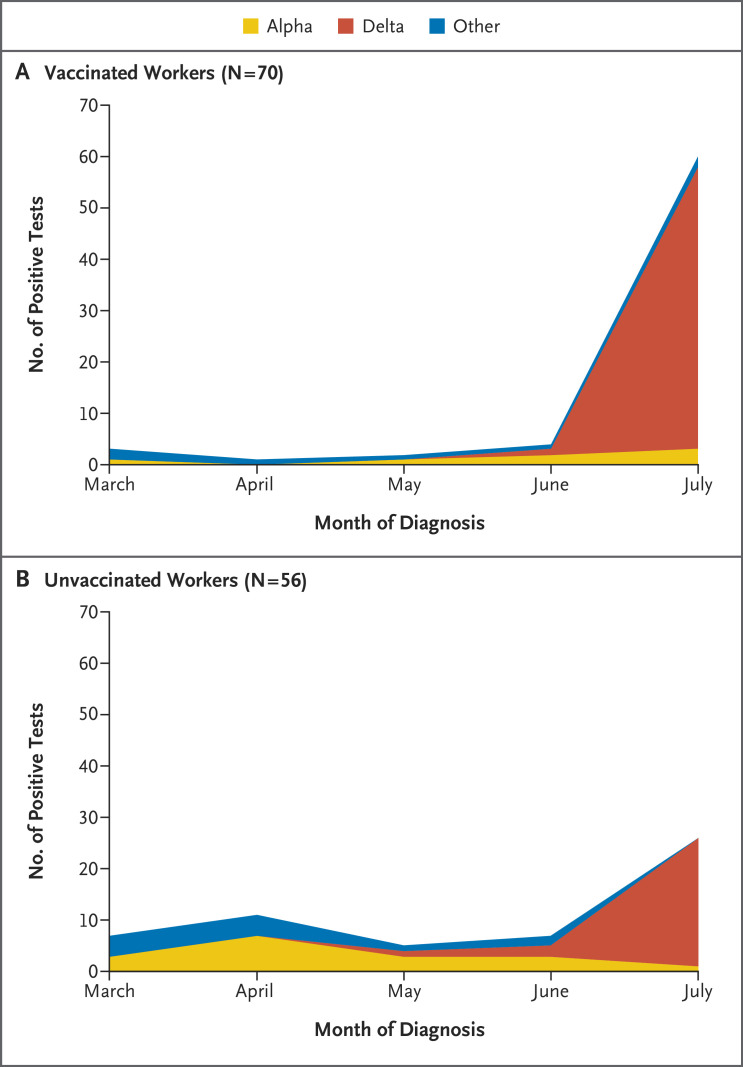
To the Editor: In December 2020, the University of California San Diego Health (UCSDH) workforce experienced a dramatic increase in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. Vaccination with mRNA vaccines began in mid-December 2020; by March, 76% of the workforce had been fully vaccinated, and by July, the percentage had risen to 87%. Infections had decreased dramatically by early February 2021.1 Between March and June, fewer than 30 health care workers tested positive each month. However, coincident with the end of California’s mask mandate on June 15 and the rapid dominance of the B.1.617.2 (delta) variant that first emerged in mid-April and accounted for over 95% of UCSDH isolates by the end of July (Figure 1), infections increased rapidly, including cases among fully vaccinated persons. Institutional review board approval was obtained for use of administrative data on vaccinations and case-investigation data to examine mRNA SARS CoV-2 vaccine effectiveness.
Figure 1. SARS-CoV-2 Variants among Symptomatic Health Workers.
Shown is the distribution of the B.1.1.7 (alpha), delta, and other SARS-CoV-2 variants according to vaccination status and month of diagnosis among health workers at University of California San Diego Health, March through July 2021. The number of workers indicates those who were symptomatic and had available variant data, and the number of positive tests indicates those that included data on variants.
UCSDH has a low threshold for SARS-CoV-2 testing, which is triggered by the presence of at least one symptom during daily screening or by an identified exposure, regardless of vaccination status. From March 1 to July 31, 2021, a total of 227 UCSDH health care workers tested positive for SARS-CoV-2 by reverse-transcriptase–quantitative polymerase-chain-reaction (RT-qPCR) assay of nasal swabs; 130 of the 227 workers (57.3%) were fully vaccinated. Symptoms were present in 109 of the 130 fully vaccinated workers (83.8%) and in 80 of the 90 unvaccinated workers (88.9%). (The remaining 7 workers were only partially vaccinated.) No deaths were reported in either group; one unvaccinated person was hospitalized for SARS-CoV-2–related symptoms.
Vaccine effectiveness was calculated for each month from March through July; the case definition was a positive PCR test and one or more symptoms among persons with no previous Covid-19 infection (see the Supplementary Appendix). Vaccine effectiveness exceeded 90% from March through June but fell to 65.5% (95% confidence interval [CI], 48.9 to 76.9) in July (Table 1). July case rates were analyzed according to the month in which workers with Covid-19 completed the vaccination series; in workers completing vaccination in January or February, the attack rate was 6.7 per 1000 persons (95% CI, 5.9 to 7.8), whereas the attack rate was 3.7 per 1000 persons (95% CI, 2.5 to 5.7) among those who completed vaccination during the period from March through May. Among unvaccinated persons, the July attack rate was 16.4 per 1000 persons (95% CI, 11.8 to 22.9).
Table 1. Symptomatic SARS-CoV-2 Infection and mRNA Vaccine Effectiveness among UCSDH Health Workers, March through July 2021.*.
| March | April | May | June | July | |
|---|---|---|---|---|---|
| UCSDH workforce — no. of persons | 18,964 | 18,992 | 19,000 | 19,035 | 19,016 |
| Vaccination status — no. of persons | |||||
| Fully vaccinated† | 14,470 | 15,510 | 16,157 | 16,426 | 16,492 |
| mRNA-1273 (Moderna) | 6,608 | 7,005 | 7,340 | 7,451 | 7,464 |
| BNT162b2 (Pfizer–BioNTech) | 7,862 | 8,505 | 8,817 | 8,975 | 9,028 |
| Unvaccinated | 3,230 | 2,509 | 2,187 | 2,059 | 1,895 |
| Percentage of workers fully vaccinated | 76.3 | 81.7 | 85.0 | 86.3 | 86.7 |
| Symptomatic Covid-19 | |||||
| Fully vaccinated workers | 3 | 4 | 3 | 5 | 94 |
| Unvaccinated workers | 11 | 17 | 10 | 10 | 31 |
| Percentage of cases in fully vaccinated workers | 21.4 | 19.0 | 23.1 | 33.3 | 75.2 |
| Attack rate per 1000 (95% CI) | |||||
| Fully vaccinated workers | 0.21 (0.21–0.47) | 0.26 (0.26–0.50) | 0.19 (0.21–0.40) | 0.30 (0.31–0.53) | 5.7 (5.4–6.2) |
| Unvaccinated workers | 3.4 (2.1–5.9) | 6.8 (4.5–10.6) | 4.6 (2.6–8.2) | 4.9 (2.9–8.7) | 16.4 (11.8–22.9) |
| Vaccine effectiveness — % (95% CI) | 93.9 (78.2–97.9) | 96.2 (88.7–98.3) | 95.9 (85.3–98.9) | 94.3 (83.7–98.0) | 65.5 (48.9–76.9) |
UCSDH denotes University of California San Diego Health.
Data are the total number of workers who had received two doses of an mRNA vaccine as of the last day of the month.
The SARS CoV-2 mRNA vaccines, BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna), have previously shown efficacy rates of 95% and 94.1%,2 respectively, in their initial clinical trials, and for the BNT162b2 vaccine, sustained, albeit slightly decreased effectiveness (84%) 4 months after the second dose.3 In England, where an extended dosing interval of up to 12 weeks was used, Lopez Bernal et al. reported a preserved vaccine effectiveness of 88% against symptomatic disease associated with the delta variant.4 As observed by others in populations that received mRNA vaccines according to standard Emergency Use Authorization intervals,5 our data suggest that vaccine effectiveness against any symptomatic disease is considerably lower against the delta variant and may wane over time since vaccination.
The dramatic change in vaccine effectiveness from June to July is likely to be due to both the emergence of the delta variant and waning immunity over time, compounded by the end of masking requirements in California and the resulting greater risk of exposure in the community. Our findings underline the importance of rapidly reinstating nonpharmaceutical interventions, such as indoor masking and intensive testing strategies, in addition to continued efforts to increase vaccinations, as strategies to prevent avoidable illness and deaths and to avoid mass disruptions to society during the spread of this formidable variant. Furthermore, if our findings on waning immunity are verified in other settings, booster doses may be indicated.
Supplementary Appendix
Disclosure Forms
This letter was published on September 1, 2021, and updated on September 3, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Keehner J, Abeles SR, Torriani FJ. More on SARS-CoV-2 infection after vaccination in health care workers. reply. N Engl J Med 2021;385(2):e8. [DOI] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas SJ, Moreira ED Jr, Kitchin N, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. July 28, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1). preprint.
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. August 5, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.03.21261496v1). preprint. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.