Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is a geriatric cancer. However, older adult patients are frequently underrepresented in large clinical trials.
Aims
The aim of this study is to assess the efficacy and safety of the EXTREME regimen (platinum + fluorouracil + cetuximab) in older and younger adult patients with HNSCC.
Methods and results
Patients with recurrent or metastatic HNSCC treated with the EXTREME regimen were retrospectively analyzed. We compare the efficacy and safety in older (aged ≥70 years) younger (aged <70 years) adult patients. Of the 86 patients examined in this study, 21 (24.4%) were older adults. There was no difference in overall response rate (46.9% vs 38.5%, P = .76), median progression‐free survival [5.7 months vs 5.8 months, hazard ratio (HR) 0.88, 95% confidence interval (CI) = 0.52‐1.51, P = .66] and overall survival (OS) (14.6 months vs 15.2 months, HR 0.79, 95% CI 0.43‐1.43, P = .44) in younger vs older patients. There was also no difference in the incidence of grade 3/4 adverse events between groups. The exploratory analysis for geriatric nutritional risk index (GNRI) showed the association with lower GNRI (≤98) and poor OS in older adult patients (37.7 months vs 7.0 months, HR 0.53, 95% CI 0.31‐0.89, P = .002).
Conclusions
The EXTREME regimen with optimal dose modification is safe and effective for both older and younger adult patients with HNSCC. The GNRI can be an indicator to select the older adult patients who can get benefit from the EXTREME regimen.
Keywords: biomarkers, cancer care, chemotherapy, head and neck cancer, nutrition
1. INTRODUCTION
Head and neck cancers are among the most aggressive and common malignancies. Histologically, these cancers are predominantly squamous cell carcinoma (SCC), originating from the epithelium of the oral cavity, pharynx, or larynx.1 Head and neck squamous cell carcinoma (HNSCC) was responsible for more than 650 000 cases and 330 000 deaths in 2018.2 Moreover, nearly half of the newly diagnosed patients were older than 65 years, and approximately 25%‐40% were older than 70 years.3, 4, 5
For patients with recurrent or metastatic HNSCC, treatment with fluorouracil + platinum (consisting of either cisplatin or carboplatin) exhibited preferable survival outcomes in several randomized controlled trials compare to single agent chemotherapy.6, 7 Cetuximab, an IgG1 monoclonal antibody targeting the epidermal growth factor receptor, demonstrated anti‐cancer activity by competitively inhibiting endogenous ligand binding and ligand‐dependent downstream signaling, with antibody‐dependent cell‐mediated cytotoxicity.8, 9 In the phase 3 EXTREME (ERBITUX in first‐line Treatment of REcurrent or MEtastatic head and neck cancer) trial, treatment with cetuximab in combination with fluorouracil + platinum (cisplatin or carboplatin) prolonged both progression‐free survival (PFS) [5.6 months vs 3.3 months, hazard ratio (HR) 0.54, P < .001] and overall survival (OS) (10.1 months vs 7.4 months, HR 0.80, P = .04) compared with chemotherapy alone in patients with recurrent or metastatic HNSCC.10 More recently, pembrolizumab, a monoclonal antibody targeting programmed cell death 1 (PD‐1), was approved for treatment of metastatic or recurrent HNSCC. In the phase 3 KEYNOTE‐048 trial, treatment with pembrolizumab in combination with fluorouracil + platinum resulted in longer OS than the EXTREME regimen (13.0 months vs 10.7 months, HR 0.77, P = .0034), whereas pembrolizumab monotherapy exhibited non‐inferiority to the EXTREME regimen in terms of OS [11.6 months vs 10.7 months, HR 0.85, 95% confidence interval (CI) 0.71‐1.03].11
In the above‐mentioned clinical trials, patients aged 70 years or older were frequently excluded. Indeed, in the EXTREME trial, patients aged ≥65 years constituted only 17.4% (77/442) of the study population, and the number of patients aged ≥70 years was not reported. In the KEYNOTE‐048 trial, patients aged ≥65 years constituted approximately 35% of the study population, and the number of patients aged ≥70 years was not reported for this study as well. A combined post hoc analysis of two phase 3 trials reported the tolerability and comparable efficacy of platinum‐based chemotherapy regimens in HNSCC patients aged ≥70 years.12 However, this analysis was conducted before the approval of cetuximab and immune‐checkpoint inhibitors. We therefore conducted a retrospective analysis to evaluate the efficacy and safety of the EXTREME regimen for HNSCC patients aged ≥70 years in comparison with patients <70 years of age. We also exploratory analyzed geriatric nutritional risk index (GNRI) to identify the older adult patients who can benefit from cisplatin‐based chemotherapy.
2. PATIENTS AND METHODS
We retrospectively reviewed the medical records of patients with recurrent or metastatic HNSCC treated with the EXTREME regimen from September 2013 to December 2019 at the Department of Medical Oncology of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. We defined older adults as patients aged ≥70 years and younger adults as patients aged <70 years.
Patients in both groups received either cisplatin (100 mg/m2 administrated by intravenous infusion) or carboplatin [area under the blood concentration‐time curve (AUC) 5] on day 1, along with fluorouracil (1000 mg/m2 per day administered by continuous intravenous infusion on days 1‐4) and cetuximab (intravenous infusion at a dose of 400 mg/m2 on day 1 of the first cycle, followed by 250 mg/m2 weekly) every 3 weeks. Platinum and fluorouracil were administered for up to six cycles, followed by maintenance cetuximab monotherapy every 3 weeks. The choice of the platinum agent was at the physician's discretion. For patients treated with cisplatin, oral aprepitant or intravenous fosaprepitant was administrated for antiemetic prophylaxis before each infusion. All patients also received intravenous administration of a serotonin‐3 antagonist and dexamethasone. Dose modifications or delays during the treatment schedule were allowed according to the physicians' discretion. Treatment was continued until disease progression, unacceptable toxicity despite appropriate dose reduction, and/or interruption, or the patient refused treatment. Blood samples were taken every week for routine laboratory testing.
Treatment response was evaluated by computed tomography according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (version 1.1).13 Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. The overall response rate (ORR) was defined as the percentage of patients with the best overall response of complete response (CR) or partial response (PR). The disease control rate (DCR) was the percentage of patients with a best overall response of CR, PR or stable disease (SD). PFS was defined as the time from the first day of treatment to either the first objective evidence of disease progression or death from any cause. OS was defined as the time from the first day of treatment to death by any cause. The relative dose intensity (RDI) of each agent was calculated as the ratio of the actual dose intensity to the scheduled dose intensity. The GNRI values were calculated as 1.489 × serum albumin level (g/L) + 41.7 × [actual bodyweight (ABW)/ideal bodyweight (IBW) (kg)]. If the ABW exceeded the IBW, the ABW/IBW value was set to one. The cutoff value of GNRI was set to 98 as described in the previous study.14
EZR software (R ver. 4.0.2) was used for statistical analyses.15 Differences in categorical variables were evaluated using the 2‐tailed Fisher's exact test, and differences in continuous variables were evaluated using the Mann‐Whitney U test. PFS and OS were estimated using the Kaplan‐Meier method. HRs and P‐values for differences in OS and PFS between the two age groups were calculated using the Cox proportional hazard model. Survival results are expressed as the median with 95% CI. Statistical significance was defined as P < .05.
3. RESULTS
3.1. Patient characteristics
Between September 2013 and December 2019, a total of 86 HNSCC patients received treatment with the EXTREME regimen. Baseline patient characteristics are described in Table 1. The median age of all patients was 64 years (range, 32‐77 years), and 21 (24.4%) patients were older adults (aged ≥70 years). Seventy‐five (87.2%) of the patients were male. All patients were diagnosed with SCC histologically. The most common primary tumor site was the hypopharynx (26.7%), followed by the oral cavity (24.4%), oropharynx (17.4%), and larynx (13.9%). Metastatic sites included the lung in 53 (61.6%), distant lymph nodes in 22 (25.5%), bone in 10 (11.6%), and liver in 5 (5.8%) patients. As definitive treatment for the primary tumor, 53 (61.6%) patients had previously undergone surgery, and 50 (58.1%) patients had previously undergone radiotherapy. Twenty‐two (25.6%) patients received prior cisplatin as induction chemotherapy or concurrent chemoradiotherapy. One (5%) patient was platinum‐refractory (tumor progression or recurrence within 6 months after the last dose of platinum‐containing chemotherapy as definitive therapy). Fewer patients in the younger adult group had metastatic lung disease in comparison with the older adult group (55.4% vs 81.0%, P = .04). Creatinine clearance, estimated using the Cockroft‐Gault equation, was lower in the older adult group than the younger adult group (86.2 mL/min vs 62.4 mL/min, P = .001), though there was no difference in median serum creatinine levels (0.73 mg/dL vs 0.82 mg/dL), P = .12). There were no other significant differences in the characteristics examined between the two age groups.
TABLE 1.
Patient characteristics
| Characteristics | All | Age < 70 years | Age ≥ 70 years | P‐value |
|---|---|---|---|---|
| N = 86 | N = 65 | N = 21 | ||
| Age, years, median (range) | 64 (32‐77) | 61 (32‐69) | 74 (70‐77) | |
| Sex | .28 | |||
| Male | 75 (87.2%) | 55 (84.6%) | 20 (95.2%) | |
| Female | 11 (12.8%) | 10 (15.4%) | 1 (4.8%) | |
| Primary site | .49 | |||
| Hypopharynx | 23 (26.7%) | 15 (23.1%) | 8 (38.1%) | |
| Oral cavity | 21 (24.4%) | 16 (24.6%) | 5 (23.8%) | |
| Oropharynx | 15 (17.4%) | 11 (16.9%) | 4 (19.0%) | |
| Larynx | 12 (13.9%) | 11 (16.9%) | 1 (4.8%) | |
| Nasopharynx | 5 (5.8%) | 5 (7.7%) | 0 (0.0%) | |
| Others | 10 (11.6%) | 7 (10.7%) | 3 (14.3%) | |
| ECOG PS | .31 | |||
| 0 | 47 (54.6%) | 38 (58.4%) | 9 (42.9%) | |
| ≥1 | 39 (45.4%) | 27 (41.6%) | 12 (57.1%) | |
| Extent of disease | .34 | |||
| Locoregional recurrence | 15 (17.4%) | 13 (20.0%) | 2 (9.5%) | |
| Metastatic disease | 71 (82.6%) | 52 (80.0%) | 19 (90.5%) | |
| Metastatic sites | ||||
| Lung | 53 (61.6%) | 36 (55.4%) | 17 (81.0%) | .04 |
| Distant lymph node | 22 (25.5%) | 19 (29.2%) | 3 (14.3%) | .25 |
| Bone | 10 (11.6%) | 9 (13.8%) | 1 (4.8%) | .44 |
| Liver | 5 (5.8%) | 4 (6.2%) | 1 (4.8%) | 1.00 |
| Target lesions | 62 (72.1%) | 49 (75.4%) | 13 (61.9%) | .27 |
| Prior surgery | 53 (61.6%) | 38 (58.5%) | 15 (71.4%) | .32 |
| Prior radiotherapy | 50 (58.1%) | 40 (61.5%) | 10 (47.6%) | .31 |
| Prior chemotherapy with cisplatin | 22 (25.6%) | 19 (29.2%) | 3 (14.3%) | .25 |
| Serum creatinine (mg/dl), median (range) | 0.76 (0.33‐1.48) | 0.73 (0.33–1.48) | 0.82 (0.40‐1.16) | .12 |
| CCr (Cockcroft‐Gault Equation) (ml/min), median (range) | 80.7 (41.9‐151.3) | 86.2 (46.8‐151.3) | 62.4 (41.9‐94.8) | .001 |
| Smoking history | .40 | |||
| None | 19 (22.1%) | 14 (21.5%) | 5 (23.8%) | |
| <20 pack‐year | 19 (22.1%) | 17 (26.2%) | 2 (9.5%) | |
| ≥20 pack‐year | 47 (54.7%) | 33 (50.8%) | 14 (66.7%) | |
| Unknown | 1 (1.2%) | 1 (1.5%) | 0 (0.0%) | |
| Alcohol use | .17 | |||
| None | 29 (33.7%) | 23 (35.4%) | 6 (28.6%) | |
| Occasional use | 5 (6.2%) | 4 (6.2%) | 1 (4.8%) | |
| <2 drinks/day | 9 (10.5%) | 4 (6.2%) | 5 (23.8%) | |
| ≥2 drinks/day | 37 (43.0%) | 28 (43.1%) | 9 (42.9%) | |
| Unknown | 6 (7.0%) | 6 (9.2%) | 0 (0.0%) |
Abbreviations: CCr, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status.
3.2. Treatment delivery
Carboplatin was administered to 16 patients (24.6%) of the younger adult group and 12 patients (57.2%) of the older adult group (P < .01). The initial chemotherapy dose was reduced for 2 (3.1%) patients in the younger adult group and 11 (52.3%) patients in the older adult group (P < .001). The reduced doses were 80 mg/m2 for cisplatin, AUC 4 for carboplatin, and 800 mg/m2 for fluorouracil, respectively. The median number of platinum + fluorouracil treatment cycles was 5 (range, 1‐6) for all patients, 5 (range, 1‐6) in younger adults, and 4 (range, 1‐6) in older adults. Reasons for not completing 6 cycles of platinum + fluorouracil included adverse events (25 patients, 29.1%), disease progression (21 patients, 24.4%), patient refusal (three patients, 3.5%), and conversion surgery (one patient, 1.2%). The frequency of discontinuation of the platinum + fluorouracil regimen was slightly higher in the older adult group (52% vs 76%, P = .08), although the difference was not statistically significant. The median RDI of cisplatin, carboplatin, and fluorouracil was 77.9%, 80.8%, and 80.0%, respectively. Although the cumulative dose of carboplatin and fluorouracil was higher in younger adult patients, the median RDI did not differ between the two age groups. A total of 67 (77.9%) patients received subsequent chemotherapy (eg, nivolumab and taxane) after failure of the EXTREME regimen (Table 2).
TABLE 2.
Treatment delivery
| Age < 70 years | Age ≥ 70 years | ||
|---|---|---|---|
| N = 65 | N = 21 | P‐value | |
| Platinum agent | <.01 | ||
| Cisplatin | 49 (75.4%) | 9 (42.8%) | |
| Carboplatin | 16 (24.6%) | 12 (57.2%) | |
| No. of cycles delivered, median (range) | 5 (1–6) | 4 (1–6) | .24 |
| Initial dose reduction | 2 (3.1%) | 11 (52.3%) | <.001 |
| Discontinuation of chemotherapy | 34 (52.3%) | 16 (76.2%) | .08 |
| Reason for discontinuation | |||
| Adverse events | 18 (52.9%) | 7 (43.8%) | .78 |
| Disease progression | 14 (41.1%) | 7 (43.8%) | .38 |
| Patients' refusal | 1 (2.9%) | 2 (12.5%) | .15 |
| Conversion surgery | 1 (2.9%) | 0 (0.0%) | 1.00 |
| Cumulative dose (mg/m2), median (range) | |||
| Cisplatin | 420 (100‐600) | 400 (100–600) | .59 |
| Carboplatin | 1818 (734‐3240) | 1710 (275‐2125) | <.05 |
| Fluorouracil | 17 600 (3000‐24 000) | 14 400 (1920‐24 000) | <.03 |
| Relative dose intensity (%) | |||
| Cisplatin | 79.5 (53.7‐100.0) | 71.0 (63.0‐72.9) | .06 |
| Carboplatin | 83.4 (46.2‐100.8) | 80.0 (62.6‐100.0) | .45 |
| Fluorouracil | 81.3 (46.3‐100.8) | 78.8 (63.0‐80.4) | .10 |
| Subsequent treatment | |||
| Nivolumab | 26 (40.0%) | 8 (38.1%) | 1.00 |
| Taxane | 12 (18.5%) | 4 (19.0%) | 1.00 |
| Cetuximab | 11 (16.9%) | 2 (9.5%) | .51 |
| Others | 3 (4.6%) | 1 (4.8%) | 1.00 |
3.3. Efficacy
As of the data collection cutoff of July 20, 2020, the median follow‐up time for all enrolled patients was 13.2 months (range, 1.2‐72.0 months). PFS and OS events were observed in 80 (93.0%) and 62 (72.1%) patients, respectively. Treatment response was evaluated in 62 (72.1%) patients who had measurable target lesions. Among the 62 patients, 49 were younger and 13 patients were older adult patients. The ORR was 45.2% (28/62, 95% CI 32.5%‐58.3%), and the DCR was 77.4% (48/62, 95% CI 65.0%‐87.1%). The median PFS and OS were 5.7 (95% CI 4.4‐6.8) months and 14.8 (95% CI 11.2‐17.6) months, respectively. There was no significant difference in ORR [46.9% (23/49) vs 38.5% (5/13), P = .76] or DCR [77.5% (38/49) vs 76.9% (10/13), P = 1.00] between younger adult and older adult patients. In addition, no differences were observed for median PFS (younger: 5.7 months vs older: 5.8 months, HR 0.88, 95% CI 0.52‐1.51, P = .66) (Figure 1A) and OS (younger: 14.6 months vs older: 15.2 months, HR 0.79, 95% CI 0.43‐1.43, P = .44) (Figure 1B) between younger and older adult patients. The age‐adjusted ORR was higher in patients who received cisplatin than in patients who received carboplatin (56.1% vs 23.8%, P = .04, Cochran‐Mantel‐Haenszel test). There was no significant difference in PFS (cisplatin 5.4 months vs 6.5 months, P = .68; carboplatin 4.1 months vs 5.7 months, P = .29; fluorouracil 4.7 months vs 6.5 months, P = .22) and ORR (cisplatin 50% vs 60%, P = .75; carboplatin 20% vs 43%, P = .33; fluorouracil 35% vs 55%, P = .20) between the higher RDI (≥80%) group and lower RDI (<80%) group. The ORR was 44.4% (12/27), 46.7% (14/30), 40% (2/5), and 41% (7/17) in patients with both locoregional and distant disease, with only distant disease, with only locoregional disease, and with only lung metastasis, respectively. No significant difference in ORR was observed according to the extent of disease.
FIGURE 1.
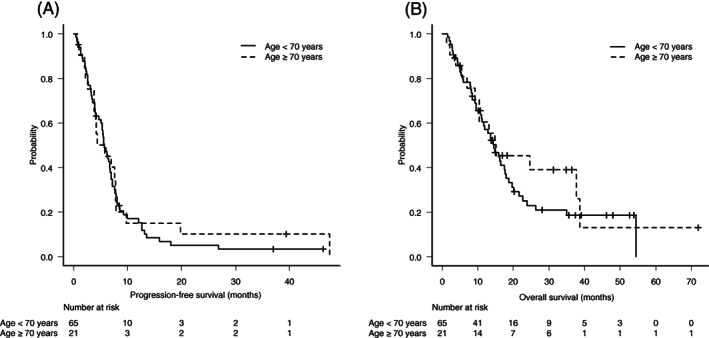
Kaplan–Meier curves for progression‐free survival (A) and overall survival (B) according to two age groups
3.4. Toxicity
Common adverse events resulting from EXTREME regimen treatment are summarized in Table 3. The most common grade 3 or 4 adverse events included neutropenia in 36 patients (41.9%), leukopenia in 31 (36.0%), nausea in seven (8.1%), and anorexia in six (7.0%) patients. Febrile neutropenia was observed in two patients (2.3%). Diarrhea, stomatitis, and increased serum creatinine levels were observed in 16 (18.6%), 28 (32.6%), and 12 (13.9%) patients, although none of these events were classified as grade 3 or higher. There were no treatment‐related deaths during the study period. The incidence of grade 3 nausea was lower in patients with habitual alcohol use (≥2 drinks/day) than those with not (0.0% vs 17.5%, P = .04). The younger adult patients had a higher incidence of all‐grade nausea than the older adult patients (66.7% vs 33.3%, P = .01). There was no difference in the incidence of other treatment‐related adverse events between the two age groups.
TABLE 3.
Adverse events related with the EXTREME regimen
| Any grade | Grade 3/4 | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Age < 70 | Age ≥ 70 | All | Age < 70 | Age ≥ 70 | |||
| N = 86 | N = 65 | N = 21 | P‐value | N = 86 | N = 65 | N = 21 | P‐value | |
| Hematological | ||||||||
| Leukopenia | 61 (70.9%) | 48 (73.8%) | 13 (61.9%) | .41 | 31 (36.0%) | 25 (38.5%) | 6 (28.6%) | .45 |
| Neutropenia | 63 (73.3%) | 49 (75.4%) | 14 (66.7%) | .57 | 36 (41.9%) | 30 (46.2%) | 6 (28.6%) | .21 |
| Anemia | 74 (86.0%) | 57 (87.7%) | 17 (81.0%) | .48 | 2 (2.3%) | 2 (3.1%) | 0 (0.0%) | 1.00 |
| Thrombocytopenia | 47 (54.7%) | 35 (53.8%) | 12 (57.1%) | 1.00 | 2 (2.3%) | 1 (1.5%) | 1 (4.8%) | .43 |
| Non‐hematological | ||||||||
| Nausea | 51 (59.3%) | 44 (67.7%) | 7 (33.3%) | .01 | 7 (8.1%) | 6 (9.2%) | 1 (4.8%) | 1.00 |
| Vomiting | 6 (7.0%) | 6 (9.2%) | 0 (0.0%) | .33 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Anorexia | 62 (72.1%) | 46 (70.8%) | 16 (76.2%) | .78 | 6 (7.0%) | 5 (7.7%) | 1 (4.8%) | 1.00 |
| Diarrhea | 16 (18.6%) | 10 (15.4%) | 6 (28.6%) | .20 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Stomatitis | 28 (32.6%) | 20 (30.8%) | 8 (38.1%) | .60 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Rash acneiform | 66 (76.7%) | 49 (75.4%) | 17 (81.0%) | .77 | 4 (4.7%) | 4 (6.2%) | 0 (0.0%) | .57 |
| Febrile neutropenia | 2 (2.3%) | 1 (1.5%) | 1 (4.8%) | .43 | ||||
| Creatinine increased | 12 (13.9%) | 10 (15.4%) | 2 (9.5%) | .72 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Peripheral sensory neuropathy | 25 (29.1%) | 20 (30.8%) | 5 (23.8%) | .59 | 1 (1.2%) | 1 (1.5%) | 0 (0.0%) | 1.00 |
4. EXPLORATORY ANALYSIS FOR GNRI
In 21 older adult patients, the median GNRI value was 99.3 (range, 87.3‐114.1). Patients with GNRI >98 had a longer median OS (37.7 months vs 7.0 months, HR 0.53, 95% CI 0.31‐0.89, P = .002) than patients with GNRI ≤98. The median PFS was modestly longer in patients with GNRI >98 than patients with GNRI ≤98 (6.4 months vs 3.2 months, HR 0.50, 95% CI 0.19‐1.31, P = .16) (Figure 2A,B). A trend indicating that GNRI might affect the incidence of grade 3 or 4 neutropenia (15% vs 50%, P = .15) was seen, although significance was not observed in this exploratory analysis. In 65 younger adult patients, the median PFS (5.8 months vs 5.7 months, P = .63) and OS (11.6 months vs 15.9 months, P = .25) were not significantly different between GNRI >98 patients and GNRI ≤98 patients.
FIGURE 2.
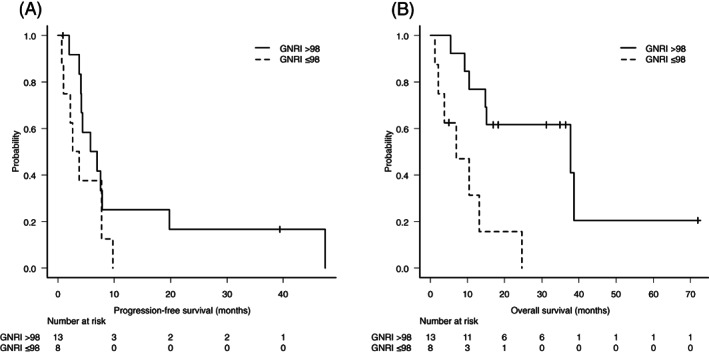
Kaplan–Meier curves for progression‐free survival (A) and overall survival (B) according to geriatric nutritional risk index (GNRI) in older adult patients
5. DISCUSSION
To the best of our knowledge, this is the first study to assess the efficacy and safety of the EXTREME regimen in the treatment of older adult (aged ≥70 years) patients with recurrent or metastatic HNSCC in comparison with younger adult (aged <70 years) patients. We found that PFS, OS, ORR, and safety were comparable in the older and younger adult patients treated with the EXTREME regimen. In addition, we found that GNRI can be a prognostic marker for older adult patients with HNSCC treated with the EXTREME regimen.
The EXTREME regimen has long been a standard therapy for patients with recurrent or metastatic HNSCC. The standard initial dose of cisplatin for the EXTREME regimen is relatively higher (100 mg/m2) than that of cisplatin‐containing standard regimens for other cancers.10, 16, 17, 18, 19, 20, 21 Given the current lack of sufficient evidence of the safety of administering the high dose of cisplatin in the EXTREME regimen to patients aged ≥70 years, patients in this age group are frequently excluded from randomized trials.
There are few reports describing systemic chemotherapy for patients with HNSCC aged ≥70 years. In a combined analysis of two phase three trials of cisplatin‐containing chemotherapy without cetuximab for patients with HNSCC, the older adult patients (aged ≥70 years) experienced comparable efficacy but a greater incidence of toxic effects compared with younger adult patients (aged <70 years).12 In that trial, the incidence of severe (grade 3 or more) thrombocytopenia (12% vs 26%, P = 0.0003) and diarrhea (3% vs 17%, P < .0001) was significantly higher in the older adult patients, whereas grade 3 or higher thrombocytopenia (2.3%) and diarrhea (0%) were rare in both the older and younger patients in our cohort. This discrepancy could be explained by ethnic differences in fluorouracil tolerability.22 Most of the patients enrolled in the previous cohort were fluorouracil‐intolerant non‐Asians, whereas all of the patients in our cohort were Japanese. Moreover, it is conceivable that administration of antiemetics such as aprepitant and olanzapine could have reduced the incidence of nausea and vomiting in the present study compared to the previous study, which was conducted in the early 2000s.23, 24 The lower incidence of nausea in the older adult patients is compatible with the results of a risk factor analysis of the phase 3 trial of aprepitant.25 No grade 3/4 renal toxicity was observed in the present study in either the younger or older adult patients, whereas it was observed in 2% (younger) and 8% (older) of patients in the previous report. Addition of magnesium sulfate to the hydration may have protected renal function in the present study.26 Further improvements in supportive therapies that reduce the incidence of severe toxicity associated with chemotherapy could enhance the safety of platinum‐based chemotherapy regimens for older adult patients.
A recent phase 2 trial of carboplatin + fluorouracil + cetuximab for select patients with HNSCC aged ≥70 years demonstrated preferable ORR (38%) and OS (14.7 months), similar to our results.27 That trial did not use cisplatin, however, with all of the patients receiving carboplatin instead. The toxicity profiles of the present study suggest that cisplatin + fluorouracil + cetuximab is tolerable for select patients aged ≥70 years, although the RDI of cisplatin was slightly lower in the older adult patients (79.5% vs 71.0%, P = .06). Another study reported that the ORR of a carboplatin‐based regimen was inferior to that of a cisplatin‐based regimen for patients with HNSCC (32% vs 21%).6 Indeed, the ORR was higher for cisplatin than carboplatin in our cohort (56.1% vs 23.8%, P = .04). Therefore, if a patient's general condition allows, cisplatin with optimal dose modification may be preferable to carboplatin for first‐line chemotherapy in patients with HNSCC, even those aged ≥70 years.
The median OS both in the present study (14.8 months) and in the previous study for older adult patients (14.7 months)27 was longer than the OS reported in the EXTREME trial (10.1 months).10 One possible explanation for this is the approval of nivolumab as the later line treatment. Indeed, 40% (34/86) of the patients received immune‐checkpoint inhibitors for the second or third line therapy, and the median OS of these 34 patients was 24.6 months (range, 14.6‐Not reached). Although, we should consider the selection bias, the prognostic impact of later line immune‐checkpoint inhibitors cannot be ignored. There was no significant difference in OS between the patients who previously underwent radiotherapy (N = 50) and the patients who did not (N = 36) (16.4 months vs 13.6 months, P = .37), and also no difference in OS between the patients who were previously administrated cisplatin (N = 22) and the patients who were not (N = 64) (14.6 months vs 14.8 months). It is suggested that the prognostic impact is greater in the later line therapy than the previous therapy.
According to the results of the KEYNOTE‐048 trial, current standard first‐line chemotherapy for patients with recurrent or metastatic HNSCC is pembrolizumab with or without platinum + fluorouracil. Programmed death ligand 1 (PD‐L1) expression on tumor cells is a biomarker for predicting the treatment response to pembrolizumab in a variety of cancers, including HNSCC.28 In the KEYNOTE‐048 trial, the combined positive score (CPS) was employed to evaluate PD‐L1 expression. Pembrolizumab monotherapy was superior to the EXTREME regimen in the PD‐L1‐positive (CPS ≥20 and CPS ≥1) population but non‐inferior to the EXTREME regimen among the total population in terms of OS. Furthermore, pembrolizumab + platinum + fluorouracil was superior to the EXTREME regimen among the total population in terms of OS. However, no direct comparison between pembrolizumab monotherapy and pembrolizumab + chemotherapy was performed in the KEYNOTE‐048 trial. The median PFS of pembrolizumab monotherapy was 2.3 months (95% CI 2.2‐3.3 months) among the total population and 3.4 months (95% CI 3.2‐3.8) among the population with PD‐L1 CPS ≥20. The ORR of pembrolizumab monotherapy was 17% among the total population and 23% among the population with PD‐L1 CPS ≥20. Although the preferable OS may have been due to delayed onset of the tumor‐reduction effect of the immune‐checkpoint inhibitor, approximately half of the patients receiving pembrolizumab monotherapy exhibited disease progression at the first evaluation. Moreover, in PD‐L1‐negative (CPS < 1) patients, the reported ORR was only 4.5% (95% CI 0.6‐15.5). For the population with PD‐L1 CPS < 1 (approximately 15% of the patients enrolled in KEYNOTE‐048), pembrolizumab monotherapy exhibited insufficient antitumor activity. Although pembrolizumab monotherapy is safer than pembrolizumab + chemotherapy in older adult patients, concomitant use of chemotherapy is preferable for some patients, such as those with PD‐L1 CPS < 1 or massive tumor volume who require a higher ORR. The results of the present study appear to suggest that platinum‐based chemotherapy is tolerable in select older adult patients, and in the era of immune‐checkpoint inhibitors, this can be informative for salvaging patients who might not obtain adequate benefit from immune‐checkpoint inhibitors.
In the present study, the older adult patients required an initial dose reduction more frequently than the younger adult patients, and the RDI of cisplatin was slightly lower in the older adult patients. However, there was no significant difference in PFS, OS, and ORR between the two age groups. There was also no difference in the incidence of treatment‐related adverse events and treatment discontinuation due to adverse events between the two age groups. The optimal initial dose of chemotherapy, especially cisplatin‐based regimens for older adult patients, has not been established. A recent prospective, phase 3 trial showed non‐inferiority of reduced‐dose to standard‐dose chemotherapy for older and/or frail gastroesophageal cancer patients.29 Similarly, the results of the present study indicate that a decrease in RDI due to age might not cause a critical decrease in the survival benefit of the EXTREME regimen for HNSCC patients.
Nutritional status has increasingly been recognized a prognostic factor in cancer patients. The GNRI was first developed as a nutrition marker to predict morbidity and mortality in older adult non‐cancer patients.14 The lower GNRI has been reported to be associated with poor survival outcomes in several solid cancers.30, 31, 32 We found that the lower GNRI (≤98) was associated with poor OS in older adult HNSCC patients treated with the EXTREME regimen. This trend could not be observed in younger adult patients, possibly because GNRI was originally designed for geriatric patients. Nutritional status can reflect not only nutrition but also patients' general condition, inflammation, and tumor activity. It is suggested that GNRI might be a useful marker in selecting older adult patients with HNSCC who can tolerate and can benefit from the EXTREME regimen. Therefore, we propose that the older adult HNSCC patient who had GNRI >98 can be tried the EXTREME regimen. Previous report described the initial dose reduction of the EXTREME regimen (80 mg/m2 for cisplatin and 800 mg/m2 for fluorouracil) for the Japanese patients.33 Considering our results and the previous report, 80% of target RDI might be enough. The older adult patients with GNRI ≤98 might not tolerate intensive chemotherapy even with a reduced dosage, and less intensive treatment such as paclitaxel‐based chemotherapy or immune‐checkpoint inhibitors might be better for these patients.
There are several limitations to this study. First, this was a retrospective analysis, in which the initial dose of chemotherapy, choice of platinum agent, and dose modifications were based on each physician's judgment. Thus, we cannot exactly provide how to modify the EXTREME regimen for the older adult patients. As we mentioned above, initial dose reduction with 20% can be acceptable modification. The older adult patients analyzed in the present study were in relatively good general condition which can cause selection bias. Second, the incidence of treatment‐related toxicity may have been underestimated due to the characteristics of the retrospective design. Third, a small sample size from a single‐institute study is an obvious limitation. To resolve these issues and validate our results, further prospective studies will be needed.
In conclusion, the results of present study suggest that the EXTREME regimen with either cisplatin or carboplatin is effective and tolerable for the treatment of patients with recurrent or metastatic HNSCC aged ≥70 years as well as patients aged <70 years. Optimal dose reduction and/or interruption might further enhance the safety of the EXTREME regimen for older adult patients. The GNRI can supportively be used for selecting older adult patients who can get benefit from the EXTREME regimen.
CONFLICT OF INTEREST
NF reports personal fees from Eisai. YS reports personal fees from ONO Pharmaceutical Co., Ltd., Bristol‐Myers Squibb Company, MSD K.K., TAIHO Pharmaceutical Co., Ltd. JT reports personal fees from Eisai. ST reports grants, personal fees from ONO Pharmaceutica, Bristol‐Myers Squibb, MSD, AstraZeneca, Chugai, and BAYER. The authors report no other conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
Naoki Fukuda: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; visualization; writing‐original draft; writing‐review and editing. Mayu Yunokawa: Conceptualization; supervision; writing‐review and editing. Yu Fujiwara: Investigation; writing‐review and editing. Xiaofei Wang: Investigation; writing‐review and editing. Akihiro Ohmoto: Investigation; writing‐review and editing. Naomi Hayashi: Investigation; writing‐review and editing. Tetsuya Urasaki: Investigation; writing‐review and editing. Yasuyoshi Sato: Investigation; supervision; writing‐review and editing. Kenji Nakano: Investigation; supervision; writing‐review and editing. Makiko Ono: Investigation; supervision; writing‐review and editing. Junichi Tomomatsu: Investigation; supervision; writing‐review and editing. Hiroki Mitani: Supervision; writing‐review and editing. Shunji Takahashi: Investigation; supervision; writing‐review and editing.
ETHICS STATEMENT
This study was approved by the institutional review board of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (No. 2019‐1003). This study was conducted in accordance with the Helsinki Declaration of 1964 and later versions. Given the retrospective nature of this study, the requirement for informed consent from the patients was waived by our hospital's institutional review board.
ACKNOWLEDGMENTS
We thank all members of the Department of Head and Neck Oncology at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research for patient referral. We also thank the members of the Department of Pathology at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research for their assistance in patient diagnosis.
Fukuda N, Yunokawa M, Fujiwara Y, et al. Comparison of the efficacy and safety of the EXTREME regimen for treating recurrent or metastatic head and neck squamous cell carcinoma in older and younger adult patients. Cancer Reports. 2021;4:e1322. 10.1002/cnr2.1322
DATA ACCESSIBILITY
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9‐22. [DOI] [PubMed] [Google Scholar]
- 2.Syrigos KN, Karachalios D, Karapanagiotou EM, Nutting CM, Manolopoulos L, Harrington KJ. Head and neck cancer in the elderly: an overview on the treatment modalities. Cancer Treat Rev. 2009;35:237‐245. [DOI] [PubMed] [Google Scholar]
- 3.Neve M, Jameson MB, Govender S, Hartopeanu C. Impact of geriatric assessment on the management of older adults with head and neck cancer: a pilot study. J Geriatr Oncol. 2016;7:457‐462. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 5.Surveillance Epidemiology and End Results (SEER): SEER Cancer Statistics Review, 1975–2015. https://seer.cancer.gov/csr/1975_2015/. Accessed July 29, 2020.
- 6.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous‐cell carcinoma of the head and neck: a southwest oncology group study. J Clin Oncol. 1992;10:1245‐1251. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs C, Lyman G, Velez‐García E, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10:257‐263. [DOI] [PubMed] [Google Scholar]
- 8.Galizia G, Lieto E, De Vita F, et al. Cetuximab, a chimeric human mouse anti‐epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654‐3660. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311‐1318. [PubMed] [Google Scholar]
- 10.Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 11.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet. 2019;394:1915‐1928. [DOI] [PubMed] [Google Scholar]
- 12.Argiris A, Li Y, Murphy BA, Langer CJ, Forastiere AA. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin‐based chemotherapy. J Clin Oncol. 2004;22:262‐268. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 14.Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr. 2005;82:777‐783. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078‐2092. [DOI] [PubMed] [Google Scholar]
- 17.Baka S, Califano R, Ferraldeschi R, et al. Phase III randomised trial of doxorubicin‐based chemotherapy compared with platinum‐based chemotherapy in small‐cell lung cancer. Br J Cancer. 2008;99:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med. 2002;346:85‐91. [DOI] [PubMed] [Google Scholar]
- 19.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068‐3077. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi W, Narahara H, Hara T, et al. S‐1 plus cisplatin versus S‐1 alone for first‐line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215‐221. [DOI] [PubMed] [Google Scholar]
- 21.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376:687‐697. [DOI] [PubMed] [Google Scholar]
- 22.Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26:2118‐2123. [DOI] [PubMed] [Google Scholar]
- 23.Gralla RJ, de Wit R, Herrstedt J, et al. Antiemetic efficacy of the neurokinin‐1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high‐dose cisplatin: analysis of combined data from two phase III randomized clinical trials. Cancer. 2005;104:864‐868. [DOI] [PubMed] [Google Scholar]
- 24.Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting. N Engl J Med. 2016;375:134‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard‐of‐care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin‐based chemotherapy. Support Care Cancer. 2010;18:1171‐1177. [DOI] [PubMed] [Google Scholar]
- 26.Bodnar L, Wcislo G, Gasowska‐Bodnar A, Synowiec A, Szarlej‐Wcisło K, Szczylik C. Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: a randomised phase II study. Eur J Cancer. 2008;44:2608‐2614. [DOI] [PubMed] [Google Scholar]
- 27.Guigay J, Auperin A, Mertens C, et al. Personalized treatment according to geriatric assessment in first‐line recurrent and/or metastatic (R/M) head and neck squamous cell cancer (HNSCC) patients aged 70 or over: ELAN (ELderly heAd and neck cancer) FIT and UNFIT trials. Ann Oncol. 2019;30(suppl 5):v450. [Google Scholar]
- 28.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): a randomised, open‐label, phase 3 study. Lancet. 2019;393:156‐167. [DOI] [PubMed] [Google Scholar]
- 29.Hall P, Swinson DE, Waters JS, et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): the GO2 phase III trial. J Clin Oncol. 2019;37(15_suppl):400. [Google Scholar]
- 30.Gu W, Zhang G, Sun L, et al. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle. 2015;6:222‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migita K, Matsumoto S, Wakatsuki K, et al. The prognostic significance of the geriatric nutritional risk index in patients with esophageal squamous cell carcinoma. Nutr Cancer. 2018;70:1237‐1245. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto T, Hatakeyama S, Narita S, et al. Impact of nutritional status on the prognosis of patients with metastatic hormone‐naïve prostate cancer: a multicenter retrospective cohort study in Japan. World J Urol. 2019;37:1827‐1835. [DOI] [PubMed] [Google Scholar]
- 33.Sano D, Fujisawa T, Tokuhisa M, et al. Real‐world treatment outcomes of the EXTREME regimen as first‐line therapy for recurrent/metastatic squamous cell carcinoma of the head and neck: a multi‐center retrospective cohort study in Japan. Anticancer Res. 2019;39:6819‐6827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


