Abstract
Objectives
Obstructive sleep apnoea (OSA) has received much attention as a risk factor for perioperative complications and 68.5% of OSA patients remain undiagnosed before surgery. Faciocervical characteristics may screen OSA for Asians due to smaller upper airways compared with Caucasians. Thus, our study aimed to explore a machine-learning model to screen moderate to severe OSA based on faciocervical and anthropometric measurements.
Design
A cross-sectional study.
Setting
Data were collected from the Shanghai Jiao Tong University School of Medicine affiliated Ruijin Hospital between February 2019 and August 2020.
Participants
A total of 481 Chinese participants were included in the study.
Primary and secondary outcome
(1) Identification of moderate to severe OSA with apnoea–hypopnoea index 15 events/hour and (2) Verification of the machine-learning model.
Results
Sex-Age-Body mass index (BMI)-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference (SABIHC2) model was set up. The SABIHC2 model could screen moderate to severe OSA with an area under the curve (AUC)=0.832, the sensitivity of 0.916 and specificity of 0.749, and performed better than the STOP-BANG (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male gender) questionnaire, which showed AUC=0.631, the sensitivity of 0.487 and specificity of 0.772. Especially for asymptomatic patients (Epworth Sleepiness Scale <10), the SABIHC2 model demonstrated better predictive ability compared with the STOP-BANG questionnaire, with AUC (0.824 vs 0.530), sensitivity (0.892 vs 0.348) and specificity (0.755 vs 0.809).
Conclusion
The SABIHC2 machine-learning model provides a simple and accurate assessment of moderate to severe OSA in the Chinese population, especially for those without significant daytime sleepiness.
Keywords: public health, sleep medicine, adult anaesthesia
Strengths and limitations of this study.
This is the first study to assess the screening value of faciocervical measurements in predicting moderate to severe obstructive sleep apnoea (OSA).
Support vector machine, a well-known machine-learning method, was used to set up the model to screen moderate to severe OSA in the Chinese population.
Instead of most questionnaires based on symptoms and comorbidities, the Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference model which is entirely based on measurements, avoids the confounding effect of symptoms and comorbidities.
The cross-sectional design of the study makes it difficult to establish the causal relationship between variables.
The study is a single-centre study with a relatively small sample size, and further confirmation with large-sample, multicentre, prospective clinical trial is needed in the future.
Introduction
Obstructive sleep apnoea (OSA) is a common breathing sleep disorder that affects about 936 million adults globally,1 of which about 80% are estimated to be undiagnosed.2 In the Chinese population, approximately 175 million adults have mild to severe OSA, of whom about 65 million adults have moderate to severe OSA.1 In addition to cardiovascular injury and metabolic syndrome, OSA has recently been regarded as a risk factor for perioperative complications, including hypoxaemia, pneumonia, pulmonary embolism, unplanned transfer to the intensive care unit and even death, especially for those who received abdominal or vascular surgery.3 The rates of postoperative cardiovascular events show a rise in moderate to severe OSA (25.1%) compared with no or mild OSA (16.8%).4 Thirty-eight per cent of surgical patients had moderate to severe OSA, of which 68.5% were not diagnosed before surgery.5 Guidelines have been recommended to develop a local protocol for screening possible OSA patients before elective surgery.6 7 The most common screening scales, including STOP-BANG (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference and male gender), Berlin questionnaire and OSA50, are mainly based on symptoms and comorbidities,8 which might lead to missed diagnoses for those without significant daytime sleepiness.
Several factors contribute to the pathogenesis of OSA, including obesity, faciocervical anatomy and alteration in pharyngeal muscle function, etc.9 Asians have relatively smaller upper airways compared with Caucasians.10 Only in Asians, smaller upper airways are predictors of upper airway collapsibility, and an anatomic imbalance between tongue and mandible volume influenced upper airway collapsibility among Caucasians.11 The above evidence prompts faciocervical characteristics that may predict OSA for Asians, such as thyromental distance (TMD), thyrosternum distance (TSD), maximum interincisal distance (MID) and Mallampati test score, which have been widely used in predicting difficult intubation.12 However, no studies assessed whether faciocervical characteristics are suitable for predicting OSA. Thus, it is necessary to evaluate the relationship between faciocervical characteristics and OSA.
During the past two decades, machine-learning models have provided simple but effective approaches for improving diagnostic accuracy.13 Support vector machine (SVM) is a well-known classification technique and has achieved great success in bioinformatics applications. Thus, the study aimed to build a new model via SVM and monitor its effectiveness in screening OSA.
Methods
Study design
Participants with suspected OSA (snoring, witnessed apnoea or excessive daytime sleepiness, etc) were enrolled in the study from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine between February 2019 and August 2020. All participants underwent anthropometric measurements and subsequent overnight polysomnography (PSG). Participants were grouped according to their Apnoea-Hypopnoea Index (AHI) gain into the following: (1) no or mild OSA (subjects without moderate to severe OSA: AHI<15 events/hour), (2) moderate to severe OSA (moderate to severe OSA: AHI≥15 events/hour). Exclusion criteria: (1) patients showing complications with severe respiratory diseases, such as severe chronic obstructive pulmonary disease, interstitial lung disease, or acute asthma; (2) patients showing complications with serious cardiovascular diseases such as acute myocardial infarction, acute heart failure or chronic congestive heart failure (grades III and IV); (3) patients with mental illnesses who could not cooperate with the examination; (4) patients who receiving non-invasive positive pressure ventilation therapy and (5) patients who might have other sleep disorders under clinical evaluation.
Written informed consent was obtained from all participants. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Clinical characteristics and anthropometric measurements
Age, sex, height, weight, body mass index (BMI), neck circumference (NC), waist circumference (WC), Epworth Sleepiness Scale (ESS) and STOP-BANG questionnaire were recorded. The STOP-BANG questionnaire is a scoring model consisting of eight questions and its scores are based on yes/no answers (score: 1/0). The eight questions included snoring, tiredness, observed apnoea, high blood pressure, BMI, age, NC and gender. Faciocervical measurements including Mallampati score, MID, TMD and TSD were measured. The Mallampati score was evaluated when participants were asked to sit upright and open their mouths as wide as possible. In grade I, the entire uvula, faucial pillar and soft palate are visible. In grade II, part of the uvula and palate are visible. In grade III, the soft palate is visible, but the uvula is obscured.14 MID was recorded by asking the participant to sit upright and open the mouth as wide as possible.15 The TMD was measured as the straight distance between the thyroid notch and the lower border of the mental prominence, while the head was fully extended, and the mouth closed.16 TSD was measured as the distance between the thyroid notch and the upper border of the sternum. The distance was rounded to the nearest 0.5 cm. The ratios of height to TMD (H/TMD) and height to TSD (H/TSD) were calculated.16
Polysomnography
All participants underwent overnight PSG. No coffee, tea, caffeine-containing products or sedative hypnotics were taken before sleep. The PSG monitoring included electroencephalography, electrooculography, chin electromyography, electrocardiography, measurements of thoracal and abdominal movements, and airflow pressure and thermistor (Alice 5, Philips Respironics, USA), with the addition of oxygen saturation (Nonin, Herrsching, Germany). Sleep recordings were scored according to the American Academy of Sleep Medicine (AASM) 2007 criteria.17 The diagnosis of moderate to severe OSA was defined by the presence of an obstructive AHI≥15 events/hour according to AASM guidelines.
Statistical analysis
Continuous variables with a normal distribution are presented as mean±SD, while values without a normal distribution are presented as median (25–75th percentiles). Categorical variables are presented as numbers and percentages. Independent samples t-test and Mann-Whitney U test were used to determine differential risk factors between the two groups. The χ2 test and Fisher’s exact test were used to compare the categorical data, as appropriate. Logistic regression analyses were performed to calculate the OR. Receiver operating characteristic (ROC) analysis was used to determine the area under the curve (AUC) with a 95% CI, sensitivity and specificity. All tests were two sided and used a significance level of 0.05. All analyses were performed using the SPSS software (V.24.0; SPSS).
Model construction via SVM
SVM is a representative machine-learning algorithm for classification, which could be viewed as a nonlinear regression model. We used the SVM to capture the potential hyperplane that maximises the margin between moderate or severe OSA and no or mild OSA. The SVM has the following format:
Where c is the output of the model based on the new data x, which could be regarded as the classifier (0 or 1), xi (i=1,…, n) is the training dataset, αi and b are parameters determined by the algorithm.18 We used the Gaussian radial basis function as the kernel function, with γ of 0.23 and the box constraint set to 20. Based on the results of the statistical analysis, sex, age, NC, WC, BMI, MID and H/TSD were set as independent variables (p<0.05). We performed a significant principal component taking into account the strong collinearity among parameters, such as BMI and WC. Five principal components were selected according to the accumulative variance contribution of more than 90% and scree plot.19
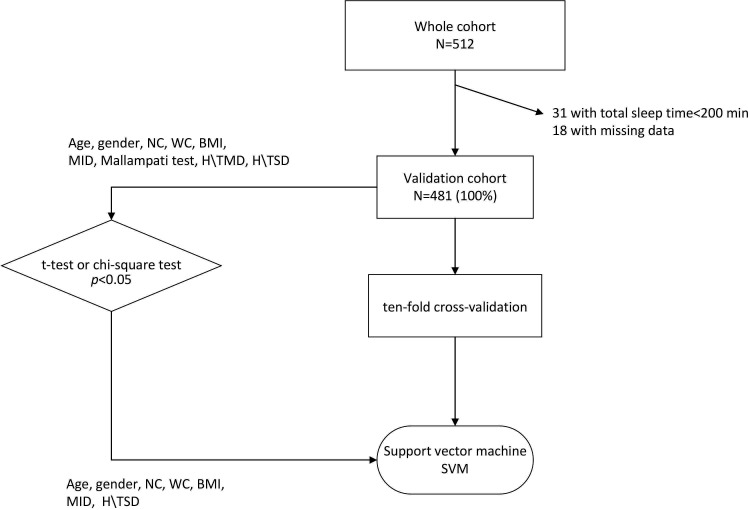
The performance of the SVM methods was evaluated by the 10-fold cross-validation. The participants were stratified sampling into 10 subsamples: one formed the test dataset for verifying the effectiveness of the model, and the others formed the training dataset to predict moderate to severe OSA for the model. The SVM model, namely SABIHC, that is, sex, age, BMI, MID, H/TSD, NC and WC (2 Cs) was estimated by the training dataset and then applied to predict OSA in the testing dataset. The flow chart including screening, randomisation and algorithm is presented in figure 1.
Figure 1.
Flow chart showing screening, randomisation and algorithm. A total of 481 participants were enrolled in our study after excluding 31 patients. Age, sex, neck circumference (NC), waist circumference (WC), body mass index (BMI) and faciocervical measurements (maximum interincisal distance (MID), height to thyrosternum distance (H/TSD)) were potential factors related to OSA due to the significant difference between the two groups (p<0.05). We chose the following parameters to set up the SABIHC2 model based on training dataset, whose name refers to sex, age, BMI, MID, H/TSD, NC and WC (2 Cs). Then, the model was verified on testing dataset. H/TMD, ratio of height to thyromegaly distance; OSA, obstructive sleep apnoea; SABIHC2, Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference; SVM, support vector machine.
The screening performance was assessed using sensitivity, specificity, AUC, negative likelihood ratio (−LR), positive likelihood ratio (+LR) and accuracy. Visual optimal hyperplanes for the SVM are shown in figure 2. Finally, we compared the difference in predictive power between the STOP-BANG questionnaire and the SVM model. Analyses were performed using the Scikit-learn package (V.0.20.3) based on Python (V.3.5.1).
Figure 2.
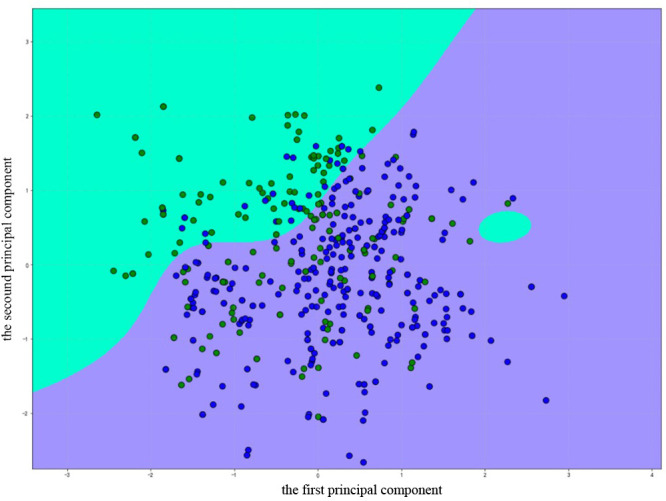
Optimal hyperplane for support vector machine (SVM). The green area means the SVM predicting Apnoea–Hypopnoea Index (AHI) <15 events/hour, the purple means the SVM predicts the AHI ≥15 events/hour. Green dots represent no or mild obstructive sleep apnoea (OSA) (AHI <15 events/hour), blue dots represent moderate to severe OSA (AHI ≥15 events/hour). The accuracy of SABIHC2 model based on all patients was 0.857, of which 0.916 for moderate to severe OSA (blue dots in purple area), and 0.748 for no or mild OSA (green dots in green area). The boundary was obtained from the SVM classifier and the figure was created using python. SABIHC2, Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
A total of 512 participants were recruited for the study at first, among which 31 patients were excluded due to short total sleep time (<200 min, 13 cases) or missing data (18 cases). Finally, 481 participants (325 males and 156 females; aged between 14 and 77 years) were enrolled in our study (figure 1). The participants were divided into no or mild OSA (AHI<15 events/hour, n=171, mean AHI 7.01±4.47 events/hour) and moderate to severe OSA (AHI≥15 events/hour, n=310, mean AHI 42.4±20.7 events/hour). Increased BMI, NC, WC, older age and a higher percentage of males were found in moderate to severe OSA (p<0.001). Meanwhile, they had a higher Microarousal index (32.5±13.2 vs 16.4±8.3 events/hour) and lower lowest pulse oxygen saturation (72.1±13.3 vs 87.13%±6.3%) than no or mild OSA (p<0.001) (table 1).
Table 1.
Subject demographics, crude and adjusted associations between morphometric variables and moderate to severe OSA
| All patients (n=481) | AHI<15 (n=171) | AHI≥15 (n=310) | P value* | Crude OR (95% CI)† | P value‡ | Adjusted OR for BMI (95% CI)† | P value‡ | |
| General characteristics | ||||||||
| Male (%) | 325 (67.6%) | 93 (54.39%) | 232 (74.84%) | <0.001 | 0.401 (0.270 to 0.595) | <0.001 | 0.463 (0.308 to 0.697) | <0.001 |
| Age | 47.32±12.94 | 44.05±13.80 | 49.11±12.08 | <0.001 | 1.032 (1.016 to 1.048) | <0.001 | 1.037 (1.021 to 1.054) | <0.001 |
| NC | 39.05±4.17 | 37.63±3.74 | 39.83±4.20 | <0.001 | 1.146 (1.090 to 1.205) | <0.001 | 1.121 (0.054 to 1.193) | <0.001 |
| WC | 94.97±11.32 | 90.58±11.93 | 97.39±10.20 | <0.001 | 1.109 (1.041 to 1.081) | <0.001 | 1.074 (1.041 to 1.107) | <0.001 |
| BMI | 26.26±4.16 | 25.18±4.16 | 26.85±4.04 | <0.001 | 1.109 (1.056 to 1.166) | <0.001 | N/A | N/A |
| Faciocervical measurements | ||||||||
| MID | 4.88±0.88 | 4.76±0.79 | 4.94±0.92 | 0.028 | 1.275 (1.025 to 1.585) | 0.029 | 1.298 (1.037 to 1.626) | 0.023 |
| Mallampati test=1 | 195 (40.5%) | 77 (45.02%) | 118 (38.06%) | 0.12 | N/A | N/A | N/A | N/A |
| Mallampati test=2 | 142 (29.5%) | 54 (31.58%) | 88 (28.39%) | 0.589 (0.371 to 0.938)§ | 0.026§ | 0.679 (0.421 to 1.095)§ | 0.113§ | |
| Mallampati test=3 | 144 (29.9%) | 40 (23.40%) | 104 (33.55%) | 0.627 (0.381 to 1.031)§ | 0.066§ | 0.713 (0.428 to 1.186)§ | 0.192§ | |
| H/TMD | 18.98±2.75 | 18.69±2.30 | 19.14±2.95 | 0.085 | 1.064 (0.991 to 1.142) | 0.086 | 1.071 (0.998 to 1.150) | 0.057 |
| H/TSD | 18.92±2.97 | 17.49±2.06 | 19.70±3.10 | <0.001 | 1.448 (1.311 to 1.599) | <0.001 | 1.458 (1.417 to 1.614) | <0.001 |
Data are presented as mean± SD or n (%).
*t-test or χ2 test as appropriate.
†ORs are depicted for moderate to severe OSA relative to no or mild OSA.
‡Logistic regression.
§ORs of Mallampati test are depicted for Mallampati test=2 (or 3) relative to Mallampati test=1.
AHI, Apnoea–Hypopnoea Index; BMI, body mass index; H/TMD, ratio of height to thyromegaly distance; H/TSD, ratio of height to thyrosternum distance; MID, maximum interincisal distance; N/A, not applicable; NC, neck circumference; OSA, obstructive sleep apnoea; WC, waist circumference.
Associations between faciocervical characteristics and OSA
Greater MID (4.94 vs 4.76, p=0.028) and H/TSD (19.70 vs 17.49, p<0.001) were found in the moderate to severe group compared with no or mild OSA. MID (OR=1.275, adjusted OR=1.298) and H/TSD (OR=1.448, adjusted OR=1.458) are associated with moderate to severe OSA, even after controlling for BMI. No significant intergroup differences were found in Mallampati test scores and H/TMD (p>0.05) (table 1).
Setup and predictive accuracy of the Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference model
Age, sex, NC, WC, BMI and faciocervical measurements (MID, H/TSD) were chosen as covariates because a significant difference was found between the two groups. We set up the Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference (SABIHC2) model, whose name refers to sex, age, BMI, MID, H/TSD, NC and WC (2 Cs). The AUC was 0.832 (95% CI 0.790 to 0.875), with a sensitivity of 0.916 and specificity of 0.749. Corresponding LR+and LR− were 3.649 and 0.112, respectively (table 2, figure 3A). The accuracy of the SABIHC2 model was 0.857, of which 0.916 for the moderate to severe group (blue dots in the purple area; figure 2), and 0.748 for no or mild OSA (green dots in the green area; figure 2).
Table 2.
Performance of SABIHC2 model
| Sensitivity | Specificity | AUC | 95% CI | +LR | −LR | |
| SABIHC2 | 0.916 | 0.749 | 0.832 | 0.790 to 0.875 | 3.649 | 0.112 |
| STOP-BANG | 0.487 | 0.772 | 0.631 | 0.581 to 0.682 | 2.136 | 0.665 |
AUC, area under the curve; +LR, positive likelihood ratio; −LR, negative likelihood ratio; SABIHC2, Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference; STOP-BANG, snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male gender.
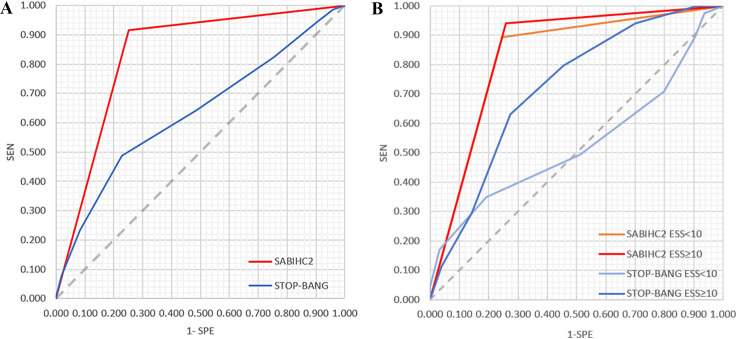
Figure 3.
Receiver operating characteristic (ROC) curve of SABIHC2 model and Stop-Bang. (A) The ROC curve of SABIHC2 model and STOP-BANG. The AUC of the SABIHC2 model was 0.832 (95% CI 0.790 to 0.875), with a sensitivity of 0.916 and specificity of 0.749. The AUC of STOP-BANG questionnaire was 0.631 (95% CI 0.581 to 0.682), with a sensitivity of 0.487 and specificity of 0.772. (B) The ROC curve of SABIHC2 model and STOP-BANG based on asymptomatic patients (Epworth Sleepiness Scale, ESS<10) and sleepiness patients (ESS≥10). In asymptomatic patients, SABIHC2 model (orange line) remarkably demonstrated better predictive ability than Epworth Sleepiness Scale questionnaire (soft blue line), with AUC (0.824 vs 0.530), sensitivity (0.892 vs 0.348) and specificity (0.755 vs 0.809). Similarly, SABIHC2 model (red line) had higher predictive power than STOP-BANG questionnaire (dark blue line) in sleepiness patients (ESS≥10), with sensitivity (0.941 vs 0.632), and specificity (0.740 vs 0.727) and AUC (0.841 vs 0.720). AUC, area under the curve; ESS, Epworth Sleepiness Scale; SABIHC2, Sex-Age-Body mass index-maximum Interincisal distance-ratio of Height to thyrosternum distance-neck Circumference-waist Circumference; SEN, sensitivity; SPE, specificity; STOP-BANG, snoring, tiredness, observed apnea, high blood pressure, body mass index, age, neck circumference and male gender.
Discriminative ability of the SABIHC2 model and STOP-BANG questionnaire
To compare the predictive ability between the SABIHC2 model and the STOP-BANG questionnaire, we calculated the ROC curve of STOP-BANG for moderate to severe OSA. The AUC was 0.631 (95% CI 0.581 to 0.682), with a sensitivity of 0.487 and specificity of 0.772. (table 2, figure 3A).
As the STOP-BANG questions are based on the symptoms and comorbidities and may result in missed diagnoses, we further compared whether there are differences in the predictive capacity of the SABIHC2 model and STOP-BANG questionnaire both for the symptomatic (ESS ≥10) and asymptomatic patients (ESS <10). For asymptomatic patients, the SABIHC2 model demonstrated better predictive ability than the STOP-BANG questionnaire, with AUC (0.824 vs 0.530), sensitivity (0.892 vs 0.348) and specificity (0.755 vs 0.809). Similarly, the SABIHC2 model had higher predictive power than the STOP-BANG questionnaire in patients experiencing sleepiness (ESS ≥10), with AUC (0.841 vs 0.720), sensitivity (0.941 vs 0.632) and specificity (0.740 vs 0.727) (figure 3B).
Discussion
In the current study, we measured a broad range of anthropometric variables and assessed the association with moderate to severe OSA. Sex, age, BMI, MID, H/TSD, NC and WC were significant determining factors. We also developed the SABIHC2 predictive model, which provides a simple and accurate assessment of moderate to severe OSA.
Faciocervical measurements are essential factors related to OSA. The majority of previous studies focused on the soft-tissue of the upper airway. A significant correlation between the oropharyngeal soft tissue (Mallampati test and tonsillar enlargement, etc) and AHI was reported.20 21 However, the Mallampati test was not a significant parameter in our study. It is possible that the association between soft tissue and OSA is weak in Asian populations.11 No previous studies explored the relationship between moderate to severe OSA and faciocervical measurements, such as MID, H/TMD and H/TSD, which have been frequently used to evaluate difficult intubation. Herein, we found that MID and H/TSD were strongly associated with moderate to severe OSA, and H/TSD showed a significant correlation with moderate to severe OSA, even controlling for BMI. The result may suggest that H/TSD is a potential factor for moderate to severe OSA in Chinese subjects.
As indicated previously, the overall scarcity and labour and financially onerous nature of PSG has prompted exploration of other suitable screening approaches, ranging from questionnaires to simplified multichannel recording. The STOP-BANG is one of the most widely used questionnaires. Some limitations of the STOP-BANG questionnaire should be considered. First, it is based on simplified categories (0 or 1) and this might reduce its screening accuracy.22 Second, the evaluation is based on symptoms and comorbidities, which may lead to the omission of diagnosis for snorers without significant daytime sleepiness or hypertension.23 Thus, we developed a new screening tool based on faciocervical measurements by machine learning.
Considering non-linear relationships and data interaction effects,24 the machine-learning algorithm (SVM) was used to find the complex linkages between variables. Distinct from previous machine-learning models which were based on age, sex, NC, WC and BMI,25 26 we included the structural faciocervical measurements to construct the SABIHC2 model. This showed much better performance than the STOP-BANG in screening for OSA.
The results confirmed that the SABIHC2 model performed better than the STOP-BANG questionnaire in predicting moderate to severe OSA, especially for asymptomatic patients (ESS <10), which prompted that it may be particularly useful for this cohort of patients. As most models work, we are planning to develop a software or application in the future, to allow healthcare worker-friendly installation and application.
To our knowledge, this is the first study examining the predictive power of faciocervical measurements, which may become a new index to predict OSA besides sex, age and BMI. However, several limitations should be mentioned. First, the study cohort was based on the Chinese population. The results obtained are hard to generalise for other populations. Second, the cross-sectional design of the study makes it difficult to establish the causal relationship between variables. Third, the sample size was relatively small, and it was a single-centre study, which may affect the validation of the machine-learning algorithm model.
In conclusion, we confirmed that faciocervical measurements are associated with moderate to severe OSA in the Chinese population. The machine-learning model called the SABIHC2 model was set up based on faciocervical and anthropometric measurements. The model is more effective than the STOP-BANG questionnaire in predicting moderate to severe OSA, especially for those without significant daytime symptoms.
Supplementary Material
Acknowledgments
The authors thank Peizhe Liu for his assistance with the SVM algorithm.
Footnotes
Contributors: LZ and YRY drafted the manuscript, had full access to all the data and took responsibility for the integrity of the data and the accuracy of the data analysis. SQL contributed to data analysis and interpretation. QYL and SQL contributed to study conception and design. HPL, YNL, NL, XWS and YJD contributed to data collection, critical revision. CXL and QYL contributed to final approval of the manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China (founding no. 81770084, 81700084, 81570082) and National Key R&D Programme of China (2018YFC1311900).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi: 10.5061/dryad.qnk98sfhg
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the ethics committee of the Shanghai Jiao Tong University School of Medicine at Ruijin Hospital, with the following reference number: 2018-107.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687–98. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705–6. 10.1093/sleep/20.9.705 [DOI] [PubMed] [Google Scholar]
- 3.Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012;141:436–41. 10.1378/chest.11-0283 [DOI] [PubMed] [Google Scholar]
- 4.Chan MTV, Wang CY, Seet E, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA 2019;321:1788–98. 10.1001/jama.2019.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh M, Liao P, Kobah S, et al. Proportion of surgical patients with undiagnosed obstructive sleep apnoea. Br J Anaesth 2013;110:629–36. 10.1093/bja/aes465 [DOI] [PubMed] [Google Scholar]
- 6.American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea . Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task force on perioperative management of patients with obstructive sleep apnea. Anesthesiology 2014;120:268–86. 10.1097/ALN.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 7.Dalesio NM, Hendrix CW, McMichael DH, et al. Effects of obesity and leptin deficiency on morphine pharmacokinetics in a mouse model. Anesth Analg 2016;123:1611–7. 10.1213/ANE.0000000000001578 [DOI] [PubMed] [Google Scholar]
- 8.Kee K, Dixon J, Shaw J, et al. Comparison of commonly used questionnaires to identify obstructive sleep apnea in a high-risk population. J Clin Sleep Med 2018;14:2057–64. 10.5664/jcsm.7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 2015;1:15015. 10.1038/nrdp.2015.15 [DOI] [PubMed] [Google Scholar]
- 10.Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012;17:213–22. 10.1111/j.1440-1843.2011.02082.x [DOI] [PubMed] [Google Scholar]
- 11.Schorr F, Kayamori F, Hirata RP, et al. Different craniofacial characteristics predict upper airway Collapsibility in Japanese-Brazilian and white men. Chest 2016;149:737–46. 10.1378/chest.15-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth D, Pace NL, Lee A, et al. Airway physical examination tests for detection of difficult airway management in apparently normal adult patients. Cochrane Database Syst Rev 2018;5:CD008874. 10.1002/14651858.CD008874.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obermeyer Z, Emanuel EJ. Predicting the future — big data, machine learning, and clinical medicine. N Engl J Med 2016;375:1216–9. 10.1056/NEJMp1606181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon SM, Sampangiramaiah S, Mathew M. Cross sectional observational study performed to see for relation of Mallampati score and extended Mallampati score with body mass index. J Clin Diagn Res 2017;11:Ug01–3. 10.7860/JCDR/2017/23937.9865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Herder C, Schmeck J, Appelboom DJK, et al. Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ 2004;329:955–9. 10.1136/bmj.329.7472.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt HJ, Kirmse M, Radespiel-Troger M. Ratio of Patient’s Height to Thyromental Distance Improves Prediction of Difficult Laryngoscopy. Anaesth Intensive Care 2002;30:763–5. 10.1177/0310057X0203000607 [DOI] [PubMed] [Google Scholar]
- 17.Sleep-Related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of sleep medicine Task force. Sleep 1999;22:667–89. [PubMed] [Google Scholar]
- 18.Liu W-T, Wu H-tieng, Juang J-N, et al. Prediction of the severity of obstructive sleep apnea by anthropometric features via support vector machine. PLoS One 2017;12:e0176991. 10.1371/journal.pone.0176991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Björklund M. Be careful with your principal components. Evolution 2019;73:2151–8. 10.1111/evo.13835 [DOI] [PubMed] [Google Scholar]
- 20.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med 2000;162:740–8. 10.1164/ajrccm.162.2.9908123 [DOI] [PubMed] [Google Scholar]
- 21.Schwab RJ, Leinwand SE, Bearn CB, et al. Digital morphometrics: a new upper airway phenotyping paradigm in OSA. Chest 2017;152:330–42. 10.1016/j.chest.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung F, Abdullah HR, Liao P. Stop-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149:631–8. 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- 23.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014;44:1600–7. 10.1183/09031936.00032314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linnen D, et al. Statistical modeling and Aggregate-Weighted scoring systems in prediction of mortality and ICU transfer: a systematic review. J Hosp Med 2019;14:161–9. 10.12788/jhm.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mencar C, Gallo C, Mantero M, et al. Application of machine learning to predict obstructive sleep apnea syndrome severity. Health Informatics J 2020;26:298–317. 10.1177/1460458218824725 [DOI] [PubMed] [Google Scholar]
- 26.Bozkurt S, Bostanci A, Turhan M. Can statistical machine learning algorithms help for classification of obstructive sleep apnea severity to optimal utilization of polysomnography resources? Methods Inf Med 2017;56:308–18. 10.3414/ME16-01-0084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi: 10.5061/dryad.qnk98sfhg