CASE
A 79-year-old man with a medical history of hypertension presented to the hospital with acute-on-chronic back pain. Ten weeks prior to presentation, he developed severe, persistent lower back pain while working on a construction project, which progressed until he required a cane and ultimately a walker for mobility. He had previously been active with construction projects, biking, and hiking. Two weeks prior to presentation, he was evaluated by a neurosurgeon, and magnetic resonance imaging (MRI) revealed L4-L5 disc enhancement, which was concerning for discitis. An outpatient vertebral bone biopsy was planned, but due to worsening pain, he presented to the emergency department for expedited evaluation.
On initial evaluation, he reported low back and buttock pain and worsening ambulation requiring a walker. He denied weight loss, fever, chest pain, shortness of breath, or incontinence. On physical exam, he had severe tenderness in the bilateral paraspinal muscles of the lower back, with normal neurological function and normal vital signs. On cardiovascular exam, he had a faint systolic murmur (first noted several years prior to hospitalization with unremarkable workup). He had no petechiae or skin changes. A complete blood count showed a normal white blood cell count of 6.9 × 103 cells/μl (reference, 4.0 to 11.0 × 103 cells/μl). Erythrocyte sedimentation rate was elevated at 79 mm/h (reference, ≤20 mm/h), and C-reactive protein was elevated at 25.1 mg/liter (reference, ≤7.4 mg/liter). Biopsy of the L4-L5 disc space and bone revealed no pathological change, no organisms seen on Gram stain, and no growth on aerobic, anaerobic, or fungal tissue cultures.
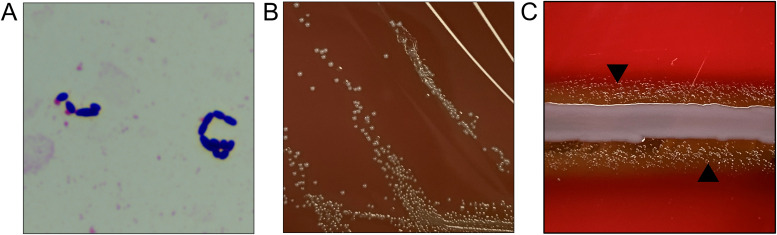
Two sets of blood cultures were drawn at the time of biopsy, each consisting of a Bactec Plus Aerobic/F (Becton, Dickinson, and Company, Franklin Lakes, NJ) and a Bactec Lytic/10 Anaerobic/F (BD) blood bottle. On the second day of hospitalization, all four bottles flagged positive after 16 to 18 hours of incubation. Gram stains from all cultures revealed Gram-positive cocci, some pleomorphic and slightly elongated in appearance (Fig. 1A). These results prompted empirical therapy with vancomycin. All bottles were subcultured to blood agar, and per the laboratory workflow, one aerobic bottle was tested on the ePlex blood culture ID panel (GenMark Diagnostics, Carlsbad, CA) for Gram-positive organisms, but no targets were detected. Prompted by the molecular panel result, all bottles were also subcultured to chocolate agar, and 24 hours later, gray colonies were visualized on the chocolate agar with “greening” of the media, but no growth was observed on blood agar (Fig. 1B). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) with the Vitek MS using library v3.2 (bioMérieux, Marcy-l'Étoile, France) identified the colonies as Abiotrophia defectiva. With this result, antibiotic therapy was changed to ceftriaxone and gentamicin. Repeat blood cultures were positive until hospital day 4, remaining negative thereafter. Susceptibility testing performed by broth microdilution at a reference laboratory and interpreted by Clinical and Laboratory Standards Institute (CLSI)-indicated intermediate susceptibility to penicillin (MIC, 0.5 μg/ml) and susceptibility to both ceftriaxone (MIC, 1 μg/ml) and vancomycin (MIC, 1 μg/ml).
FIG 1.
Overview of Abiotrophia defectiva phenotypic growth. (A) Gram stain from the aerobic blood culture bottle showing slightly elongated Gram-positive cocci. Magnification, ×1,000. (B) Colony growth of A. defectiva on chocolate agar. (C) Satellite growth of A. defectiva (arrowheads) in proximity to hemolytic staphylococcal streak.
Given the persistence of positive blood cultures, transthoracic echocardiogram was performed and showed severe aortic valve regurgitation with leaflet thickening. Subsequent transesophageal echocardiogram confirmed aortic valve regurgitation with compromise of the left coronary cusp and mitral valve regurgitation, which was concerning for endocarditis involving the aortic and mitral valves. Surgery was performed on hospital day 19, with successful replacement of the mitral and aortic valves. Two additional weeks of gentamicin and 4 weeks of ceftriaxone were completed with resolution of valvular regurgitation on postoperative echocardiogram. In retrospect, despite an unremarkable lumbar disc biopsy, his initial presentation of low back pain with disc space enhancement on MRI was thought to be due to infective lumbar discitis resulting from the earlier bacteremia and exacerbated by the disability caused by severe pain. His back pain improved with antibiotics and ongoing physical therapy.
DISCUSSION
Abiotrophia defectiva is a fastidious Gram-positive coccus, previously labeled as a “nutritionally variant streptococci,” which grows as satellite colonies around supporting microorganisms such as around a staphylococcal streak (Fig. 1C) (1, 2). Through 16S rRNA gene sequencing, this grouping has been reclassified to include four species which span two genera, Abiotrophia defectiva, Granulicatella adiacens, Granulicatella elegans, and Granulicatella balaenopterae (1). These organisms are commensal oral, intestinal, and urogenital microbiota and rarely cause bacteremia and endocarditis, comprising only ∼3% of total endocarditis cases (1, 3). Ultimately, despite the negative biopsy, our patient was felt to most likely have had an infective discitis given the constellation of other findings, consistent with prior reports of discitis-associated endocarditis caused by Abiotrophia (1, 4) and Granulicatella species (5, 6). In addition, there are a few reports of involvement of these as pathogens in brain and pancreatic abscesses, as well as in osteomyelitis and wound infections (1, 2). Population-based studies have demonstrated that among this group of organisms, Abiotrophia defectiva has the highest propensity to cause infective endocarditis (7).
Laboratory diagnosis of infective endocarditis is made through a multipronged approach, including cultures, serology, histopathology, and molecular methods, with blood cultures as the cornerstone (8). The organisms most likely to cause infectious endocarditis include staphylococci, streptococci, and enterococci. But Gram-negative bacilli are also known to cause disease, such as organisms included in the “HACEK” grouping (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella species), Enterobacterales, and more fastidious organisms (Coxiella burnetii, Tropheryma whipplei, and Bartonella species). Abiotrophia and Granulicatella grow well in modern blood culture bottles, are routinely recovered with standard incubation on solid media (35 to 37°C in 5 to 10% CO2 for 18 to 24 hours), and often provide the first evidence of infective endocarditis. If surgical intervention is pursued, histopathologic analysis and culture of the explanted heart valve are recommended, which can confirm valve involvement and help inform duration of antimicrobials, respectively (9). In our case, culture of the valve was negative, likely explained by the use of antimicrobial therapy prior to surgery. Abiotrophia and Granulicatella may also cause “culture-negative” endocarditis when antimicrobial therapy is administered during or prior to the drawing of blood cultures. Treatment-induced culture-negative endocarditis is more common than involvement of an uncultivable pathogen (8). In such cases, 16S rRNA gene PCR with sequencing from valve tissue can be a useful tool in identifying the causative agent (8).
On Gram stain, Abiotrophia and Granulicatella species typically appear as Gram-positive cocci in pairs or chains but can be pleomorphic. As in our case, they can appear more like coccobacilli or stain as Gram variable (1, 2). Gram stain pleomorphism can be particularly apparent with suboptimal nutritional conditions (1). Rapid molecular blood culture identification panels have become increasingly integrated into the workup of positive blood cultures. However, the most common commercially available panels (FilmArray blood culture identification [BCID] panel [BioFire Diagnostics LLC, Salt Lake City, UT], ePlex BCID-Gram positive [BCID-GP] [GenMark Diagnostics, Carlsbad, CA], and Verigene blood culture-GP [BC-GP] [Nanosphere, Northbrook, IL]), which include pan-streptococcal targets, do not reliably detect Abiotrophia and Granulicatella species due to their genetic distinction from Streptococcus species. While sequence analysis of targets for these molecular assays does not predict cross-reactivity of Abiotrophia or Granulicatella, rare cases of cross-reactivity with staphylococcal or streptococcal targets are reported in the product inserts. Gram stains consistent with a streptococcal-like morphology that are not detected by these panels should raise suspicions for Abiotrophia or Granulicatella, among other organisms.
Abiotrophia and Granulicatella species can be distinguished from other streptococcal-like Gram-positive organisms by their growth patterns and biochemical reactions (Table 1). Abiotrophia and Granulicatella species are catalase-negative, facultative anaerobes and demonstrate both pyrrolidonyl aminopeptidase (PYR) and leucine aminopeptidase (LAP) activity (1). Due to their unique nutritional requirements, these organisms are unable to grow independently on routine media, such as tryptic soy agar (TSA) with 5% sheep blood agar (1). Specifically, Abiotrophia and Granulicatella species require supplemental l-cysteine or pyridoxal hydrochloride for growth and are most often recovered in the clinical laboratory on enriched chocolate agar. For this reason, routine subculture to chocolate agar in addition to blood agar is standard in many laboratories. To enhance recovery of these fastidious organisms but avoid excess media usage, the current workflow in our laboratory consists of progressive subculturing based on Gram stain morphology and molecular panel results. When the Gram stain morphology resembles streptococci, positive bottles are subcultured to blood agar, and the initial positive bottle is tested via a rapid molecular identification panel. If no targets on the panel are detected, all previously positive bottles and any subsequent bottles with similar Gram stain morphology are subcultured to chocolate agar. Isolate identification is possible by a variety of methods, including manual and automated systems. Abiotrophia defectiva and Granulicatella adiacens are included in the current FDA-cleared databases of both MALDI-TOF MS manufacturers.
TABLE 1.
Growth, biochemical, and susceptibility patterns of frequently isolated Streptococcus-like bacteriaa
| Bacteria | Growth on: |
Reaction to biochemical: |
Susceptibilityb to: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| BAP | CHOC | Staph satellite streak | NaCl | PYR | LAP | PCN | CEF | VANC | |
| A. defectiva | − | + | + | − | + | + | R | S | S |
| G. adiacens | − | + | + | − | + | + | V | V | S |
| G. elegans | − | + | + | − | + | + | S | S | S |
| Aerococcus | + | + | − | + | +/−c | +/−c | S | S | S |
| Gemella | + | + | − | − | + | +/−c | S | S | S |
| Globicatella | + | + | − | + | + | − | Vd | Vd | Sc |
| Leuconostoc | + | + | − | +/− | − | − | S | Vd | R |
| Pediococcus | + | + | − | +/− | − | + | S | Sd | R |
| Viridans streptococci | + | + | − | − | +/−c | + | V | V | S |
Based on data from references 1 and 2. Abbreviations: BAP, blood agar; CHOC, chocolate agar; NaCl, 6.5% NaCl broth; PYR, pyrrolidonyl arylamidase activity; LAP, leucine aminopeptidase activity; PCN, penicillin; CEF, ceftriaxone; VANC, vancomycin; S, susceptible; V, variable; R, resistant; +, positive; −, no growth or reaction.
bGeneralized susceptibility patterns. Refer to organism-specific resources or antibiograms for more specific information.
cSpecies-dependent variability.
dNo CLSI interpretative criteria.
Antimicrobial susceptibility testing of these organisms presents several challenges. Because of the unique growth requirements of Abiotrophia and Granulicatella species, broth microdilution is the preferred method of antimicrobial susceptibility testing. To facilitate their growth, the CLSI M45 guidance document (10) recommends using 2.5 to 5% lysed horse blood (LHB) and 10 μg/ml pyridoxal HCl for susceptibility testing of Abiotrophia and Granulicatella species. These unique reagent requirements, combined with the relative infrequency of isolation, often limit susceptibility testing for Abiotrophia and Granulicatella species to reference labs. However, it is critical that susceptibility testing be performed when isolated from normally sterile sites to allow for effective tailoring of antimicrobial therapy. The antimicrobial susceptibility patterns of Abiotrophia and Granulicatella species vary widely despite their historical pairing. Additionally, their patterns diverge from Streptococcus species and have elicited three recent comprehensive studies reporting susceptibility profiles for these organisms (11–13). Across these reports, susceptibility to penicillin was lowest for Abiotrophia defectiva (10 to 24%) but still infrequent for Granulicatella adiacens (34 to 40%). Susceptibility to ceftriaxone varied greatly between Abiotrophia defectiva (92 to 100%) and Granulicatella adiacens (22 to 48%). These reports also differed in their comparison of meropenem susceptibility but theorized that this could have been due to regional differences or the sample size of the populations tested (13). Isolates were 100% susceptible to vancomycin across all of the studies, and MICs <4 μg/ml to gentamicin were in line with American Heart Association guidelines which support the empirical addition of gentamicin in the treatment of Abiotrophia and Granulicatella species (9, 11–13). In conclusion, routine susceptibility testing should be performed for these isolates to guide the appropriate therapy.
SELF-ASSESSMENT QUESTIONS
-
Abiotrophia defectiva grows best on which media?
-
a.
TSA media with 5% sheep blood agar
-
b.
MacConkey agar
-
c.
Enriched chocolate agar
-
d.
Columbia CNA media with 5% sheep blood agar
-
a.
-
Abiotrophia and Granulicatella species are most reliably susceptible to which antimicrobial?
-
a.
Vancomycin
-
b.
Meropenem
-
c.
Gentamicin
-
d.
Ceftriaxone
-
a.
-
What is the prevalence of infective endocarditis caused by Abiotrophia and Granulicatella species?
-
a.
<5%
-
b.
10%
-
c.
20%
-
d.
>25%
-
a.
Footnotes
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.03094-20. in this issue.
Contributor Information
Kyle G. Rodino, Email: Kyle.Rodino@pennmedicine.upenn.edu.
Carey-Ann D. Burnham, Washington University School of Medicine
REFERENCES
- 1.Christensen JJ, Nielsen XC, Ruoff KL. 2019. Aerococcus, Abiotrophia, and other aerobic catalase‐negative, Gram‐positive cocci. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW. Manual of clinical microbiology, 12th ed, vol 1. ASM Press, Washington, DC. [Google Scholar]
- 2.Procop GW, Church DL, Hall GS, Janda WM, Koneman EW, Schreckenberger PC, Woods GL. 2020. Koneman's color atlas and textbook of diagnostic microbiology, 7th ed.Jones & Bartlett Learning, Burlington, MA. [Google Scholar]
- 3.Téllez A, Ambrosioni J, Llopis J, Pericàs JM, Falces C, Almela M, Garcia de la Mària C, Hernandez-Meneses M, Vidal B, Sandoval E, Quintana E, Fuster D, Tolosana JM, Marco F, Moreno A, Miro JM, Miró JM, Ambrosioni J, Pericàs JM, Téllez A, Hernandez-Meneses M, Moreno A, Garcia de la Mària C, Garcia-Gonzalez J, Marco F, Almela M, Vila J, Quintana E, Sandoval E, Paré JC, Falces C, Pereda D, Cartañá R, Ninot S, Azqueta M, Sitges M, Vidal B, Pomar JL, Castella M, Tolosana JM, Ortiz J, Fita G, Rovira I, Fuster D, Ramírez J, Brunet M, Soy D, Castro P, Llopis J, Hospital Clinic Infective Endocarditis Investigators. 2018. Epidemiology, clinical features, and outcome of infective endocarditis due to Abiotrophia species and Granulicatella species: report of 76 cases, 2000-2015. Clin Infect Dis 66:104–111. 10.1093/cid/cix752. [DOI] [PubMed] [Google Scholar]
- 4.Miraclin AT, Perumalla SK, Daniel J, Sathyendra S. 2017. Abiotrophia defectiva endarteritis with infective spondylodiscitis in an adult patient with patent ductus arteriosus. BMJ Case Rep 2017:bcr2017219295. 10.1136/bcr-2017-219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uehara K, Chikuda H, Higurashi Y, Ohkusu K, Takeshita K, Seichi A, Tanaka S. 2013. Pyogenic discitis due to Abiotrophia adiacens. Int J Surg Case Rep 4:1107–1109. 10.1016/j.ijscr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath CH, Bowen SF, McCarthy JS, Dwyer B. 1998. Vertebral osteomyelitis and discitis associated with Abiotrophia adiacens (nutritionally variant streptococcus) infection. Aust N Z J Med 28:663. 10.1111/j.1445-5994.1998.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 7.Berge A, Kronberg K, Sunnerhagen T, Nilson BHK, Giske CG, Rasmussen M. 2019. Risk for endocarditis in bacteremia with Streptococcus-like bacteria: a retrospective population-based cohort study. Open Forum Infect Dis 6:ofz437. 10.1093/ofid/ofz437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesman RM, Pritt BS, Maleszewski JJ, Patel R. 2017. Laboratory diagnosis of infective endocarditis. J Clin Microbiol 55:2599–2608. 10.1128/JCM.00635-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, FowlerVG, Jr., Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
- 11.Alberti MO, Hindler JA, Humphries RM. 2015. Antimicrobial susceptibilities of Abiotrophia defectiva, Granulicatella adiacens, and Granulicatella elegans. Antimicrob Agents Chemother 60:1411–1420. 10.1128/AAC.02645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mushtaq A, Greenwood-Quaintance KE, Cole NC, Kohner PC, Ihde SM, Strand GJ, Harper LW, Virk A, Patel R. 2016. Differential antimicrobial susceptibilities of Granulicatella adiacens and Abiotrophia defectiva. Antimicrob Agents Chemother 60:5036–5039. 10.1128/AAC.00485-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasidthrathsint K, Fisher MA. 2017. Antimicrobial susceptibility patterns among a large, nationwide cohort of Abiotrophia and Granulicatella clinical isolates. J Clin Microbiol 55:1025–1031. 10.1128/JCM.02054-16. [DOI] [PMC free article] [PubMed] [Google Scholar]