Craske, & Knowlton, 2013; 2019), adolescence (Barfield et al., 2017; Hinton, Li, Allen, & Gourley, 2019), and adulthood (Braun & Hauber, 2013; Dias-Ferreira et al., 2009; Fournier, d’Arripe-Longueville, & Radel, 2017; Schwabe, Tegenthoff, Höffken, & Wolf, 2010; 2012; Schwabe & Wolf, 2010) can predispose both humans and rodents toward habit formation. Although not evaluated in the current study, intragastric gavage may be stressful itself, as noted in previous studies (Bonnichsen, Dragsted, & Hansen, 2005; Chakraborty & Chattarji, 2019; Õkva et al., 2006; Walker et al., 2012), with this stress response potentially contributing to the observed shifts in habit formation in the present study. It seems unlikely, however, since the shifts from goal-directed to habitual behavior were only observed following ethanol exposure during late adolescence, with no effects of gavage evident in water-exposed animals or animals exposed during adolescence. In addition, intragastric gavage elicits a stress response similar to that of a routine cage change (Õkva et al., 2006) therefore any gavage-associated stress likely had a minimal effect on the changes to habit formation observed in the current study.
The current study had a few limitations that should be considered. All groups consumed more g/kg of the devalued sucrose pellets in the pre-feeding session, a finding that could drive reductions in lever pressing following this access period. To control for this difference in consumption between devalued and non-devalued conditions, we conducted correlations with g/kg consumed and lever pressing during extinction, with no meaningful relationships emerging. Interestingly, negative correlations for only the non-devalued condition were evident in early-exposed rats, but not in the other groups. Although the meaning of these correlations is unclear, these animals displayed goal-directed behavior and hence this may further represent a goal-directed phenotype. In contrast, responding following reward devaluation was not correlated with the amount consumed for any group. Thus, lever pressing during extinction after the devalued access period was not directly associated with amount consumed and therefore may be attributed to a greater familiarity of these sucrose pellets. Another important consideration is that early and late adolescent-exposure groups were tested at different ages in adulthood. A number of age of exposure differences were noted, particularly in males, findings that may have been associated with age when behavioral testing was conducted, since these differences were evident regardless of adolescent exposure condition. A final limitation that should be noted is that rats underwent two extinction trials, and it is possible that responses during the second extinction were reduced due to this repeated extinction testing. To control for this possibility, we retrained animals for two days following the first extinction in order to re-establish responding. Furthermore, analyses between test days did not reveal differences as a result of repeated testing. Thus, we are confident that prior testing did not influence subsequent responding.
In humans, prolonged ethanol consumption can promote the development of habitual drinking that is more dependent on drinking cues rather than the reinforcing properties of ethanol (Barker & Taylor, 2014). The current study found that repeated binge ethanol exposure during adolescence led to a greater sensitivity to develop habits in adulthood. Our findings were dependent upon the age of exposure, with late AIE increasing habit formation, an effect not evident following early adolescent exposure. The shift toward habit formation following late AIE may promote the habitual use of ethanol. Additional studies are needed to determine whether such AIE exposure leads to the development of habitual ethanol consumption and to determine the mechanisms underlying these effects.
Supplementary Material
Supplemental Figure 1. Correlations of lever pressing during extinction with amount of sucrose pellets consumed during the free access period. Early adolescent-exposed male (A) and female (C) rats had lower responding when consuming more of the non-devalued sucrose pellets. However, this was not seen for the devalued condition. Both male and female animals exposed during late adolescence (B and D) had no significant correlations between lever pressing and amount consumed.
Highlights.
Exposure to ethanol in early adolescence led to persistent goal-directed behavior.
Late adolescent ethanol exposure increased habit formation in adulthood.
Adolescent ethanol-induced change to habit formation was not sex-dependent.
Acknowledgments
This work was supported by the National Institute of Alcoholism and Alcohol Abuse Neurobiology of Adolescent Drinking in Adulthood project (U01 AA019972) and the Development and Neuroadaptation in Alcohol and Addictions training grant (T32 AA025606). The findings, conclusions, and suggestions are those of the authors and do not necessarily reflect those of the funding agencies listed above. We also thank Drs. Varlinskaya and Werner for their contributions in the revision of this publication.
Footnotes
Declarations of interest
None.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–531. doi: 10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Aoki C, Romeo RD, & Smith SS (2017). Adolescence as a critical period for developmental plasticity. Brain Research, 1654(Pt B), 85–86. doi: 10.1016/j.brainres.2016.11.026 [DOI] [PubMed] [Google Scholar]
- Baker STE, Lubman DI, Yücel M, Allen NB, Whittle S, Fulcher BD, et al. (2015). Developmental changes in brain network hub connectivity in late adolescence. The Journal of Neuroscience, 35(24), 9078–9087. doi: 10.1523/JNEUROSCI.5043-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, Gerber KJ, Zimmermann KS, Ressler KJ, Parsons RG, & Gourley SL (2017). Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS Biology, 15(11), e2003000. doi: 10.1371/journal.pbio.2003000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Bryant KG, Osborne JI, & Chandler LJ (2017). Age and sex interact to mediate the effects of intermittent, high-dose ethanol exposure on behavioral flexibility. Frontiers in Pharmacology, 8, 450. doi: 10.3389/fphar.2017.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, & Taylor JR (2014). Habitual alcohol seeking: Modeling the transition from casual drinking to addiction. Neuroscience and Biobehavioral Reviews, 47, 281–294. doi: 10.1016/j.neubiorev.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, & Smith RF (2006). Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiology and Behavior, 88(4–5), 466–472. doi: 10.1016/j.physbeh.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Bonnichsen M, Dragsted N, & Hansen AK (2005). The welfare impact of gavaging laboratory rats. Animal Welfare, 14(3), 223–227. [Google Scholar]
- Braun S, & Hauber W (2013). Acute stressor effects on goal-directed action in rats. Learning and Memory, 20(12), 700–709. doi: 10.1101/lm.032987.113 [DOI] [PubMed] [Google Scholar]
- Broadwater M, & Spear LP (2013). Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behavioural Brain Research, 256, 10–19. doi: 10.1016/j.bbr.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, et al. (2018). Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addiction Biology, 23(2), 810–823. doi: 10.1111/adb.12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, & Roesch MR (2015). From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiology of Learning and Memory, 117, 51–59. doi: 10.1016/j.nlm.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carzoli KL, Sharfman NM, Lerner MR, Miller MC, Holmgren EB, & Wills TA (2019). Regulation of NMDA receptor plasticity in the BNST following adolescent alcohol exposure. Frontiers in Cellular Neuroscience, 13, 440. doi: 10.3389/fncel.2019.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P, & Chattarji S (2019). Interventions after acute stress prevent its delayed effects on the amygdala. Neurobiology of Stress, 10, 100168. doi: 10.1016/j.ynstr.2019.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA (2018). Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiology & Behavior, 187, 2–5. doi: 10.1016/j.physbeh.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Coleman LG, He J, Lee J, Styner M, & Crews FT (2011). Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism: Clinical and Experimental Research, 35(4), 671–688. doi: 10.1111/j.1530-0277.2010.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Liu W, Oguz I, Styner M, & Crews FT (2014). Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacology, Biochemistry, and Behavior, 116, 142–151. doi: 10.1016/j.pbb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A, Polín E, Miguéns M, Pérez-García C, Pérez V, Ruiz-Gayo M, et al. (2019). Intermittent-excessive and chronic-moderate ethanol intake during adolescence impair spatial learning, memory and cognitive flexibility in the adulthood. Neuroscience, 418, 205–217. doi: 10.1016/j.neuroscience.2019.08.051 [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, & Janak PH (2012). Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry, 72(5), 389–395. doi: 10.1016/j.biopsych.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, et al. (2019). Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcoholism: Clinical and Experimental Research, 43(9), 1806–1822. doi: 10.1111/acer.14154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Grant KA, & Lovinger DM (2018). Synaptic adaptations to chronic ethanol intake in male rhesus monkey dorsal striatum depend on age of drinking onset. Neuropharmacology, 131, 128–142. doi: 10.1016/j.neuropharm.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer CA, Kim EU, Saalfield J, Werner DF, Varlinskaya EI, & Spear LP (2018). Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology, 235(10), 3065–3077. doi: 10.1007/s00213-018-5003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasque S, & Galván A (2017). Frontostriatal development and probabilistic reinforcement learning during adolescence. Neurobiology of Learning and Memory, 143, 1–7. doi: 10.1016/j.nlm.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Desikan A, Wills DN, & Ehlers CL (2014). Ontogeny and adolescent alcohol exposure in Wistar rats: Open field conflict, light/dark box and forced swim test. Pharmacology, Biochemistry, and Behavior, 122, 279–285. doi: 10.1016/j.pbb.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science, 325(5940), 621–625. doi: 10.1126/science.1171203 [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, & Spear LP (2005). Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research, 29(10), 1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa [DOI] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, & Juraska JM (2016). Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse, 70(9), 361–368. doi: 10.1002/syn.21909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, & Brusco A (2006). A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: An immunohistochemical study. Experimental Neurology, 200(2), 438–459. doi: 10.1016/j.expneurol.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Fernandez GM, Lew BJ, Vedder LC, & Savage LM (2017). Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience, 348, 324–334. doi: 10.1016/j.neuroscience.2017.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GM, & Savage LM (2017). Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience, 361, 129–143. doi: 10.1016/j.neuroscience.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H, Bright N, Gallo M, Pajser A, & Pickens CL (2017). Relationship of low doses of alcohol voluntarily consumed during adolescence and early adulthood with subsequent behavioral flexibility. Behavioural Pharmacology, 28(7), 531–544. doi: 10.1097/FBP.0000000000000331 [DOI] [PubMed] [Google Scholar]
- Fournier M, d’Arripe- Longueville F, & Radel R (2017). Effects of psychosocial stress on the goal-directed and habit memory systems during learning and later execution. Psychoneuroendocrinology, 77, 275–283. doi: 10.1016/j.psyneuen.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Galaj E, Kipp BT, Floresco SB, & Savage LM (2019). Persistent alterations of accumbal cholinergic interneurons and cognitive dysfunction after adolescent intermittent ethanol exposure. Neuroscience, 404, 153–164. doi: 10.1016/j.neuroscience.2019.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience, 26(25), 6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology, 39(11), 2570–2583. doi: 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, & Blakemore SJ (2014). The influence of puberty on subcortical brain development. NeuroImage, 88, 242–251. doi: 10.1016/j.neuroimage.2013.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom EM, Hawley WR, Bromley-Dulfano SS, Marino SE, Stathopoulos NG, & Dohanich GP (2012). Learning strategy is influenced by trait anxiety and early rearing conditions in prepubertal male, but not prepubertal female rats. Neurobiology of Learning and Memory, 98(2), 174–181. doi: 10.1016/j.nlm.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Hauser SR, Knight CP, Truitt WA, Waeiss RA, Holt IS, Carvajal GB, et al. (2019). Adolescent intermittent ethanol increases the sensitivity to the reinforcing properties of ethanol and the expression of select cholinergic and dopaminergic genes within the posterior ventral tegmental area. Alcoholism: Clinical and Experimental Research, 43(9), 1937–1948. doi: 10.1111/acer.14150 [DOI] [PubMed] [Google Scholar]
- Hinton EA, Li DC, Allen AG, & Gourley SL (2019). Social isolation in adolescence disrupts cortical development and goal-dependent decision-making in adulthood, despite social reintegration. ENeuro, 6(5), ENEURO.0318-19.2019. doi: 10.1523/ENEURO.0318-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops D, Reynolds LM, Restrepo-Lozano JM, & Flores C (2018). Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence. ENeuro, 5(1), ENEURO.0372-17.2017. doi: 10.1523/ENEURO.0372-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel C, Kastman EK, Glenn CR, & Somerville LH (2017). Development of corticostriatal connectivity constrains goal-directed behavior during adolescence. Nature Communications, 8(1), 1605. doi: 10.1038/s41467-017-01369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Liput DJ, Homanics GE, & Lovinger DM (2020). Age-dependent impairment of metabotropic glutamate receptor 2-dependent long-term depression in the mouse striatum by chronic ethanol exposure. Alcohol, 82, 11–21. doi: 10.1016/j.alcohol.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Cservenka A, & Nagel BJ (2016). Binge drinking impacts dorsal striatal response during decision making in adolescents. NeuroImage, 129, 378–388. doi: 10.1016/j.neuroimage.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, & Willing J (2017). Pubertal onset as a critical transition for neural development and cognition. Brain Research, 1654(Pt B), 87–94. doi: 10.1016/j.brainres.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, et al. (2017). Chronic ethanol during adolescence impacts corticolimbic dendritic spines and behavior. Alcoholism: Clinical and Experimental Research, 41(7), 1298–1308. doi: 10.1111/acer.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Carzoli KL, Sharfman NM, Henderson T, Holmgren EB, Lerner MR, et al. (2020). Adolescent alcohol exposure produces sex differences in negative affect-like behavior and group I mGluR BNST plasticity. Neuropsychopharmacology, 45(8), 1306–1315. doi: 10.1038/s41386-020-0670-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EU, Varlinskaya EI, Dannenhoffer CA, & Spear LP (2019). Adolescent intermittent ethanol exposure: Effects on pubertal development, novelty seeking, and social interaction in adulthood. Alcohol, 75, 19–29. doi: 10.1016/j.alcohol.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Larsen B, & Luna B (2015). In vivo evidence of neurophysiological maturation of the human adolescent striatum. Developmental Cognitive Neuroscience, 12, 74–85. doi: 10.1016/j.dcn.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, & Luna B (2018). Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience and Biobehavioral Reviews, 94, 179–195. doi: 10.1016/j.neubiorev.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36(4), 1065–1073. doi: 10.1016/j.neuroimage.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Berini CR, Ghee SM, & Reichel CM (2016). Extended cocaine-seeking produces a shift from goal-directed to habitual responding in rats. Physiology & Behavior, 164(Pt A), 330–335. doi: 10.1016/j.physbeh.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton DM, Gonzales BJ, & Citri A (2019). Dorsal striatal circuits for habits, compulsions and addictions. Frontiers in Systems Neuroscience, 13, 1–14. doi: 10.3389/fnsys.2019.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Rivier C, Lau C, Im S, Vaughan J, & Lee S (2013). Adolescent alcohol exposure alters the rat adult hypothalamic-pituitary-adrenal axis responsiveness in a sex-specific manner. Neuroscience, 235, 174–186. doi: 10.1016/j.neuroscience.2012.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag AC, Stringfield SJ, Reissner KJ, Boettiger CA, & Robinson DL (2017). Sex and Adolescent Ethanol Exposure Influence Pavlovian Conditioned Approach. Alcoholism: Clinical and Experimental Research, 41(4), 846–856. doi: 10.1111/acer.13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallya AP, Wang HD, Lee HNR, & Deutch AY (2019). Microglial pruning of synapses in the prefrontal cortex during adolescence. Cerebral Cortex, 29(4), 1634–1643. doi: 10.1093/cercor/bhy061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Fromme K (2012). Age of first use and delay to first intoxication in relation to trajectories of heavy drinking and alcohol-related problems during emerging adulthood. Alcoholism: Clinical and Experimental Research, 36(11), 1991–1999. doi: 10.1111/j.1530-0277.2012.01812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, L’Insalata A, Butler ER, McKee A, & Krishnan-Sarin S (2018). Age at drinking onset, age at first intoxication, and delay to first intoxication: Assessing the concurrent validity of measures of drinking initiation with alcohol use and related problems. Addictive Behaviors, 79, 195–200. doi: 10.1016/j.addbeh.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannessian CMC, Finan LJ, Schulz J, & Hesselbrock V (2015). A long-term longitudinal examination of the effect of early onset of alcohol and drug use on later alcohol abuse. Substance Abuse, 36(4), 440–444. doi: 10.1080/08897077.2014.989353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Õkva K, Tamoševičiute E, Čižiute A, Pokk P, Rukšenas O, & Nevalainen T (2006). Refinements for intragastric gavage in rats. Scandinavian Journal of Laboratory Animal Science, 33(4), 243–252. [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, & Zhang H (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiology of Disease, 82, 607–619. doi: 10.1016/j.nbd.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, & Schulenberg JE (2013). Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Research: Current Reviews, 35(2), 193–200. [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, & Terry-McElrath YM (2019). Prevalence of high-intensity drinking from adolescence through young adulthood: National data from 2016-2017. Substance Abuse: Research and Treatment, 13, 1178221818822976. doi: 10.1177/1178221818822976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TK, Craske MG, & Knowlton BJ (2013). The effect of early-life stress on memory systems supporting instrumental behavior. Hippocampus, 23(11), 1025–1034. doi: 10.1002/hipo.22174 [DOI] [PubMed] [Google Scholar]
- Patterson TK, Craske MG, & Knowlton BJ (2019). Enhanced avoidance habits in relation to history of early-life stress. Frontiers in Psychology, 10, 1876. doi: 10.3389/fpsyg.2019.01876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, & Gremel CM (2018). Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nature Communications, 9(1), 211. doi: 10.1038/s41467-017-02615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, et al. (2013). Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: Radial-arm maze performance and operant food reinforced responding. PLoS One, 8(5), e62940. doi: 10.1371/journal.pone.0062940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Paye G, Fabi JL, Zhang J, Risinger MO, Palmer KN, et al. (2018). Sex differences in photic entrainment and sensitivity to ethanol-induced chronodisruption in adult mice after adolescent intermittent ethanol exposure. Alcoholism: Clinical and Experimental Research, 42(11), 2144–2159. doi: 10.1111/acer.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfield J, & Spear L (2015). Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Developmental Cognitive Neuroscience, 16, 174–182. doi: 10.1016/j.dcn.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Kyzar EJ, Gavin DP, Zhang H, Chen Y, Krishnan HR, et al. (2019). Altered amygdala DNA methylation mechanisms after adolescent alcohol exposure contribute to adult anxiety and alcohol drinking. Neuropharmacology, 157, 107679. doi: 10.1016/j.neuropharm.2019.107679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Peña-Oliver Y, Ripley TL, & Stephens DN (2014). Repeated ethanol exposure during early and late adolescence: Double dissociation of effects on waiting and choice impulsivity. Alcoholism: Clinical and Experimental Research, 38(10), 2579–2589. doi: 10.1111/acer.12535 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, & Wolf OT (2010). Concurrent glucocorticoid and noradrenergic activity shifts instrumental behavior from goal-directed to habitual control. The Journal of Neuroscience, 30(24), 8190–8196. doi: 10.1523/JNEUROSCI.0734-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, & Wolf OT (2012). Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. The Journal of Neuroscience, 32(30), 10146–10155. doi: 10.1523/JNEUROSCI.1304-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, & Wolf OT (2010). Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology, 35(7), 977–986. doi: 10.1016/j.psyneuen.2009.12.010 [DOI] [PubMed] [Google Scholar]
- Sey NYA, Gómez-A A, Madayag AC, Boettiger CA, & Robinson DL (2019). Adolescent intermittent ethanol impairs behavioral flexibility in a rat foraging task in adulthood. Behavioural Brain Research, 373, 112085. doi: 10.1016/j.bbr.2019.112085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, & Laiks LS (2018). Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87(Pt A), 11–21. doi: 10.1016/j.pnpbp.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, & Casey BJ (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20(2), 236–241. doi: 10.1016/j.conb.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2015). Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiology & Behavior, 148, 122–130. doi: 10.1016/j.physbeh.2015.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Rockville, MD: SAMHSA. Retrieved from https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf [Google Scholar]
- Towner TT, Fager M, & Spear LP (2020). Adolescent but not adult Sprague-Dawley rats display goal-directed responding after reward devaluation. Developmental Psychobiology, 62, 368–379. doi: 10.1002/dev.21912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner TT, & Varlinskaya EI (2020). Adolescent ethanol exposure: Anxiety-like behavioral alterations, ethanol intake, and sensitivity. Frontiers in Behavioral Neuroscience, 14, 45. doi: 10.3389/fnbeh.2020.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Hata J, Komaki Y, Seki F, Yamada C, Okahara N, et al. (2017). Mapping orbitofrontal-limbic maturation in non-human primates: A longitudinal magnetic resonance imaging study. NeuroImage, 163, 55–67. doi: 10.1016/j.neuroimage.2017.09.028 [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Westhoff B, de Vos F, Wierenga LM, & Crone EA (2019). A three-wave longitudinal study of subcortical-cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Human Brain Mapping, 40(13), 3769–3783. doi: 10.1002/hbm.24630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Hosová D, Towner T, Werner DF, & Spear LP (2020). Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behavioural Brain Research, 378, 112292. doi: 10.1016/j.bbr.2019.112292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Kim EU, & Spear LP (2017). Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Research, 1654(Pt B), 145–156. doi: 10.1016/j.brainres.2016.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, & Spear LP (2014). Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol, 48(5), 433–444. doi: 10.1016/j.alcohol.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Bohnsack JP, Kusumo H, Liu W, Pandey SC, & Crews FT (2020). Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise. Addiction Biology, 25(2), e12731. doi: 10.1111/adb.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, & Spear LP (2007). Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism: Clinical and Experimental Research, 31(7), 1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, & Spear L (2009). Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and Alcoholism, 44(6), 547–554. doi: 10.1093/alcalc/agp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, et al. (2012). A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicology and Applied Pharmacology, 260(1), 65–69. doi: 10.1016/j.taap.2012.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, & Hingson R (2013). The burden of alcohol use: excessive alcohol consumption and related consequences among college students. Alcohol Research : Current Reviews, 35(2), 201–218. [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Nickel MM, & Bielak JT (2018). Oral binge-like ethanol pre-exposure during juvenile/adolescent period attenuates ethanol-induced conditioned place aversion in rats. Alcohol and Alcoholism, 53(5), 518–525. doi: 10.1093/alcalc/agy040 [DOI] [PubMed] [Google Scholar]
- Willing J, Cortes LR, Brodsky JM, Kim T, & Juraska JM (2017). Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Developmental Psychobiology, 59(5), 583–589. doi: 10.1002/dev.21525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, & Shippenberg TS (2010). Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. The Journal of Neuroscience, 30(46), 15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP (1997). Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology, 30(2), 141–150. doi: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Correlations of lever pressing during extinction with amount of sucrose pellets consumed during the free access period. Early adolescent-exposed male (A) and female (C) rats had lower responding when consuming more of the non-devalued sucrose pellets. However, this was not seen for the devalued condition. Both male and female animals exposed during late adolescence (B and D) had no significant correlations between lever pressing and amount consumed.