Abstract
Background
Intraoperative EEG suppression duration has been associated with postoperative delirium and mortality. In a clinical trial testing anaesthesia titration to avoid EEG suppression, the intervention did not decrease the incidence of postoperative delirium, but was associated with reduced 30-day mortality. The present study evaluated whether the EEG-guided anaesthesia intervention was also associated with reduced 1-yr mortality.
Methods
This manuscript reports 1 yr follow-up of subjects from a single-centre RCT, including a post hoc secondary outcome (1-yr mortality) in addition to pre-specified secondary outcomes. The trial included subjects aged 60 yr or older undergoing surgery with general anaesthesia between January 2015 and May 2018. Patients were randomised to receive EEG-guided anaesthesia or usual care. The previously reported primary outcome was postoperative delirium. The outcome of the current study was all-cause 1-yr mortality.
Results
Of the 1232 subjects enrolled, 614 subjects were randomised to EEG-guided anaesthesia and 618 subjects to usual care. One-year mortality was 57/591 (9.6%) in the guided group and 62/601 (10.3%) in the usual-care group. No significant difference in mortality was observed (adjusted absolute risk difference, –0.7%; 99.5% confidence interval, –5.8% to 4.3%; P=0.68).
Conclusions
An EEG-guided anaesthesia intervention aiming to decrease duration of EEG suppression during surgery did not significantly decrease 1-yr mortality. These findings, in the context of other studies, do not provide supportive evidence for EEG-guided anaesthesia to prevent intermediate term postoperative death.
Clinical trial registration
Keywords: burst suppression, depth of anaesthesia, electroencephalogram suppression, postoperative death, postoperative delirium, postoperative falls, postoperative mortality, quality of life
Editor's key points.
-
•
Burst suppression in the EEG during anaesthesia has been associated with delirium and mortality, but it is not clear whether avoidance of burst suppression improves these outcomes.
-
•
In the previously performed Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) study, titration of anaesthetic agents to avoid burst suppression was not associated with reduced incidence of delirium, but was associated with significant reduction in 30-day mortality.
-
•
The authors now report the results of a 1 yr follow-up study involving the same subjects.
-
•
There was no significant difference in 1-yr mortality rate in subjects in whom the EEG-based intervention was applied compared with those receiving standard care.
Although many subjects undergo surgery to prolong their lives or to improve their quality of life, some subjects fail to achieve these goals. In both high- and low-income countries, roughly one in 200 elective surgical subjects does not survive until hospital discharge.1 Of surgical subjects above age 65 yr, about 10% die within the first year.2 A growing body of evidence has demonstrated associations between markers of deep hypnosis and postoperative morbidity and mortality. Intraoperative EEG suppression and time spent with low bispectral index (BIS) readings have both been identified as independent predictors of 1-yr postoperative death in observational cohorts.3,4 Intraoperative EEG suppression has also been linked to postoperative delirium after both cardiac and noncardiac surgery,5,6 and subjects who experience delirium in the hospital are known to be at increased risk for death after discharge.7 Based on these reported associations, several randomised controlled trials have investigated the impact of interventions intended to reduce intraoperative exposure to EEG suppression or low BIS readings.8, 9, 10, 11, 12
One of the trials to study this topic was the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) trial.9 ENGAGES was a single-centre RCT comparing an EEG-guided anaesthesia intervention vs usual care. No significant difference was observed between the two groups with respect to the primary outcome of postoperative delirium. However, the EEG-guided group had a statistically significant reduction in 30-day mortality (four of 614 subjects, 0.7%) compared with the usual care group (19 of 618 subjects, 3.1%).9 The primary purpose of the present analysis was to determine whether this effect on postoperative mortality persisted at 1 yr followup. As secondary outcomes, the effect of the EEG guidance intervention on quality of life and falls at 1 yr was also studied. The effects of postoperative delirium on each of these outcomes were also assessed.
Methods
This is a 1-yr analysis of the single-centre ENGAGES RCT.9 One-year mortality is an unplanned secondary outcome of the trial, added owing to the unexpected finding of reduced 30-day mortality in the EEG guidance group. One-year quality of life and falls are pre-specified secondary outcomes. Examining the associations of postoperative delirium with quality of life and falls were pre-specified analyses, whereas examining the association of postoperative delirium with 1-yr mortality was unplanned because 1-yr mortality was not a planned secondary outcome of the trial. The trial was registered at ClinicalTrials.gov (NCT02241655), and detailed analytic plans for this manuscript were made available on that website before conducting the analyses. The Human Research Protection Office at Washington University approved this study (#201407128), and all subjects provided written informed consent. This report follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines and the extension for reporting pragmatic trials.13,14
Patient population
Recruitment occurred at Barnes-Jewish Hospital, a large academic medical centre in St. Louis, MO, USA. Patients aged 60 yr or older scheduled for surgery with general anaesthesia and anticipated hospital stay of at least 2 days were eligible for inclusion. Patients unable to complete interviews in English, with delirium at baseline, with a history of intraoperative awareness, or scheduled for a second surgery within 5 days of the initial surgery were excluded.
Trial conduct
As described,9,15 subjects were randomised to the EEG guidance protocol or to usual care in a 1:1 ratio, stratified by cardiac vs noncardiac surgery and presence or absence of falls in the 6 months before enrolment. Further details about the randomisation procedures are available in the primary manuscript.9 A BIS Quatro (Medtronic Corp., Minneapolis, MN, USA) sensor was applied to each patient's forehead to display a single channel of frontal EEG. BIS uses a proprietary algorithm to generate a number between 0 and 100, with lower numbers intended to represent greater depth of anaesthesia. Clinicians caring for subjects in the usual care group were blinded to the EEG data, except the signal quality index. Clinicians caring for subjects in the intervention group viewed the unfiltered frontal EEG waveform and the processed EEG parameters on the bedside vital sign monitor. These clinicians were instructed to titrate the volatile anaesthetic primarily to minimise EEG suppression and secondarily to minimise periods of BIS readings <40. Clinicians in the operating room could not be blinded to randomisation assignment, but subjects and research staff assessing outcomes were blinded.
Delirium assessment
Postoperative delirium was measured on postoperative days 1 through 5 using in-person delirium assessment by study personnel and using chart review. The in-person assessment was conducted once per day using the Confusion Assessment Method (CAM) for non-intubated subjects16 and the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) for intubated subjects.17 Chart review was conducted each day using the validated Chart Abstraction for Delirium tool.18 Incident delirium was defined as a positive in-person assessment or positive chart review at any time point during postoperative days 1 through 5. Duration of delirium was defined as the number of days with positive delirium assessments. Severe delirium was defined as a CAM-S score of 10 or greater (on a scale of 0–19) or a CAM-ICU-7 score of 6 or greater (on a scale of 0–7).19,20
Outcomes
The primary outcome in this analysis was all-cause mortality at 1 yr after surgery. Patients who returned the survey regarding the 1-yr secondary outcomes (see below) were known to be alive. Patients who did not return the survey were followed up by telephone to encourage survey completion; vital status was assessed during that phone call. Secondary outcomes were self-reported quality of life and falls at 1 yr after surgery. Quality of life was measured preoperatively and 1 yr after surgery using the physical composite summary and mental composite summary from the Veterans RAND 12-Item (VR-12) Health Survey.21 Each summary score takes a value between 0 and 100, with higher values representing better quality of life. Patients completed a survey at each time point that included a question about whether they had fallen in the past 6 months (preoperative survey) or since surgery (postoperative survey). The question defined a fall as ‘an event in which you lost your balance and landed on the floor or ground or lower level’, consistent with the definition used by the Prevention of Falls Network Europe.22
Statistical analysis
All analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).23 P values <0.05 were considered as providing suggestive evidence, whereas P values <0.005 were considered as providing more compelling evidence.24 All tests were two-sided. The sample size of the ENGAGES trial was selected to achieve >90% power to detect an 8% absolute difference in the incidence of postoperative delirium between the intervention and control groups with α=0.05.9 The sample size was therefore fixed at 1232 in this secondary analysis. Similar previous studies at our institution have reported 1 yr mortality of 10%.25,26 Assuming that ENGAGES would also demonstrate an overall 10% 1 yr mortality, we estimated that we would have 80% power to detect a difference in mortality of at least 4.8% between the intervention and control groups at α=0.05. Normally distributed variables were described using mean and standard deviation, whereas non-normally distributed variables were described using median and inter-quartile range (IQR).
The primary analysis followed the intention-to-treat principle. Survival was compared between the groups using a Cox proportional hazards model and using a logistic regression model for 1 yr mortality. Patients who were lost to follow-up before 1 yr were right-censored at the time of last follow-up in the Cox model and excluded from the logistic regression. Each model was adjusted for age, sex, ASA physical status, number of comorbid conditions documented in the anaesthesiology preoperative assessment, history of falls, evidence of baseline abnormal cognition (preoperative Short Blessed Test score >4),27 baseline poor functional capacity (<4 metabolic equivalents), cardiac surgery, length of anaesthesia, and units of packed red blood cells transfused intraoperatively. Missing values for abnormal Short Blessed Test score (n=82) and poor functional capacity (n=52) were imputed using multiple imputation with chained equations (R package MICE28). Patients missing outcome data were excluded from the analysis. To examine whether enhanced clinician compliance with the intervention would have changed outcomes, post hoc sensitivity analyses were performed removing 25% of intervention group subjects in each stratum with the most EEG suppression, longest duration of BIS <40, and highest end-tidal anaesthetic concentration.
The secondary analyses followed similar methods. The incidence of postoperative falls was compared between the two groups using logistic regression. Because the VR-12 physical and mental composite summaries did not follow a normal distribution, median regression was used to compare each postoperative quality of life measure between the two groups, adjusting for preoperative quality of life. The regression models for these secondary outcomes were adjusted for the same covariates as the primary analysis, and analogous sensitivity analyses were conducted for these outcomes. Patients missing preoperative VR-12 scores were excluded from the quality of life analysis. To further explore the associations between postoperative delirium and these outcomes, the regressions were repeated using incident delirium, days of delirium, and severe delirium as predictors. At the suggestion of a reviewer, regressions using peak delirium severity were also added.
Results
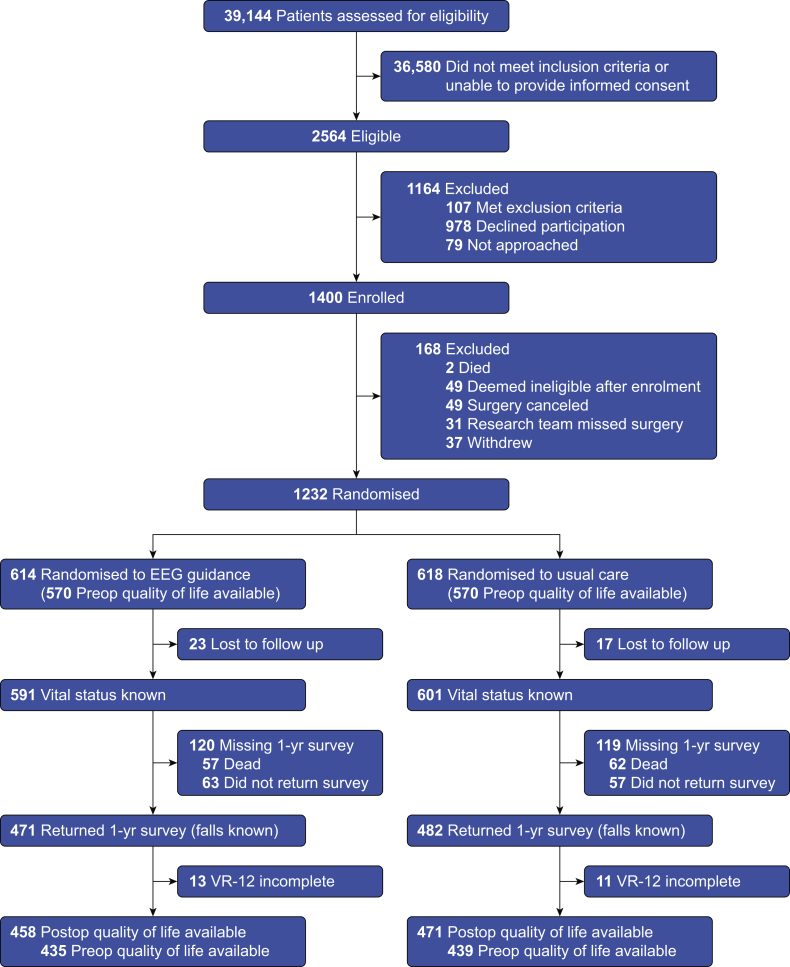
Between January 2015 and May 2018, 1232 subjects were enrolled, of whom 614 were randomised to the EEG-guided anaesthesia intervention and 618 were randomised to usual care. Follow-up ended in June 2019, and vital status at 1 yr was known for 1192 subjects (97% of those randomised). Missing data were nearly equally distributed between the two groups (Fig. 1). The groups included in the primary analysis had similar demographic characteristics, comorbid health conditions, and performance on preoperative evaluations of cognition (Table 1).
Fig 1.
Flow of subjects in the trial. VR-12, Veterans RAND 12-Item Health Survey.
Table 1.
Characteristics and management of subjects with known vital status at 1 yr. Data are presented as n (%) or as median [inter-quartile range]. Percentages do not add up to 100% because of rounding. METs, metabolic equivalents; PCS-12, Veterans RAND 12-Item Health Survey physical composite summary; MCS-12, Veterans RAND 12-Item Health Survey mental composite summary.
| Feature | Usual care (n=601) | EEG guidance (n=591) |
|---|---|---|
| Age (yr) | 69 [65–76] | 69 [65–74] |
| Male sex | 330 (55%) | 323 (55%) |
| ASA physical status | ||
| 1 | 2 (<1%) | 4 (1%) |
| 2 | 96 (16%) | 84 (14%) |
| 3 | 291 (48%) | 306 (52%) |
| 4 | 212 (35%) | 197 (33%) |
| Number of comorbid conditions | 4 [3–6] | 4 [3–6] |
| Hypertension | 438 (73%) | 444 (75%) |
| Diabetes mellitus | 171 (28%) | 180 (30%) |
| Coronary artery disease | 219 (36%) | 226 (38%) |
| History of cancer | 314 (52%) | 285 (48%) |
| Current cancer | 184 (31%) | 166 (28%) |
| History of falls | 129 (21%) | 130 (22%) |
| Functional capacity <4 METs | 284 (50%) | 288 (50%) |
| Abnormal Short Blessed Test (score >4) | 109 (20%) | 105 (19%) |
| Type of surgery | ||
| Cardiac | 228 (38%)a | 225 (38%) |
| Gastrointestinal | 60 (10%) | 60 (10%) |
| Gynaecologic | 55 (9%) | 35 (6%) |
| Hepatobiliary | 74 (12%) | 82 (14%) |
| Thoracic | 44 (7%) | 48 (8%) |
| Urologic | 55 (9%) | 47 (8%) |
| Vascular | 57 (9%) | 58 (10%) |
| Other | 28 (5%) | 36 (6%) |
| Preoperative PCS-12 | 38.2 [29.6–47.7] | 38.1 [28.1–48.1] |
| Preoperative MCS-12 | 57.0 [47.7–61.5] | 56.7 [47.8–61.1] |
| Anaesthesia Length (min) | 312 [234–398] | 317 [237–399] |
| Median end-tidal anaesthetic concentration (minimum alveolar concentration units) | 0.80 [0.71–0.86] | 0.69 [0.62–0.77] |
| Duration of EEG suppression (min) | 11 [1–54] | 7 [1–23] |
| Duration of bispectral index <40 (min) | 57 [15–129] | 32 [9–80] |
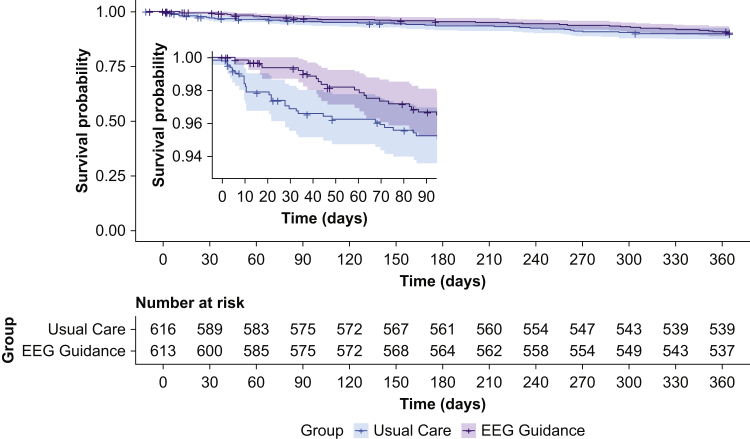
The 1-yr mortality incidence was 57/591 (9.6%) in the intervention group and 62/601 (10.3%) in the usual care group. No significant difference in mortality was observed between the two groups (adjusted absolute difference, –0.7%; 99.5% confidence interval [CI], –5.8% to 4.3%; P=0.68; see Supplementary Table S1 for 95% CI). Survival curves are shown in Figure 2. The adjusted hazard ratio in a Cox proportional hazards model was 0.85 (99.5% CI, 0.51–1.44; P=0.39). Full coefficients for all adjusted primary analyses are shown in Supplementary Tables S2–6 in the online supplementary material. Results were similar in the subset undergoing cardiac surgery and the subset undergoing noncardiac surgery (Supplementary Table S7). There was no significant association between the intervention and mortality in sensitivity analyses removing subjects in the intervention group with the most EEG suppression, most time with BIS <40, or highest end-tidal anaesthetic concentration (Table 2).
Fig 2.
Survival curves for subjects in the EEG guidance and usual care groups. Inset: survival curves for the first 90 days in greater detail. In a Cox proportional hazards model, the hazard for death did not differ significantly between the EEG-guided group and the usual care group (adjusted hazard ratio=0.85; 99.5% confidence interval, 0.51–1.44; P=0.39).
Table 2.
Effect sizes for EEG guidance intervention in primary analyses and sensitivity analyses. ∗Effect sizes for mortality and falls are absolute risk differences (marginal effects from logistic regression models). Effect sizes for PCS-12 and MCS-12 are differences in medians from quantile regression. See Supplementary Table S1 for 95% confidence interval (CI). †Models were adjusted for age, sex, ASA physical status, number of comorbid conditions, history of falls, cardiac surgery, abnormal preoperative Short Blessed Test (score >4), preoperative functional capacity <4 metabolic equivalents, length of anaesthesia, and units of packed red blood cells transfused intraoperatively. ‡Post-hoc analyses. In each of these sensitivity analyses, patients in the EEG guidance group in the top quartile of duration of EEG suppression, duration of BIS <40, or median end-tidal anaesthetic concentration within their randomisation stratum (stratified by preoperative history of falls and cardiac vs noncardiac surgery) were excluded. ¶All models for postoperative PCS-12 were adjusted for the preoperative PCS-12 value. All models for postoperative MCS-12 were adjusted for the preoperative MCS-12 value. IQR, inter-quartile range; PCS-12, Veterans RAND 12-Item Health Survey physical composite summary; MCS-12, Veterans RAND 12-Item Health Survey mental composite summary; BIS, bispectral index; MAC, minimum alveolar concentration.
| Analysis | n | Incidence (%) or median [IQR] |
Effect size (99.5% CI)∗ | P value | |
|---|---|---|---|---|---|
| Usual care | EEG guidance | ||||
| Mortality | |||||
| Primary – unadjusted | 1192 | 62/601 (10.3%) | 57/591 (9.6%) | –0.7% (–5.6% to 4.2%) | 0.70 |
| Primary – adjusted† | 1192 | 62/601 (10.3%) | 57/591 (9.6%) | –0.7% (–5.8% to 4.3%) | 0.68 |
| Sensitivity – most EEG Suppression‡ | 1040 | 62/600 (10.3%) | 32/438 (7.3%) | –3.1% (–8.4% to 2.2%) | 0.10 |
| Sensitivity – most BIS <40‡ | 1046 | 62/600 (10.3%) | 44/444 (9.9%) | –0.4% (–5.7% to 4.9%) | 0.82 |
| Sensitivity – highest MAC‡ | 1055 | 62/600 (10.3%) | 48/453 (10.6%) | 0.3% (–5.1% to 5.6%) | 0.89 |
| Falls | |||||
| Primary – unadjusted | 953 | 135/482 (28.0%) | 136/471 (28.9%) | 0.9% (–7.3% to 9.1%) | 0.77 |
| Primary – adjusted | 953 | 135/482 (28.0%) | 136/471 (28.9%) | 1.1% (–7.2% to 9.4%) | 0.71 |
| Sensitivity – most EEG suppression | 843 | 135/481 (28.1%) | 99/362 (27.3%) | –0.7% (–9.5% to 8.0%) | 0.82 |
| Sensitivity – most BIS <40 | 832 | 135/481 (28.1%) | 103/351 (29.3%) | 1.3% (–7.6% to 10.2%) | 0.69 |
| Sensitivity – highest MAC | 838 | 135/481 (28.1%) | 101/357 (28.3%) | 0.2% (–8.6% to 9.0%) | 0.94 |
| PCS-12¶ | |||||
| Primary – unadjusted | 902 | 43.0 [30.6–51.9] | 43.7 [32.8–52.2] | 0.8 (–1.7 to 3.4) | 0.36 |
| Primary – adjusted | 902 | 43.0 [30.6–51.9] | 43.7 [32.8–52.2] | –0.1 (–2.5 to 2.4) | 0.93 |
| Sensitivity – most EEG suppression | 798 | 42.9 [30.6–51.8] | 45.3 [33.4–53.0] | 1.3 (–1.2 to 3.8) | 0.13 |
| Sensitivity – most BIS <40 | 790 | 42.9 [30.6–51.8] | 44.2 [32.9–51.3] | 0.8 (–2.0 to 3.6) | 0.43 |
| Sensitivity – highest MAC | 790 | 42.9 [30.6–51.8] | 43.4 [33.2–51.6] | 0.5 (–2.1 to 3.1) | 0.59 |
| MCS-12¶ | |||||
| Primary – unadjusted | 902 | 58.4 [50.7–61.9] | 58.0 [51.2–62.0] | 0.2 (–1.2 to 1.5) | 0.74 |
| Primary – adjusted | 902 | 58.4 [50.7–61.9] | 58.0 [51.2–62.0] | 0.1 (–1.4 to 1.6) | 0.86 |
| Sensitivity – most EEG suppression | 798 | 58.5 [50.7–61.9] | 57.9 [51.5–61.9] | 0.04 (–1.4 to 1.4) | 0.94 |
| Sensitivity – most BIS <40 | 790 | 58.5 [50.7–61.9] | 58.0 [51.5–62.2] | 0.3 (–1.3 to 1.8) | 0.64 |
| Sensitivity – highest MAC | 790 | 58.5 [50.7–61.9] | 58.2 [51.4–62.2] | 0.3 (–1.2 to 1.7) | 0.59 |
Of the 1073 subjects alive and not lost to follow-up at 1 yr, 953 subjects (89%) completed the survey with information about falls and quality of life. Postoperative falls were reported by 136 of 471 subjects (28.9%) in the intervention group and 135 of 482 subjects (28.0%) in the usual care group. Incidence of falls did not differ between the groups (adjusted absolute risk difference, 1.1%; 99.5% CI, –7.2% to 9.4%; P=0.71). Median 1-yr quality of life physical composite summary (PCS-12) was 43.7 (IQR, 32.8 to 52.2) in the intervention group and 43.0 (IQR, 30.6–51.9) in the usual care group. Median 1-yr mental composite summary (MCS-12) was 58.0 (IQR, 51.2–62.0) in the intervention group and 58.4 (IQR, 50.7–61.9) in the usual care group. Adjusting for preoperative values, neither the postoperative PCS-12 (adjusted difference in medians, –0.1; 99.5% CI, –2.5 to 2.4; P=0.93) nor the postoperative MCS-12 (adjusted difference in medians, 0.1; 99.5% CI, –1.4 to 1.6; P=0.86) was significantly different between the two groups. No significant differences were seen for either outcome in the sensitivity analyses, as shown in Table 2.
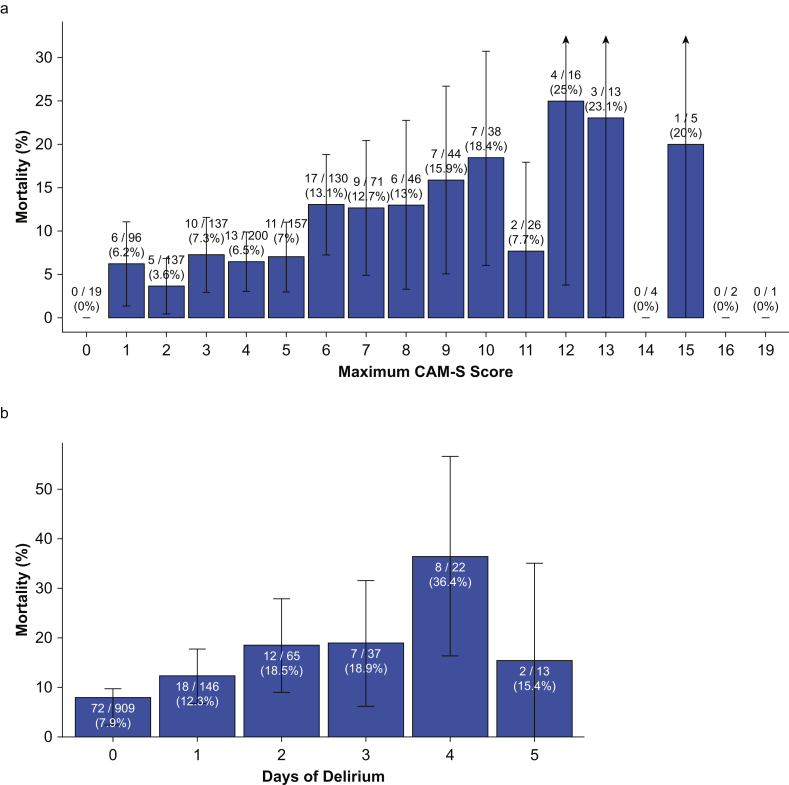
Associations between postoperative delirium and the outcomes are shown in Table 3 (see Supplementary Table S8 for 95% CIs). Subjects with postoperative delirium had an increased risk for 1-yr death in an unadjusted analysis, but this association was no longer significant at P<0.005 after adjusting for potential confounding variables. However, subjects with higher maximum CAM-S scores (higher peak delirium severity) and longer duration of postoperative delirium had increased incidence of 1 yr death (Fig. 3). Results were similar in the subsets undergoing cardiac surgery (Supplementary Table S9) and undergoing noncardiac surgery (Supplementary Table S10). Longer duration of postoperative delirium was also associated with increased falls in an unadjusted analysis, but this association was no longer significant at P<0.005 after adjusting for potential confounding variables. Neither postoperative delirium nor duration of delirium was associated with postoperative PCS-12 or MCS-12.
Table 3.
Effect sizes for associations between delirium measures and outcomes. ∗Models were adjusted for age, sex, ASA class, number of comorbid conditions, history of falls, cardiac surgery, abnormal preoperative Short Blessed Test (score >4), preoperative functional capacity <4 metabolic equivalents, length of anaesthesia, and units of packed red blood cells transfused intraoperatively. †Effect sizes for mortality and falls are absolute risk differences (marginal effects from logistic regression models). Effect sizes for PCS-12 and MCS-12 are differences in medians from quantile regression. See Supplementary Table S8 for 95% confidence interval (CI). ‡All models for postoperative PCS-12 were adjusted for the preoperative PCS-12 value. All models for postoperative MCS-12 were adjusted for the preoperative MCS-12 value. IQR, inter-quartile range; PCS-12, Veterans RAND 12-Item Health Survey physical composite summary; MCS-12, Veterans RAND 12-Item Health Survey mental composite summary; CAM-S, Confusion Assessment Method – Severity; NA, not available.
| Incidence (%) or median [IQR] |
Unadjusted |
Adjusted∗ |
||||
|---|---|---|---|---|---|---|
| With predictor | Without predictor | Effect size (99.5% CI)† | P | Effect size (99.5% CI)† | P | |
| Mortality | ||||||
| Any delirium | 47/283 (16.6%) | 72/909 (7.9%) | 7.4% (2.3%–12.5%) | <0.001 | 4.6% (–0.9% to 10.2%) | 0.020 |
| Severe delirium | 18/118 (15.3%) | 86/1039 (8.3%) | 5.6% (–0.8% to 12.1%) | 0.014 | 3.9% (–3.2% to 10.9%) | 0.124 |
| Max CAM-S (per point) | N/A | N/A | 1.1% (0.4%–1.8%) | <0.001 | 0.9% (0.1%–1.6%) | 0.002 |
| Days of delirium (per day) | N/A | N/A | 3.1% (1.2%–5.0%) | <0.001 | 2.3% (0.2%–4.4%) | 0.002 |
| Falls | ||||||
| Any delirium | 73/203 (36.0%) | 198/750 (26.4%) | 9.1% (–0.4% to 18.5%) | 0.007 | 5.5% (–4.7% to 15.8%) | 0.130 |
| Severe delirium | 34/84 (40.5%) | 234/852 (27.5%) | 11.9% (–1.4% to 25.1%) | 0.012 | 10.7% (–3.4% to 24.8%) | 0.033 |
| Max CAM-S (per point) | N/A | N/A | 1.9% (0.6%–3.2%) | <0.001 | 1.3% (–1.7% to 2.8%) | 0.013 |
| Days of delirium (per day) | N/A | N/A | 5.3% (1.1%–9.5%) | <0.001 | 4.2% (–0.3% to 8.7%) | 0.009 |
| PCS-12‡ | ||||||
| Any delirium | 39.5 [28.6–48.6] | 45.3 [32.8–53.1] | –1.8 (–5.4 to 1.8) | 0.157 | –2.1 (–6.0 to 1.7) | 0.122 |
| Severe delirium | 39.1 [26.2–49.6] | 44.6 [32.8–52.5] | –2.4 (–6.6 to 1.9) | 0.116 | –2.4 (–8.4 to 3.6) | 0.267 |
| Max CAM-S (per point) | N/A | N/A | –0.3 (–0.8 to 0.1) | 0.037 | –0.5 (–1.0 to 0.0) | 0.006 |
| Days of delirium (per day) | N/A | N/A | –1.0 (–2.6 to 0.7) | 0.105 | –1.5 (–3.0 to 0.1) | 0.007 |
| MCS-12‡ | ||||||
| Any delirium | 55.5 [45.1–60.9] | 58.9 [52.5–62.2] | –1.3 (–3.3 to 0.7) | 0.061 | –1.2 (–3.7 to 1.4) | 0.200 |
| Severe delirium | 52.9 [40.3–60.2] | 58.5 [51.8–62.0] | –2.5 (–7.8 to 2.8) | 0.181 | –2.0 (–7.0 to 2.9) | 0.248 |
| Max CAM-S (per point) | N/A | N/A | –0.4 (–0.6 to –0.1) | <0.001 | –0.3 (–0.7 to 0.1) | 0.035 |
| Days of delirium (per day) | N/A | N/A | –0.8 (–2.2 to 0.6) | 0.098 | –0.6 (–2.1 to 0.8) | 0.232 |
Fig 3.
Association between postoperative delirium characteristics and 1-yr mortality. Associations between (a) maximum CAM-S score and 1-yr mortality and between (b) number of days of postoperative delirium and 1-yr mortality. Labels on the bars show number of deaths in each group and the total number of patients in the group. Error bars show 95% confidence interval around 1-yr mortality rate in each group. CAM-S, Confusion Assessment Method – Severity.
Discussion
In this report of secondary 1-yr outcomes from the ENGAGES RCT, there was no significant association between an EEG-guided anaesthesia intervention and mortality 1 yr postoperatively. The EEG-guidance intervention was also not associated with decreased falls or with improved quality of life 1 yr after surgery. In post hoc exploratory analyses, increasing peak delirium severity and increased duration of postoperative delirium were both associated with increases in the incidence of death within the first year after surgery.
The absence of an effect of the EEG guidance intervention on 1-yr mortality is consistent with the existing literature. The Balanced Anaesthesia Study did not find a significant decrease in 1-yr mortality with a BIS target of 50 vs a BIS target of 35 (0.8% absolute risk reduction [95% CI, –0.5% to 2.0%]).10 Of note, a greater reduction in volatile agent administration was achieved in the Balanced Anaesthesia Study than in ENGAGES, but there was still no significant effect on 1-yr mortality. The DeLiT (Dexamethasone, Light anaesthesia, Tight glucose control) trial, which randomised noncardiac surgical subjects to light (BIS 55) vs deep (BIS 35) anaesthesia, was stopped early because of futility.11 The B-Aware, B-Unaware, BIS or Anesthetic Gas to Reduce Explicit Recall (BAG-RECALL), Cognitive Dysfunction after Anesthesia (CODA), and Surgery Depth of Anaesthesia Cognitive Outcome (SuDoCo) trials all randomised subjects to BIS monitoring vs either usual care or an active comparator.8,12,29, 30, 31 None of these trials actively randomised subjects to deep vs light anaesthesia, even though many achieved group separation with respect to BIS readings or other surrogates for hypnotic depth. None found differences in mortality between the control and intervention arms.25,26,32 The Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients (STRIDE) trial, which randomised hip fracture subjects to light vs heavy propofol sedation during spinal anaesthesia,33 showed no significant difference in 1-yr mortality.34
Inadequate power may have played a role in the failure of any of these trials to detect an effect on 1-yr mortality. As recently described in an independent discussion of the STRIDE trial, in order to detect an absolute mortality decrease from 10% to 9% (close to the estimated [non-significant] difference found in this trial) an adequately powered trial would require 23 000 subjects per group at α=0.005 or 13 500 subjects per group at the more conventional α=0.05.35 However, these studies had greater precision for continuous outcomes such as quality of life and disability ratings than for uncommon events such as mortality. In the present study, EEG guidance still had no significant effect on postoperative quality of life. The Balanced Anaesthesia Study used the WHODAS 2.0 disability instrument and did not present interval estimates on the differences between groups, but based on the information in their manuscript, the difference between groups is likely bounded with 99% confidence at less than 1 point on a 48-point scale. Although a quantitative model connecting disability scores to mortality is lacking, it seems implausible for EEG guidance or lighter anaesthetic depth to have a precise null effect on survivor quality of life and disability but to have a meaningful effect on mortality. A similar argument applies to falls and the other postoperative complications measured by the Balanced Anaesthesia Study.
Given that the EEG guidance intervention in the ENGAGES trial was associated with reduced 30-day mortality but no significant effect on 1-yr mortality, three explanations are plausible. First, deep anaesthesia and EEG suppression may accelerate death among subjects who were already moving on that trajectory. Even though a similar proportion of subjects in both groups ended up dying within 1 yr, increased EEG suppression in the usual care group may have caused some subjects to die in the first few months who otherwise would have died later in the year. If this is the case, the EEG guidance intervention could provide benefit to subjects such as allowing them to spend more time with family or achieve other personal goals. Second, EEG suppression may have a causal relationship with mortality, but the intervention did not sufficiently decrease EEG suppression duration for the effect to persist at 1 yr. An intervention that caused a larger difference between the groups may have produced a different result. Third, it is possible that the association between EEG guidance and 30 day mortality reported in our previous publication represented a type 1 error, meaning there is no association between the intervention and mortality at all.
Although the reported association between duration of postoperative delirium and 1-yr postoperative mortality was an exploratory analysis and should be interpreted with caution, the findings are consistent with the published literature. In a prospective study of patients older than 50 yr undergoing surgery with postoperative ICU admission, subjects who experienced postoperative delirium were more likely to die within 6 months than subjects who did not experience delirium.36 A similar association between postoperative delirium and 6-month mortality has been reported after hip fracture surgery.37 However, these observational associations do not necessarily imply that prevention of postoperative delirium will prevent deaths. There may be a non-causal relationship in which certain subjects are at risk for both delirium and death, either because of their preoperative phenotype or because of perioperative events.
This study has several notable strengths. First, this study focuses on outcomes that are important to subjects. Second, the EEG guidance intervention changed anaesthetic management, as evidenced by the differing durations of EEG suppression and low BIS readings between the intervention and usual care groups. Third, quality of life was measured using a widely used, validated questionnaire, and falls were explicitly defined using standard terminology from the Prevention of Falls Network Europe. Fourth, methodologic plans for this analysis were publicly published at ClinicalTrials.gov before the analysis being performed, enhancing transparency. Fifth, an excellent follow-up rate (97%) was achieved for vital status.
This study also has limitations. First, follow-up rates were imperfect for both quality of life and falls, potentially limiting power or creating bias. Second, this trial was conducted at a single centre, so the findings may not generalise to other locations. Third, reliance on patient report to detect falls could have introduced a recall bias. However, this bias would be expected to impact the intervention and usual care groups equally. Fourth, the difference in average end-tidal anaesthetic concentration between the two groups was modest (0.80 vs 0.69 minimum alveolar concentration). It is possible that an intervention causing greater separation in anaesthetic dosing could have resulted in different outcomes. Fifth, EEG suppression was quantified using the BIS monitor's calculated suppression ratio, which may underestimate the duration of EEG suppression.38
In conclusion, decreasing duration of EEG suppression during surgery after an EEG-guided anaesthesia intervention did not significantly decrease 1-yr mortality in this secondary analysis of an RCT. The intervention also had no effect on 1-yr quality of life or falls. These findings, in the context of other previously published studies, do not provide evidence to support the use of EEG-guided anaesthesia to prevent postoperative death.
Authors' contributions
Conceptualisation: BAF, CRK, TSW, ABA, SLM, SLS, EJL, MSA
Formal analysis: BAF, CRK, ABA
Data curation: AMM, TPB, JO, DP, HRM, AK
Investigation: BAF, CRK, AMM, TSW, TPB, JO, DP, HRM, BAT, TJG, DAE, BJP, TWS, SLS, EJL, MSA
Methodology: BAF, CRK, AMM, TSW, TPB, DP, HRM, ABA, AK, SLM, SLS, EJL, MSA
Visualisation: BAF, CRK
Resources: SLS, EJL, MSA
Project administration: SLM
Supervision: SLM, SLS, EJL, MSA
Funding acquisition: SLS, EJL, EJL, MSA
Writing original draft: BAF, CRK, HRM, ABA, MSA
Reviewing and editing: BAF, CRK, AMM, TSW, TPB, JO, DP, HRM, ABA, AK, SLM, BAT, TJG, DAE, BJP, TWS, SLS, MSA
Acknowledgements
The authors thank all members of the ENGAGES Research Group for their contributions to this study.
Handling editor: Tony Absalom
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.04.036.
Contributor Information
Bradley A. Fritz, Email: bafritz@wustl.edu.
ENGAGES Research Group:
Arbi B. Abdallah, Ginika Apakama, Amrita Aranake-Chrisinger, Michael S. Avidan, Jacob Bolzenius, Thaddeus P. Budelier, Jamila Burton, Victoria Cui, Daniel A. Emmert, Bradley A. Fritz, Shreya Goswami, Thomas J. Graetz, Shelly Gupta, Katherine Jordan, Alex Kronzer, Hannah R. Maybrier, Sherry L. McKinnon, Angela M. Mickle, Maxwell R. Muench, Matthew R. Murphy, Jordan Oberhaus, Ben J. Palanca, Daniel Park, Aamil Patel, James W. Spencer, Tracey W. Stevens, Patricia Strutz, Catherine M. Tedeschi, Brian A. Torres, Emma R. Trammel, Ravi T. Upadhyayula, Troy S. Wildes, Anke C. Winter, Nan Lin, Eric Jacobsohn, Tamara Fong, Jackie Gallagher, Sharon K. Inouye, Eva M. Schmitt, Emily Somerville, Susan Stark, Eric J. Lenze, Spencer J. Melby, and Jennifer Tappenden
Investigators and Staff of the ENGAGES Research Group
Department of Anesthesiology, Washington University School of Medicine, St. Louis, MO, USA: Arbi Ben Abdallah; Ginika Apakama; Amrita Aranake-Chrisinger; Michael S. Avidan; Jacob Bolzenius; Thaddeus P. Budelier; Jamila Burton; Victoria Cui; Daniel A. Emmert; Bradley A. Fritz; Shreya Goswami; Thomas J. Graetz; Shelly Gupta; Katherine Jordan; Alex Kronzer; Hannah R. Maybrier; Sherry L. McKinnon; Angela M. Mickle; Maxwell R. Muench; Matthew R. Murphy; Jordan Oberhaus; Ben J. Palanca; Daniel Park; Aamil Patel; James W. Spencer; Tracey W. Stevens; Patricia Strutz; Catherine M. Tedeschi; Brian A. Torres; Emma R. Trammel; Ravi T. Upadhyayula; Troy S. Wildes; Anke C. Winter. Department of Mathematics, Washington University in St. Louis, St. Louis, MO, USA: Nan Lin. Department of Anesthesiology, University of Manitoba, Winnipeg, Canada: Eric Jacobsohn. Department of Medicine, Beth Israel-Deaconess Medical Center, Boston, MA, USA: Tamara Fong; Jackie Gallagher; Sharon K. Inouye; Eva M. Schmitt. Program in Occupational Therapy, Washington University School of Medicine, St. Louis, MO, USA: Emily Somerville; Susan Stark. Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA: Eric J.Lenze. Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA: Spencer J. Melby; Jennifer Tappenden.
Declarations of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all authors had financial support from the National Institutes of Health for the submitted work; SLS reported grants from the National Institutes of Health, the National Institute of Disability, Independent Living and Rehabilitation Research, and the Department of Housing and Urban Development; EJL reported grants from PCORI, Takeda, the McKnight Brain Research Foundation, the Taylor Family Institute, the Barnes-Jewish Foundation, Lundbeck, Janssen Pharmaceuticals, Alkermes, Aptinyx Inc., and the US Food and Drug Administration; the other authors reported no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 yr; no other relationships or activities that could appear to have influenced the submitted work.
Funding
US National Institute on Aging (grant number UH3 AG050312). US National Institute for General Medical Sciences (grant number T32 GM108539).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.The International Surgical Outcomes Study group Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–609. doi: 10.1093/bja/aew316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monk T.G., Saini V., Weldon B.C., Sigl J.C. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 3.Willingham M., Ben Abdallah A., Gradwohl S. Association between intraoperative electroencephalographic suppression and postoperative mortality. Br J Anaesth. 2014;113:1001–1008. doi: 10.1093/bja/aeu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorrilla-Vaca A., Healy R.J., Wu C.L., Grant M.C. Relation between bispectral index measurements of anesthetic depth and postoperative mortality: a meta-analysis of observational studies. Can J Anaesth. 2017;64:597–607. doi: 10.1007/s12630-017-0872-6. [DOI] [PubMed] [Google Scholar]
- 5.Soehle M., Dittmann A., Ellerkmann R.K., Baumgarten G., Putensen C., Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. doi: 10.1186/s12871-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritz B.A., Kalarickal P.L., Maybrier H.R. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–242. doi: 10.1213/ANE.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witlox J., Eurelings L.S.M., de Jonghe J.F.M., Kalisvaart K.J., Eikelenboom P., van Gool W.A. Delirium in elderly tgcq and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 8.Chan M.T., Cheng B.C., Lee T.M., Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 9.Wildes T.S., Mickle A.M., Ben Abdallah A. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the engages randomized clinical trial. JAMA. 2019;321:473–483. doi: 10.1001/jama.2018.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Short T.G., Campbell D., Frampton C. Anaesthetic depth and complications after major surgery: an international, randomised controlled trial. Lancet. 2019;394:1907–1914. doi: 10.1016/S0140-6736(19)32315-3. [DOI] [PubMed] [Google Scholar]
- 11.Abdelmalak B.B., Bonilla A., Mascha E.J. Dexamethasone, light anaesthesia, and tight glucose control (delit) randomized controlled trial. Br J Anaesth. 2013;111:209–221. doi: 10.1093/bja/aet050. [DOI] [PubMed] [Google Scholar]
- 12.Radtke F.M., Franck M., Lendner J., Kruger S., Wernecke K.D., Spies C.D. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110:98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 13.Schulz K.F., Altman D.G., Moher D. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwarenstein M., Treweek S., Gagnier J.J. Improving the reporting of pragmatic trials: an extension of the consort statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wildes T., Winter A., Maybrier H. Protocol for the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) study: a pragmatic, randomised clinical trial. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 17.Ely E.W., Margolin R., Francis J. Evaluation of delirium in critically ill tgcq: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Inouye S.K., Leo-Summers L., Zhang Y., Bogardus S.T., Jr., Leslie D.L., Agostini J.V. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 19.Inouye S.K., Kosar C.M., Tommet D. The CAM-S: Development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160:526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan B.A., Perkins A.J., Gao S. The CAM-ICU-7 Delirium Severity Scale: a novel delirium severity instrument for use in the intensive care unit. Crit Care Med. 2017;45:851–857. doi: 10.1097/CCM.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selim A.J., Rogers W., Fleishman J.A. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12) Qual Life Res. 2009;18:43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 22.Lamb S.E., Jorstad-Stein E.C., Hauer K., Becker C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 23.Klebanoff M.A., Cole S.R. Use of multiple imputation in the epidemiologic literature. Am J Epidemiol. 2008;168:355–357. doi: 10.1093/aje/kwn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin D.J., Berger J.O., Johannesson M. Redefine statistical significance. Nat Hum Behav. 2018;2:6–10. doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 25.Kertai M.D., Pal N., Palanca B.J. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware trial. Anesthesiology. 2010;112:1116–1127. doi: 10.1097/ALN.0b013e3181d5e0a3. [DOI] [PubMed] [Google Scholar]
- 26.Kertai M.D., Palanca B.J., Pal N. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware trial. Anesthesiology. 2011;114:545–556. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 27.Blessed G., Tomlinson B.E., Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myles P.S., Leslie K., McNeil J., Forbes A., Chan M. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004;363:1757–1763. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 30.Avidan M.S., Zhang L., Burnside B.A. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–1108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 31.Avidan M.S., Jacobsohn E., Glick D. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 32.Leslie K., Myles P.S., Forbes A., Chan M.T. The effect of bispectral index monitoring on long-term survival in the B-Aware trial. Anesth Analg. 2010;110:816–822. doi: 10.1213/ANE.0b013e3181c3bfb2. [DOI] [PubMed] [Google Scholar]
- 33.Sieber F.E., Neufeld K.J., Gottschalk A. Effect of depth of sedation in older tgcq undergoing hip fracture repair on postoperative delirium: the STRIDE randomized clinical trial. JAMA Surg. 2018;153:987–995. doi: 10.1001/jamasurg.2018.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieber F., Neufeld K.J., Gottschalk A. Depth of sedation as an interventional target to reduce postoperative delirium: mortality and functional outcomes of the strategy to reduce the incidence of postoperative delirium in elderly tgcq randomised clinical trial. Br J Anaesth. 2019;122:480–489. doi: 10.1016/j.bja.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlisides P.E., Ioannidis J.P., Avidan M.S. Hypnotic depth and postoperative death: a Bayesian perspective and an independent discussion of a clinical trial. Br J Anaesth. 2019;122:421–427. doi: 10.1016/j.bja.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson T.N., Raeburn C.D., Tran Z.V., Angles E.M., Brenner L.A., Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 37.Bellelli G., Mazzola P., Morandi A. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62:1335–1340. doi: 10.1111/jgs.12885. [DOI] [PubMed] [Google Scholar]
- 38.Muhlhofer W.G., Zak R., Kamal T. Burst-suppression ratio underestimates absolute duration of electroencephalogram suppression compared with visual analysis of intraoperative electroencephalogram. Br J Anaesth. 2017;118:755–761. doi: 10.1093/bja/aex054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.