Abstract
Most of the DNA-based methods for genetic typing of Staphylococcus aureus strains generate complex banding patterns. Therefore, we have developed a binary typing procedure involving strain-differentiating DNA probes which were generated on the basis of randomly amplified polymorphic DNA (RAPD) analysis. We present and validate the usefulness of 15 DNA probes, according to generally accepted performance criteria for molecular typing systems. RAPD analysis with multiple primers was performed on 376 S. aureus strains of which 97% were methicillin resistant (MRSA). Among the 1,128 RAPD patterns generated, 66 were selected which identified 124 unique DNA fragments. From these amplicons, only 12% turned out to be useful for isolate-specific binary typing. The nature of the RAPD-generated DNA fragments was investigated by partial DNA sequence analysis. Several homologies with known S. aureus sequences and with genes from other species were discovered; however, 87% of the probe sequences are of previously unknown origin. The locations of most of the DNA probes on the chromosome of S. aureus NCTC 8325 were determined by hybridization. Seven fragments were randomly dispersed along the genome, five were clustered within the 2500- to 2600-kb position of the genome, and the remaining four did not recognize complementary sequences in S. aureus NCTC 8325. A total of 103 S. aureus strains (69% MRSA) were used for the validation of the binary typing technique. The 15 DNA probes provided stable epidemiological markers, both in vitro (type consistency after serial passages on culture media) and in vivo (comparison of sequential isolates recovered from cases of persistent colonization). The discriminatory power of binary typing (D = 0.998) exceeded that of pulsed-field gel electrophoresis (D = 0.966) and RAPD analysis (D = 0.949). Reproducibility, measured by analyzing multiple strains belonging to a multitude of different epidemiological clusters, was comparable to that of other genotyping techniques used. Contribution of the DNA probes to the discriminatory power of the system was analyzed by comparison of dendrograms. This study demonstrates that binary typing is a robust tool for the genetic typing of S. aureus isolates.
Staphylococcus aureus has remained a prime pathogen of nosocomial and community-acquired infections. Worldwide, the increasing prevalence of multiresistant S. aureus has become an additional problem (4, 20, 25). Consequently, the epidemiology of S. aureus infections needs to be studied, and for this purpose multiple typing techniques based on the detection of DNA polymorphisms have been developed and optimized (3, 22). Nucleotide sequence variations among S. aureus strains can be identified by a number of techniques, varying from pulsed-field gel electrophoresis (PFGE) (39, 46) to randomly amplified polymorphic DNA (RAPD) analysis (37). However, these techniques generate complex banding patterns which lack generally accepted interpretation criteria (8, 36). Consequently, comparison of large numbers of fingerprints is very tedious and has little validity beyond the individual laboratory (8, 38, 42). Therefore, we have sought to develop less tedious typing systems that can be interpreted unequivocally. We have identified relatively unique domains within the staphylococcal genome on the basis of RAPD analysis that could be targets for such a typing system. Strain-specific DNA probes which produce a simple binary output were isolated by using hybridization assays. This collection of probes thus constitutes a so-called library typing system that can elucidate genetic polymorphism and clonal relatedness among S. aureus strains (45, 46).
In this study, the DNA probes were sequenced and homologies with known sequences and their locations on the physical map of S. aureus NCTC 8325 (29) were determined. The performance of this binary typing system was validated by using the evaluation criteria as proposed by Struelens et al. (33), Arbeit (3), and Maslow et al. (22). The performance criteria include the stability, discriminatory power, and reproducibility of the typing system.
MATERIALS AND METHODS
Bacteria.
Strains of S. aureus (n = 463) were pooled from 11 collections previously used for several purposes (Table 1). For cultivation, bacteria from glycerol stocks, stored at −80°C, were inoculated on Columbia III agar (Becton Dickinson, Etten-Leur, The Netherlands) supplemented with 5% sheep blood and incubated at 37°C for 24 h. All strains were identified as S. aureus by standard microbiological methods (19). Methicillin resistance was determined by “direct-colony suspension” inoculation of the strains on Mueller Hinton agar (Oxoid CM 337; Brunswig Chemie, Amsterdam, The Netherlands) in the presence of a disk containing 5 μg of methicillin (Oxoid; Brunswig Chemie, Amsterdam, The Netherlands) and after 16 to 18 h of incubation at 35°C. Zone diameters were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (26).
TABLE 1.
Characterization of the S. aureus collections used in this study
| Collection | Collection code(s)a | Geographic originb | No. of strains | Original purpose for strain collection in this study | Description of collections | Reference(s) or source |
|---|---|---|---|---|---|---|
| 1 | United States (CDC)a | 59 | Multicenter collection of MRSA (63%) and MSSA (37%) strains | 35 | ||
| 2 | Portugal | 184 | Nationwide disseminated MRSA strains from hospitals | 1 | ||
| 3 | Worldwide | 66 | Generation of strain-specific DNA probes | Worldwide collection of MRSA strains | 20 | |
| 4 | Italy | 49 | Genetically unrelated MRSA strains (determined by PFGE), isolated from hospitalized patients (five centers), Sicily, Italy | 41 | ||
| 5 | Australia | 18 | Genetically unrelated MRSA strains (determined by PFGE), obtained from four different hospitals | 41 | ||
| 6 | K2 | United States | 26 | Determination of discriminatory power of the genotyping methods (see Table 4) | Community-acquired MRSA strains | 46 |
| 7 | SA, SB, SC | United States (CDC) | 14 | Selection of geographically diverse strains from collection 1 | 35 | |
| 8 | NC | The Netherlands | 10 | Stability experiment for binary typing probes (see Table 3) | Strains isolated from persistant nasal carriers | 43 |
| 9 | RIVM | The Netherlands | 2 | Selection of 1 MRSA strain (Va) and 1 MSSA strain (Ia), from collection no. 10 | ||
| 10 | RIVM | The Netherlands | 49 | Epidemiological applications of the diverse genotyping systems (see Table 5) | MRSA and MSSA strains from 10 outbreaks in 10 Dutch hospitals | This study |
| 11 | United Kingdom (NCTC) | 2 | Mapping of the strain-specific DNA probes (see Fig. 1) | S. aureus NCTC 8325 and 8325-4 | 27, 29 |
K2, community-acquired MRSA strains from a New York City hospital; SA, SB, SC, S. aureus strains from the CDC collection; NC, nasal carrier; RIVM, National Institute of Public Health and the Environment (Bilthoven, The Netherlands).
CDC, Centers for Disease Control and Prevention; NCTC, National Collection of Type Cultures (Central Public Health Laboratory, London, United Kingdom).
Binary typing.
Binary typing was performed as described previously by van Leeuwen et al. (45, 46). However, we increased the overall number of strain-specific DNA probes from 5 to 15. The same procedures were used for the generation of the DNA probes as described before (45).
(i) Generation of the strain-specific DNA probes.
In short, after RAPD analysis DNA fingerprints were compared visually, and unique, strain-differentiating amplicons were selected and subsequently cloned into a TA cloning vector (Invitrogen, Leek, The Netherlands) and then transformed into Escherichia coli JM 109 cells. Inserts were amplified from the recombinant plasmids with M13 and T7 primers. Cloned fragments were characterized by DNA sequencing with dye-terminator chemistry by using a 373 DNA sequencing system (Perkin-Elmer, Foster City, Calif.). The insert sequences were compared with all entries in the data bank of the National Center for Biotechnology Information (NCBI) and were analyzed for nucleotide and protein sequence similarities with Basic Local Alignment Search Tool (BLASTN and BLASTP, respectively [2]).
(ii) Implementation of the binary typing system.
Labeling, hybridization, and detection of the cloned DNA fragments were performed with enhanced chemiluminescence (ECL) direct labeling and detection systems, according to the manufacturer’s protocols (Amersham Life Science, Buckinghamshire, United Kingdom), in order to use them as probes. The hybridization characteristics of the DNA probes were defined by prescreening these probes on a Southern blot containing 14 genetically unrelated staphylococcal strains (Table 1, collection 7). DNA probes displaying differential hybridization were added to the binary typing system. Hybridization of the 15 different DNA probes was scored with a 1 or a 0 according to the presence or absence of the hybridization signal, respectively, and the resulting binary code was transformed into a decimal number. This number is further represented as the binary type.
RAPD analysis.
RAPD analysis was carried out essentially as described before (37). Fingerprints were scored visually in which a single band difference defined a novel RAPD type. The three-letter codes are based on ERIC-2, AP-1, and AP-7 priming (45) and can only be compared within each group and not across the different groups of organisms represented in Tables 3, 4, and 5.
TABLE 3.
Stability of the strain-differentiating DNA probes determined with S. aureus strains obtained from persistent nasal carriers
| Persistent carrier (sex)a | Strainb | Isolation date | Binary code (type)c | Result by indicated genotyping techniqued:
|
||||
|---|---|---|---|---|---|---|---|---|
| RAPD analysis | PFGE | spa polymorphism | Coagulase gene polymorphism | Presence/absence of cna | ||||
| 099 (F) | NC-711 | 1988 | 001010110101111 (5551) | CCC | J | 9 | F | − |
| NC-220 | September 1995 | 001010110101111 (5551) | CCC | J | 9 | F | − | |
| 060 (F) | NC-1740 | 1988 | 101010011100101 (21733) | FCC | M | 9 | D | − |
| NC-1733 | February 1995 | 101010011100101 (21733) | FCC | M | 5 | D | − | |
| 038 (F) | NC-1288 | 1988 | 000000011100111 (231) | EDE | G | 7 | B | − |
| NC-105 | June 1995 | 000000011100111 (231) | EDE | G | 7 | B | − | |
| 076 (F) | NC-705 | 1988 | 000010011100101 (1253) | AEA | A | 7 | A | + |
| NC-054 | May 1995 | 000010011100101 (1253) | AEA | A | 7 | A | + | |
| 145 (M) | NC-714 | 1988 | 000000010100101 (165) | BBB | B | 8 | B | + |
| NC-063 | May 1995 | 000000010100101 (165) | BBB | B | 8 | B | + | |
Persistency defined as 10 identical culture results from longitudinal sampling over 3 months (43).
NC, strain from nasal carrier. See Table 1, collection 8, for more details.
Overall results after hybridization with 15 strain-specific DNA probes (AW-1 through AW-15, respectively). The decimal number represents the binary type. See Materials and Methods for more information.
Results (except for presence/absence of cna) are given as codes for each technique. See Materials and Methods for more information.
TABLE 4.
Analysis of the discriminatory power of the binary typing method compared with other genotyping techniques, estimated on the basis of the typing results for epidemiologically unrelated MRSA strains from New York City and geographically diverse S. aureus strains from the United Statesa
| Strain | Binary code | Binary type | Result by indicated genotyping technique
|
|||
|---|---|---|---|---|---|---|
| PFGE | RAPD analyses | mecA-Tn554 probing | Methicillin resistanceb | |||
| K2-01 | 001110111110111 | 7671 | J | HMC | IV:M | R |
| K2-02 | 110001011100101 | 25317 | K | AAK | II:F | R |
| K2-06 | 001110111100111 | 7655 | L | NHB | III:NH | R |
| K2-07 | 000100100100111 | 2343 | M | CCC | II:NH | R |
| K2-12 | 001110100100111 | 7463 | J | HMC | IV:M | R |
| K2-13 | 101110111110101 | 24053 | M | NBB | II:NH | R |
| K2-19 | 001100100100101 | 6437 | J | MMC | IV:M | R |
| K2-20 | 011011111100001 | 14305 | N | NHB | II:NH | R |
| K2-21 | 111110011100001 | 31969 | O | NBB | II:NH | R |
| K2-22 | 000010111111111 | 1535 | P | BBB | I:unique | R |
| K2-24 | 001100000110111 | 6199 | Q | NNA | I:NH | R |
| K2-30 | 001110100110011 | 7475 | J | GOC | I:A | R |
| K2-31 | 011111110111101 | 16317 | R | NHB | II:NH | R |
| K2-32 | 001110100111111 | 7487 | J | HMC | IV:M | R |
| K2-34 | 011110111111101 | 15869 | S | NHB | II:NH | R |
| K2-38 | 011110111110101 | 15861 | T | BIB | I:A | R |
| K2-40 | 011110111110001 | 15857 | T | BBB | I:A | R |
| K2-44 | 001100101110011 | 6515 | U | MNA | I:E | R |
| K2-45 | 001110001100011 | 7267 | J | HMC | IV:M | R |
| K2-47 | 011110011100001 | 15585 | V | NHB | II:NH | R |
| K2-50 | 001111111101111 | 8175 | V | BBB | I:A | R |
| K2-51 | 101111111111101 | 24573 | V | BBB | I:A | R |
| K2-52 | 001111010110111 | 7863 | J | HMC | IV:M | R |
| K2-56 | 011110011110011 | 15603 | W | HMC | Unique:M | R |
| K2-57 | 000100110111101 | 2493 | X | JAE | I:D | R |
| K2-65 | 101110110100011 | 23971 | Y | PPH | Unique:E | R |
| SA-04 | 111010000000000 | 29696 | E | FSL | NH:X | S |
| SA-06 | 101110000100000 | 23584 | C | TTM | II:NH | I |
| SA-07 | 111110011100000 | 31968 | B | TTM | NH:NH | S |
| SA-08 | 111110011100001 | 31969 | G | VSL1 | I:NH | R |
| SA-11 | 100100001000000 | 18496 | F | VSL1 | II:NH | R |
| SA-12 | 111110001100000 | 31840 | A | TTM | I:A | R |
| SA-13 | 111110000110000 | 31792 | A | TTM | I:A | R |
| SA-14 | 100100000100000 | 18464 | H | TTM1 | NH:NH | S |
| SA-17 | 111110000010000 | 31760 | A | TTM | I:A | R |
| SB-02 | 000001101011111 | 863 | I | WUN | NH:NH | S |
| SB-09 | 111101110011111 | 31647 | I | WVN | NH:Z | S |
| SB-13 | 111101110011111 | 31647 | I | WVN | NH:NH | S |
| SC-06 | 101111110000111 | 24455 | Z | JXO | NH:NH | S |
| SC-08 | 100000000011000 | 16408 | Z | JXO | NH:NH | S |
| Total number of types | 38 | 25 | 19 | 11c | 3 | |
| D | 0.998 | 0.96 | 0.949 | 0.848 | ||
See Table 1 for more information on the origins of the strains used.
R, resistant; I, intermediately resistant; S, susceptible.
The mecA-Tn554 typing data for the MSSA strains (n = 8) were deleted.
TABLE 5.
Survey of geno- and phenotypic results for epidemic outbreak strains of MRSA and MSSA from Dutch hospitals and nursing homes
| Cluster codea | Epidemiological data
|
Isolation date (mo/yr) | Binary codeb | Binary typec | Phage typed | Ribotypee | Result for indicated typing method:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Patient | Isolate source | PFGEf | RAPD analysisg | Coagulase gene polymorphismh | cnai | spaj | mecAk | mecIk |
mecR1k
|
|||||||
| 5′ | 3′ | ||||||||||||||||
| MSSA | |||||||||||||||||
| Ia | A | 1 | Groin | 11-96 | 101001111111101 | 21501 | A | A | AAA | −/− | 11 | − | − | + | − | ||
| Ib | A | 1 | Sputum | 11-96 | 101001111111101 | 21501 | A | A | AAA | A | −/− | 11 | − | − | + | − | |
| Ic | A | 1 | Blood | 11-96 | 101001111111101 | 21501 | A | A | AAA | A | −/− | 11 | − | − | + | − | |
| Id | A | 2 | Sputum | 11-96 | 101001111111101 | 21501 | A | A | AAA | A | −/− | 11 | − | − | + | − | |
| Ie | A | 3 | Blood | 07-96 | 101001111111101 | 21501 | A | A | AAA | A | −/− | 11 | − | − | + | − | |
| IIa | B2 | 1 | Pus | 12-96 | 001000000100000 | 4128 | A | B | BBB | NR | +/+ | 5 | − | − | + | − | |
| IIb | B1 | 1 | Pus | 10-96 | 001000000100000 | 4128 | A | B | B1BB | NR | +/+ | 5 | − | − | + | − | |
| IIc | B1 | 2 | Pus | 08-96 | 001000000100000 | 4128 | A | B1 | B1BB | NR | +/+ | 10 | − | − | + | − | |
| IId | B2 | 3 | Pus | 11-96 | 001000010100000 | 4256 | A | B2 | B1BB | NR | +/+ | 11 | − | − | + | − | |
| IIe | B2 | 4 | Pus | 10-96 | 001000000100000 | 4128 | A | B | BBB | NR | +/+ | 5 | − | − | + | − | |
| IIIa | B1 | 5 | Pus | 11-96 | 000000110101011 | 427 | B | C | CCC | NR | −/− | 10 | − | − | + | − | |
| IIIb | B3 | 6 | Urine | 10-96 | 000000110101011 | 427 | B | C | CCC | NR | −/− | 10 | − | − | + | − | |
| IIIc | B3 | 6 | Urine | 10-96 | 000000110101011 | 427 | B | C | CCC | NR | −/− | 10 | − | − | + | − | |
| IIId | B3 (B1)l | 7 | Pus | 12-95 | 010000110101011 | 8619 | B | C1 | CCC | NR | −/− | 10 | − | − | + | − | |
| IIIe | B1 | 8 | Urine | 02-96 | 010000110101011 | 8619 | B | C1 | CCC | NR | −/− | 10 | − | − | + | − | |
| IVa | C | 1 | Nose | 11-96 | 001011010100010 | 5794 | A | D | DDD | B | −/+ | 7 | − | − | + | − | |
| IVb | D (C)m | 2 | Exit site | 04-96 | 001001010100010 | 4770 | A | D | DDD | B | +/+ | 9 | − | − | + | − | |
| IVc | C | 3 | Nose | 07-96 | 001011010100010 | 5794 | A | D1 | DDD1 | B | −/+ | 11 | − | − | + | − | |
| IVd | C | 4 | Pus | 08-96 | 001011010100010 | 5794 | A | D1 | DDD1 | B | −/+ | 11 | − | − | + | − | |
| MRSA | |||||||||||||||||
| Va | En | 1 | Unknown | 01-96 | 110111111111111 | 28671 | XVI-3 | A | E1 | EEE1 | C | +/+ | 10 | + | + | + | + |
| Vb | E | 2 | Unknown | 01-96 | 110111111111111 | 28671 | XVI-3 | A | E1 | EEE1 | C | +/+ | 10 | + | + | + | + |
| Vc | E | 3 | Unknown | 01-96 | 110111111111111 | 28671 | XVI-3 | A | E1 | EEE1 | C | +/+ | 10 | + | + | + | + |
| Vd | E | 4 | Unknown | 01-96 | 110111111111111 | 28671 | XVI-3 | A | E | EEE1 | C | +/+ | 10 | + | + | + | + |
| Ve | E | 5 | Unknown | 01-96 | 110111111111111 | 28671 | XVI-3 | A | E1 | EEE1 | C | +/+ | 10 | + | + | + | + |
| VIa | F1o | 1 | Unknown | 10-96 | 001110010000000 | 7296 | XVI-4 | A | F | FFB | NR | +/+ | 9 | + | − | + | − |
| VIb | F1 | 2 | Unknown | 05-96 | 001110010000000 | 7296 | XVI-4 | A | F | FFB | NR | +/+ | 9 | + | − | + | − |
| VIc | F1 | 3 | Nose | 05-96 | 001110010000000 | 7296 | XVI-4 | A | F | FFB | NR | +/+ | 9 | + | − | + | − |
| VId | F1 | 4 | Unknown | 07-96 | 001110010000000 | 7296 | XVI-4 | A | F | FFB | NR | +/+ | 9 | + | − | + | − |
| VIe | F1 | 5 | Unknown | 04-96 | 001110010000000 | 7296 | XVI-4 | A | F | FFB | NR | +/+ | 9 | + | − | + | − |
| VIIa | F2o | 1 | Nose | 10-96 | 001110000000000 | 7168 | XI-6 | A | F1 | FFB | NR | +/+ | 9 | + | − | + | − |
| VIIb | F2 | 2 | Nose | 10-96 | 001110000000000 | 7168 | XI-6 | A | F1 | FFB | NR | +/+ | 9 | + | − | + | − |
| VIIc | F2 | 3 | Nose | 10-96 | 001110000000000 | 7168 | XI-6 | A | F1 | FFB | NR | +/+ | 9 | + | − | + | − |
| VIId | F2 | 4 | Nose | 10-96 | 001110000000000 | 7168 | XI-6 | A | F1 | FFB | NR | +/+ | 9 | + | − | + | − |
| VIIe | F2 | 5 | Nose | 10-96 | 001110000000000 | 7168 | XI-6 | A | F1 | FFB | NR | +/+ | 9 | + | − | + | − |
| VIIIa | G | 1 | Sputum | 01-96 | 100111110111101 | 20413 | Z-72 | A | G | GGF | D | −/− | 10 | + | − | + | − |
| VIIIb | G | 2 | Unknown | 03-96 | 100111110111101 | 20413 | Z-72 | A | G | GGF | D | −/− | 10 | + | − | + | − |
| VIIIc | G | 3 | Unknown | 04-96 | 100111110111101 | 20413 | Z-72 | A | G | GGF | D | −/− | 10 | + | − | + | − |
| VIIId | G | 4 | Pus | 04-96 | 100111110111101 | 20413 | Z-72 | A | G | GGF | D | −/− | 10 | + | − | + | − |
| VIIIe | G | 5 | Nose | 04-96 | 100111110111101 | 20413 | Z-72 | A | G | GGF | D | −/− | 10 | + | − | + | − |
| IXa | H | 1 | Urine | 01-96 | 101110010101101 | 23725 | III-29 | A | H | HAA | D | −/− | 9 | + | − | + | − |
| IXb | H | 1 | Urine | 01-96 | 101110010101101 | 23725 | III-29 | A | H | HAA | D | −/− | 9 | + | − | + | − |
| IXc | H | 2 | Pus | 01-96 | 101110010101101 | 23725 | III-29 | A | H | HAA | D | −/− | 8 | + | − | + | − |
| IXd | H | 3 | Pus | 01-96 | 101110010101101 | 23725 | III-29 | A | H | HAA | D | −/− | 9 | + | − | + | − |
| IXe | H | 4 | Pus | 01-96 | 101110010101101 | 23725 | III-29 | A | H | HAA | D | −/− | 9 | + | − | + | − |
| X1 | I | 1 | Ven. line | 01-93 | 001111110101011 | 8107 | III-29 | A | I | IAA | D | −/− | 10 | + | − | + | − |
| X2 | I | 2 | Nose | 01-93 | 001111110101011 | 8107 | III-29 | A | I | IAA | D | −/− | 10 | + | − | + | − |
| X3 | I | 3 | Wound | 02-93 | 001111110101011 | 8107 | III-29 | A | I | IAA | D | −/− | 10 | + | − | + | − |
| X4 | I | 4 | Bile | 02-93 | 001111110101011 | 8107 | III-29 | A | I | IAA | D | −/− | 10 | + | − | + | − |
| X5 | I | 5 | Wound | 02-93 | 001111110101011 | 8107 | III-29 | A | I | IAA | D | −/− | 10 | + | − | + | − |
See Table 1, collection 10 for more details.
Overall results after hybridization with 15 strain-specific DNA probes (AW-1 through AW-15, respectively).
Binary code transformed into a decimal number.
MSSA strains have identical phage types within a cluster. Types were dropped on account of the extensive code.
Results with EcoRI-digested genomic DNA.
After SmaI macrorestriction analysis.
The three letter code summarizes the typing results per primer used (first letter, primer ERIC-2; second letter, primer AP-1; third letter, primer AP-7).
RFLP analysis of the coagulase gene and subsequent digestion of the amplicon with AluI. NR, no restriction sites on the amplicon.
PCR result with cna gene-specific primers is presented before the shill, and the plus or minus after the shill describes the hybridization result with the PCR-generated, cna-specific DNA probe.
Analysis of the so-called X region by PCR. Amplicons were digested with RsaI. The number of direct repeats in this region was determined after electrophoresis.
−, absence of the mec-specific DNA regions analyzed by PCR; +, presence of the mec-specific DNA regions analyzed by PCR.
Patient removed from hospital B3 to an annex, hospital B1.
Patient was transferred from nursing home D to hospital C.
Outbreak in a hospital on the Netherlands Antilles.
F1 and F2 are subdivisions in different locations of the same hospital.
PFGE.
Restriction with SmaI (Boehringer, Mannheim, Germany) of genomic staphylococcal DNA and subsequent separation of the DNA macrorestriction fragments was performed by contour-clamped homogeneous electric field (CHEF) PFGE as described before (39). Macrorestriction profiles were interpreted as described by Tenover et al. (36), and each pattern is presented as a roman letter.
Mec-A–Tn554 probe typing.
Genomic staphylococcal DNA was digested with ClaI endonuclease (Pharmacia Biotech, Roosendaal, The Netherlands) according to the manufacturer’s instructions. Generation of target-specific probes and hybridization was done as described before (20).
Coagulase gene PCR.
Coagulase gene polymorphism was determined by PCR as described previously (32). The amplified part of the coagulase gene was digested with the restriction endonuclease AluI (Boehringer) according to the manufacturer’s protocol. Restriction fragment length polymorphism (RFLP) patterns were visually interpreted and indexed by roman lettering.
PCR analysis of the mec regulator genes mecI and mecR1.
PCR was performed as described before (34). Three sets of specific primers were used to amplify the different regions of the mec regulator genes, i.e., mecI and the 5′ end (transmembrane part) and the 3′ end (penicillin-binding part) of mecR1.
cna probe.
The presence or absence of the S. aureus collagen adhesin (cna) was used as an additional genotypic marker for the differentiation of S. aureus strains. Probing was performed essentially as described by Smeltzer et al. (31).
spa gene.
Staphylococcal protein A (Spa) gene polymorphism was determined by PCR as described previously (11). The so-called X region, a repetitive part within the gene, was amplified, and subsequently, the amplicon was digested with the restriction endonuclease RsaI (Boehringer), resulting in two fragments composed of 214 and 35 bases and a third fragment containing the repetitive DNA. The number of 24-bp repeats was calculated by comparison with a 100-bp molecular weight marker (Pharmacia Biotech).
Phage typing.
Phage typing was performed at the Dutch National Institute of Public Health and the Environment by using the international set of typing phages and a set of typical Dutch phages (28, 47). Different phage patterns were given different type designations.
Ribotyping.
Conventional ribotyping with EcoRI was performed by methods described previously (13). Restriction fragments were Southern blotted onto Hybond N+ membranes (Amersham) (30), and the S. aureus 16S rRNA gene, amplified by PCR, was used as a probe. Hybridization was detected by using an ECL kit (Amersham).
MecA PCR.
All S. aureus strains were investigated for the presence of the mecA gene by PCR as described before (24).
Physical mapping.
Genomic DNAs from S. aureus NCTC 8325 (29) and 8325-4 (27) were digested with SmaI (Boehringer), SgrAI (Boehringer Mannheim), and AscI (New England Biolabs, Leusden, The Netherlands) according to the manufacturers’ protocols. Macrorestriction fragments were separated by PFGE and subsequently transferred onto Hybond N+ membranes (Amersham) for Southern hybridization (30). Probing with the 15 strain-specific DNA fragments was done as described above under “Binary typing.”
Statistical analysis.
The discriminatory power of binary typing and other genotyping formats used in this study, defined as the average probability that different genotypes will be assigned to two unrelated strains in the population of a given genus, was calculated by using the formula of the Simpson index of diversity as explained by Hunter and Gaston (17, 18). The contribution of the DNA probes to the discriminatory power of the binary typing system was analyzed by cluster analysis and comparison of the dendrograms. First, all of the probes (n = 15) were used to characterize 40 unique (Table 1, collections 6 and 7) and 10 outbreak clusters (Table 1, collection 10) of S. aureus strains. The percentages of similarity of the hybridization patterns were calculated with Dice coefficient and with unweighted pair group mathematical analysis to display relatedness hierarchies among the strains. Subsequently, the procedure was repeated after discarding the DNA probe that had the lowest level of discrimination.
RESULTS
Selection of the strain-specific DNA probes.
RAPD analysis with multiple primers was performed on 376 S. aureus strains (Table 1, collections 1 through 5) of which 97% were methicillin-resistant. One hundred and twenty-four amplicons were selected from 66 RAPD patterns. Overall, 98 DNA fragments (79%) were successfully cloned, and from those a total number of 17 clones displayed a strain-specific character after hybridization with EcoRI-digested DNA from the 14 epidemiologically unrelated S. aureus strains (Table 1, collection 7 [38% MRSA]). However, 3 of the 17 clones shared the same DNA sequence, and two of these were consequently discarded. The remaining 80 fragments hybridized with DNA of all strains either at single (n = 42) or multiple sites (n = 14) or recognized the digested DNA from their source strain (n = 24) only. The latter fragments were not included since these fragments did not contribute significantly to the discriminatory power of the system.
Characterization of the DNA probes.
The origin and the nature of the 15 RAPD-generated DNA probes are outlined in Table 2. Sequence data were obtained from both termini (M13 and T7), and the DNA sequences were analyzed separately for homology by using the BLAST program with the nucleotide and protein sequence data bank, including the unfinished microbial genomes data bank (NCBI). A large proportion of these sequences did not match with known DNA elements (87%) for the nucleotide sequence data bank and 80% for the protein data bank). Probe AW-3 (M13 terminus) appeared to have a low score (BLAST score of 36) with the gene product encoded by hrmA of Nostoc sp. in the protein sequence database. Probe AW-4 (both termini) displayed a high score (1571) with the S. aureus multiresistance plasmid pSH6 for insertion sequences IS256 and IS257 in the search of the nucleotide sequence data bank and a high score (593) with IS257 transposase in the search of the protein sequence data bank. The M13 terminus of probe AW-5 displayed a low homology score (89) with the lysostaphin precursor of Staphylococcus simulans. Finally, probe AW-8 (both termini) appeared to have a high level of similarity (BLAST score 573) with the yqeV gene, a hypothetical protein, and part of the polycystronic locus of the Bacillus subtilis dnaK operon.
TABLE 2.
Summary of the demographic data and the sequence homologies from the RAPD-generated S. aureus DNA fragments with strain-specific characteristics (n = 15)
| Probe code | Origin | Source strain code | Mc resultb | Probe size (bp) | Specificityc | Nucleotide sequence homologyd
|
Protein sequence homologyd
|
||
|---|---|---|---|---|---|---|---|---|---|
| M13 | T7 | M13 | T7 | ||||||
| AW-1 | CDCa | SA-08 | R | 1,200 | 13 | NH | NH | NH | NH |
| AW-2 | CDC | SA-01 | R | 600 | 8 | NH | NH | NH | NH |
| AW-3 | CDC | SA-06 | I | 550 | 7 | NH | NH | HrmA | NH |
| AW-4 | CDC | SA-02 | R | 700 | 6 | S. aureus plasmid pSH6 | S. aureus plasmid pSH6 | S. aureus transposase IS257 | S. aureus transposase IS257 |
| AW-5 | Portugal | HPV107 | R | 400 | 3 | NH | NH | Lysostaphin precursor of S. simulans | NH |
| AW-6 | United States | BK1591 | R | 350 | 5 | NH | NH | NH | NH |
| AW-7 | Italy | 246D | R | 1,200 | 4 | NH | NH | NH | NH |
| AW-8 | Australia | WBG8217 | R | 350 | 5 | B. subtilis yqeV | B. subtilis yqeV | B. subtilis hypothetical protein YqeV | B. subtilis hypothetical protein YqeV |
| AW-9 | United States | BK1457 | R | 1,500 | 5 | NH | NH | NH | NH |
| AW-10 | United States | BK1461 | R | 400 | 6 | NH | NH | NH | NH |
| AW-11 | Australia | WBG8231 | R | 1,500 | 6 | NH | NH | NH | NH |
| AW-12 | CDC | SB-18 | R | 350 | 4 | NH | NH | NH | NH |
| AW-13 | Italy | 85CCH | R | 350 | 4 | NH | NH | NH | NH |
| AW-14 | United States | BK1563 | R | 650 | 4 | NH | NH | NH | NH |
| AW-15 | Italy | 76CCH | R | 300 | 5 | NH | NH | NH | NH |
CDC, Center for Disease Control and Prevention.
Mc; methicillin susceptibility disk-diffusion test; R, resistant; I, intermediate.
Number of strains hybridizing with a particular probe. The probes were tested on 14 CDC strains (Table 1, collection 7).
NH; no homology. Cloned DNA sequences of insert termini (M13 and T7) were analyzed for sequence homologies with nucleotide and protein sequences in the NCBI data bank by using the BLAST computer program (2).
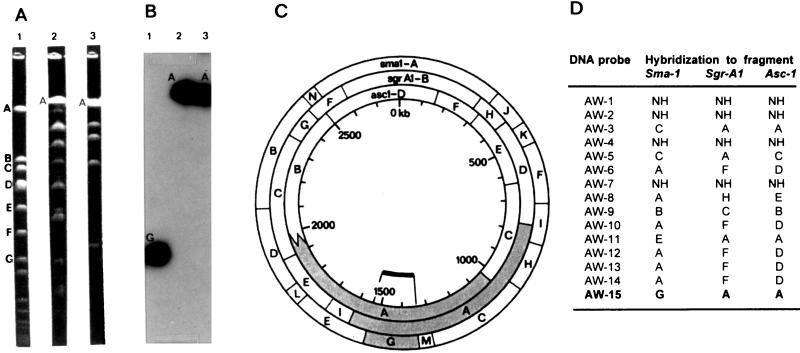
The locations of the strain-specific DNA probes were determined on the physical map of the S. aureus NCTC 8325 genome (Fig. 1) and on the restriction fragments of S. aureus NCTC 8325-4. Four of the 15 DNA probes (AW-1, AW-2, AW-4, and AW-7) failed to hybridize to either of the two staphylococcal genomes. Five probes (AW-6, AW-10, AW-12, AW-13, and AW-14) were found to be physically clustered in the same DNA region (position 2500 to 2600 kb), while the remaining seven probes were found to be scattered on the physical map of S. aureus NCTC 8325.
FIG. 1.
Physical mapping of the DNA probes on S. aureus NCTC 8325 (29) restriction fragments. (A) PFGE macrorestriction patterns of S. aureus NCTC 8325 digested with SmaI, SgrAI, and AscI (lanes 1, 2, and 3, respectively). Restriction fragments are coded by descending molecular size. (B) Example of hybridization results with probe AW-15 to PFGE patterns of S. aureus NCTC 8325. Lanes are the same as for panel A. (C) Hybridization results of probe AW-15 depicted on the physical map of S. aureus NCTC 8325. (D) Mapping results of the 15 strain-specific DNA probes (AW-1 through AW-15) to the macro-restriction fragments of the S. aureus NCTC 8325 genome. NH, no hybridization of the strain-specific DNA probe to the macrorestriction fragments.
Stability experiments.
The in vivo stability of the binary typing system was assessed by testing sequential isolates of S. aureus from five individuals who were previously classified as being persistent nasal carriers (Table 1, collection 8) (43). The 15 DNA probes uniformly and correctly identified each of the two S. aureus strains isolated from five persistent nasal carriers in 1988 and 1995, respectively, in accordance with the other genotyping techniques (Table 3). Moreover, we tested the in vitro stability of the DNA probes by serial passage (50×) of strains Ia and Va (Table 1, collection 9). Again, all descendent isolates were shown to be identical, i.e., their binary types did not change with serial passages (data not shown).
Discriminatory power.
We compared the discriminatory power of our binary typing system with that of generally accepted typing systems included PFGE, RAPD analysis, and mecA-Tn554 probing. Comparative analysis of the discriminatory power of these genotyping systems is displayed in Table 4 and is expressed by the Simpson index of diversity (D). RFLP analysis of the mecA gene and Tn554 generated 11 unique patterns from the 40 epidemiologically unrelated strains (Table 1, collections 6 and 7) and had the lowest score (D = 0.848). Due to the absence of the mecA gene, the typing data of the MSSA strains were deleted for the D value determination. PFGE and RAPD analysis differentiated the collection into 25 and 19 subtypes with D values of 0.966 and 0.949, respectively. However, the binary typing system distinguished 38 unique genotypes and had a D score of 0.998. Only two binary types, 31969 and 31647, were each found twice in the collection. Type 31647 was found to be identical by PFGE, RAPD analysis, and mecA gene polymorphism, but one strain (SB-13) lacked Tn554. Types 31969 and 31647 reportedly also share a single phage type (35). Strains K2-21 and SA-08 share binary type 31969 but clearly differed by the other genotyping systems (Table 4). It has to be emphasized that a common clonal origin for some of the (even epidemiologically unrelated) strains described in the present communication cannot be fully excluded.
Reproducibility.
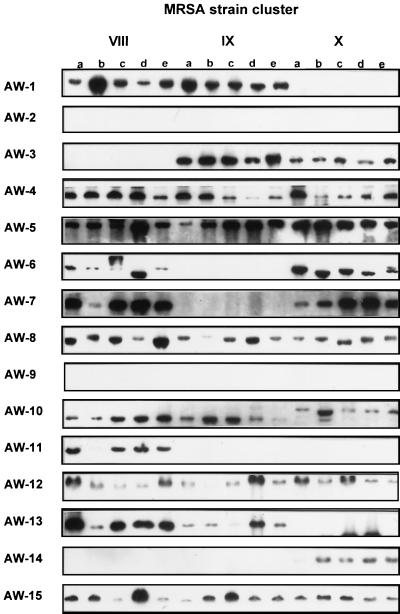
In order to test the reproducibility of our binary typing system, we tested 10 different clusters of epidemiologically related S. aureus strains (four to five strains per outbreak; four MSSA and six MRSA clusters). A representative illustration of the binary typing hybridization results is outlined in Fig. 2. The genetic relatedness of the strains within a cluster was primarily defined on the basis of epidemiological data and possession of identical phage types within the cluster. The reproducibility of the binary typing technique was calculated as the number of isolates correctly assigned to the same type within a cluster divided by the total number of strains tested. Overall, 45 of 49 (92%) strains were correctly typed, i.e., 30 of 30 MRSA and 15 of 19 MSSA strains (Table 5). Interestingly, the nonconcordant MSSA strains also showed genetic variation by one or more of the other genotyping systems applied to the same set of strains. Thus, binary typing is similarly sensitive to such variation in the genome of S. aureus.
FIG. 2.
Representative example of binary typing results obtained with the complete panel of strain-specific DNA probes (AW-1 through AW-15) from three MRSA clusters VIII, IX, and X (n = 15, collection 10). Each cluster encompassed five strains (a, b, c, d, and e) and displayed identical hybridization results.
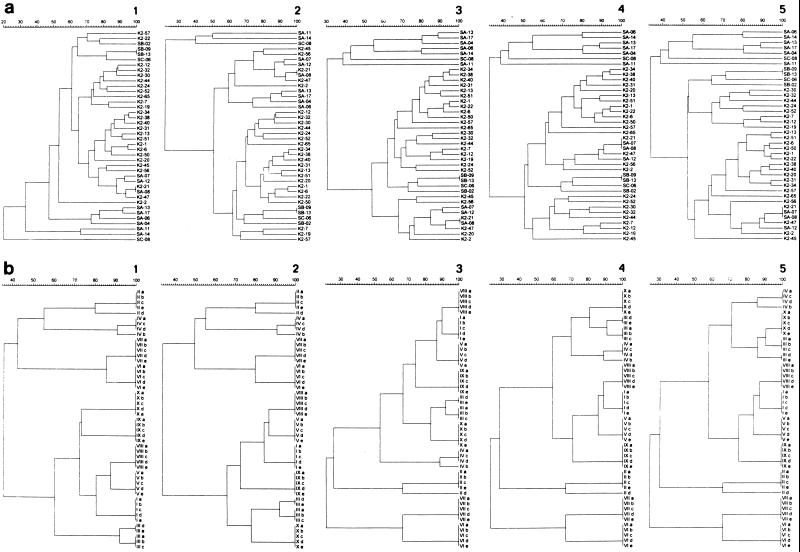
The contribution of the DNA probes to the discriminatory power of the system was analyzed by comparison of the dendrograms. Increasing subtraction of the distinct DNA probes reduced the resolution of the binary typing system among the genotypic results of the unique S. aureus strains (Fig. 3a). A similar effect on the epidemiological concordance among the hybridization patterns of the S. aureus outbreak cluster strains was noticed after subtraction of the same DNA probes (Fig. 3b). Consequently, none of the probes can be discarded from the binary typing system.
FIG. 3.
(a) Dendrograms displaying the grouping of 40 unique S. aureus strains (Table 1, collections 6 and 7) on the basis of hybridization scores after binary typing with all DNA probes (dendrogram 1); deletion of probe AW-4 (dendrogram 2); deletion of probes AW-4 and AW-3 (dendrogram 3); deletion of probes AW-4, AW-3, and AW-15 (dendrogram 4); and deletion of probes AW-4, AW-3, AW-15, and AW-6 (dendrogram 5). (b) Dendrograms presenting the similarity percentages of the hybridization patterns of 10 outbreak clusters of S. aureus strains (Table 1, collection 10) obtained with the complete panel of DNA probes comprising the binary typing system (dendrogram 1); after deletion of probe AW-4 (dendrogram 2); after deletion of probes AW-4 and AW-3 (dendrogram 3); after deletion of probes AW-4, AW-3, and AW-15 (dendrogram 4); and after deletion of probes AW-4, AW-3, AW-15, and AW-6 (dendrogram 5).
DISCUSSION
Whole genomes of bacteria are currently being sequenced at high rates, and information can be derived from analysis and comparison of these chromosomes. Essential paralogous regions as well as narrowly distributed gene families can be identified. The latter groups may be genus, species, or even strain specific. For instance, the genome of Mycoplasma genitalium commits about 5% of its content to a single species-specific domain, encoding an adhesin gene (10). Another type of DNA variability was observed after completion of the Haemophilus influenzae DNA sequence (9). Repeats in the genes encoding enzymes involved in lipopolysaccharide biosynthesis and iron acquisition and a gene encoding an adhesin display clear heterogeneity (16, 40). The E. coli genome highlights novel insertion sequence elements, phage remnants, and many DNA fragments of unusual composition, indicating genome plasticity and horizontal gene transfer (5). Many bacterial virulence genes are found as discrete DNA fragments, present in pathogenic organisms but absent from nonpathogenic members of the same genus or species, e.g., the “pathogenicity islands” of uropathogenic E. coli or enteropathogenic E. coli (14, 23). Unfortunately, only a single genome sequence of a gram-positive bacterium is known. The B. subtilis genome contains phage-type elements as well, again indicating DNA flexibility (21). Based on theoretical comparative analysis, many DNA elements contributing to DNA variation can be pinpointed. No experimental studies have been described as yet, however. Practically, the genome variability of S. aureus strains can be visualized on the basis of RAPD analysis and the use of the amplicons thereof as probes. We describe here an approach for isolating species-specific DNA elements for a bacterium for which the whole genome sequence is not in the public domain. The aim of the present study was to validate the use of strain-differentiating DNA probes for the genotyping of S. aureus and to develop a new typing format, providing a simple binary output based on the use of RAPD-generated DNA probes. Such probes can detect sequence variation between genomes without prior knowledge of the target DNA sequence, as has been presented before (45, 46). We now have extended the number of DNA probes to 15 and have shown the typing system to have a very high index of reproducibility, stability (100%), and discriminatory power (D = 0.998). Hybridization studies revealed that only 12% of the RAPD amplicons, visually selected for uniqueness, exhibited the desired genetic typing characteristics for S. aureus strains. Primer site variation may be the origin of the remaining 88% of the differentiating amplicons. The nature of the DNA probes used in this study remains largely unknown. In one case (probe AW-4) homology with a mobile genetic element, IS257, was found. IS257 is an insertion sequence identified as commonly occurring in staphylococcal plasmids (7). These plasmids often code for diverse resistance determinants. The investigation of further alignments awaits publication of the whole S. aureus genome sequence.
The locations of the DNA probes on the physical map of S. aureus NCTC 8325 (29) (Fig. 1) and S. aureus NCTC 8325-4, a derivative of 8325 cured of phages P11, P12, and P13 (27), were determined. Some probes (n = 4) were neither on the physical map of S. aureus NCTC 8325 nor present on the restriction fragments of NCTC 8325-4. The remainder of the probes recognized elements on both genomes, which argues against a putative relationship with the prophage sequences that are present in S. aureus NCTC 8325 but not in S. aureus NCTC 8325-4. Seven probes showed random locations and five clustered together around the 2500- to 2600-kb region of the S. aureus NCTC 8325 genome. These latter probes all share a nucleotide sequence of 80 bp, but the main part of their nucleotide sequence was totally different. It is possible that this DNA region is part of a direct repeat and spacer region, which can be used to generate sequence variation patterns between genomes (44). The location of these probes coincides with that of several potentially variable elements: essential genes for recombination between genomes (recA) or DNA repair (uvr), virulence factors (hla), and diverse Tn551 insertion sites (29). The probes that hybridized to DNA regions scattered throughout the genome seemed to have no linkage with variable DNA sequences, except for one probe (AW-15) which is located in the vicinity of resistance determinants (mec region), virulence factors (spa), and the origin of replication.
The stability of the binary typing system was evaluated with sequential isolates recovered from healthy individuals who were shown to be persistent nasal carriers of S. aureus (43). The persistent carriers were monitored in 1988 and 1995, and similarities of the genotypes among these two sampling periods were determined with binary typing, PFGE, RAPD analysis, coagulase and protein A gene polymorphism, and the absence or presence of the cna gene. All genomic characterization techniques (Table 3), including the 15 epidemiological markers of the binary typing system, indicated a high degree of genomic stability over the years, except for the spa gene typing (persistent carrier 060). During laboratory storage and replication, mutations and transpositional recombination may occur (6), and the stability of the epidemiological markers for the staphylococcal genome can be measured by in vitro stability. The in vitro stability of the binary typing system was estimated by comparing the genomes of strains before and after 50 serial passages of strains on culture media. All DNA probes generated identical results after repeated testing (data not shown).
The Simpson index of diversity (D) expresses the discriminatory power of a genotyping system (17, 18). We calculated the D value for binary typing and compared this with the results of other frequently used techniques (Table 4, PFGE, RAPD analysis, mecA-Tn554 probing). Certain systems, such as PFGE or RAPD analysis with multiple primers, generate complex banding patterns, and the Simpson index was calculated on the basis of the similarity level, defining a genotype (36). Hunter (18) proposed that the standardized discrimination index determines the discrimination index of a typing method that has a reproducibility of 95%; this is designated D95. Both binary typing and PFGE exceed the level of D95, and consequently these methods can be used as a single method. Less discriminating systems such as RAPD analysis and mecA-Tn554 probing can be used in combination to obtain a significant D95 index (18, 33).
The probability of clonal linkage among epidemic strains determined to be similar by diverse genotyping techniques can be expressed at the level of reproducibility. In fact an application of in vivo stability, i.e., comparison of sequential isolates, recovered along the course of a well-documented outbreak (33). The whole-genome characterization techniques binary typing, PFGE, and RAPD analysis display adequate reproducibility among the related genomes of the epidemic MRSA strains (Table 5, clusters V through X). Only the number of repeats within the spa gene remain unstable within genetically related strains, and no concordance is demonstrated for analyzing presence versus absence of specific genes (cna, mecA, mecI, and mecR1). Strains originated from different locations of hospital F (Table 5, clusters VI and VII) are genetically related, as shown by the genotyping results.
Conclusion and future developments.
The binary typing method described herein provides a reproducible, high-resolution molecular typing system strategy that may in the end be preferred over other means of genotyping. This method generates a simply binary output which is to be preferred over the complex banding patterns generated by most other genotyping systems. Furthermore, an important advantage of the binary typing system compared to other genotyping systems is that the system essentially comprises an assay procedure that is amenable to extensive automation and does not require variation in electrophoretic conditions such as voltage, time of run, and temperature, etc. (38, 42). Moreover, DNA hybridization can be performed by using an enzyme-linked immunosorbent assay-like technique, allowing implementation of this approach in most routine microbiological laboratories. It is theoretically also possible to develop specific DNA probes to determine virulence factors and resistance determinants for additional diagnostic information (15). In principle, this technique can be extrapolated easily to other bacterial species (12). The binary typing system satisfies the requirements of the accepted performance criteria and promises to become a technically simple and fast library typing system.
ACKNOWLEDGMENTS
Marly Sijmons is acknowledged for practical assistance with DNA sequencing of the probes. M. van den Bergh (St. Radboud Ziekenhuis, Nijmegen, Netherlands), W. Grubb (Curtin University of Technology, Perth, Australia), B. Kreiswirth (PHRI, New York, N.Y.), H. de Lencastre (Rockefeller University, New York, N.Y.), R. Roberts (Cornell Medical Center, New York, N.Y.), S. Stefani (Universita di Catania, Catania, Italy), F. Tenover (CDC, Atlanta, Ga.), and E. IJzerman (Streeklaboratorium voor de Volksgezondheid, Haarlem, Netherlands) are thanked for providing the bacterial strain collections.
This study was partially funded by the Dutch Ministry of Economic Affairs (BTS 97134).
REFERENCES
- 1.Aires de Sousa M, Sanches I S, van Belkum A, van Leeuwen W, Verbrugh H, de Lencastre H. Characterization of methicillin-resistant Staphylococcus aureus isolates from Portuguese hospitals by multiple genotyping systems. Microb Drug Res. 1996;2:331–341. doi: 10.1089/mdr.1996.2.331. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Arbeit R D. Laboratory procedures for the epidemiologic analysis of staphylococci. In: Archer G L, Crossley K B, editors. Staphylococci and staphylococcal diseases. New York, N.Y: Churchill Livingstone; 1998. pp. 203–286. [Google Scholar]
- 4.Ayliffe G A J. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997;24:S74–S79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Brikun I, Suziedelis K, Berg D E. DNA sequence divergence among derivatives of Escherichia coli K-12 detected by arbitrary primer PCR (random amplified polymorphic DNA) fingerprinting. J Bacteriol. 1994;176:1673–1682. doi: 10.1128/jb.176.6.1673-1682.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne M E, Littlejohn T G, Skurray R A. Transposons and insertion sequences in the evolution of multi-resistant Staphylococcus aureus. In: Novick R P, editor. Molecular biology of staphylococci. New York, N.Y: VCH publishers; 1990. pp. 165–174. [Google Scholar]
- 8.Cookson B D, Aparicio P, Deplano A, Struelens M, Goering R, Marples R. Inter-centre comparison of pulsed-field gel electrophoresis for the typing of methicillin-resistant Staphylococcus aureus. J Med Microbiol. 1996;44:179–184. doi: 10.1099/00222615-44-3-179. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage E R, Bult C J, Tomb J, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 10.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, Fleischmann R D, Bult C J, Kerlavage A R, Sutton G, Kelley J M, Fritchman J L, Weidman J F, Small K V, Sandusky M, Fuhrmann J, Nguyen D, Utterback T R, Saudeck D M, Phillips C A, Merrick J M, Tomb J, Dougherty B A, Bott K F, Hu P, Lucier T S, Peterson S N, Smith H O, Hutchinson III C A, Venter J C. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 11.Frénay H M E, Theelen J P G, Schouls L M, Vanden Broucke-Grauls C M J E, van Leeuwen W J, Mooi F R. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. J Clin Microbiol. 1994;32:846–847. doi: 10.1128/jcm.32.3.846-847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giesendorf B A J, Quint W G V, Vandamme P, van Belkum A. Generation of DNA probes for detection of microorganisms by polymerase chain reaction fingerprinting. Zentralbl Bakteriol. 1996;283:417–430. doi: 10.1016/s0934-8840(96)80121-6. [DOI] [PubMed] [Google Scholar]
- 13.Grimont F, Lefevre M, Ageron E, Grimont P A D. rRNA gene restriction patterns of Legionella species: a molecular identification system. Res Microbiol. 1989;140:615–626. doi: 10.1016/0923-2508(89)90193-9. [DOI] [PubMed] [Google Scholar]
- 14.Groisman E A, Ochman H. Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996;87:791–794. doi: 10.1016/s0092-8674(00)81985-6. [DOI] [PubMed] [Google Scholar]
- 15.Hermans P W M, Saha S K, van Leeuwen W J, Verbrugh H A, van Belkum A, Goessens W H F. Molecular typing of Salmonella typhi strains from Dhaka (Bangladesh) and development of DNA probes identifying plasmid-encoded multidrug-resistant isolates. J Clin Microbiol. 1996;34:1373–1379. doi: 10.1128/jcm.34.6.1373-1379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood D W, Deadman M E, Allen T, Masoud H, Martin A, Brisson J R, Fleischmann R, Venter J C, Richards J C, Moxon E R. Use of the complete genome sequence information of Haemophilus influenzae strain Rd to investigate lipopolysaccharide biosynthesis. Mol Microbiol. 1996;22:951–965. doi: 10.1046/j.1365-2958.1996.01545.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter P R. Reproducibility and indices of discriminatory power of microbial typing methods. J Clin Microbiol. 1990;28:1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloos W E, Bannerman T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R P, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 282–298. [Google Scholar]
- 20.Kreiswirth B, Konblum J, Arbeit R D, Eisner W, Maslow J N, McGreer A, Low D E, Novick R P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- 21.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Crummings N J, Daniel R A, Denizot F, Devine K M, Düsterhöft A, Ehrlich S D, Emmerson P T, Entian K D, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim S Y, Glaser P, Goffeau A, Golightly E J, Grandi G, Guiseppi G, Guy B J, Haga K, Haiech J, Harwood C R, Hénault A, Hilbert H, Holsappel S, Hosono S, Hullo M F, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee S M, Levine A, Liu H, Masuda S, Mauël C, Médigue C, Medina N, Mellado R P, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park S H, Parro V, Pohl T M, Portetelle D, Porwollik S, Prescott A M, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror S J, Serror P, Shin B S, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Tognoni A, Tosato V, Uchiyama S, Vandenbol M, Vannier F, Vassarotti A, Viari A, Wanbutt R, Wedler E, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yashumoto K, Yata K, Yoshida K, Yoshikawa H F, Zumstain E, Yoshikawa H, Danchin A. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 22.Maslow J N, Mulligan M E, Arbeit R D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993;17:153–164. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- 23.Mel S F, Mekalanos J. Modulation of horizontal gene transfer in pathogenic bacteria by in vivo signals. Cell. 1996;87:795–798. doi: 10.1016/s0092-8674(00)81986-8. [DOI] [PubMed] [Google Scholar]
- 24.Murakami K, Minamide W, Wada K, Nakumara E, Teraoka H, Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musser J M, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; 8th informational supplement. 18, no. 1. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 27.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 28.Parker M T. Phage typing of Staphylococcus aureus. Methods Microbiol. 1978;11:15–21. [Google Scholar]
- 29.Pattee P A. Genetic and physical map of Staphylococcus aureus NCTC 8325. In: O’Brien S J, editor. Genetic maps. 6th ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 2.106–2.113. [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Schmeltzer M S, Pratt F L, Gillaspy A F, Young L A. Genomic fingerprinting for epidemiological differentiation of Staphylococcus aureus clinical isolates. J Clin Microbiol. 1996;34:1364–1372. doi: 10.1128/jcm.34.6.1364-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzkopf A, Karch H. Genetic variation in Staphylococcus aureus coagulase genes: potential and limits for use as epidemiological marker. J Clin Microbiol. 1994;32:2407–2412. doi: 10.1128/jcm.32.10.2407-2412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struelens M J the members of the European Study Group on Epidemiological Markers (ESGEM), of the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki E, Kuwakara-Arai K, Richardson J F, Hiramatsu K. Distribution of mec regulator genes in methicillin-resistant Staphylococcus aureus clinical strains. Antimicrob Agents Chemother. 1993;37:1219–1226. doi: 10.1128/aac.37.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenover F C, Arbeit R, Archer G, Biddle J, Byrne S, Goering R, Hancock G, Ann Hébert G, Hill B, Hollis R, Jarvis W R, Kreiswirth B, Eisner W, Maslow J, McDougal L K, Miller J M, Mulligan M, Pfaller M A. Comparison of traditional and molecular methods for typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994;32:407–415. doi: 10.1128/jcm.32.2.407-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Belkum A, Bax R, van Straaten P C J, Quint W G V, Veringa E. PCR fingerprinting for epidemiological studies of Staphylococcus aureus. J Microbiol Methods. 1994;20:235–247. [Google Scholar]
- 38.Van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, VandenBroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, VanEechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Belkum A, van Leeuwen W, Verkooyen R, Saçilik S C, Cokmus C, Verbrugh H. Dissemination of a single clone of methicillin-resistant Staphylococcus aureus among Turkish hospitals. J Clin Microbiol. 1997;35:978–981. doi: 10.1128/jcm.35.4.978-981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Belkum A, Scherer S, van Leeuwen W, Willemse D, van Alphen L, Verbrugh H. Variable number of tandem repeats in clinical strains of Haemophilus influenzae. Infect Immun. 1997;65:5017–5027. doi: 10.1128/iai.65.12.5017-5027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Belkum A, Hermans P W M, Licciardello L, Stefania S, Grubb W, van Leeuwen W, Goessens W H F. Polymerase chain reaction-mediated typing of microorganisms: tracking dissemination of genes and genomes. Electrophoresis. 1998;19:602–607. doi: 10.1002/elps.1150190424. [DOI] [PubMed] [Google Scholar]
- 42.Van Belkum A, van Leeuwen W, Kaufmann M E, Cookson B, Forey F, Etienne J, Goering R, Tenover F, Steward C, O’Brien F, Grubb W, Tassios P, Legakis N, Morvan A, El Sohl N, de Ryck R, Struelens M, Salmenlinna S, Vuopio-Varkila J, Kooistra M, Talens A, Witte W, Verbrugh H. Assessment of resolution and intercenter reproducibility of results of genotyping Staphylococcus aureus by pulsed-field gel electrophoresis of SmaI macrorestriction fragments: a multicenter study. J Clin Microbiol. 1998;36:1653–1659. doi: 10.1128/jcm.36.6.1653-1659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VandenBergh, M. F. Q., E. P. F. IJzerman, A. van Belkum, and H. A. Verbrugh. Eight-year follow-up of Staphylococcus aureus nasal carriage: (re)defining the persistant carrier state. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 44.Van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenbach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Leeuwen W B, Sijmons M, Sluijs J, Verbrugh H, van Belkum A. On the nature and use of randomly amplified DNA from Staphylococcus aureus. J Clin Microbiol. 1996;34:2770–2777. doi: 10.1128/jcm.34.11.2770-2777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Leeuwen W, van Belkum A, Kreiswirth B, Verbrugh H. Genetic diversification of methicillin-resistant Staphylococcus aureus as a function of prolonged geographic dissemination and as measured by binary typing and other genotyping methods. Res Microbiol. 1998;149:497–507. doi: 10.1016/s0923-2508(98)80004-1. [DOI] [PubMed] [Google Scholar]
- 47.Van Leeuwen W J, Rost J A. Additional set of typing phages of Staphylococcus aureus strains of human strains not typeable with the international basic set of phages. Zentralbl Bakteriol Mikrobiol Hyg Abt 1 Suppl. 1976;5:1013–1019. [Google Scholar]