Abstract
Background:
Down syndrome (DS) is one of the most common birth defects in the US associated with high levels of overweight and obesity. Unique characteristics of adults with DS that may contribute to the high levels of obesity are: high rates of hypothyroidism, poor muscle tone, altered gait, and lower resting metabolic rate. Due to these factors, it is unknown if the same weight management interventions that are effective in adults with intellectual disabilities (IDD) without DS, are as effective in those with DS. Therefore, the purpose of this secondary analysis was to compare changes in weight, diet, and physical activity between participants with DS and non-DS related IDD participating in an 18-month weight management trial.
Methods:
We used propensity score methods to adjust baseline variables of overweight/obese adults with and without DS participating in an 18-month effectiveness trial with 6 months weight loss and 12 months weight maintenance. Participants followed one of two reduced calorie diet plans, obtain 150 min of moderate-to-vigorous intensity physical activity (MVPA) per week, and log dietary intake daily. A health educator held monthly at-home visits with participants and a caregiver to give feedback on intervention compliance.
Results:
Out of the 124 participants that met criteria for inclusion, 21 were diagnosed with DS and 103 with non-DS related IDD. Twenty out of 21 participants with DS were successfully matched. Clinically significant weight loss was seen at 18 months in participants with DS (−5.2%) and non-DS related IDD (−6.8%), with no difference between groups (p=0.53). Significant reductions in EI were seen across the 18-month intervention in both DS and non-DS related IDD groups with between group differences at 12 months only (1119 vs. 1492 kcals/day, respectively; p=0.003). Although MVPA did not increase in either group across the intervention, those with non-DS related IDD had higher levels of MVPA compared to those with DS across the 18 months.
Conclusion:
Participants with DS lost a clinically significant amount of weight across the 18-month intervention. Compared to those with non-DS related IDD, those with DS lost similar amounts of weight, had similar decreases in EI, and participated in less MVPA across the 18-month intervention. Although individuals with DS have physiological factors that may contribute to obesity, weight management interventions designed for individuals with IDD may be equally effective in this population.
INTRODUCTION
Approximately 1% of the global population is diagnosed with an intellectual or developmental disability (IDD).(Maulik et al., 2011) A common cause of IDD is Down syndrome (DS), or trisomy 21, which is also associated with physical growth delays and low muscle tone. (Organization, 2014) Characteristically, adults with IDD have a high prevalence of overweight and obesity at levels (BMI ≥ 25 kg/m2) equal to (Stancliffe et al., 2011) or greater than the general population (Hsieh et al., 2013, Robertson et al., 2014, Melville et al., 2007). However, adults with DS experience disproportionate prevalence of overweight and obesity (≈89%) (Braunschweig et al., 2004) compared to both adults with non-DS related IDD (Melville et al., 2005) and their typically developing counterparts.(Hales et al., 2018) This high level of obesity is a major public health concern as obesity is associated with increased risk for many serious diseases and health conditions. (Alexander et al., 2016, Rimmer et al., 2004)
It has been well established that lack of regular physical activity, high levels of sedentary behaviour (Bartlo and Klein, 2011, Sundahl et al., 2015, Dixon-Ibarra et al., 2013), and unhealthy dietary choices (Draheim et al., 2007, Adolfsson et al., 2008, Stancliffe et al., 2011, Robertson et al., 2014) are associated with weight gain. However, individuals with DS may be unequally predisposed to excessive weight gain as this population has a significantly lower resting metabolic rate (RMR) relative to body size, poor body composition related to hypotonia, high rates of hypothyroidism, increased leptin levels, poor postural stability and gait, and lower respiratory capacity compared to typically developed adults and individuals with non-DS related IDD (Allison et al., 1995, Luke et al., 1994, Bertapelli et al., 2016, Roizen and Patterson, 2003, Basil et al., 2016, Agiovlasitis et al., 2009). These physiological factors may contribute to unsuccessful weight management interventions, however, it is unknown if weight management interventions in adults with DS can promote clinically significant weight loss.
In adults with DS, there is limited information on the efficacy of weight management interventions. The existing interventions include adults with multiple types of IDDs and are not DS specific.(Spanos et al., 2013, Doherty et al., 2018, Willems et al., 2018). We identified one weight management intervention that included only individuals with DS. In this 12-month intervention, Curtin et al. (Curtin et al., 2013) examined the effect of adding parent training to a multi-component weight loss program for adolescents and young adults with DS. This program resulted in clinically insignificant weight loss at both 6 and 12 months. Across the 12-month study, participants in the parent group lost only 2% of their initial body weight and participants in the education only group experienced a 4% weight gain, suggesting that weight management interventions in adults with DS may be ineffective at promoting clinically significant weight loss. However, these results may not be generalisable to most adults with DS, as participants in this study were young (13–26 yrs.) and living at home with a parent. Furthermore, the effectiveness of this intervention program in individuals without IDD is unknown.
Additional research is needed to determine if weight management interventions in adults with DS can promote clinically significant weight loss. Data from a recently completed 18-month weight management intervention in adults with IDD was used to examine changes in weight, dietary intake, and physical activity in adults with DS and compare these results to adults with non-DS related IDDs who followed the same intervention.
METHODS
Overview of study design
This is a secondary analysis from a recently completed weight management trial in adults with IDD. A detailed description of the rationale, design and methods (Donnelly et al., 2013) and the main outcomes (Ptomey et al., 2017) have been previously published. Briefly, 150 overweight/obese adults with mild to moderate IDD (21% with DS) and a caregiver who agreed to be their study partner and support them during the intervention, enrolled into an 18-month effectiveness trial with 6 months of weight loss followed by 12 months of weight maintenance to compare two dietary approaches for weight management. Participants were randomly assigned to either an enhanced Stop Light Diet (eSLD) or a conventional diet (CD). Following the 6-month weight loss period, both groups were encouraged to continue following their diet at a level of energy intake estimated to result in weight maintenance.
Participants
The study took place from June 2011– May 2014 in the greater Kansas City Metropolitan area. Participants were community-dwelling overweight and obese adults, 18 years of age or older with a diagnosis of mild to moderate IDD as determined by a Community Service Provider operating in the state of Kansas under the auspices of a Community Developmental Disability Organization (CDDO). To be included in the study, participants had to be overweight or obese (BMI≥25kg/m2), reside in a supported living environment either at home or with no more than 4 residents, and have a caregiver who agreed to support them during the program. Individuals were excluded if they had a diagnosis of any of the following: uncontrolled hypertension, severe heart disease, cancer, HIV, severe depression, or an eating disorder. Individuals were also excluded if they were on a special diet (e.g. vegan, gluten-free) or had participated in a weight reduction program within the past 6 months. Participants were required to reside within a 50-mile radius of the Kansas City Metropolitan area. Written informed consent, approved by the IRB at the University of Kansas Medical Center, was obtained from either the participant (self as guardian) or their legal guardian and their study partner. Randomization, was completed after written consent and written physician clearance were obtained. Participants were computer randomized, stratified by the number of adults with IDD in a residence (alone, 1–2, or 3–5), with equal allocation to the eSLD and CD groups. Those with values for weight for at least two time points are included in this analysis.
Intervention
Overview
All participants were randomised to either the eSLD or CD. All participants were assigned a health educator, who visited them to deliver the intervention. At baseline, the participant and study partner attended a 90-minute at-home diet orientation session conducted by their health educator. Participants were provided detailed instruction on dietary requirements and the study protocol. Subsequent monthly follow-up education sessions were conducted throughout the 18-month intervention in the participants’ home.
Weight Loss Diets (Months 0–6).
Details regarding both the eSLD and CD are presented elsewhere. (Ptomey et al., 2017, Donnelly et al., 2013) Briefly, the eSLD encouraged consumption of high volume, lower calorie (100–300 kcal), portion-controlled meals (PCMs; entrées/shakes) and fruits and vegetables. (Academy of Nutrition and Dietetics, 2011) Participants were asked to consume a minimum daily total of 2 entrées (~200 to 300 kcal each with saturated fat ≤ 3g and sodium <600 mg), 2 shakes (~100 kcal each), 5 one-cup servings of fruits and vegetables, and ad libitum non-caloric beverages. Participants desiring foods replacing or in addition to these recommendations were encouraged to select additional green and yellow foods from the Stop Light Diet chart developed by Epstein. (Epstein and Squires, 1988) Participants in the CD group were educated to follow the recommendations found on the USDA website, ChooseMyPlate.gov, (U.S. Department of Agriculture, 2013) which helps consumers carry out Dietary Guidelines for Americans. (Desalvo et al., 2016, Guenther et al., 2014) Participants’ energy needs were estimated using the equation of Mifflin-St Jeor (Mifflin et al., 1990) multiplied by 1.4 to 1.6 to account for physical activity. A deficit of 500–700 kcal/day was prescribed.
Weight Maintenance Diet (months 7 to 12)
Energy intake for weight maintenance was estimated using the same methods used for weight loss. Participants in the eSLD diet were encouraged, but not required, to continue to use 14 PCMS per week, and continue to eat a minimum of 5 fruits and vegetables per day. Participants in the CD diet were provided examples of meal plans consisting of suggested amounts of grains, proteins, fruits and vegetables, dairy, and fats based on their maintenance energy needs.
Physical activity
Participants were encouraged to accumulate a minimum of 30 min./day of moderate-intensity physical activity 5 days/week (150 min./week). Brisk walking was the primary form of exercise recommended as it is inexpensive, safe, fits easily into the daily routine, and can be performed alone or with others. Pedometers (Omron HJ-320, Lake Forest, IL) were provided to all participants as both a motivational tool and to self-monitor physical activity.
Weekly tracking and monthly meetings
Participants, with assistance from their study partner, were asked to complete weekly data recording cards that were specific to their study group throughout the 18-month study. Participants in the eSLD group were asked to check the pictorial representations of the number of entrees, shakes, fruits and vegetables, and other green, yellow or red foods consumed each day. Similarly, participants in the CD group were asked to check the number of daily servings of vegetables, fruits, dairy, meat and grains. The tracking sheets for both groups contained space for recording daily pedometer steps and minutes of non-walking physical activity. All participants were visited once monthly by their health educator. During these monthly home visits, the health educator would assess body weight and compliance with the intervention, provide feedback to participants, answer any questions from participant or study partner, and problem solve if there were any issues related to following the diet or physical activity goals.
Outcomes assessments
Overview.
All Outcome variables were assessed at baseline, 6, 12, and 18 months. All outcomes were assessed at the participant’s home during a single visit by study staff blinded to intervention condition.
Down Syndrome Status and Demographic Information.
At baseline only, basic demographic information (age, race/ethnicity, sex, education level) was collect from participants, with the help of a caregiver, using a questionnaire. Diagnosis of Down syndrome was collected from the CDDO serving the participant.
Anthropometrics.
Participants were weighed to the nearest 0.25 kg, in duplicate, on a calibrated digital scale (Belfour model #PS6600, Saukville, WI) between 8 and 10 AM, following an overnight fast (~12 hrs.), wearing a standard hospital gown. Standing height was measured, in duplicate to the nearest 0.1 cm using a portable stadiometer (#Invicta Plastics Limited, model IP0955, Leicester, UK). The average of the 2 measures of weight and height were used to calculate BMI (weight (kg)/height (m2)).
Energy and macronutrient intake.
Proxy-assisted 3-day food records were obtained to assess energy and macronutrient content of the diet. Participants and their study partner were instructed to record all food and beverage consumption on 3 consecutive days (2 weekdays and 1 weekend day). Records were reviewed with the participants and their study partner by a registered dietitian nutritionist (RDN) who used portion guides to help estimate portion sizes. The portion guides used in the interviews were 3-dimensional models consisting of a variety of items intended to provide a reference and improve recall accuracy (i.e., glasses, mugs, bowls, circles, thickness sticks, chip bags, drink bottles, a 12-inch ruler, measuring cups and spoons, a grid, wedges, geometric shapes, and diagrams of chicken pieces) (Wright et al., 2007). Records were removed from analysis if the RDN felt the record could not be considered reliable (participant forgot one or more meals, or claimed they could not remember anything consumed that day). The records were entered into the Nutrient Data System for Research (NDS-R; University of Minnesota, Minneapolis, MN; version 2014) (Nurtition Coordinating Center, 2014, Schakel, 2001) for analysis.
Physical activity.
Participants were asked to wear a portable accelerometer (Actigraph GT1X, Pensacola, FL) on a belt over the non-dominant hip for 7 consecutive days. Accelerometer data was collected in 1-min epochs with a minimum of 8 hours constituting a valid monitored day. Participants were required to have at least one valid day to be included in the accelerometer data analysis. No minimum criteria for number of weekdays or weekend days were required. Non-wear time was identified as ≥60 consecutive minutes with 0 counts/min, with allowance for 1–2 minutes of accelerometer counts between 0 and 100 (Troiano et al., 2008). Data were processed using a custom SAS program. Sedentary time was defined valid wear time with accelerometer readings <100 counts/min.(Troiano et al., 2008) Data are reported as proportion of total wear time spent in sedentary, light physical activity (LPA; 100 to 2020 counts/min), and moderate-vigorous intensity physical activity (MVPA; >2020 counts/min) (Troiano et al., 2008). On average, approximately 3 valid days with over 11 hours of wear time of accelerometer data were available.
Statistical Analysis
A propensity score for the probability of having DS or non-DS related IDDs was estimated for each individual using a logistic regression model including the following baseline variables as covariates: age, sex, race/ethnicity, BMI, and original study randomization group. We created a propensity score-matched baseline cohort by attempting to match each participant with DS to an individual with non-DS related IDD. A nearest-neighbor 1:1-greedy matching algorithm was applied to match participants on the basis of the logit of their propensity score, with a caliper width equal to 0.2 times the standard deviation of the logit of the propensity score. (Austin, 2011) Balance of baseline covariates between those with DS and non-DS related IDD in the matched sample was assessed using standardised differences, with standardised differences of <0.1 for each covariate being used to indicate good balance.(Austin, 2009)
Sample characteristics were summarised using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Intent to treat (ITT) analyses were performed using all matched participants. Missing data were handed by using maximum likelihood estimation under the assumption of at least missing at random. Analyses used multi-level linear mixed models (SAS PROC MIXED) to examine differences in weight change between DS and non-DS related IDD groups during weight loss (0–6 months), weight maintenance (7–18 months), and across the entire intervention (0–18 months). Cohen’s d effect sizes, defined as small = 0.2, medium = 0.5 and large = 0.8, were calculated for group differences. (Cohen, 1988) Models included random effect for matched pairs to account for covariance between matched participants as well as fixed effects for diagnosis (DS and non-DS related IDD), time (treated as a categorical variable; baseline, 6, 12, 18 months), and the group*time interaction. Several error covariance structures were assessed to account for statistical covariance among repeated measurements and a spatial exponential working covariance was used because the Bayesian Information Criterion was smaller. These same methods were used to assess overall changes in physical activity and energy intake outcomes. The residuals of continuous outcome variables were checked for normality. Where evidence of departure from normality was apparent the log of the outcomes were used for analyses, however, results are presented in their original scale for ease of interpretation. Outcome values are presented as adjusted means and standard error unless otherwise stated. The McNemar test was used to compare proportion of individuals achieving clinically significant weight loss (> 5%) between groups. Statistical significance was defined as p < 0.05 and all analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 150 overweight/obese adults with mild to moderate IDD participated in an 18 month weight loss intervention. Twenty-six participants were excluded who did not have weight values for at least two time points. The analytic cohort included 124 participants (21 with DS and 103 with non-DS related IDDs). Of the 124 participants, 21 were diagnosed with DS and the other 103 were diagnosed with non-DS related IDD. Table 1 shows the demographic characteristics of the study population. Prior to propensity score matching there was noticeable dissimilarities in baseline covariates between groups indicated by a standardised difference >0.1. Those with DS were more likely to be female and been randomised to eSLD diet. Using propensity score matching 109 participants with similar propensity scores were identified and 20 out of 21 participants with DS were successfully matched to those with non-DS related IDD, indicated by a standardised difference <0.1% between the two groups (Table 1).
Table 1.
Distribution of variables and standardised difference pre and post matching
| non-DS IDD | DS | Standardised Difference | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| Distribution of variables pre-matching | |||||||
| Age (yrs.) | 103 | 36.5 | 12.5 | 21 | 36.5 | 9.6 | 0.003 |
| BMI (kg/m2) | 103 | 37.0 | 7.9 | 21 | 36.8 | 8.1 | 0.033 |
| Female (n, %) | 56 | 54.4 | 14 | 66.7 | 0.254 | ||
| Race/Ethnicity (n, %) | 0.056 | ||||||
| Non-Hispanic White | 83 | 83.6 | 18 | 85.7 | |||
| Non-Hispanic Black | 13 | 12.6 | 1 | 4.8 | |||
| Other | 7 | 6.8 | 2 | 9.5 | |||
| Randomization (n, %) | 0.192 | ||||||
| eSLD | 54 | 52.4 | 13 | 61.9 | |||
| CD | 49 | 47.6 | 8 | 38.1 | |||
| Distribution of variables post-matching | |||||||
| Age (yrs.) | 20 | 36.6 | 10.2 | 20 | 36.8 | 9.8 | 0.020 |
| BMI (kg/m2) | 20 | 37.2 | 6.9 | 20 | 37.1 | 8.1 | 0.009 |
| Female (n, %) | 13 | 65.0 | 13 | 65.0 | 0.000 | ||
| Race/Ethnicity (n, %) | 0.000 | ||||||
| Non-Hispanic White | 18 | 90.0 | 18 | 90.0 | |||
| Non-Hispanic Black | 0 | 0.0 | 0 | 0.0 | |||
| Other | 2 | 10.0 | 2 | 10.0 | |||
| Randomization (n, %) | 0.000 | ||||||
| eSLD | 13 | 65.0 | 13 | 65.0 | |||
| CD | 7 | 35.0 | 7 | 35.0 | |||
Note: IDD = intellectual disabilities, DS = Down Syndrome, SD= standard deviation, BMI = body mass index, yrs. = years, kg = kilograms, m = meters, eSLD = enhanced stop light diet, CD= conventional diet.
Body weight and BMI.
Changes in total body weight and BMI following weight loss (0–6 months) and weight maintenance (7–18 months) are presented in Table 2 and Figure 1.
Table 2.
Changes in weight and BMI during weight loss, maintenance, and weight loss + maintenance in adults with Down syndrome and those with non-DS related IDD groups.
| non-DS IDD | DS | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | 95% CI | Mean | 95% CI | p-value | ||
| Weight Loss (0–6 months) | |||||||
| Weight (kg) | −5.2 | −7.4 | −3.0 | −5.1 | −7.3 | −2.9 | 0.94 |
| Weight (%) | −5.8 | −8.0 | −3.6 | −5.8 | −8.0 | −3.6 | 0.99 |
| BMI (kg/m2) | −1.9 | −2.8 | −0.9 | −1.9 | −2.8 | −1.0 | 0.94 |
| Weight Maintenance (7–18 months) | |||||||
| Weight (kg) | −1.9 | −5.1 | 1.4 | 0.4 | −2.8 | 3.5 | 0.34 |
| Weight (%) | −1.0 | −4.1 | 2.1 | 0.6 | −2.4 | 3.6 | 0.47 |
| BMI (kg/m2) | −0.6 | −2.0 | 0.8 | 0.1 | −1.2 | 1.4 | 0.48 |
| Weight Loss + Maintenance (0–18 months) | |||||||
| Weight (kg) | −7.1 | −11.0 | −3.2 | −4.7 | −8.5 | −1.0 | 0.39 |
| Weight (%) | −6.8 | −10.3 | −3.3 | −5.2 | −8.6 | −1.9 | 0.53 |
| BMI (kg/m2) | −2.5 | −4.1 | −0.8 | −1.8 | −3.4 | −0.2 | 0.58 |
Note: IDD = intellectual disabilities, DS = Down Syndrome, CI = confidence interval BMI = body mass index
Figure 1.
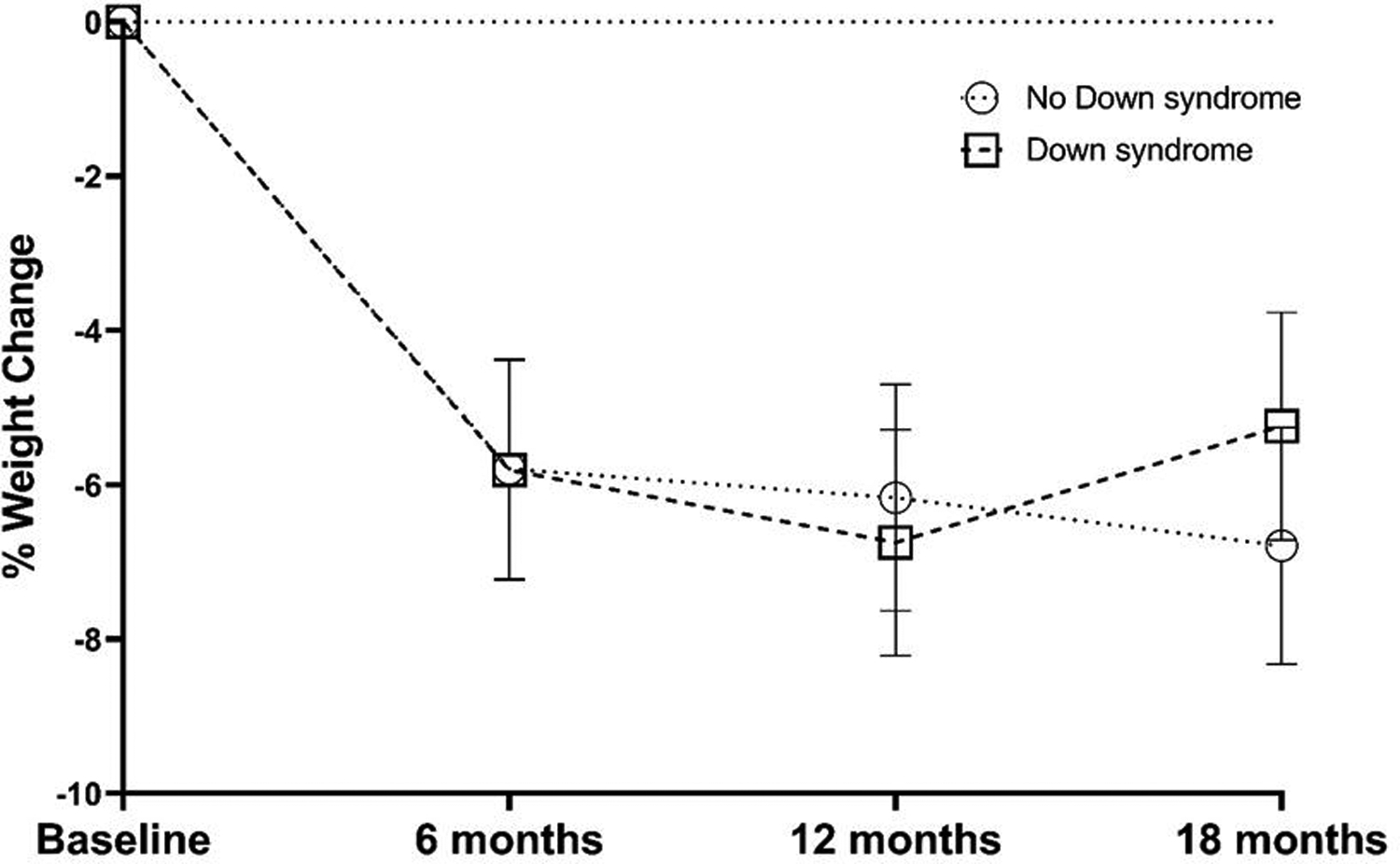
Percent weight change in adults with Down syndrome and those with non-DS related IDD groups
Weight loss (0–6 months).
Weight decreased in both participants with DS (−5.1 kg., 95% CI = −7.3 to −2.9; −5.8%) and non-DS related IDD (−5.2 kg., 95% CI = −7.4 to −3.0; −5.8%). Weight loss at 6 months did not differ significantly between groups (p=0.94). The proportion of participants achieving clinically significant weight loss of >5% among participants completing the 6-month weight loss intervention was not significantly different between DS (45%) and non-DS related IDD groups (50%; p=0.71). Similar to the results for body weight, there were no significant differences between groups for change in BMI (p=0.95) at 6 months. The between-group effect for weight change outcomes were near zero (Cohen’s d range: 0.003–0.02).
Weight maintenance (7–18 months).
Weight slightly decreased from 7–18 months in those with non-DS related IDD (−1.9kg. 95% CI = −5.1 to 1.4; −1.0%) and remained relatively unchanged in those with DS (+0.4 kg., 95% CI = −2.8 to 3.5; +0.6%); however, the between group difference was not statistically significant (p=0.34). The between-group effect for weight change outcomes were small to medium (Cohen’s d range: 0.22–0.30)
Weight loss + maintenance (0–18 months).
Weight loss at 18 months did not differ significantly between the DS (−4.7 kg 95% CI = −8.5 to −1.0; −5.2%) and non-DS related IDD groups (−7.1 kg 95% CI = −11.0 to −3.2; −6.8%; p = 0.39). The proportion of participants achieving clinically significant weight loss of >5% among participants completing the 18-month weight loss intervention was not significantly different between DS (25%) and non-DS related IDD groups (30%; p=0.74). Similar to the results for body weight, there were no significant differences between groups for change in BMI (p=0.58) across the 18 months. The between-group effect for weight change outcomes were small (Cohen’s d range: 0.18–0.27)
Energy Intake.
Energy and macronutrient intake over the 18-month intervention are presented in Table 3. Mixed modeling results revealed that total energy intake (kcal/day) decreased in both DS and non-DS related IDD groups. There was a significant time effect (p<0.0001) and group*time interaction (p=0.04), however, there was no significant group effect. At month 12, energy intake was significantly lower in those with non-DS related IDD compared to those with DS (p = 0.003). There were no significant between- or within-group differences (group or time effects) or group*time interactions for proportion of calories consumed from carbohydrates or fat. Proportion of calories consumed of protein significantly increased over the 18 months in both DS and non-DS related IDD groups (time effect p=0.001), however there were no significant group or group*time effects.
Table 3.
Adjusted means and 95% CI for energy intake across the 18-month study in individuals with DS and those with non-DS related IDD.
| non-DS IDD | DS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | 95% CI | Mean | 95% CI | Group | Time | Group*Time | ||
| Energy Intake (kcal/day) | 0.09 | <.0001 | 0.04 | ||||||
| Baseline | 1721 | 1532 | 1910 | 1810 | 1621 | 1999 | |||
| 6 months | 1453 | 1253 | 1654 | 1350 | 1158 | 1543 | |||
| 12 months | 1119 | 904 | 1335 | 1492 | 1295 | 1688 | |||
| 18 months | 1337 | 1116 | 1559 | 1387 | 1187 | 1588 | |||
| Carbohydrate Intake (%) | 0.34 | 0.83 | 0.72 | ||||||
| Baseline | 52.3 | 47.8 | 56.8 | 50.7 | 46.2 | 55.2 | |||
| 6 months | 51.2 | 46.4 | 56.0 | 52.1 | 47.5 | 56.7 | |||
| 12 months | 55.7 | 50.3 | 61.0 | 51.3 | 46.6 | 56.0 | |||
| 18 months | 53.3 | 47.7 | 58.8 | 51.6 | 46.7 | 56.5 | |||
| Fat Intake (%) | 0.51 | 0.27 | 0.37 | ||||||
| Baseline | 31.9 | 28.4 | 35.4 | 33.0 | 29.5 | 36.6 | |||
| 6 months | 30.8 | 27.0 | 34.6 | 28.4 | 24.8 | 32.0 | |||
| 12 months | 28.3 | 24.1 | 32.5 | 32.1 | 28.4 | 35.8 | |||
| 18 months | 28.9 | 24.6 | 33.2 | 30.1 | 26.3 | 33.9 | |||
| Protein Intake (%) | 0.57 | <0.01 | 0.92 | ||||||
| Baseline | 17.5 | 15.2 | 19.7 | 18.2 | 16.0 | 20.4 | |||
| 6 months | 20.1 | 17.7 | 22.4 | 21.5 | 19.2 | 23.8 | |||
| 12 months | 17.7 | 15.1 | 20.3 | 18.1 | 15.8 | 20.4 | |||
| 18 months | 19.9 | 17.2 | 22.6 | 19.8 | 17.4 | 22.2 | |||
Note: IDD = intellectual disabilities, DS = Down syndrome, CI = confidence interval
Physical Activity.
Table 4 presents the time spent in sedentary, LPA and MVPA assessed by accelerometer, expressed as a percentage of wear time. There were no significant effects for group, time, or group*time interaction for sedentary time or LPA. For MVPA, there was no significant time effect. Significant group effect (p<0.001)) and group*time interaction (p=0.04) was observed. Significantly greater MVPA was observed in those with non-DS related IDD at months 6 (p = 0.001), 12 (p = 0.035), and 18 (p < 0.001) compared to those with DS.
Table 4.
Adjusted means and 95% CI for physical activity across the 18-month study in individuals with DS and those with non-DS related IDD.
| non-DS IDD | DS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | 95% CI | Mean | 95% CI | Group | Time | Group*Time | ||
| Sedentary (% of wear time) | 0.23 | 0.45 | 0.09 | ||||||
| Baseline | 63.6 | 57.7 | 69.6 | 64.0 | 58.1 | 69.9 | |||
| 6 months | 62.6 | 56.5 | 68.6 | 67.5 | 61.6 | 73.4 | |||
| 12 months | 61.5 | 55.4 | 67.6 | 62.8 | 56.6 | 69.0 | |||
| 18 months | 58.6 | 52.1 | 65.0 | 68.1 | 61.7 | 74.5 | |||
| LPA (% of wear time) | 0.38 | 0.55 | 0.12 | ||||||
| Baseline | 34.3 | 28.7 | 40.0 | 34.6 | 28.9 | 40.2 | |||
| 6 months | 35.8 | 30.1 | 41.6 | 31.4 | 25.8 | 37.1 | |||
| 12 months | 36.1 | 30.3 | 41.9 | 36.4 | 30.5 | 42.3 | |||
| 18 months | 38.4 | 32.3 | 44.6 | 31.3 | 25.2 | 37.4 | |||
| MVPA (% of wear time) | <0.001 | 0.44 | 0.04 | ||||||
| Baseline | 1.5 | 0.9 | 2.4 | 0.8 | 0.5 | 1.3 | |||
| 6 months | 1.7 | 1.0 | 2.9 | 0.5 | 0.3 | 0.9 | |||
| 12 months | 1.2 | 0.7 | 1.9 | 0.5 | 0.3 | 0.9 | |||
| 18 months | 1.7 | 1.0 | 3.0 | 0.4 | 0.2 | 0.7 | |||
Note: IDD = intellectual disabilities, DS = Down syndrome, CI = confidence interval, LPA = light physical activity, MVPA = moderate to vigorous physical activity
DISCUSSION
The results of this study found that adults with DS enrolled in an 18-month weight loss intervention lost a clinically significant amount of weight at both 6 and 18 months. Compared to those with non-DS related IDD, adults with DS lost similar amounts of weight, had similar decreases in energy intake, and obtained significantly less MVPA. This study suggests that although adults with DS may have physiological factors that contribute to obesity and their ability to lose weight, they can lose a clinically meaningful amount of weight in a weight loss intervention targeting adults with IDD, as well as respond in a similar manner to adults with non-DS related IDD.
Previous literature indicates that adults with IDD are capable of achieving clinically significant weight loss in response to multi-component weight loss/maintenance interventions, like the one used in this trial, that are tailored to their cognitive abilities, and include an energy reduced diet, increased physical activity and behavioral strategies. (Melville et al., 2011, Spanos et al., 2015, Saunders et al., 2011, Martinez-Zaragoza et al., 2015, Spanos et al., 2013) This study indicates that adults with DS are also capable of achieving clinically significant weight loss in response to a multi-component weight loss intervention. These results are in contrast to the previously detailed 12-month weight loss intervention by Curtin et al (Curtin et al., 2013) in which adolescents and young adults with DS had minimal weight loss. However, the intervention by Curtin et al. targeted a younger population who may still be growing and who rely on parent support for grocery shopping, preparation of meals, and opportunities for physical activity. We are unaware of other trials that have examine changes in weight, dietary intake, and physical activity in adults with DS enrolled in a weight loss program.
It is of interest to note that adults with DS and those without DS lost a similar amount of weight while consuming a similar energy intake (~1300–1400 kcals/day). It is believed that compared to those without DS, including those with non-DS related IDD, adults with DS have lower energy needs and would need a lower energy intake to elicit a similar weight loss. For example, Allison et al (Allison et al., 1995) compared 13 adults with DS (29.7 ± 10.2 years, 31% female) to 77 typically developing adults (36.6 ± 10.4 years, 53% female), and found that after controlling for sex, free fat mass, fat mass, age, and height, adults with DS had 20% lower RMR then those without DS (p=0.007). Using this 20% estimate we would have expected that individuals with DS would have needed to have consumed ~1100 kcals per day to have the same weight loss as someone without DS consuming ~1400 kcals per day. However, our results may be skewed by inherit limitations to our measure of energy intake with 3-day food records. While 3-day food records are commonly used in the general population (Mcclung et al., 2018) as well as those with IDD, (Ptomey et al., 2018a) they are an imperfect measurement as they rely on self-report and a person’s ability to correctly describe what was consumed (Goris et al., 2000). In the general population diet records have been shown to underestimate energy intake by approximately 20% compared to direct measures like doubly labeled water (Subar et al., 2003), with some researchers arguing that self-report measures should not be used to measure energy intake (Subar et al., 2015). Additionally, 3-day food records only show a snap-shot of time and may not be representative of the entire intervention period. Thus, our energy intake results should be taken cautiously and future studies should examine changes in energy intake across a weight loss intervention in adults with DS using newer methods of energy intake assessment such as image-assisted dietary assessments or double labeled water.(Mcclung et al., 2018)
In the current trial, the baseline level of physical activity was extremely low in both groups, but in adults with DS, MVPA actually decreased over time. The lack of success of our approach for increasing physical activity suggests alternative strategies for increasing physical activity need to be developed and evaluated in all adults with IDD in general, but specifically in adults with DS. In this trial, walking was promoted as the predominate mode of physical activity. However, in adults with DS, walking may not be the best form of physical activity due to poor gait and stability challenges inherent to DS, (Smith and Ulrich, 2008, Agiovlasitis et al., 2009) and could have been a contributing factor for the decrease in MVPA seen across time. Interventions that focus on functional movement and dance (Mcguire et al., 2019, Gutiérrez-Vilahú et al., 2016, Ptomey et al., 2018b) have been found to be successful at increasing MVPA in adults with DS, and should be included in future weight loss and physical activity interventions.
Strength of this analysis include the use of data collected from an 18-month weight loss intervention trial tailored to the cognitive abilities of adults with IDD, intervention delivery was supervised by a member of the investigative team to ensure intervention fidelity, and it includes both a weight loss period (6 months) and a 12 month follow-up. Limitations of the study include small sample size (n=40), the fact that not all participants who participated in the original trial were analyzed due to propensity score matching, and an inability to control for unobserved confounding variables. In an effort to balance demographics of individuals with DS and non-DS related IDD, a propensity-score method was employed to reduce biases to enable comparison between groups. However, only limited variables were available to perform matching on so it is possible that important confounders are not controlled for. Finally, the main intervention was not specifically designed or powered to detect differences in weight between individuals with DS and non-DS. Adequately powered case-control trials, matching on a wider range of confounders, such as living situation, education level, presence/absence of study partner support, and using modern assessment of both dietary intake and physical activity are required to compare changes in weight, dietary intake, and physical activity between adults with DS and adults with non-DS IDD. Additionally, interventions that examine methods to increase MVPA in adults with DS are warranted.
In summary, although adults with DS may have physiological factors that may contribute to obesity and their ability to lose weight, they can still lose a clinically meaningful amount of weight in a manner similar to those with non-DS related IDD. The weight loss seen in adults with DS exceeded the 3–5% sustained weight loss threshold associated with clinically meaningful health benefits such as a decreases in triglycerides, blood glucose, hemoglobin A1c, and the risk of developing type 2 diabetes(Jensen et al., 2014). Thus, weight loss interventions in adults with DS can be effective and should be recommended to help reduced the risk of obesity related health conditions.
Acknowledgements and Funding:
National Institutes of Diabetes, Digestive and Kidney Diseases (R01- DK83539). We acknowledge HMR for providing the pre-packaged meals
Footnotes
Disclosures: No author has any conflicts of interest to declare.
Clinical Trails Number: NCT01724905
REFERENCES
- Academy of Nutrition and Dietetics. 2011. How effective (in terms of client adherence and weight loss and maintenance) are meal replacements (liquid meals, meal bars, frozen prepackaged meals)? [Online]. Available: http://www.adaevidencelibrary.com/evidence.cfm?evidence_summary_id=250141 [Accessed].
- Adolfsson P, Sydner YM, Fjellstrom C, Lewin B & Andersson A 2008. Observed dietary intake in adults with intellectual disability living in the community. Food Nutr Res, 52 10.3402/fnr.v52i0.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agiovlasitis S, Mccubbin JA, Yun J, Mpitsos G & Pavol MJ 2009. Effects of down syndrome on three-dimensional motion during walking at different speeds. Gait & posture, 30, 345–350. [DOI] [PubMed] [Google Scholar]
- Alexander M, Petri H, Ding Y, Wandel C, Khwaja O & Foskett N 2016. Morbidity and medication in a large population of individuals with down syndrome compared to the general population. Developmental Medicine & Child Neurology, 58, 246–254. [DOI] [PubMed] [Google Scholar]
- Allison DB, Gomez JE, Heshka S, Babbitt RL, Geliebter A, Kreibich K & Heymsfield SB 1995. Decreased resting metabolic rate among persons with down syndrome. Int J Obes Relat Metab Disord, 19, 858–61. [PubMed] [Google Scholar]
- Austin PC 2009. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine, 28, 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC 2011. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical statistics, 10, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlo P & Klein PJ 2011. Physical activity benefits and needs in adults with intellectual disabilities: Systematic review of the literature. Am J Intellect Dev Disabil, 116, 220–32. 10.1352/1944-7558-116.3.220. [DOI] [PubMed] [Google Scholar]
- Basil JS, Santoro SL, Martin LJ, Healy KW, Chini BA & Saal HM 2016. Retrospective study of obesity in children with down syndrome. J Pediatr, 173, 143–148. [DOI] [PubMed] [Google Scholar]
- Bertapelli F, Pitetti K, Agiovlasitis S & Guerra-Junior G 2016. Overweight and obesity in children and adolescents with down syndrome—prevalence, determinants, consequences, and interventions: A literature review. Res Dev Disabil, 57, 181–192. [DOI] [PubMed] [Google Scholar]
- Braunschweig CL, Gomez S, Sheean P, Tomey KM, Rimmer J & Heller T 2004. Nutritional status and risk factors for chronic disease in urban-dwelling adults with down syndrome. Am J Ment Retard, 109, 186–93. . [DOI] [PubMed] [Google Scholar]
- Cohen J 1988. Statistical power analysis for the behaviors science.(2nd). New Jersey: Laurence Erlbaum Associates, Publishers, Hillsdale, [Google Scholar]
- Curtin C, Bandini LG, Must A, Gleason J, Lividini K, Phillips S, Eliasziw M, Maslin M & Fleming RK 2013. Parent support improves weight loss in adolescents and young adults with down syndrome. J Pediatr, 163, 1402–8.e1. 10.1016/j.jpeds.2013.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desalvo KB, Olson R & Casavale KO 2016. Dietary guidelines for americans. Jama, 315, 457–458. [DOI] [PubMed] [Google Scholar]
- Dixon-Ibarra A, Lee M & Dugala A 2013. Physical activity and sedentary behavior in older adults with intellectual disabilities: A comparative study. Adapt Phys Activ Q, 30, 1–19. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Jones SP, Chauhan U & Gibson JM 2018. An integrative review of multicomponent weight management interventions for adults with intellectual disabilities. Journal of Applied Research in Intellectual Disabilities, 31, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JE, Saunders RR, Saunders M, Washburn RA, Sullivan DK, Gibson CA, Ptomey LT, Goetz JR, Honas JJ, Betts JL, Rondon MR, Smith BK & Mayo MS 2013. Weight management for individuals with intellectual and developmental disabilities: Rationale and design for an 18 month randomized trial. Contemp Clin Trials, 36, 116–24. 10.1016/j.cct.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim CC, Stanish HI, Williams DP & Mccubbin JA 2007. Dietary intake of adults with mental retardation who reside in community settings. Am J Ment Retard, 112, 392–400. 10.1352/0895-8017(2007)112[0392:dioawm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Epstein L & Squires S 1988. The stoplight diet for children: An eight-week program for parents and children, Boston, MA, Little Brown & Company. [Google Scholar]
- Goris AH, Westerterp-Plantenga MS & Westerterp KR 2000. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am J Clin Nutr, 71, 130–4. [DOI] [PubMed] [Google Scholar]
- Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO & Carroll RJ 2014. The healthy eating index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for americans. J Nutr, 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Vilahú L, Massó-Ortigosa N, Costa-Tutusaus L, Guerra-Balic M & Rey-Abella F 2016. Effects of a dance program on static balance on a platform in young adults with down syndrome. Adapted Physical Activity Quarterly, 33, 233–252. [DOI] [PubMed] [Google Scholar]
- Hales CM, Fryar CD, Carroll MD, Freedman DS & Ogden CL 2018. Trends in obesity and severe obesity prevalence in us youth and adults by sex and age, 2007–2008 to 2015–2016. Jama, 319, 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K, Rimmer JH & Heller T 2013. Obesity and associated factors in adults with intellectual disability. J Intellect Disabil Res, 10.1111/jir.12100. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM & Yanovski SZ 2014. 2013 aha/acc/tos guideline for the management of overweight and obesity in adults: A report of the american college of cardiology/american heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol, 63, 2985–3023. 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Luke A, Roizen NJ, Sutton M & Schoeller DA 1994. Energy expenditure in children with down syndrome: Correcting metabolic rate for movement. J Pediatr, 125, 829–838. [DOI] [PubMed] [Google Scholar]
- Martinez-Zaragoza F, Campillo-Martinez JM & Ato-Garcia M 2015. Effects on physical health of a multicomponent programme for overweight and obesity for adults with intellectual disabilities. J Appl Res Intellect Disabil, 10.1111/jar.12177. [DOI] [PubMed] [Google Scholar]
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T & Saxena S 2011. Prevalence of intellectual disability: A meta-analysis of population-based studies. Res Dev Disabil, 32, 419–436. [DOI] [PubMed] [Google Scholar]
- Mcclung HL, Ptomey LT, Shook RP, Aggarwal A, Gorczyca AM, Sazonov ES, Becofsky K, Weiss R & Das SK 2018. Dietary intake and physical activity assessment: Current tools, techniques, and technologies for use in adult populations. Am J Prev Med, 55, e93–e104. [DOI] [PubMed] [Google Scholar]
- Mcguire M, Long J, Esbensen AJ & Bailes AF 2019. Adapted dance improves motor abilities and participation in children with down syndrome: A pilot study. Pediatric Physical Therapy, 31, 76–82. [DOI] [PubMed] [Google Scholar]
- Melville C, Cooper SA, Mcgrother C, Thorp C & Collacott R 2005. Obesity in adults with down syndrome: A case–control study. Journal of Intellectual Disability Research, 49, 125–133. [DOI] [PubMed] [Google Scholar]
- Melville CA, Boyle S, Miller S, Macmillan S, Penpraze V, Pert C, Spanos D, Matthews L, Robinson N, Murray H & Hankey CR 2011. An open study of the effectiveness of a multi-component weight-loss intervention for adults with intellectual disabilities and obesity. Br J Nutr, 105, 1553–62. 10.1017/s0007114510005362. [DOI] [PubMed] [Google Scholar]
- Melville CA, Hamiltom S, Hankey CR, Miller SL & Boyle S 2007. The prevalence and determinants of obesity in adults with intellectual disabilities. Obesity Reviews, 8, 223–230. [DOI] [PubMed] [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA & Koh YO 1990. A new predictive equation for resting energy expenditure in healthy individuals. American Journal of Clinical Nutrition, 51, 241–247. [DOI] [PubMed] [Google Scholar]
- Nurtition Coordinating Center 2014. Ndsr 2014 user manual. Minneapolis, MN: University of Minnesota. [Google Scholar]
- Organization, W. H. 2014. Definition: Intellectual disability. Retrieved from World Health Organization. Regional Office for Europe website: http://www.euro.who.int/en/what-we-do/health-topics/noncommunicable-diseases/mental-health/news/news/2010/15/childrens-right-to-family-life/definitionintellectual-disability,
- Ptomey LT, Saunders RR, Saunders M, Washburn RA, Mayo MS, Sullivan DK, Gibson CA, Goetz JR, Honas JJ & Willis EA 2017. Weight management in adults with intellectual and developmental disabilities: A randomized controlled trial of two dietary approaches. Journal of Applied Research in Intellectual Disabilities, [DOI] [PubMed] [Google Scholar]
- Ptomey LT, Steger FL, Lee J, Sullivan DK, Goetz JR, Honas JJ, Washburn RA, Gibson CA & Donnelly JE 2018a. Changes in energy intake and diet quality during an 18-month weight-management randomized controlled trial in adults with intellectual and developmental disabilities. J Acad Nutr Diet, 118, 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptomey LT, Szabo AN, Willis EA, Greene JL, Danon JC, Washburn RA, Forsha DE & Donnelly JE 2018b. Remote exercise for adults with down syndrome. Translational Journal of the American College of Sports Medicine, 3, 60–65. [PMC free article] [PubMed] [Google Scholar]
- Rimmer JH, Heller T, Wang E & Valerio I 2004. Improvements in physical fitness in adults with down syndrome. American Journal on Mental Retardation, 109, 165–174. [DOI] [PubMed] [Google Scholar]
- Robertson J, Emerson E, Baines S & Hatton C 2014. Obesity and health behaviours of british adults with self-reported intellectual impairments: Cross sectional survey. BMC Public Health, 14, 219. 10.1186/1471-2458-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizen NJ & Patterson D 2003. Down’s syndrome. The Lancet, 361, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Saunders RR, Saunders MD, Donnelly JE, Smith BK, Sullivan DK, Guilford B & Rondon MF 2011. Evaluation of an approach to weight loss in adults with intellectual or developmental disabilities Am J Intellect Dev Disabil, 49, 103–112. [DOI] [PubMed] [Google Scholar]
- Schakel SF 2001. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products—a research perspective. Journal of Food Composition and Analysis, 14, 315–322. [Google Scholar]
- Smith BA & Ulrich BD 2008. Early onset of stabilizing strategies for gait and obstacles: Older adults with down syndrome. Gait & posture, 28, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos D, Hankey CR & Melville CA 2015. The effectiveness of a weight maintenance intervention for adults with intellectual disabilities and obesity: A single stranded study. J Appl Res Intellect Disabil, 10.1111/jar.12181. [DOI] [PubMed] [Google Scholar]
- Spanos D, Melville CA & Hankey CR 2013. Weight management interventions in adults with intellectual disabilities and obesity: A systematic review of the evidence. Nutr J, 12, 132. 10.1186/1475-2891-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancliffe RJ, Lakin KC, Larson S, Engler J, Bershadsky J, Taub S, Fortune J & Ticha R 2011. Overweight and obesity among adults with intellectual disabilities who use intellectual disability/developmental disability services in 20 u.S. States. Am J Intellect Dev Disabil, 116, 401–18. 10.1352/1944-7558-116.6.401. [DOI] [PubMed] [Google Scholar]
- Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V, Reedy J & Krebs-Smith SM 2015. Addressing current criticism regarding the value of self-report dietary data. J Nutr, 145, 2639–45. 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S & Ballard-Barbash R 2003. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The open study. American Journal of Epidemiology, 158, 1–13. [DOI] [PubMed] [Google Scholar]
- Sundahl L, Zetterberg M, Wester A, Rehn B & Blomqvist S 2015. Physical activity levels among adolescent and young adult women and men with and without intellectual disability. J Appl Res Intellect Disabil, 10.1111/jar.12170. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T & Mcdowell M 2008. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc, 40, 181–8. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. 2013. Choose myplate [Online]. Washington DC. Available: www.choosemyplate.gov [Accessed]. [Google Scholar]
- Willems M, Waninge A, Hilgenkamp TI, Van Empelen P, Krijnen WP, Van Der Schans CP & Melville CA 2018. Effects of lifestyle change interventions for people with intellectual disabilities: Systematic review and meta-analysis of randomized controlled trials. Journal of Applied Research in Intellectual Disabilities, [DOI] [PubMed] [Google Scholar]
- Wright JD, Borrud LG, Mcdowell MA, Wang CY, Radimer K & Johnson CL 2007. Nutrition assessment in the national health and nutrition examination survey 1999–2002. J Am Diet Assoc, 107, 822–9. 10.1016/j.jada.2007.02.017. [DOI] [PubMed] [Google Scholar]


